Abstract
Detection of high on-treatment platelet reactivity (HPR) by point-of-care tests has not been validated after successful fibrinolysis for ST-elevation myocardial infarction. We assessed the validity of the point-of-care VerifyNow P2Y12 (VN) and INNOVANCE PFA P2Y (PFA) tests on HPR compared to light transmittance aggregometry (LTA) in these patients. The HPR was identified in 10 (34.5%) patients, 15 (51.7%) patients, and 14 (50%) patients using LTA, VN, and PFA, respectively. Discrepancies were observed between the tests despite significant correlations between platelet reactivity measures by LTA and VN (r = 0.74; P < .0001) and LTA and PFA (r = .75; P < .0001). Compared to LTA, VN and PFA were associated with a 92% and 53% and 92% and 64% positive predictive value (PPV) and negative predictive value (NPV), respectively, in detecting HPR. When combined, VN and PFA results yielded 90% and 100% PPV and NPV values if discrepancies between the 2 tests were considered as non-HPR. The VN or PFA identify patients without HPR correctly but overestimate the proportion of HPR patients. The association of the 2 tests, in case of HPR, improves the accuracy of the detection of HPR.
Keywords: clopidogrel, fibrinolysis, platelet aggregation, point-of-care testing, ST-segment elevation myocardial infarction
Introduction
Primary percutaneous coronary intervention (PCI) is considered as the preferred reperfusion strategy in patients with ST-elevation myocardial infarction (STEMI), when performed within 2 hours following first medical contact.1 However, most patients present to non-PCI capable hospitals worldwide.2 In patients with STEMI managed <12 hours after symptom-onset, prehospital fibrinolysis (FL) in association with routine angiography and PCI performed 3 to 24 hours after FL is a valuable alternative with similar early and late mortality rates compared with primary PCI.3 Adjuvant antiplatelet therapy including aspirin and clopidogrel (loading dose of 300 mg in patients aged ≤75 years and 75 mg in those >75 years) is recommended at the time of FL.4,5
Light transmittance aggregometry (LTA) is the gold standard for determining the on-treatment platelet reactivity but it requires specific equipment and competence. Point-of-care (POC) platelet function assays were developed for use in routine clinical practice. VerifyNow P2Y12 (VN) and INNOVANCE PFA P2Y (PFA) are rapid, easy to perform, and commonly used bedside tests, well correlated with LTA in different settings.6–10 However, they have not been validated in the detection of on-treatment high platelet reactivity (HPR) in the highly prothrombotic setting of post-FL, at the time of recommended PCI.
The paradoxical prothrombotic status observed after FL11 associated with the variability of platelet response and slow onset of action of clopidogrel12 could promote HPR, which has been reported to be associated with increased risk of stent thrombosis13 and ischemic events14 after PCI. Few studies have reported HPR prevalence early post-FL.15,16 All such studies have used the VN assay only despite the absence of validation of the test in comparison with the reference method. Moreover, the variability of the results of different platelet function tests in assessing response to clopidogrel and aspirin17,18 appears important in the setting of post-FL and justifies studies comparing such tests.
Our study aimed to assess the accuracy of HPR detected by VN and PFA at the time of PCI in patients with STEMI successfully treated by FL in comparison with LTA.
Methods
Patient Selection
We prospectively included consecutive patients with STEMI successfully treated by FL admitted to our center between November 2014 and November 2016. Successful FL was defined as the resolution of both ST-segment elevation (>50%) and chest pain <90 minutes after FL.1 All patients had early routine post-thrombolysis angiography with subsequent PCI if indicated. Patients were selected at the time of coronary angiography during modified opening hours (6 am-2 pm, Monday-Friday) as needed for access to LTA. Exclusion criteria were contraindications or history of allergy to anticoagulant/antithrombotic therapy, hematocrit <30% or thrombocytopenia <100.000/dL, oral anticoagulation, use of glycoprotein IIb/IIIa inhibitors, clinical instability, and the absence of reperfusion criteria leading to rescue PCI. All patients provided written informed consent, and the study was approved by the institutional ethics committee.
Pharmacological Regimen
All patients received 250 mg intravenous (IV) aspirin, per os clopidogrel (loading dose of 300 mg if age <75 years and 75 mg if age ≥75 years), followed by 75 mg of clopidogrel and 75 mg of aspirin daily as recommended in the regional STEMI network protocol. Anticoagulation was performed with enoxaparin (30 mg IV bolus followed 15 minutes later by 1 mg/kg subcutaneously (SC) every 12 hours in patients <75 years; no IV bolus and an SC dose of 0.75 mg/kg in patients ≥75 years) or unfractionated heparin (60 U/kg IV bolus with a maximum of 4000 U followed by an IV infusion of 12 U/kg with a maximum of 1000 U/h). The fibrinolytic agent was tenecteplase, administered with a weight and age-adjusted dose (half weight-adjusted dose in patients ≥75 years).19
Platelet Function Tests
Blood samples were drawn at the beginning of the coronary angiogram directly from the arterial sheath for platelet reactivity analysis. A second VN test was performed 12 to 24 hours after the initial test, on a sample drawn by venipuncture, after a 5 mL discard sample to avoid spontaneous platelet activation. All blood samples were drawn into vacutainer tubes containing sodium citrate 3.2% (Becton-Dickinson, San Jose, California) and analyzed within 2 hours after extraction.
