Abstract
The development of coagulation abnormalities is common in patients with sepsis. Sepsis-associated coagulopathy (SAC) is typically diagnosed by prothrombin time (PT) prolongation or elevated international normalized ratio (INR) in conjunction with reduced platelet count. INR is also used to monitor warfarin-treated patients. However, due to the different natures of SAC and warfarin anticoagulation, it is likely that the same INR value provides different information in these two patient populations. The purpose of this study was to compare measures of coagulation function and clotting factor levels in patients with SAC to those observed in patients receiving warfarin anticoagulation. Deidentified plasma samples were collected at baseline from patients diagnosed with SAC and from patients receiving warfarin. These plasma samples were evaluated for PT/INR, activated partial thromboplastin time (aPTT), fibrinogen, and functional and immunologic levels of factors VII, IX, and X. Both aPTT and fibrinogen correlated with INR in patients with SAC, but not in patients treated with warfarin. Factors VII, IX, and X showed an inverse relationship with INR in the anticoagulated patients; however, no relationship between factor level and INR was observed in patients with SAC. Distinct patterns of coagulopathy were observed in patients with SAC and patients receiving warfarin anticoagulation, and equivalent INR values were associated with distinct coagulation profiles in the two patient groups. These results suggest that an abnormal INR provides different information about the coagulation status in patients with disseminated intravascular coagulation than in patients receiving warfarin. This may indicate that an equivalently increased INR predicts different bleeding risks in these two patient groups.
Keywords: blood coagulation factors, disseminated intravascular coagulation, warfarin, INR
Introduction
Sepsis is a severe systemic response to infection characterized by an overwhelming inflammatory response. This syndrome is a significant cause of morbidity and mortality both within the United States and worldwide, with “septicemia” listed by the Centers for Disease Control as the 11th most common cause of death in the United States in 2010.1 Sepsis occurs across a wide spectrum of severity, and estimates of mortality are variable, ranging from 14.7% to 29.9%,2,3 to up to 80% for more severe forms of sepsis.4
The patient’s own immune system plays a key role in the pathology of sepsis, both in the initial stage of hyperimmune response and in the subsequent phase of immune paralysis. While robust activation of the immune and inflammatory systems is necessary for the eradication of bacteria, this excessive response may also prove detrimental to the host.3–6 In addition to contributing to the development of blood pressure collapse, shock, and organ failure, the excessive inflammation observed in sepsis contributes to the development of coagulopathy.
Extensive cross talk occurs between inflammation and coagulation.7 In sepsis, bacterial components, particularly lipopolysaccharide, elicit a vigorous inflammatory response. This includes production of high levels of inflammatory cytokines, such as interleukin (IL)-6, IL-1β, and tumor necrosis factor α, which induce tissue factor (TF) expression on intravascular cells such as monocytes, macrophages, and the endothelium.8–14 The introduction of TF into the circulation activates the extrinsic pathway of the coagulation cascade and is generally considered to be the major initiator of coagulation in this patient population.15,16 Consequently, a significant number of patients hospitalized with sepsis develop coagulation abnormalities.17
The coagulation abnormalities observed in patients with sepsis range from perturbations in laboratory values to severe overt disseminated intravascular coagulation (DIC), an acquired coagulation disorder with high mortality that is characterized by both thrombotic and bleeding complications.3,16,18,19 In DIC, widespread activation of coagulation leads to fibrin deposition in the microvasculature and subsequent organ failure. This process consumes platelets and coagulation factors, leading to a risk of significant and potentially fatal bleeding. Development of DIC significantly increases the risk of death in patients with sepsis,16–18,20–22 with mortality due to DIC often estimated at 40% or above.16
Disseminated intravascular coagulation is graded through the application of a scoring system published by the International Society of Thrombosis and Hemostasis (ISTH).23 This algorithm assigns patients with a predisposing condition such as sepsis with a DIC score based on reduced platelet count, prolonged international normalized ratio (INR), elevated fibrin degradation products (ie, D-dimer), and decreased fibrinogen. In clinical practice, however, simplified schemes are often used to identify patients with sepsis-associated coagulopathy (SAC), a less rigidly defined term that indicates the presence of coagulation dysfunction of variable severity in patients with sepsis. Typically, identification of SAC is made on the basis of an elevated prothrombin time (PT) or INR in conjunction with thrombocytopenia.
As INR is one of the key parameters used clinically to identify patients with SAC, it is important to understand the appropriate interpretation of this measure in this specific patient population. Prolonged PT or elevated INR is generally indicative of a hypocoagulable state; however, patients presenting with SAC with an elevated INR are at risk of complications due to both thrombosis and bleeding.
Elevated PT or INR is reported in 90% or more of patients with sepsis with severe disease.24–26 Prolonged PT and elevated INR are associated with increased mortality and poor clinical outcome in patients with sepsis25,27 as well as in other critically ill or injured patient populations.28,29 Elevated PT–INR (typically INR ≥1.2) is often a component of the inclusion criteria for clinical trials in patients with SAC.30,31 The majority of the elevated INRs within this patient population have been reported to fall into the range of 1.6 to 2.5.29 Other changes in global coagulation parameters, including activated partial thromboplastin time (aPTT)17,24–26,32,33 and whole-blood coagulability as measured by thromboelastography,26,32,33 are also often reported in patients with sepsis as well as in other critically ill patient populations.
