Abstract
It has been well established that angiopoietin 2 (Ang-2), a glycoprotein involved in activation of the endothelium, plays an integral role in the pathophysiology of sepsis and many other inflammatory conditions. However, the role of Ang-2 in sepsis-associated coagulopathy (SAC) specifically has not been defined. The aim of this study was to measure Ang-2 plasma levels in patients with sepsis and suspected disseminated intravascular coagulation (DIC) in order to demonstrate its predictive value in SAC severity determination and 28-day mortality outcome. Plasma samples were collected from 102 patients with sepsis and suspected DIC at intensive care unit (ICU) admission. The Ang-2 plasma levels were quantified using a sandwich enzyme-linked immunosorbent assay method. The International Society on Thrombosis and Haemostasis DIC scoring system was used to compare the accuracy of Ang-2 levels versus clinical illness severity scores in predicting SAC severity. Mean Ang-2 levels in patients with sepsis and DIC were significantly higher in comparison to healthy controls (P < 0.0001), and median Ang-2 levels showed a downward trend over time (P = 0.0008). Baseline Ang-2 levels and clinical illness severity scores were higher with increasing severity of disease, and Ang-2 was a better predictor of DIC severity than clinical illness scores. This study demonstrates that Ang-2 levels are significantly upregulated in SAC, and this biomarker can be used to risk stratify patients with sepsis into non-overt DIC and overt DIC. Furthermore, the Ang-2 level at ICU admission in a patient with sepsis and suspected DIC may provide a predictive biomarker for mortality outcome.
Keywords: angiopoietin, sepsis-associated coagulopathy, DIC
Introduction
The Society of Critical Care Medicine and the European Society of Intensive Care Medicine created a task force in 2014 to define sepsis as life-threatening organ dysfunction caused by a dysregulated host response to infection.1 Disseminated intravascular coagulation (DIC) is defined by the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis (ISTH) as an acquired syndrome characterized by the intravascular activation of coagulation with loss of localization arising from different infectious and non-infectious insults.2 Coagulation abnormalities occur in many cases of sepsis, and approximately 35% of cases of sepsis are complicated by DIC.3 Comorbidity of sepsis and DIC is termed sepsis-associated coagulopathy (SAC) or sepsis-associated DIC. The incidence of SAC increases with clinical progression from systemic inflammatory response syndrome to severe sepsis and septic shock, and the mortality rate in patients with both severe sepsis and DIC is almost 2-fold higher than those with severe sepsis alone.2 At this point in time, there is no individual biomarker that can be used as a reliable predictor of mortality and disease severity for patients with SAC. Angiopoietin 2 (Ang-2) is a promising endothelial biomarker that has been studied in critical illnesses.4 It is a glycoprotein that binds to the Tie2 tyrosine kinase receptor in the endothelium to promote a proinflammatory state.5–15 The literature shows that Ang-2 levels correlate with disease severity and mortality outcomes in patients with sepsis; however, its correlation in patients with sepsis-associated DIC is lacking.
In critical illness such as sepsis and DIC, an inflammatory milieu causes endothelial cell (EC) dysfunction. Therapies targeting coagulative and immune responses in SAC, including recombinant tissue factor (TF) pathway inhibitor,3 antithrombin concentrates,16 and recombinant human-activated protein C,17 have had limited success due to their failure to address the complex multiorgan response to critical illness. A better target in the treatment of sepsis and SAC may be the vascular endothelium specifically, as it is the coordinator of the various immune, coagulative, and inflammatory processes that occur in sepsis-associated DIC. The Ang-Tie2 system, a non-redundant regulator of the endothelium, may thus be a good target for SAC therapy and is the focus of the present study.