Light transmittance aggregometry
Platelet-rich plasma (PRP) was obtained after blood centrifuging at 100 × g for 10 minutes. The remaining blood was further centrifuged at 2300 × g for 15 minutes to prepare platelet-poor plasma (PPP). The LTA was performed using an APACT 4004 aggregometer (ELITechGroup, France). The PRP samples were prewarmed in the instrument at 37°C for 5 minutes and 100% transmission was set using autologous PPP and 0% transmission was set with PRP. Platelet aggregation was measured in PRP after addition of adenosine-5-diphosphate (ADP; final concentration 10 µmol/L). Testing was performed for exactly 496 seconds and the maximal platelet aggregation (MPA) was recorded as the highest value achieved during this observation period. Inhibition of platelet aggregation (IPA at time t = IPAt) was calculated using the formula IPAt = [1−(MPAt/MPA0)] × 100%, where MPA0 is the MPA at baseline. The HPR was defined as an IPAt <30%.
VerifyNow P2Y12
The VN assay (Accumetrics, San Diego, California) is a whole-blood, cartridge-based, light transmission-based optical detection system designed to measure platelet aggregation. The method has been described elsewhere.20 Results from the device are reported as P2Y12 reaction units (PRU), percent inhibition, and a baseline value (BASE) for platelet function, claimed by the manufacturer to be independent of the level of P2Y12 blockade. The percent inhibition (IPA) is calculated as: [(1−PRU/BASE) × 100]. The HPR was defined as a PRU ≥208.14
INNOVANCE PFA P2Y
The PFA-100 system (Siemens Healthcare Diagnostics Products GmbH, Marburg, Germany) is a system for the assessment of high-shear stress-dependent platelet function by a procedure simulating the complex process of primary hemostasis in vitro. This test has been described previously.7 The time required to occlude the central aperture (diameter, 100 μm) is defined as the closure time (CT). The HPR was defined as a CT <106 seconds as recommended by the manufacturer.
The mean coefficient of variation of test precision has been reported to be 3.2%, 6.8%, and 9.3% in patients with coronary artery disease for VN, LTA, and PFA, respectively.21
Clinical Follow-Up
Patients or their general practitioner were contacted in December 2016 to assess clinical follow-up. Major adverse clinical events (MACEs) were defined as the composite of all-cause death, new myocardial infarction, stroke, and urgent revascularization. Minor and major bleedings were defined according to the Thrombolysis In Myocardial Infarction definition.22
Statistical Analysis
The inclusion of 30 patients was considered to assume a normal distribution. Groups were defined as non-HPR group or HPR group based on the results of each test at the beginning of the coronary angiography. The distribution of continuous variables was tested for normality using the Kolmogorov-Smirnov test. With the exception of the VN IPA and the PFA CT, all other variables were normally distributed. Continuous variables were expressed as mean (SD) or median (interquartile range) and compared between groups using the Student t test or the Wilcoxon-Cox test when applicable. Categorical variables were expressed as n (%) and compared between groups using the χ2 test. The correlation between different measures was assessed using Pearson or Spearman correlation tests when applicable. Sensitivity, specificity, negative predictive value (NPV) and positive predictive value (PPV) were calculated based on binary definitions of HPR in comparison to LTA. Such parameters were calculated for VN, PFA, and both VN and PFA with discrepancies considered as non-HPR.
A P value of <.05 was considered statistically significant. R software version 3.4.1 (2017-06-30) for MacOS (R Foundation for Statistical Computing, Vienna, Austria) was used for statistical analysis.
Results
A total of 30 patients were included but the measure with the VN assay failed in 1 patient due to a technical problem, LTA was not realized in 1 patient, and PFA in 2 other patients because of a >2-hour delay after sample collection.
The HPR was identified in 10 (34.5%), 15 (51.7%), and 14 (50%) patients using LTA, VN, and PFA, respectively. Patients’ clinical characteristics and treatments (Table 1) were comparable between groups identified by HPR on VN, except for a hemoglobin concentration lower in the HPR group (13.9 [1] g/dL vs 15.1 [1.1] g/dL; P = .006). Baseline characteristics of the patients were comparable between groups identified by HPR on PFA or LTA.
Table 1.
Baseline Characteristics With and Without HPR According to Each Platelet Function Test.