Despite the clear evidence that significant changes to the overall coagulation profile occur in sepsis, changes in the levels of individual coagulation factors in patients with SAC are less well established. Reduced levels of coagulation factors including factors (F) II, FV, FVII, FX, and FXII compared to normal individuals have been reported in patients with SAC.24 However, these results demonstrated no discernible relationship to standard coagulation tests and are highly variable between studies.24,32,33
The PT/INR was designed to monitor the anticoagulation status in patients treated with warfarin and is widely used clinically for this purpose. Patients treated with warfarin are typically considered appropriately anticoagulated with an INR of between 2 and 3, and regular adjustments to drug dosage are made to maintain the INR within this range. A study of the relationship of INR to the risk of severe bleeding in patients on warfarin revealed that the INRs associated with severe bleeding in this population are more elevated than those observed in patients with sepsis, with a mean INR of 5.9 at the time of the bleed and 3.0 at the clinic visit prior to the bleed.34 A study of the relationship of serial INR levels to severe bleeding in patients receiving warfarin anticoagulation found that warfarin-treated patients hospitalized with severe bleeding showed an elevated INR compared to nonbleeding patients (5.9 ± 5.9 vs 2.3 ± 0.7) as well as higher INRs before the event of the bleed (3.0 ± 1.2 vs 2.1 ± 0.8).34
The difference in INR levels at which bleeding occurs in patients treated with warfarin and patients with SAC and the fact that patients with SAC with an elevated INR indicative of hypocoagulability experience both thrombotic and bleeding complications suggest that the information provided by this common laboratory test may be significantly different in these two patient populations. The purpose of this study was to compare the relationship of laboratory coagulation tests and levels of individual coagulation factors with INR in patients with SAC to the relationships observed in warfarin-treated patients.
Materials and Methods
Control Plasma Samples
Frozen, citrated plasma samples from healthy individuals, ages 18 to 55, nonsmokers, with no known medical conditions, were purchased from George King Biomedical (Overland Park, Kansas) and stored at −80°C prior to analysis.
Deidentified Patients Treated With Warfarin and DIC Samples
Citrated, deidentified plasma samples were collected from the clinical laboratory under an institutional review board–approved protocol. Samples were collected from discarded specimens and no modification was made to patient care during this sample collection. Limited information was available to accompany each specimen including diagnosis and treatment. Samples were collected from patients receiving warfarin anticoagulation (n = 112) and patients with SAC, defined as overt or nonovert DIC by the ISTH scoring criteria (n = 78).
Clotting Assays: PT and aPTT and Fibrinogen Assays
Prothrombin time, aPTT, and fibrinogen were measured using standard operating procedures on an ACL-300 or ACL-ELITE automated coagulation analyzer (Instrumentation Laboratory, Bedford, Massachusetts). This instrument uses an optical method to detect clot formation in a plasma sample. For aPTT, Platelin (Diagnostica Stago, Parsippany, New Jersey) was used along with 0.025 M CaCl2 to recalcify the citrated plasma. For PT/INR and fibrinogen, Recombiplastin (Instrumentation Laboratory) was used.
Coagulation Factor Antigenic Levels
Antigenic levels of FVII, FIX, and FX were measured using commercially available enzyme-linked immunosorbent assay (ELISA) kits (Hyphen BioMed, Neuville-Sur-Oise, France), performed according to the manufacturer’s instructions.
Coagulation Factor Activity Assays
Activity levels of individual coagulation factors were evaluated using modified 1-step PT or aPTT methods. Clot formation in these tests was evaluated mechanically using an ST4 coagulation analyzer (Diagnostica Stago).
For FVII, a 1-step PT assay was used. Patient samples were diluted 1:10 in Owren Veronal buffer (Dade Bennng Siemens: Erlangen, Germany). 50 μl of factor-deficient plasma (Aniara, Westchester, Ohio) and 50 μL of diluted patient sample were warmed to 37°C in a cuvette with a metal mixing ball for 180 seconds. 100 μl of Dade Innovin PT reagent (Siemens Healthcare Diagnostics, Newark, Delaware) was added and the time to clot was recorded. Factor VII level was calculated in each sample relative to normal human plasma based on a standard curve.
For FIX and FX, a 2-step aPTT assay was used. Patient samples were diluted 1:20 in Owren Veronal buffer. 50 μl of diluted sample, 50 μL of aPTT reagent, and 50 μL of factor-deficient plasma (Aniara) were warmed to 37°C in a cuvette with a metal mixing ball for 300 seconds. 50 μl of CaCl2 was added and the time to clot was recorded. Factor IX and FX levels were calculated in each sample relative to normal human plasma.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism software (La Jolla, California). Patients were categorized into groups based on INR levels, with groups of INR of less than 1.5, 1.5 to 1.9, 2 to 2.9, and 3 or greater. For both patient populations, variability of every parameter based on INR was assessed using the Kruskal-Wallis 1-way analysis of variance (ANOVA) and Dunn multiple comparison test, with α = .05 as the cutoff for significance. Plots of test result or factor level versus INR were also created for both patient groups, and trend line fit was assessed. Spearman correlation coefficients were determined for relationships between coagulation factor levels and coagulation test results for both patient groups.