The role of angiopoietins in many critical illnesses such as sepsis,4–6,10–13,18–28 acute lung injury (ALI),15,29–33 acute liver failure,25,34 acute kidney injury (AKI),5,35–37 and cardiovascular disease38–42 has been investigated extensively. Associations between high Ang-2 levels and clinical measures of sepsis severity and organ dysfunction such as Organ Failure Index,19 fluid overload,25 Multiple Organ Dysfunction Syndrome (MOD) score,10 Acute Physiology and Chronic Health Evaluation (APACHE II and III), and Sequential Organ Failure Assessment (SOFA) scores12–14,18,27 have been found. Perhaps most importantly, Ang-2 predicts 28-day survival in patients with sepsis.12–14,23,25
However, there is limited understanding of the role of Ang-2 in SAC specifically.43 Further research into its role could be beneficial because of the known upregulation and release of Ang-2 in inflammatory states such as DIC. Disruption of the endothelial-lined vasculature in DIC causes exposure of subendothelial TF. Ensuing TF-dependent coagulation causes systemic thrombin formation in the microvasculature, causing tissue hypoxia that leads to organ ischemia, injury, and eventual necrosis.2 Thrombin also causes rapid release of pre-stored Ang-2 from endothelial Weibel-Palade bodies, and tissue hypoxia upregulates Ang-2 transcription.44–46
The present investigation is a multicenter cohort study with 4 main objectives: First, this study investigated whether plasma levels of Ang-2 in a cohort of patients with sepsis and suspected DIC are significantly different from Ang-2 levels in healthy controls; second, whether a trend in median Ang-2 level over time is present; third, whether baseline (day 0) mean Ang-2 levels can predict 28-day mortality, and whether or not Ang-2 is a better predictor than clinical illness severity scores; and fourth, whether baseline median Ang-2 levels and clinical illness severity scores are significantly different between patients with different severities of DIC and if either can effectively stratify patient risk.
We hypothesized that patients with sepsis and suspected DIC will have higher Ang-2 levels than healthy controls and that Ang-2 will decrease over time as patients receive standard of care. We suspected that non-survivors and those with higher DIC scores will have higher Ang-2 levels and illness severity scores than survivors and less critically ill patients. Also, we hypothesized that Ang-2 will outperform illness severity scores in mortality outcome prediction and DIC severity stratification.
Materials and Methods
Plasma samples from adult patients admitted to the intensive care unit (ICU) at the University of Utah or an associated community hospital with a diagnosis of sepsis and suspected DIC were acquired between 2008 and 2012 under an institutional review board (IRB)-approved protocol by Matthew Rondina, MD, at the University of Utah.47–49 Citrated whole blood samples were collected upon ICU admission at baseline (day 0) as well as on days 4 and 8 for those remaining in the ICU at those times. Plasma samples were stored at −80°C prior to analysis. Transfer of samples and accompanying de-identified clinical information was approved by the Loyola University Chicago Institutional Review Board (LU number 207958). Baseline (day 0) samples and accompanying data were available from 102 patients. Of these patients, 56 had day 4 samples and data available and 30 had day 8 samples and data available. Mortality data, but not DIC severity data, was missing for 1 patient; therefore, n = 101 for baseline mortality data and n = 102 for baseline DIC severity data (Tables 1 -3).
Table 1.
Patient Demographics.