| Baseline Patient Characteristics | All | VN | PFA | LTA | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (n = 30) | HPR (n = 15) | No HPR (n = 14) | P | HPR (n = 14) | No HPR (n = 14) | P | HPR (n = 10) | No HPR (n = 19) | P | |
| Age (years) | 57.4 (12) | 56 (12.5) | 57.9 (11.5) | .67 | 56.5 (14.1) | 59.7 (10) | .5 | 61.2 (14.2) | 55.2 (10.8) | .26 |
| Sex (male) | 26 (86.7%) | 12 (80%) | 13 (92.9%) | .32 | 13 (92.9) | 12 (85.7) | .5 | 9 (90) | 16 (84.2) | .67 |
| Systemic hypertension | 10 (33.3%) | 6 (40%) | 4 (28.6%) | .52 | 6 (42.9) | 4 (28.6) | .4 | 5 (50) | 5 (26.3) | .20 |
| Hyperlipidemia | 13 (43.3%) | 7 (46.7%) | 6 (42.9%) | .84 | 5 (35.7) | 7 (50) | .4 | 3 (30) | 9 (47.4) | .37 |
| Diabetes mellitus | 3 (10%) | 2 (13.3%) | 0 | .16 | 3 (21.4) | 0 | .07 | 1 (10) | 2 (10.5) | .96 |
| Active smoker | 19 (63.3%) | 11 (73.3%) | 8 (57.1%) | .36 | 10 (71.4) | 7 (50) | .25 | 6 (60) | 13 (68.4) | .65 |
| Body mass index, kg/m2 | 25.5 (4.7) | 25.5 (5.8) | 25.5 (3.5) | .98 | 26.4 (5.6) | 24.9 (3.8) | .42 | 26.7 (6.4) | 24.8 (3.6) | .4 |
| Past history of | ||||||||||
| Myocardial infarction | 2 (6.7%) | 0 | 2 (14.3%) | .13 | 0 | 2 (14.3) | .14 | 1 (10) | 1 (5.3) | .63 |
| PCI | 2 (6.7%) | 0 | 2 (14.3%) | .13 | 0 | 2 (14.3) | .14 | 1 (10) | 1 (5.3) | .63 |
| Treatment prior to index event | ||||||||||
| Aspirin | 1 (3.3%) | 0 | 1 (7.1%) | .29 | 0 | 1 (7.1) | .3 | 1 (10) | 0 | .16 |
| Clopidogrel | 2 (6.7%) | 0 | 2 (14.3%) | .13 | 0 | 2 (14.3) | .14 | 1 (10) | 1 (5.3) | .63 |
| Proton-pump inhibitor | 5 (16.7%) | 2 (13.3%) | 3 (21.4%) | .56 | 2 (14.3) | 3 (21.4) | .6 | 3 (30) | 2 (10.5) | .19 |
| Statins | 6 (20%) | 4 (26.7%) | 2 (14.3%) | .41 | 3 (21.4) | 2 (14.3) | .6 | 2 (20) | 4 (21.1) | .95 |
| Anterior wall STEMI | 5 (16.7%) | 3 (20%) | 2 (14.3%) | .68 | 2 (14.3) | 2 (14.3) | 1 | 2 (20) | 3 (15.8) | .77 |
| Inferior wall STEMI | 23 (76.7%) | 12 (80%) | 10 (71.4%) | .59 | 12 (85.7) | 10 (71.4) | .36 | 8 (80) | 14 (73.7) | .70 |
| Other infarct location | 3 (10%) | 1 (6.7%) | 2 (14.3%) | .5 | 1 (7.1) | 2 (14.3) | .54 | 1 (10) | 2 (10.5) | .96 |
| Left ventricular ejection fraction, % | 52.5 (8.7) | 51.7 (8.4) | 53.4 (9.5) | .61 | 51.8 (9.3) | 53.4 (8.6) | .6 | 51.5 (8.8) | 53.1 (8.9) | .65 |
| Systolic blood pressure, mm Hg | 146 (29) | 142.9 (31.4) | 147.9 (27.8) | .65 | 144.1 (31.8) | 149.6 (29) | .6 | 157.2 (23.5) | 139.8 (31.2) | .1 |
| Heart rate, beats/min | 78 (18) | 79 (21) | 77 (16) | .76 | 78 (22) | 77 (15) | .9 | 84 (22) | 76 (16) | .35 |
| Killip class ≥II | 2 (6.7%) | 1 (6.7%) | 1 (7.1%) | .96 | 1 (7.1) | 1 (7.1) | 1 | 1 (10) | 1 (5.3) | .63 |
| GRACE score | 129.6 (26.6) | 128.9 (27.6) | 129.4 (27.3) | .96 | 129.4 (28.8) | 130.1 (27.3) | .95 | 133.4 (30.5) | 127.3 (25.7) | .59 |
| CRUSADE score | 27.7 (12.5) | 26.3 (14.2) | 27.9 (10) | .73 | 30.1 (12.9) | 27 (12.2) | .5 | 30.6 (12.7) | 26.8 (12.5) | .45 |
| Time points, min | ||||||||||
| Pain to FMC | 112.5 (81) | 107.2 (60.2) | 109.6 (97.9) | .94 | 113 (71.5) | 107 (96.3) | .8 | 110.7 (73.8) | 116.8 (87.1) | .84 |