Results
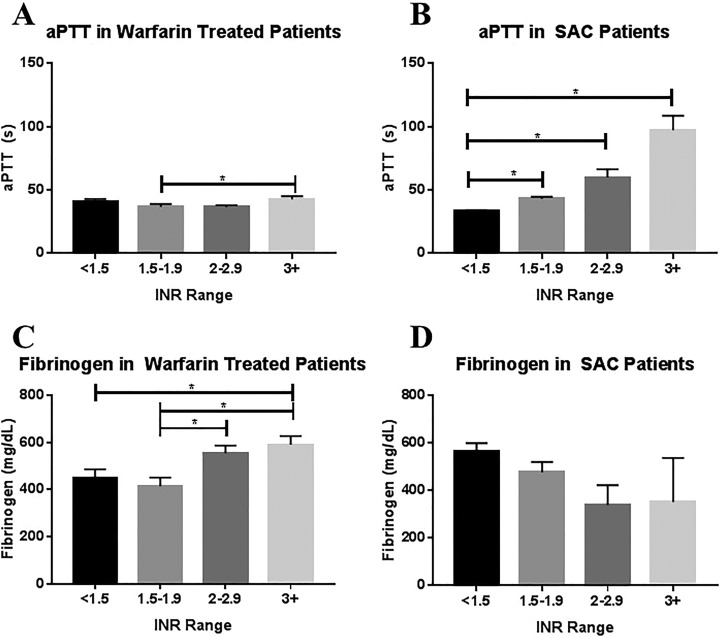
The aPTT was measured in both warfarin-treated patients and patients with SAC, as shown in Table 1 and Figure 1A and B. Overall, significant variation in aPTT based on INR was observed in both patients treated with warfarin (P = .019) and patients with SAC (P < .0001). In patients treated with warfarin, a significant difference was observed between patients with a subtherapeutic INR of 1.5 to 1.9 and patients with a supertherapeutic INR of greater than 3 (P = .034; Figure 1A). In patients with SAC, aPTT showed a stepwise increase with increasing INR, although statistical significance was only reached between patients with an INR of less than 1.5 and patients with an INR of 1.5 to 1.9 (P = .0001), 2 to 2.9 (P < .0001), and 3 or greater (P = .001; Figure 1B). Although the aPTT values for patients with an INR of less than 2 were similar between the two patient populations, the maximum observed aPTTs were markedly higher in the patient with SAC population than in patients treated with warfarin. Scatterplots of aPTT versus INR were also generated for both patients treated with warfarin and patients with SAC, and linear fit was assessed. For patients treated with warfarin, the fit was poor fit (R 2 = 0.005) and the slope was not significantly nonzero (P = .444). For patients with SAC, the R 2 was 0.6789 and the slope was significantly nonzero (P < .0001).
Table 1.
Relationship of aPTT and Fibrinogen to INR in Warfarin-Treated Patients and Patients With SAC.
| INR Range | Warfarin-Treated Patients | Patients With SAC | Warfarin-Treated Patients | Patients With SAC | ||||
|---|---|---|---|---|---|---|---|---|
| aPTT (seconds), Mean ± SEM | aPTT (seconds), Median | aPTT (seconds), Mean ± SEM | aPTT (seconds), Median | FIB (mg/dL), Mean ± SEM | FIB (mg/dL), Median | FIB (mg/dL), Mean ± SEM | FIB (mg/dL), Median | |
| <1.5 | 40.4 ± 2.5 | 38.2 | 33.5 ± 0.7 | 32.9 | 447 ± 39 | 430 | 563 ± 36 | 515 |
| 1.5-1.9 | 36.8 ± 2.2 | 33.5 | 43.2 ± 1.6 | 43.4 | 415 ± 36 | 361 | 478 ± 43 | 472 |
| 2-2.9 | 36.6 ± 1.5 | 33.7 | 60.0 ± 6.5 | 55.1 | 555 ± 38 | 582 | 339 ± 84 | 223 |
| ≥3 | 43.7 ± 2.4 | 40.8 | 97.1 ± 11.6 | 92.9 | 590 ± 38 | 588 | 352 ± 186 | 195 |
Abbreviations: aPTT, activated partial thromboplastin time; FIB, fibrinogen; INR, international normalized ratio; SAC, sepsis-associated coagulopathy; SEM, standard error of the mean.
Figure 1.