| Parameters | All | Survivors, n = 86 | Non-survivors, n = 15 | a P | Sepsis n = 20 | Sepsis + Non-overt DIC, n = 58 | Sepsis + Overt DIC, n = 24 | b P |
|---|---|---|---|---|---|---|---|---|
| Demographics | ||||||||
| Age, mean (SD), years | 56.8 (1.8) | 55.3 (2.0) | 65.4 (4.9) | 0.0336 | 53.8 (4.0) | 56.4 (2.4) | 61.1 (4.2) | 0.2315 |
| Weight, mean (SD), kg | 89.6 (2.7) | 88.9 (2.9) | 93.1 (7.9) | 0.5685 | 93.1 (7.5) | 91.7 (3.6) | 80.9 (4.0) | 0.2845 |
| BMI (SD) | 30.9 (0.9) | 30.9 (1.0) | 31.2 (2.0) | 0.7280 | 31.8 (2.4) | 32.3 (1.1) | 27.1 (1.9) | 0.1076 |
| Gender, n (%) | ||||||||
| Male | 53 (52.5) | 45 (52.3) | 8 (53.3) | – | 11 (55) | 32 (55.2) | 11 (45.8) | – |
| Female | 48 (47.5) | 41 (47.7) | 7 (46.7) | – | 9 (45) | 26 (44.8) | 13 (54.2) | – |
| Race, n (%) | ||||||||
| White | 86 (85.1) | 73 (84.9) | 13 (86.7) | – | 19 (95) | 47 (81.0) | 21 (87.5) | – |
| Hispanic | 9 (8.9) | 7 (8.1) | 2 (13.3) | – | 1 (5) | 6 (10.3) | 2 (8.3) | – |
| Comorbidities and medications, n | ||||||||
| Cirrhosis | 6 | 4 | 2 | – | 2 | 3 | 1 | – |
| Recent/active cancer | 8 | 7 | 1 | – | 1 | 4 | 3 | – |
| CVD | 20 | 15 | 5 | – | 4 | 13 | 4 | – |
| Direct thrombin inhibitor | 20 | 15 | 5 | – | 4 | 13 | 4 | – |
| Warfarin | 6 | 5 | 1 | – | 3 | 1 | 2 | – |
| Lovenox (prophylactic) | 0 | – | – | – | – | – | – | – |
| Lovenox (therapeutic) | 0 | – | – | – | – | – | – | – |
| Illness severity | ||||||||
| Length of stay, mean (SD), days | 11.1 (19.5) | 8.6 (6.7) | 26.4 (48.2) | 0.1534 | 9 (5.7) | 12.14 (24.5) | 10.05 (8.9) | 0.7143 |
| Septic shock (VP use day 0), n | 45 | 33 | 12 | – | 5 | 27 | 13 | – |
| Ventilator use at day 0, n | 48 | 43 | 5 | – | 10 | 25 | 13 | – |
| 28-Day mortality, n (%) | – | – | – | 2 (10) | 7 (12.1) | 6 (25) | – | |
Abbreviations: BMI, body mass index; CVD; cardiovascular disease; DIC, disseminated intravascular coagulation; SD, standard deviation; VP, vasopressor.
a P is a Mann-Whitney t test between means of survivors and nonsurvivors.
b P is a Kruskal-Wallis 1-way ANOVA for medians of DIC severity groups.
Table 2.
Baseline (Day 0) Clinical Illness Severity Scores Listed by Mortality Outcome (Left) and DIC Severity (Right).
| Clinical Illness Severity Scores | All | Survivors, n = 86 | Non-survivors, n = 15 | a P | Sepsis, n = 20 | Sepsis + Non-overt DIC, n = 58 | Sepsis + Overt DIC, n = 24 | b P |
|---|---|---|---|---|---|---|---|---|
| SOFA score | ||||||||
| Mean ± SEM | 5.9 ± 0.4 | 5.4 ± 0.4 | 8.5 ± 0.8 | 0.0027 | 4.2 ± 0.6 | 5.7 ± 0. | 7.5 ± 0.6 | – |
| Median (range) | 6 (0-14) | 5 (0-14) | 9 (1-13) | – | 4 (0-10) | 56 (0-14) | 8 (2-13) | 0.010 |
| MOD score | ||||||||
| Mean ± SEM | 5.2 ± 0.4 | 4.8 ± 0.4 | 7.2 ± 1.1 | 0.0445 | 3.8 ± 0.6 | 5 ± 0.5 | 6.6 ± 0.8 | – |
| Median (range) | 5 (0-15) | 5 (0-15) | 8 (1-14) | – | 4 (0-9) | 5 (0-14) | 8 (0-15) | 0.0478 |
| APACHE II score | ||||||||
| Mean ± SEM | 16.4 ± 0.8 | 23.1 ± 1.7 | 0.0003 | 16 ± 1.4 | 17.2 ± 1.0 | 18.8 ± 1.4 | – | |
| Median (range) | 16 (4-42) | 15 (4-37) | 22 (13-42) | – | 15 (5-30) | 16 (4-42) | 19 (7-30) | 0.3657 |
| APACHE III score | ||||||||
| Mean ± SEM | 79.3 ± 3.7 | 74.7 ± 4.0 | 100.8 ± 5.7 | 0.0027 | 59.6 ± 10.1 | 78.6 ± 4.5 | 90.6 ± 6.9 | – |
| Median (range) | 79 (14-150) | 73 (14-150) | 107.5 (67-127) | – | 51 (14-106) | 79.5 (15-140) | 91.5 (41-150) | 0.0786 |
Abbreviations: APACHE III, Acute Physiology and Chronic Health Evaluation III; DIC, disseminated intravascular coagulation; MOD, Multiple Organ Dysfunction Syndrome; SEM, standard error of the mean; SOFA, Sequential Organ Failure Assessment.
a P is a Mann-Whitney t test between means of survivors and nonsurvivors.
b P is a Kruskal-Wallis 1-way ANOVA for medians of DIC severity groups.