| Pain to FL | 156.1 (86.4) | 151 (65.3) | 154.1 (105.8) | .92 | 152.1 (75.8) | 156.4 (103.4) | .9 | 153.5 (73.9) | 159.4 (96) | .85 |
| FL to PCI | 1057.6 (563.4) | 1078 (648.8) | 1002.4 (484) | .72 | 1157.5 (659.9) | 1041.9 (443) | .6 | 1217.1 (703.4) | 941.4 (463.4) | .28 |
| FMC to PCI | 1101.1 (567) | 1121.6 (652.6) | 1046.9 (489) | .73 | 1196.3 (662.5) | 1091.1 (450.2) | .6 | 1259.6 (700.9) | 983.9 (470) | .28 |
| Admission biological characteristics | ||||||||||
| Hemoglobin, g/dL | 14.5 (1.2) | 13.9 (1) | 15.1 (1.1) | .006 | 14.2 (1.1) | 14.8 (1.2) | .20 | 14.2 (1.1) | 14.6 (1.3) | .46 |
| Hematocrit, % | 40.1 (10.2) | 41.3 (2.5) | 38.4 (14.7) | .49 | 42.2 (2.6) | 37.8 (14.6) | .28 | 42.7 (3.2) | 38.4 (12.4) | .17 |
| White blood cell count, G/L | 12.2 (4.5) | 12.6 (4.3) | 12 (5) | .74 | 13.8 (6.1) | 10.7 (1.8) | .08 | 12.5 (5.1) | 12.2 (4.5) | .88 |
| Platelet count, G/L | 252.4 (72.7) | 262.7 (80.8) | 243.6 (66.9) | .49 | 269.4 (73.4) | 225 (44.2) | .07 | 250.9 (72.3) | 254.9 (76.4) | .89 |
| Kaliemia, mmol/L | 4.3 (0.4) | 4.2 (0.4) | 4.3 (0.4) | .63 | 4.3 (0.4) | 4.3 (0.4) | .7 | 4.2 (0.5) | 4.3 (0.3) | .67 |
| Creatinine clearance, mL/min | 91.2 (32.2) | 99.3 (40.2) | 85 (19.2) | .23 | 93.4 (41.8) | 86.6 (21.9) | .6 | 86.4 (39.2) | 93.7 (29.6) | .61 |
| Prothrombin rate, % | 95.4 (6.9) | 96 (7.7) | 95.3 (6.4) | .8 | 95.4 (7.9) | 95.5 (6.6) | .9 | 95.8 (4.7) | 96.2 (6.9) | .86 |
| aPTT, seconds | 1.2 (0.3) | 1.2 (0.3) | 1.2 (0.3) | .77 | 1.2 (0.3) | 1.2 (0.3) | .9 | 1.1 (0.3) | 1.2 (0.3) | .76 |
| Anti-Xa, IU | 0.5 (0.2) | 0.5 (0.2) | 0.6 (0.2) | .42 | 0.55 (0.2) | 0.49 (0.3) | .5 | 0.5 (0.2) | 0.55 (0.2) | .66 |
| Adjunctive therapy to FL | ||||||||||
| Clopidogrel loading dose 300 mg | 28 (93.3%) | 14 (93.3%) | 13 (92.9%) | .96 | 13 (92.9) | 13 (92.9) | 1 | 9 (90) | 18 (94.7) | .63 |
| Enoxaparin | 27 (90%) | 12 (80%) | 14 (100%) | .08 | 12 (85.7) | 13 (92.9) | .5 | 8 (80) | 18 (94.7) | .21 |
| Unfractionated heparin | 3 (10%) | 3 (20%) | 0 | .08 | 2 (14.3) | 1 (7.1) | .5 | 2 (20) | 1 (5.3) | .21 |
Abbreviations: aPTT, activated partial thromboplastin time; CRUSADE, Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the American College of Cardiology/American Heart Association Guidelines; FL, fibrinolysis; FMC, first medical contact; GRACE, Global Registry of Acute Coronary Events; HPR, high platelet reactivity; LTA, light transmittance aggregometry; PFA, INNOVANCE PFA P2Y; PCI, percutaneous coronary intervention; STEMI, ST-elevation myocardial infarction; TL, thrombolysis; VN, VerifyNow.
Platelet Reactivity at the Time of Angiography and Correlation of the Tests
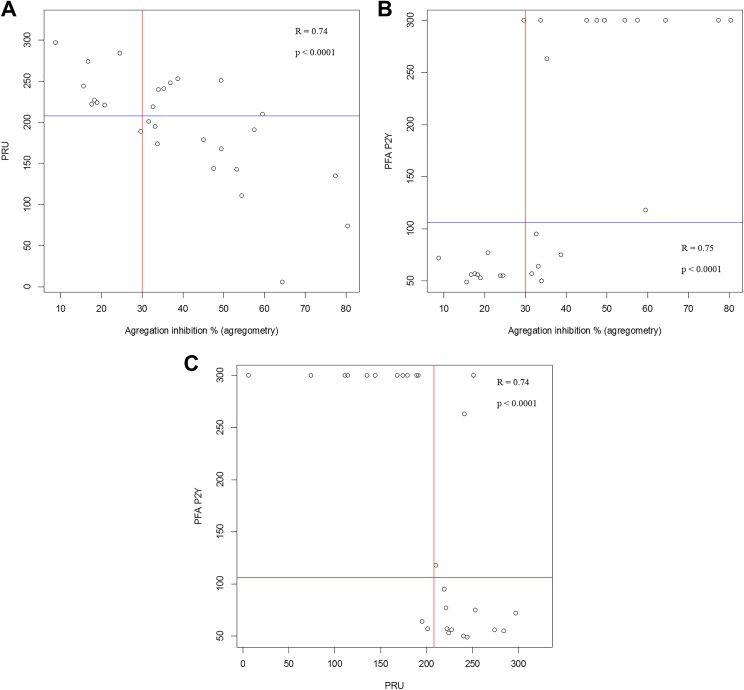
Overall, as awaited, significantly lower IPA and CT and greater PRU were observed in the HPR group regardless of the test used to define HPR (Table 2). There was a significant correlation between IPA and PRU (r = .74; P < .0001; Figure 1A), between IPA and CT (r = .75; P < .0001; Figure 1B), and between CT and PRU (r = .74; P < .0001; Figure 1C).
Table 2.