Relationship of aPTT and fibrinogen to INR in warfarin-treated patients and patients with SAC. Top, Activated partial thromboplastin time in (A) warfarin-treated patients and (B) patients with SAC stratified by INR group. Bottom, Fibrinogen in (C) warfarin-treated patients and (D) patients with SAC. Comparison was made using the Kruskal-Wallis 1-way ANOVA and Dunn multiple comparison test with α = .05 as the cutoff for significance (indicated by asterisk). ANOVA indicates analysis of variance; aPTT, activated partial thromboplastin time; INR, international normalized ratio; SAC, sepsis-associated coagulopathy.
Fibrinogen was measured in both patients treated with warfarin and patients with SAC, as shown in Table 1 and Figure 1B and C. Overall, significant variation was observed in patients treated with warfarin (P = .0005; Figure 1C), but not in patients with SAC (P = .075; Figure 1D). For patients treated with warfarin, the difference was significant between patients with an INR of less than 5 and an INR of 3 or greater (P = .0400), an INR of 1.5 to 1.9 versus an INR of 2 to 2.9 (P = .0080), and an INR of 1.5 to 1.9 versus an INR of greater than 3 (P = .0077). Scatterplots of fibrinogen versus INR were also generated for both patients treated with warfarin and patients with SAC, and linear fit was evaluated. The fit was poor for both patients treated with warfarin (R 2 = 0.05376) and patients with SAC (R 2 = 0.08381), although the slope was significantly nonzero for both the patient groups (P = .0075 for patients treated with warfarin and P = .0107 for patients with SAC).
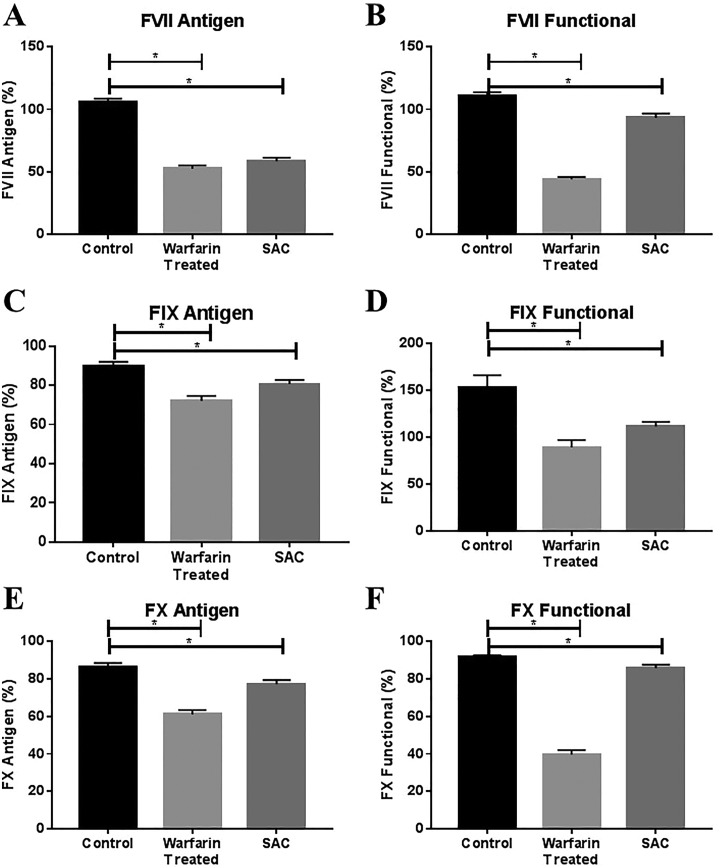
In addition, the levels of coagulation factors FVII, FIX, and FX were measured in patients treated with warfarin and patients with SAC as well as in a population of 50 healthy controls, as shown in Figure 2. Immunologic levels of all factors were determined using commercially available ELISA methods, while functional levels were determined using clot-based methods. Coagulation levels in the both patients treated with warfarin and patient with SAC populations were compared to levels in the control population. Both functional and antigenic levels of all three factors were found to be significantly reduced in both patients treated with warfarin and patients with SAC compared to healthy controls.
Figure 2.
Antigenically and functionally determined levels of coagulation factors in warfarin-treated patients and patients with SAC compared to healthy controls. For each factor, comparison was made between healthy controls and warfarin-treated patients and healthy controls and patients with SAC using the Mann-Whitney t test with P < .05 as the cutoff for significance (indicated by asterisk). INR indicates international normalized ratio; SAC, sepsis-associated coagulopathy. Panels A and B show antigenic and functional levels of FVII, respectively. Panels C and D show antigenic and functinal levels of FIX respectively. Panels E and F show antigenic and functinal levels of FX, respectively.