Table 3.
Day 0, Day 4, and Day 8 Ang-2 Levels Listed by Mortality Outcome (Left) and DIC Severity (Right).
| Ang-2 Levels, ng/mL: Day 0, Day 4, Day 8 | All | Survivors, n = 86 | Non-survivors, n = 15 | a P | Sepsis, n = 20 | Sepsis + Non-overt DIC, n = 58 | Sepsis + Overt DIC, n = 24 | b P |
|---|---|---|---|---|---|---|---|---|
| Healthy Controls | ||||||||
| n (%) | 50 | – | – | – | – | – | – | – |
| Mean ± SEM | 1.9 ± 0.2 | – | – | – | – | – | – | – |
| Median (range) | 1.6 (0.5-5.5) | |||||||
| Day 0 patients | ||||||||
| n (%) | c101 | 86 (85.1) | 15 (14.9) | – | 20 (19.6) | 58 (56.9) | 24 (23.5) | – |
| Mean ± SEM | 15.2 ± 1.9 | 12.5 ± 1.5 | 30.2 ± 8.6 | 0.0010 | 8.3 ± 2.5 | 11.7 ± 1.4 | 29.4 ± 6.3 | – |
| Median (range) | 8.3 (0.7-136.3) | 7.4 (0.7-66.2) | 19.3 (1.8-136.3) | – | 5.8 (1.0-53.6) | 8.3 (0.7-44.2) | 17.6 (1.8-136.3) | 0.0003 |
| Day 4 patients | ||||||||
| n (%) | 55 | 45 (81.8) | 10 (18.2) | – | 10 (17.9) | 36 (64.2) | 10 (17.9) | – |
| Mean ± SEM | 7.3 ± 1.1 | 6.7 ± 1.2 | 9.8 ± 2.1 | 0.0819 | 4.7 ± 1.0 | 7.9 ± 1.5 | 7.8 ± 2.3 | – |
| Median (range) | 5.7 (0.1-53.2) | 4.4 (0.1-53.2) | 9.4 (1.3-24.5) | – | 4.4 (0.1-11.3) | 6.8 (0.4-53.2) | 6.7 (1.6-24.5) | 0.5494 |
| Day 8 patients | ||||||||
| n (%) | 30 | 24 (80) | 6 (20) | – | 8 (26.7) | 22 (73.3) | 0 | – |
| Mean ± SEM | 8.5 ± 2.1 | 7.6 ± 2.5 | 11.7 ± 4.1 | 0.2264 | 2.2 ± 0.3 | 10.8 ± 2.7 | – | – |
| Median (range) | 4.9 (0.5-61.0) | 3.2 (0.9-61.0) | 11.0 (0.5-26.9) | – | 2.0 (1.1-3.2) | 8.2 (0.5-61.0) | – | – |
Abbreviations: Ang-2, Angiopoietin-2; DIC, disseminated intravascular coagulation; SEM, standard error of the mean.
a P is a Mann-Whitney t test between means of survivors and nonsurvivors.
b P is a Kruskal-Wallis 1-way ANOVA for medians of DIC severity groups.
c Mortality data, but not DIC severity data, was missing for 1 patient; therefore, n = 101 for baseline mortality data and n = 102 for baseline DIC severity data.
Demographic and clinical information for this patient cohort is provided in Table 1. Information regarding comorbidities, anticoagulant use, length of hospital stay, and ventilator use was also available for this patient population. Patient samples were collected before the regulatory approval of the direct anti-Xa agents apixaban and rivaroxaban; thus, these agents are not included in the table.
As sepsis can occur across a spectrum of severity, it is important to quantify disease severity in any study population. The presence of septic shock (defined by vasopressor use at day 0) is indicative of more severe illness. Several clinical scoring systems were used to describe the severity of critical illness in our patients. The SOFA and MOD scores (calculated based on available clinical information) incorporate measures of respiratory, hematologic, hepatic, cardiovascular, central nervous system, and renal function in order to describe clinical status. The APACHE II and III scores (calculated at ICU admission) incorporate similar but not identical criteria.50–52 A higher score is indicative of a worse prognosis (see Tables 1 and 2).