Platelet Reactivity.
| Platelet Function Test | All | VN | PFA | LTA | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (n = 30) | HPR (n = 15) | No HPR (n = 14) | P | HPR (n = 14) | No HPR (n = 14) | P | HPR (n = 10) | No HPR (n = 19) | P | |
| At coronary angiography | ||||||||||
| VerifyNow | n = 29 | n = 15 | n = 14 | n = 14 | n = 13 | n = 9 | n = 19 | |||
| PRU | 195.8 (65.2) | 243.7 (25.3) | 144.6 (54.6) | <.0001 | 238.5 (31.1) | 156.2 (65.7) | <.0001 | 242.4 ±35.4 | 178.1 (65.2) | .002 |
| Base | 214.5 (29.8) | 214.6 (24.3) | 214.3 (35.8) | .98 | 211.3 (25.4) | 217.1 (35.7) | .63 | 214.4 (28.8) | 216.3 (30.7) | .88 |
| IPA (%) | 2 (21) | 0 (0) | 25 (30.8) | <.0001 | 0 (0) | 19 (29.5) | .0002 | 0 (0) | 11 (32.5) | .014 |
| PFA | n = 28 | n = 14 | n = 13 | n = 14 | n = 14 | n = 10 | n = 17 | |||
| Closure time (sec) | 106.5 (243.3) | 64.5 (35.3) | 300 (0) | <.001 | 56.5 (15) | 300 (0) | <.001 | 56 (13.3) | 300 (205) | .002 |
| LTA | n = 29 | n = 15 | n = 13 | n = 13 | n = 14 | n = 10 | n = 19 | |||
| MPA | 51.5 (15.4) | 59.6 (11.5) | 41.4 (14.1) | .001 | 63.3 (7.0) | 39.6 (13.2) | <.0001 | 66.8 (4.4) | 43.5 (12.7) | <.001 |
| IPA (%) | 38.2 (18.6) | 28.5 (14) | 50.6 (16.6) | <.001 | 23.9 (8.8) | 52.6 (15.5) | <.0001 | 19.5 (5.7) | 48.1 (14.9) | <.001 |
| At Day 1 (VerifyNow P2Y12) | n = 26 | n = 15 | n = 11 | n = 11 | n = 13 | n = 9 | n = 17 | |||
| PRU | 189 (66.5) | 220.5 (46.9) | 146.1 (66.8) | .006 | 227.1 (36.3) | 147 (73.6) | .005 | 235.6 (35.2) | 164.3 (66.5) | .001 |
| Base | 216.5 (29.9) | 221.1 (29.5) | 210.4 (30.7) | .38 | 212.1 (31) | 222.8 (30.5) | .4 | 213.9 (33.3) | 217.9 (28.9) | .76 |
| IPA (%) | 2.5 (15.3) | 0 (8.5) | 16 (32) | .035 | 0 (0) | 16 (44.5) | .003 | 0 (0) | 13 (31) | .005 |
Abbreviations: HPR, high platelet reactivity; IPA, inhibition of platelet aggregation; LTA, light transmittance aggregometry; MPA, maximal platelet aggregation; PFA, INNOVANCE PFA P2Y; PRU, P2Y12 reaction units; VN, VerifyNow.
Figure 1.
Distribution and correlation of values derived from measurements between (A) LTA (inhibition of platelet aggregation, %) and VN (PRU), (B) LTA and PFA (closure time, seconds), and (C) PFA and VN. The lines indicate the cutoff values of each test determining high platelet reactivity (106 seconds for PFA, 208 PRU for VN, and 30% for LTA). PFA indicates INNOVANCE PFA P2Y; PRU, P2Y12 reaction units; VN, VerifyNow.
The sensitivity, specificity, PPV, and NPV of VN to detect HPR as compared to LTA were 63%, 89%, 92%, and 53%, respectively. Similarly, the sensitivity, specificity, PPV, and NPV of PFA were 70%, 90%, 92%, and 64%, respectively. The accuracy to classify HPR/non-HPR patients as assessed by LTA was 71% and 78% with the VN and the PFA, respectively.
When we combined both VN and PFA results considering the discrepancies between the 2 tests as non-HPR, PPV and NPV were increased to 90% and 100%, respectively (Table 3). The concordance between the VN and the PFA was 81%.
Table 3.
Sensitivity, Specificity, and Predictive Values of the VN and PFA Compared to LTA.
| LTA Versus | VN | PFA | VN + PFA | |
|---|---|---|---|---|
| Discrepancies Considered as non-HPR | Discrepancies Considered as HPR | |||
| Sensitivity | 0.63 | 0.70 | 0.53 | 0.75 |
| Specificity | 0.89 | 0.90 | 0.89 | 0.57 |
| PPV | 0.92 | 0.92 | 0.9 | 0.6 |
| NPV | 0.53 | 0.64 | 1 | 0.73 |
| Accuracy | 0.71 | 0.78 | 0.65 | 0.65 |
Abbreviations: HPR, high platelet reactivity; LTA, light transmittance aggregometry; NPV, negative predictive value; PFA, INNOVANCE PFA P2Y; PPV, positive predictive value; VN, VerifyNow.
VerifyNow P2Y12 at Day + 1
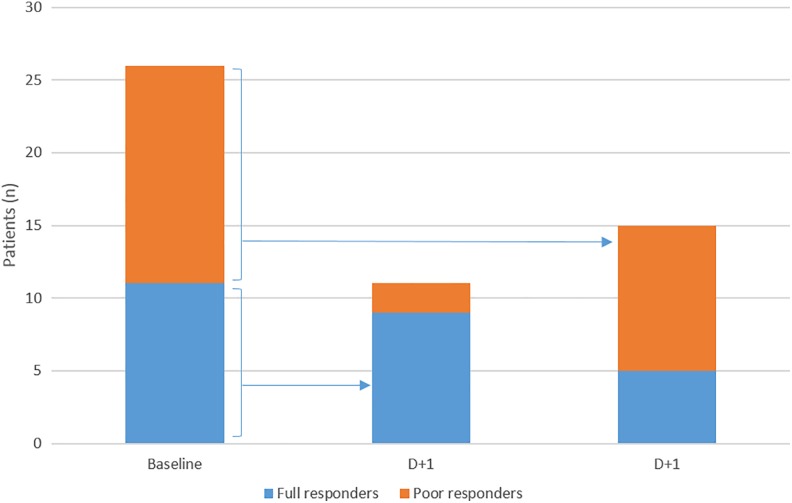
Although on-clopidogrel platelet reactivity assessed by continuous PRU did not significantly differ between baseline and day + 1 (199.5 [65.5] vs 189 [66.5]; P = .15), as outlined in Figure 2, 7 (26.9%) of 26 patients changed their responsiveness status: 5 initially HPR patients became full responders and 1 initially responder became HPR.