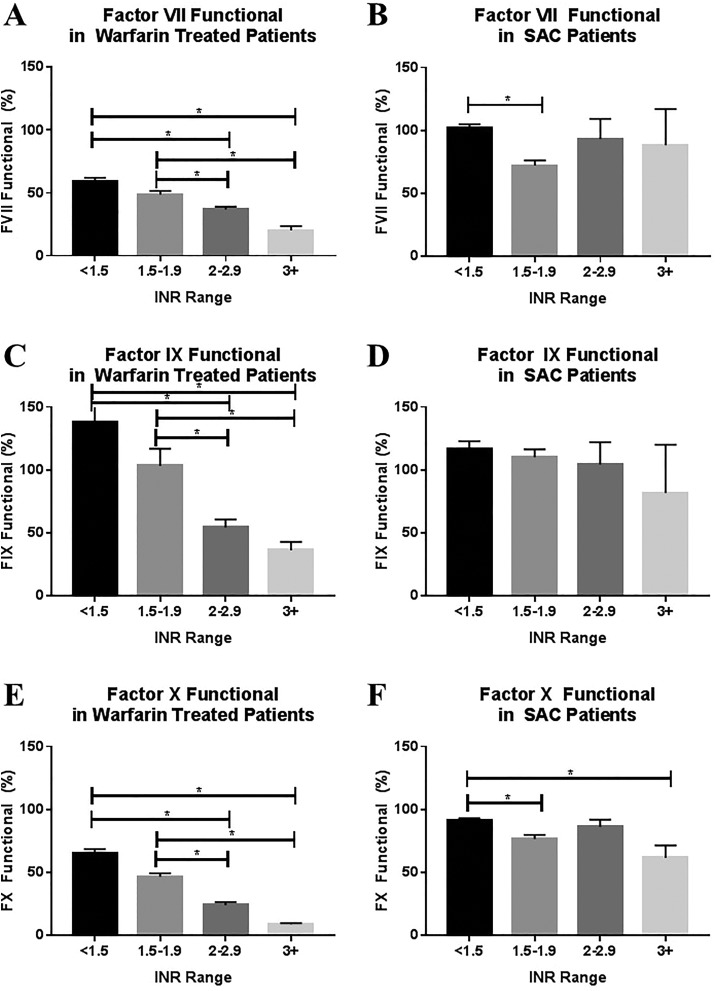
The relationship of coagulation factor level to INR was assessed for each factor in both patients with SAC and warfarin-treated patients, as shown in Figure 3 and Table 2. Differences in factor levels based on INR group were assessed using the Kruskal-Wallis ANOVA for nonparametric data, with α = .05 as the cutoff for significance. Differences between individual groups were analyzed using Dunn's multiple comparison test.
Figure 3.
Relationship of functional coagulation factor levels to INR in warfarin-treated patients and patients with SAC. Functional levels of factors VII (A and B), IX (C and D), and X (E and F) in warfarin-treated patients and patients with SAC stratified by INR group. Comparison was made using the Kruskal-Wallis 1-way ANOVA and Dunn multiple comparison test with α = .05 as the cutoff for significance. ANOVA indicates analysis of variance; INR, international normalized ratio; SAC, sepsis-associated coagulopathy.
Table 2.
Relationship Functional Levels of Factors VII, IX, and X to INR in Warfarin-Treated Patients and Patients With SAC.
| INR | Warfarin-Treated Patients | Patients With SAC | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Median | SEM | n | Mean | Median | SEM | n | ||
| Factor VII, functional (%) | <1.5 | 59.0 | 59.3 | 3.1 | 26 | 101.7 | 104.3 | 3.4 | 46 |
| 1.5-1.9 | 48.7 | 46.9 | 3.0 | 29 | 72.2 | 72.8 | 4.1 | 19 | |
| 2-2.9 | 37.0 | 34.8 | 2.1 | 33 | 93.2 | 85.9 | 16.1 | 10 | |
| ≥3 | 20.2 | 19.6 | 3.5 | 12 | 88.4 | 112.5 | 28.6 | 3 | |
| Factor IX, functional (%) | <1.5 | 85.4 | 88.6 | 4.2 | 29 | 82.3 | 84.8 | 3.1 | 46 |
| 1.5-1.9 | 72.57 | 73.77 | 4.9 | 30 | 78.3 | 77.0 | 3.4 | 19 | |
| 2-2.9 | 66.4 | 70.8 | 4.7 | 34 | 80.0 | 80.2 | 7.4 | 10 | |
| ≥3 | 53.5 | 55.6 | 4.1 | 12 | 74.8 | 81.9 | 13.8 | 3 | |
| Factor X, functional (%) | <1.5 | 65.4 | 71.6 | 3.3 | 30 | 91.3 | 92.1 | 1.8 | 46 |
| 1.5-1.9 | 46.7 | 47.1 | 2.9 | 32 | 76.7 | 80.0 | 3.3 | 19 | |
| 2-2.9 | 24.2 | 20.4 | 2.5 | 36 | 86.3 | 84.3 | 5.8 | 10 | |
| ≥3 | 9.0 | 10.4 | 0.9 | 14 | 61.7 | 53.1 | 9.9 | 3 | |
Abbreviations: INR, international normalized ratio; SAC, sepsis-associated coagulopathy; SEM, standard error of the mean.