The DIC score was calculated in all patients using the 2001 ISTH scoring algorithm for DIC subclassification into no DIC (score <3), non-overt DIC (score 3-4), and overt DIC (score ≥5; Table 4).53 The score is calculated using laboratory values, including platelet count, fibrin markers, prothrombin time (PT), and fibrinogen level. Presence of a predisposing condition is a prerequisite for the application of this scoring system; this requirement was met for all patients in this population by the presence of sepsis. The 28-day mortality, the most important outcome to measure for sepsis and DIC, was calculated for the 101 patients in which mortality data was available.
Table 4.
ISTH DIC Scoring System.
| Variable | Value | Points |
|---|---|---|
| Platelets, K/μL | >100 | 0 |
| 50-100 | 1 | |
| <50 | 2 | |
| INR | <1.3 | 0 |
| 1.3-1.7 | 1 | |
| >1.7 | 2 | |
| d-Dimer, ng/mL | <400 | 0 |
| 400-4000 | 2 | |
| >4000 | 3 | |
| Fibrinogen, mg/dL | >100 | 0 |
| <100 | 1 |
Abbreviations: DIC, disseminated intravascular coagulation; INR, international normalized ratio; ISTH, International Society on Thrombosis and Haemostasis.
Platelet counts, international normalized ratio (INR), and fibrinogen levels, all components of the DIC score, were acquired in the normal course of patient care following standard hospital protocols and were provided with patient samples. The PT/INR and fibrinogen values were not provided with some samples and were instead measured using standard procedures on an ACL-ELITE coagulation analyzer (Instrumentation Laboratories, Bedford, MA). The INR values were calculated as (patient PT/11.9seconds)1.04. The d-Dimer levels for all samples were measured using a commercially available enzyme-linked immunosorbent assay (ELISA) test (Hypehen BioMed, Neuville-Sur-Oise, France).
The Ang-2 levels were measured in all patient and control samples using a commercially available sandwich-type ELISA kit according to the manufacturer’s instructions (R&D Systems, Minneapolis, Minnesota). Optical density was measured at the wavelength of 450 nm using a spectrophotometer and SoftMaxPro software (Molecular Devices, Sunnyvale, California). Control samples, purchased from George King Bio-Medical (Overland Park, Kansas), were from 25 male and 25 female nonsmoker, non-medicated, healthy volunteers, aged 19 to 54 years, with a mean age of 32 years. Pooled normal human plasma and pooled pathological human plasma samples were included on each plate to monitor inter-assay variation. Patient and control samples were analyzed in single runs. Samples with an optical density exceeding the highest calibrator were diluted and repeated on a subsequent plate. Samples with an optical density value resulting in a calculated analyte concentration of <0 were recorded as having a concentration of 0.
All statistical analyses were performed using GraphPad Prism (version 7.02 for Windows, GraphPad Software, La Jolla, California). Multiple non-parametric Mann-Whitney tests assessed differences between healthy controls and patients at baseline (day 0), day 4, and day 8, and a single Mann-Whitney U test compared differences between survivors and non-survivors. Differences at baseline, day 4, and day 8, as well as differences among the various sepsis and ISTH DIC severity categories were assessed using non-parametric Kruskal-Wallis tests. Dunn multiple comparisons test assessed differences between mean ranks of individual groups. Receiver–operating characteristic (ROC) procedures were used to predict mortality outcome and illness severity (specifically, sepsis alone vs sepsis + overt DIC), and the area under the ROC curves (AUC) were calculated. Data is displayed as mean ± standard error of the mean (SEM) or median ± range as appropriate (Tables 1 -3). All analyses were 2 tailed, with P values <0.05 considered statistically significant.
Results
Mean Ang-2 Levels in All Patients at Baseline, Day 4, and Day 8 Were Significantly Higher Than Controls
Mean Ang-2 levels in all patients with sepsis and sepsis plus any severity of DIC at baseline, day 4, and day 8 were compared to those in normal controls (Figure 1, Table 3). The Ang-2 levels in patients on all days were significantly higher in comparison to controls (*P, ** P, and ***P <0.0001).
Figure 1.