Figure 2.
Responsiveness status of the patients at the time of angiography and day + 1 based on VerifyNow P2Y12.
Outcomes
All patients were treated with coronary stenting except one who was referred for coronary artery bypass grafting. Clinical follow-up was performed with a median of 658.3 (129.6) days. No complications were recorded during hospitalization. Outcomes were comparable between groups, without any reported death or bleeding. Two patients had an MACE, 1 in each group, based on all tests.
Discussion
Our study showed that HPR is common after successful FL, ranging from one-third to one-half of our cohort according to the test used. We found a significant correlation between LTA and both VN or PFA but also some discrepancies between POC tests and LTA in stratifying patients in HPR or non-HPR. Both PFA and VN adequately identify patients without HPR but overestimate the proportion of HPR patients. A second different POC test seems to be a useful option to improve the accuracy of the patients’ status in case of detected HPR with a first test.
Early routine angiography and PCI are recommended within a time window of 3 to 24 hours after successful FL.1 At this time window (17.6 [9.4] hours), we found high rates of HPR ranging between 32.1% and 51.9% according the test used. Two previous studies have reported 7 higher proportions of HPR early after FL reaching 67.5% and 70.7% of cases,15,16 as assessed by VN only. Enhanced platelet reactivity is reported in patients with acute coronary syndrome23,24 up to 48 hours following PCI.23 The paradoxical prothrombotic status and thrombin-induced platelet activation within the early hours after FL11,25 are also likely to participate in increasing rates of HPR. The recommended adjunctive antiplatelet therapy with clopidogrel has several drawbacks such as slow onset of action and variability of platelet response,12 especially with the lower doses26 recommended with FL. Moreover, the bioavailability of clopidogrel is impaired in patients with STEMI.27 These considerations explain the high prevalence of HPR in general and in our cohort. Our study is the first to report a simultaneous and head-to-head comparison of platelet reactivity early post-FL with LTA—considered to be the gold standard test—VN, and PFA. One-third of our cohort had LTA-detected HPR, which represents only half of the previously published rates in this setting using VN.15,16 The prevalence of HPR using PFA was similar to that found with VN in our patient population. As LTA is time-consuming and requires specialized equipment and technicians, POC platelet function tests have been developed for routine clinical practice. The VN and PFA can easily be used in the catheterization laboratory by a nonspecific staff. Our study provides details on their validity in the early post-FL setting.
We found a significant and relatively strong correlation between IPA and PRU and between IPA and CT (r = .74 and .75, respectively). The correlation between IPA and PRU is comparable to previous reports in elective PCI9 or PCI in real-life practice including ACS6 despite different study-specific LTA-ADP concentrations, cutoffs, and populations. In reference to LTA, the accuracy to classify HPR/non-HPR patients was 71% for VN and 78% between for PFA. Such values are similar to those reported previously in other settings than FL.6–8 Importantly, both VN and PFA correctly identified most patients without HPR but overestimated the proportion of HPR patients when compared to LTA. Some differences between the tests may explain these results. The PFA and VN add prostaglandin E1 to the ADP to suppress further activation of platelets through P2Y12 receptor. A variable degree of residual P2Y12 function can potentially persist despite P2Y12 inhibition and consequently modify the monitoring of clopidogrel therapy measured with ADP alone, such as in LTA. Moreover, LTA is performed in PRP, whereas VN and PFA use whole-blood samples. Such differences may explain the higher concordance to classify HPR/non-HPR (81%) observed between VN and PFA as compared to POC tests and LTA (71%). The difference in hemoglobin levels between groups may also have influenced the VN results as patients with lower hemoglobin are more likely to have HPR.28 Finally, shear stress which may influence platelet function7 is lower in LTA unlike PFA.
Our findings imply that PCI following successful FL was performed without adequate P2Y12 inhibition in a high proportion of patients. Both the early post-STEMI setting and the HPR are high-risk situations for recurrent coronary events.13,14 Hence, it is important to adequately identify patients who may benefit from alternative or intensified antiplatelet therapy29 especially in association with FL, which is on the other hand associated with a higher risk of bleeding. A reloading dose of clopidogrel distant from FL and 2 hours before PCI could enhance platelet inhibition.26,30 A delayed PCI to achieve full clopidogrel effect, although unrecommended, could also be considered. The selective use of glycoprotein IIb/IIIa inhibitors associated with reduced ischemic events without increased bleedings in poor clopidogrel-responders undergoing elective PCI31 could also be an alternative. Finally, recent more potent antiplatelet therapy could be discussed after the acute phase, as proposed in the recent European guidelines.32 Recently, ticagrelor was tested in patients with HPR post-FL and provided a more effective antiplatelet inhibition than clopidogrel.15 However, HPR was defined according only to a single VN measure in the latter study. Such strategy appears to be questionable considering our results. The bleeding risk associated with both FL and more intensive antiplatelet regimens warrants reliable identification of patients who truly benefit from such strategies.