Statistical significance was not achieved for comparison of antigenic levels of FVII between INR groups for either patient groups (data not shown). Significant variation in functional FVII based on INR was seen in both patients treated with warfarin (P < .0001) and patients with SAC (P = .0004; Figure 3A and B and Table 2). For patients treated with warfarin, significant differences were observed for patients with an INR of <1.5 versus 2 to 2.9 (P < .0001), <1.5 versus >3 (P < .0001), 1.5 to 1.9 versus 2 to 2.9 (P = .0331), and 1.5 to 1.9 versus >3 (P < .0001). For patients with SAC, the difference was only significant for patients with INRs of <1.5 versus 1.5 to 1.9. The linear fit of FVII versus INR was also evaluated. Fit was reasonably strong for warfarin-treated patients (R 2 = 0.424) with a significantly nonzero slope (P < .0001), but poor for patients with SAC (R 2 = 0.018) with a not significantly nonzero slope (P = .837).
For FIX (Figure 3C and D and Table 2), comparable results were observed for both functional and antigenic factor levels. Significant variation in functional FIX based on INR was seen in warfarin-treated patients (P < .0001), but not in patients with SAC (P = .6097). For warfarin-treated patients, significant differences were observed for patients with an INR of <1.5 versus 2 to 2.9 (P = .0008), <1.5 versus >3 (P = .0004), 1.5 to 1.9 versus 2 to 2.9 (P = .0449), and 1.5 to 1.9 versus >3 (P < .0092). The linear fit was reasonably poor for both warfarin-treated patients (R 2 = 0.165) and patients with SAC (R 2 = 0.095), with a significantly nonzero slope for both populations (P < .0001 for warfarin-treated patients and P = .006 for patients with SAC).
As with FIX, comparable results were observed for the functional and antigenic levels of FX (Figure 3E and F and Table 2). Significant variation in functional FX based on INR was seen in both warfarin-treated patients (P < .0001) and patients with SAC (P = .0003). For warfarin-treated patients, significant differences were observed for patients with an INR of <1.5 versus 2 to 2.9 (P < .0001), <1.5 versus >3 (P < .0001), 1.5 to 1.9 versus 2 to 2.9 (P = .0005), and 1.5 to 1.9 versus >3 (P < .0001). For patients with SAC, significant differences were observed for patients with an INR <15 versus 1.5 to 1.9 (P = .002) and <1.5 versus >3 (P = .036). The linear fit was reasonably good for warfarin-treated patients (R 2 = 0.5145), but poor for patients with SAC (R 2 = 0.141), with a significantly nonzero slope for both populations (P < .0001 for warfarin-treated patients and P = .0007 for patients with SAC).
The correlations between levels of all factors were analyzed for both the warfarin-treated and SAC patient groups. Spearman correlation coefficients were analyzed with α = .05 as the cutoff for significance. The observed patterns of correlations were markedly different for both warfarin-treated patients and patients with SAC. For warfarin-treated patients, significant and often strong correlations were observed between INR and both functional and antigenic levels of the coagulation factors. Correlations were stronger with functional factor levels than with antigenic factor levels. In this patient population, the levels of FVII, FIX, and FIX were highly correlated with each other as well. Strong correlations were also seen between antigenic and functional levels of all three coagulation factors. In contrast, fewer correlations were observed overall in patients with SAC, and the observed correlations were overall weaker. A strong correlation was observed between INR and aPTT in the patients with SAC, whereas no correlation was observed in the warfarin-treated patients. In the patients with SAC, both INR and aPTT correlated significantly with the functional, but not antigenic, levels of FVII, FIX, and FX, with the strongest correlations observed with functional FX for both tests. Fewer correlations between the levels of coagulation factors were observed in patients with DIC compared to warfarin-treated patients. The only strong correlation observed between coagulation factors in patients with SAC was between the functional and antigenic levels of FX. Functional and antigenic levels of FVII and FIX showed no correlations with each other.
Discussion
Markedly different relationships between INR and other laboratory coagulation tests and coagulation factor levels were observed in patients with SAC compared to patients receiving warfarin anticoagulation. In patients with SAC, increased INR was associated with increased aPTT. This supports the hypothesis that the coagulation dysfunction indicated by a given INR level is different in warfarin-treated patients, where changes in INR are the result of a targeted disruption to the coagulation cascade, than in patients with SAC, where an elevated INR is indicative of a more diffuse insult to the coagulation system.
Functional and antigenic levels of coagulation FVII, FIX, and FX were decreased in both warfarin-treated patients and patients with SAC compared to healthy controls. However, the pattern of decrease in factor levels was markedly different between the two patient groups. In warfarin-treated patients, the decrease in factor levels corresponded strongly with an increase in INR. In contrast, the factor levels in patients with SAC were uniformly low across all INR levels; additional increases in INR did not correspond with an additional drop in coagulation factor levels.
When correlations between all coagulation tests and factor levels were assessed, strikingly different patterns were observed in both patients with SAC and warfarin-treated patients. In warfarin-treated patients, both functional and antigenic levels of FVII, FIX, and FX showed strong correlations with each other. The highly correlated levels of coagulation factors in these patients are a reflection of the unified mechanism by which warfarin inhibits coagulation. These factors also correlate strongly with INR, the test designed to monitor the effects of warfarin on the coagulation cascade. In contrast, minimal significant correlations were observed between coagulation factors in patients with SAC. While coagulation factor levels were overall decreased compared to healthy controls in this patient population, the patient-to-patient variation in the nature of this decrease was high, indicated by the lack of correlation between factor levels. Furthermore, the levels of individual coagulation factors were not predictable based on INR in the SAC patient cohort.
This study supports the hypothesis that the meaning of an elevated INR is substantially different in warfarin-treated patients than in patients with SAC. In warfarin-treated patients, an elevated INR suggests a uniform and predictable reduction in the detectable and functional levels of multiple coagulation factors without an accompanying alteration in the other global coagulation parameter of aPTT. In this patient population, elevated INR is solely a measure of a specific type of hypocoagulability induced by warfarin in order to prevent thrombotic complications. In contrast, an elevated INR in patients with SAC provides different information accompanied by global coagulation dysfunction, suggested by the strong correlation between elevated INR and elevated aPTT in this patient population. However, this alteration in coagulation is not accompanied by predictable or consistent changes in levels of individual coagulation factors in this patient population. Additionally, elevated INR is accompanied not only by bleeding risk but also by thrombosis in this patient population, a risk that is not demonstrable by analysis of traditional hemostatic parameters. This study underscores the need for an improved understanding of the relationship of hemostatic laboratory parameters to the ongoing coagulation processes and the associated risks of both bleeding and thrombosis specific to the SAC patient population.
Acknowledgments
The authors would like to acknowledge the skillful assistance of the staff of the Hemostasis Research Laboratories of the Department of Pathology and the Loyola University Medical Center. The authors are thankful to Dr Eva Wojick, Chair of the Department of Pathology, for her support in facilitating this study.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was suported in part by the Achievement Rewards for College Scientists (ARCS) Foundation, Inc.'s scholar Illinois chapter 2017-2018 award.
References
- 1. Murphy SL, Xu J, Kochanek KD. Deaths: final data for 2010. Nat Vital Stat Rep. 2013;61(4):1–117. [PubMed] [Google Scholar]
- 2. Gaieski DF, Edwards JM, Kallan MJ, et al. Benchmarking the incidence and mortality of severe sepsis in the United States. Crit Care Med. 2013;41(5):1167–1174. [DOI] [PubMed] [Google Scholar]
- 3. Hawiger J, Veach R, Zienkiewicz J. New paradigms in sepsis: from prevention to protection of failing microcirculation. J Thromb Haemost. 2015;13(10):1743–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Martin GS. Sepsis, severe sepsis, and septic shock: changes in incidence, pathogens and outcomes. Expert Rev Anti Infect Ther. 2012;10(6):701–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tanaka H, Sugimoto H, Yoshioka T, et al. Role of granulocyte elastase in tissue injury in patients with septic shock complicated by multiple-organ failure. Ann Surg. 1991;213(1):81–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Annane D, Bellissant E, Cavaillon JM. Septic shock. Lancet. 2005;365(9453):63–78. [DOI] [PubMed] [Google Scholar]
- 7. Guo Y, Lip GY, Apstolakis S. Inflammation in atrial fibrillation. J Am Coll Cardiol. 2012;60(22):2263–2270. [DOI] [PubMed] [Google Scholar]
- 8. Esmon CT. The impact of the inflammatory response on coagulation. Thromb Res. 2004;114(5-6):321–327. [DOI] [PubMed] [Google Scholar]
- 9. Nawroth PP, Handley DA, Esmon CT, et al. Interleukin 1 induces endothelial cell procoagulant while suppressing cell-surface anticoagulant activity. Proc Natl Acad Sci U S A. 1986;83(10):3460–3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Osnes L, Westvik A, Joo GB, et al. Inhibition of IL-1 induced tissue factor (TF) synthesis and procoagulant activity (PCA) in purified human monocytes by IL-4, IL-10 and IL-13. Cytokine. 1996;8(11):822–827. [DOI] [PubMed] [Google Scholar]
- 11. Abraham E. Coagulation abnormalities in acute lung injury and sepsis. Am J Respir Cell Mol Biol. 2000;22(4):401–404. [DOI] [PubMed] [Google Scholar]
- 12. Levi M, van der Poll T, Buller HR. Bidirectional relation between inflammation and coagulation. Circulation. 2004;109(22):2698–2704. [DOI] [PubMed] [Google Scholar]