The studied POC tests appear to be reliable in detecting non-HPR in the early post-FL setting. A single measure with any of the POC tests appears insufficient to tailor antiplatelet therapy in those with detected HPR. Considering that platelet reactivity is enhanced early after ACS,23,24 a repetitive testing using the same device may be useful. We showed that a quarter of patients changed their responsiveness status at day 1, mostly HPR patients becoming full responders. After FL, the reported variations in the PRU levels between baseline and day 1 are discordant between studies.15,16 However, a decrease in on-clopidogrel platelet reactivity over time was showed in a larger cohort of patients treated with PCI, mostly for non-STEMI.33 Interestingly, we showed that a measure with a second different type of POC test might be useful when a first test detects HPR. Considering that the patient is HPR when the 2 tests are concordantly positive and is non-HPR when both tests are negative or discordant, which remarkably improves both NPV and PPV of the strategy. In clinical practice, POC tests are useful in tailoring antiplatelet treatment for patients at high bleeding and/or high ischemic risk. The latter situations include those with a history of stent thrombosis or complex PCI with numerous stents and/or complex bifurcation lesions. Considering the high bleeding and thrombotic risk associated with FL, and the fact that only clopidogrel can be used in concomitance with FL, the POC tests appear as ideally indicated in this setting.
Study Limitation
Our study was not powered nor designed to detect potential relationship between HPR and clinical events. Additional studies are needed to assess the clinical benefit of testing platelet reactivity and further antiplatelet regimens in patients with HPR, despite conventional recommended therapy in this setting. There was a rather lengthy period of patients recruitment due to the inclusion criteria. However, during the inclusion period FL, antithrombotic and interventional protocols were not modified in our center.
Conclusion
The HPR is a common finding after successful FL but its rates vary according to the used tests. When compared to LTA, POC tests VN and PFA correctly identify patients without HPR but overestimate the proportion of HPR patients. Hence, one measure of such POC tests detects optimally treated patients at the time of PCI following FL in a pharmacoinvasive strategy but not those requiring more aggressive antiplatelet therapy who may be more accurately identified by the association of 2 different POC tests.
Footnotes
Authors’ Note: Ethical approval to report this case series was obtained from Comité de Protection des Personnes Nord Ouest III (2012-12-18). Written informed consent was obtained from the patient(s) for their anonymized information to be published in this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Steg PG, James SK, Atar D, et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2012;33(20):2569–2619. [DOI] [PubMed] [Google Scholar]
- 2. Armstrong PW, Boden WE. Reperfusion paradox in ST-segment elevation myocardial infarction. Ann Intern Med. 2011;155(6):389–391. [DOI] [PubMed] [Google Scholar]
- 3. Roule V, Ardouin P, Blanchart K, et al. Prehospital fibrinolysis versus primary percutaneous coronary intervention in ST-elevation myocardial infarction: a systematic review and meta-analysis of randomized controlled trials. Crit Care. 2016;20(1):359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen ZM, Jiang LX, Chen YP, et al. Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet. 2005;366(9497):1607–1621. [DOI] [PubMed] [Google Scholar]
- 5. Sabatine MS, Cannon CP, Gibson CM, et al. Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with ST-segment elevation. N Engl J Med. 2005;352(12):1179–1189. [DOI] [PubMed] [Google Scholar]
- 6. Kim IS, Jeong YH, Kang MK, et al. Correlation of high post-treatment platelet reactivity assessed by light transmittance aggregometry and the VerifyNow P2Y12 assay. J Thromb Thrombolysis. 2010;30(4):486–495. [DOI] [PubMed] [Google Scholar]
- 7. Linnemann B, Schwonberg J, Rechner AR, et al. Assessment of clopidogrel non-response by the PFA-100 system using the new test cartridge INNOVANCE PFA P2Y. Ann Hematol. 2010;89(6):597–605. [DOI] [PubMed] [Google Scholar]
- 8. Tsantes A, Ikonomidis I, Papadakis I, et al. Evaluation of the role of the new INNOVANCE PFA P2Y test cartridge in detection of clopidogrel resistance. Platelets. 2012;23(6):481–489. [DOI] [PubMed] [Google Scholar]
- 9. van Werkum JW, van der Stelt CA, Seesing TH, et al. A head-to-head comparison between the VerifyNow P2Y12 assay and light transmittance aggregometry for monitoring the individual platelet response to clopidogrel in patients undergoing elective percutaneous coronary intervention. J Thromb Haemost. 2006;4(11):2516–2518. [DOI] [PubMed] [Google Scholar]
- 10. Varenhorst C, James S, Erlinge D, et al. Assessment of P2Y(12) inhibition with the point-of-care device VerifyNow P2Y12 in patients treated with prasugrel or clopidogrel coadministered with aspirin. Am Heart J. 2009;157(3):562.e1–9. [DOI] [PubMed] [Google Scholar]
- 11. Eisenberg PR, Sobel BE, Jaffe AS. Activation of prothrombin accompanying thrombolysis with recombinant tissue-type plasminogen activator. J Am Coll Cardiol. 1992;19(5):1065–1069. [DOI] [PubMed] [Google Scholar]