- 13. Bevilacqua MP, Pober JS, Majeau GR, et al. Recombinant tumor necrosis factor induces procoagulant activity in cultured human vascular endothelium: characterization and comparison with the actions of interleukin 1. Proc Natl Acad Sci U S A. 1986;83(12):4533–4537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hezi-Yamit A, Wong PW, Bien-Ly N, et al. Synergistic induction of tissue factor by coagulation factor Xa and TNF: evidence for involvement of negative regulatory signaling cascades. Proc Natl Acad Sci U S A. 2005;102(34):12077–12082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Esmon CT, Xu J, Lupu F. Innate immunity and coagulation. J Thromb Haemost. 2011;9(suppl 1):182–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gando S, Levi M, Toh CH. Disseminated intravascular coagulation. Nat Rev Dis Primers. 2016;2:1–16. [DOI] [PubMed] [Google Scholar]
- 17. Bakhtiari K, Meijers JCM, de Jonge E, et al. Prospective validation of the International Society of Thrombosis and Haemostasis scoring system for disseminated intravascular coagulation. Crit Care Med. 2004;32(12):2416–2421. [DOI] [PubMed] [Google Scholar]
- 18. Levi M, Ten Cate H. Disseminated intravascular coagulation. N Engl J Med. 1999;341(8):586–592. [DOI] [PubMed] [Google Scholar]
- 19. Koyama K, Madiowa S, Nunomiya S, et al. Combination of thrombin–antithrombin complex, plasminogen activator inhibitor-1, and protein C activity for early identification of severe coagulopathy in initial phase of sepsis: a prospective observational study. Crit Care. 2014;18(1):813–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cauchie P, Cauchie C, Zouaoui Boudjeltia K, et al. Diagnosis and prognosis of overt disseminated intravascular coagulation in a general hospital—meaning of the ISTH score system, fibrin monomers, and lipoprotein–C-reactive protein complex formation. Am J Hematol. 2006;81(6):414–419. [DOI] [PubMed] [Google Scholar]
- 21. Gando S, Saitoh D, Ogura H, et al. Natural history of disseminated intravascular coagulation diagnosed based on the newly established diagnostic criteria for critically ill patients: results of a multicenter, prospective survey. Crit Care Med. 2008;36(1):145–151. [DOI] [PubMed] [Google Scholar]
- 22. Ogura H, Gando S, Saitoh D, et al. Epidemiology of severe sepsis in Japanese intensive care units: a prospective multicenter study. J Infect Chemother. 2014;20(3):157–162. [DOI] [PubMed] [Google Scholar]
- 23. Taylor FB, Toh CH, Hoots WK, Wada H, Levi M. Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost. 2001;86(5):1327–1330. [PubMed] [Google Scholar]
- 24. Collins PW, Macchiavello LI, Lewis SJ, et al. Global tests of haemostasis in critically ill patients with severe sepsis syndrome compared to controls. Br J Haematol. 2006;135(2):220–227. [DOI] [PubMed] [Google Scholar]
- 25. Kinasewitz GT, Yan SB, Basson B, et al. Universal changes in biomarkers of coagulation and inflammation occur in patients with severe sepsis, regardless of causative micro-organism. Crit Care. 2004;8(2):R82–R90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Koami H, Sakamoto Y, Ohta M, et al. Can rotational thromboelastometry predict septic disseminated intravascular coagulation? Blood Coagul Fibrinolysis. 2015;26(7):778–783. [DOI] [PubMed] [Google Scholar]
- 27. Dhainaut JF, Shorr AF, Macias WL, et al. Dynamic evolution of coagulopathy on the first day of severe sepsis: relationship with mortality and organ failure. Crit Care Med. 2005;33(2):341–348. [DOI] [PubMed] [Google Scholar]
- 28. MacLeod JB, Lynn M, McKenney MG, et al. Early coagulopathy predicts mortality in trauma. J Trauma. 2003;55(1):39–44. [DOI] [PubMed] [Google Scholar]
- 29. Walsh TS, Stanwoth SJ, Prescott RJ, et al. Prevalence, management, and outcomes of critically ill patients with prothrombin time prolongation in United Kingdom intensive care units. Crit Care Med. 2010;38(10):1939–1946. [DOI] [PubMed] [Google Scholar]
- 30. Abraham E, Reinhart K, Opal S, et al. Efficacy and safety of tifacogin (recombinant tissue factor pathway inhibitor) in severe sepsis. J Am Med Assoc. 2003;290(2):238–247. [DOI] [PubMed] [Google Scholar]
- 31. Vincent JL, Ramesh MK, Ernest D, et al. A randomized, double-blind, placebo-controlled phase 2b study to evaluate the safety and efficacy of recombinant human soluble thrombomodulin, ART-123, in patients with sepsis and suspected disseminated intravascular coagulation. Crit Care Med. 2013;41(9):2070–2079. [DOI] [PubMed] [Google Scholar]
- 32. Daudel F, Kessler U, Folly H, et al. Thromboelastometry for the assessment of coagulation abnormalities in early and established adult sepsis: a prospective cohort study. Crit Care. 2009;13(2):R42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Johansson PI, Stensballe J, Vindelov N, et al. Hypocoagulability, as elevated by thromboelastography, at admission to the ICU is associated with increased 30-day mortality. Blood Coagul Fibrinol. 2010;21(2):168–174. [DOI] [PubMed] [Google Scholar]
- 34. Kucher N, Connolly S, Beckman JA, et al. International normalized ration increase before warfarin-associated hemorrhage. Arch Intern Med. 2004;164(19):2176–2179. [DOI] [PubMed] [Google Scholar]