- 12. O’Donoghue M, Wiviott SD. Clopidogrel response variability and future therapies: clopidogrel: does one size fit all? Circulation. 2006;114(22):e600–e606. [DOI] [PubMed] [Google Scholar]
- 13. Stone GW, Witzenbichler B, Weisz G, et al. Platelet reactivity and clinical outcomes after coronary artery implantation of drug-eluting stents (ADAPT-DES): a prospective multicentre registry study. Lancet. 2013;382(9892):614–623. [DOI] [PubMed] [Google Scholar]
- 14. Price MJ, Angiolillo DJ, Teirstein PS, et al. Platelet reactivity and cardiovascular outcomes after percutaneous coronary intervention: a time-dependent analysis of the Gauging Responsiveness with a VerifyNow P2Y12 assay: impact on Thrombosis and Safety (GRAVITAS) trial. Circulation. 2011;124(10):1132–1137. [DOI] [PubMed] [Google Scholar]
- 15. Alexopoulos D, Perperis A, Koniari I, et al. Ticagrelor versus high dose clopidogrel in ST-segment elevation myocardial infarction patients with high platelet reactivity post fibrinolysis. J Thromb Thrombolysis. 2015;40(3):261–267. [DOI] [PubMed] [Google Scholar]
- 16. Diego A, de Prado AP, Cuellas C, et al. P2Y12 platelet reactivity after thrombolytic therapy for ST-segment elevation myocardial infarction. Thromb Res. 2012;130(3):e31–e36. [DOI] [PubMed] [Google Scholar]
- 17. Gasparyan AY. Aspirin and clopidogrel resistance: methodological challenges and opportunities. Vasc Health Risk Manag. 2010;6:109–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gasparyan AY, Watson T, Lip GY. The role of aspirin in cardiovascular prevention: implications of aspirin resistance. J Am Coll Cardiol. 2008;51(19):1829–1143. [DOI] [PubMed] [Google Scholar]
- 19. Armstrong PW, Gershlick AH, Goldstein P, et al. Fibrinolysis or primary PCI in ST-segment elevation myocardial infarction. N Engl J Med. 2013;368(15):1379–1387. [DOI] [PubMed] [Google Scholar]
- 20. Luo Y, Li J, Liu X, et al. Combination of P2Y12 reaction unit and percentage of platelet inhibition assessed by VerifyNow P2Y12 assay is a useful predictor of long-term clinical outcomes in patients with acute coronary syndrome undergoing percutaneous coronary intervention. Thromb Res. 2016;139:114–120. [DOI] [PubMed] [Google Scholar]
- 21. Paniccia R, Antonucci E, Gori AM, et al. Different methodologies for evaluating the effect of clopidogrel on platelet function in high-risk coronary artery disease patients. J Thromb Haemost. 2007;5(9):1839–1847. [DOI] [PubMed] [Google Scholar]
- 22. Mehran R, Rao SV, Bhatt DL, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123(23):2736–2747. [DOI] [PubMed] [Google Scholar]
- 23. Althoff TF, Fischer M, Langer E, et al. Sustained enhancement of residual platelet reactivity after coronary stenting in patients with myocardial infarction compared to elective patients. Thromb Res. 2010;125(5):e190–e196. [DOI] [PubMed] [Google Scholar]
- 24. Geisler T, Kapp M, Gohring-Frischholz K, et al. Residual platelet activity is increased in clopidogrel- and ASA-treated patients with coronary stenting for acute coronary syndromes compared with stable coronary artery disease. Heart. 2008;94(6):743–747. [DOI] [PubMed] [Google Scholar]
- 25. Rasmanis G, Vesterqvist O, Green K, et al. Evidence of increased platelet activation after thrombolysis in patients with acute myocardial infarction. Br Heart J. 1992;68(4):374–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Savcic M, Hauert J, Bachmann F, et al. Clopidogrel loading dose regimens: kinetic profile of pharmacodynamic response in healthy subjects. Semin Thromb Hemost. 1999;25(suppl 2):15–19. [PubMed] [Google Scholar]
- 27. Heestermans AA, van Werkum JW, Taubert D, et al. Impaired bioavailability of clopidogrel in patients with a ST-segment elevation myocardial infarction. Thromb Res. 2008;122(6):776–781. [DOI] [PubMed] [Google Scholar]
- 28. Elsenberg EH, van Werkum JW, van de Wal RM, et al. The influence of clinical characteristics, laboratory and inflammatory markers on ‘high on-treatment platelet reactivity’ as measured with different platelet function tests. Thromb Haemost. 2009;102(4):719–727. [DOI] [PubMed] [Google Scholar]
- 29. Aradi D, Komocsi A, Price MJ, et al. Efficacy and safety of intensified antiplatelet therapy on the basis of platelet reactivity testing in patients after percutaneous coronary intervention: systematic review and meta-analysis. Int J Cardiol. 2013;167(5):2140–2148. [DOI] [PubMed] [Google Scholar]
- 30. Gurbel PA, Bliden KP, Hayes KM, et al. The relation of dosing to clopidogrel responsiveness and the incidence of high post-treatment platelet aggregation in patients undergoing coronary stenting. J Am Coll Cardiol. 2005;45(9):1392–1396. [DOI] [PubMed] [Google Scholar]
- 31. Cuisset T, Frere C, Quilici J, et al. Glycoprotein IIb/IIIa inhibitors improve outcome after coronary stenting in clopidogrel nonresponders: a prospective, randomized study. JACC Cardiovasc Interv. 2008;1(6):649–653. [DOI] [PubMed] [Google Scholar]
- 32. Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39(2):119–177. [DOI] [PubMed] [Google Scholar]
- 33. Campo G, Parrinello G, Ferraresi P, et al. Prospective evaluation of on-clopidogrel platelet reactivity over time in patients treated with percutaneous coronary intervention relationship with gene polymorphisms and clinical outcome. J Am Coll Cardiol. 2011;57(25):2474–2483. [DOI] [PubMed] [Google Scholar]