Plasma Ang-2 levels in patients with sepsis and DIC on ICU day 0, day 4, and day 8 compared to normal controls (mean ± SEM; Mann-Whitney U test). Ang-2 indicates Angiopoietin-2; DIC, disseminated intravascular coagulation; ICU, intensive care unit; SEM, standard error of the mean.
Plasma Levels of Ang-2 Show a Downward Trend Over Time
Serial plasma samples from all patients at baseline, day 4, and day 8 were compared to understand the effect of standard of care for patients with sepsis and suspected DIC on Ang-2 levels (Figure 2; Table 3). Blood draws on subsequent days were only performed on patients who remained in the ICU. There was a significant difference in median Ang-2 levels on different days (P = 0.0008). In particular, day 0 showed a significantly higher Ang-2 level in comparison to day 4 (*P = 0.0027) and day 8 (**P = 0.0226). However, there was no significant difference between Ang-2 levels on day 4 in comparison to day 8 (P > 0.999).
Figure 2.

Trendline of median plasma Ang-2 levels in all patients with sepsis and suspected DIC on day 0, day 4, and day 8. Ang-2 indicates Angiopoietin-2; DIC, disseminated intravascular coagulation.
Lower Baseline Ang-2 Plasma Levels and Lower Illness Severity Scores had Better Mortality Outcomes
Of the 101 patients in which mortality data was reported, 86 survived and 15 died, giving a 28-day mortality rate of 15%. Mortality was notably higher in the overt DIC group (25%) compared to patients with sepsis alone (10%; Table 1). Mean Ang-2 levels at admission and baseline clinical measures of illness severity, including SOFA score, MOD score, and APACHE II and III scores, were significantly lower in survivors compared to non-survivors (Figure 3; Tables 2 and 3).
Figure 3.
Baseline Ang-2 levels and clinical illness severity scores for the 28-day survival outcome (mean ± SEM). Ang-2 indicates Angiopoietin-2; SEM, standard error of the mean.
Figure 4 shows the ROC curves of all baseline parameters for predicting death in SAC. The AUC values (AUC, SEM, P value) for Ang-2 (0.7597, 0.06623, 0.0014), SOFA score (0.738, 0.06596, 0.0034), MOD score (0.662, 0.08023, 0.0459), APACHE II score (0.7802, 0.0535, 0.0006), and APACHE III score (0.7697, 0.06587, 0.0035) were similar. Therefore, Ang-2 was as accurate as commonly used clinical severity scores at predicting mortality.
Figure 4.

Receiver–operating characteristic (ROC) curves for prediction of 28-day mortality based on baseline measurements.
Angiopoietin 2 as a Clinical Predictor of SAC Severity
To understand the association of Ang-2 and clinical illness scores with the categorization of SAC, baseline Ang-2 levels were stratified based on the ISTH DIC severity scoring system (Figure 5). The Ang-2, SOFA scores, and MOD scores showed significant differences, while APACHE II and III scores showed no significant differences between any of the SAC severity categories (Tables 2 and 3). The Ang-2 levels (P = 0.0004), SOFA scores (P = 0.0081), and MOD scores (P = 0.0469) were significantly lower in the sepsis category in comparison to the sepsis + overt DIC category. Additionally, Ang-2 levels were significantly lower in the sepsis + non-overt DIC group in comparison to the sepsis + overt DIC group (P = 0.005). Neither Ang-2 nor any illness severity score showed a significant difference between patients with sepsis alone compared to the sepsis + non-overt DIC group.
Figure 5.
Baseline Ang-2 levels outperform clinical illness scores in differentiating patients with different severities of DIC (median ± range). Ang-2 indicates Angiopoietin-2; DIC, disseminated intravascular coagulation.
Figure 6 shows the ROC curves of all baseline parameters for predicting SAC severity (specifically, sepsis alone vs sepsis + overt DIC). The AUC values (AUC, SEM, P value) of the different parameters were not the same, as median Ang-2 (0.8292, 0.06403, 0.0002) outperformed SOFA score (0.7771, 0.06984, 0.0017), MOD score (0.7094, 0.07842, 0.0178), APACHE II score (0.6167, 0.08634, 0.1869), and APACHE III score (0.7778, 0.09547, 0.0206). This indicates that admission Ang-2 levels may be more accurate than clinical illness scores at predicting the presence of DIC in patients with sepsis.
Figure 6.
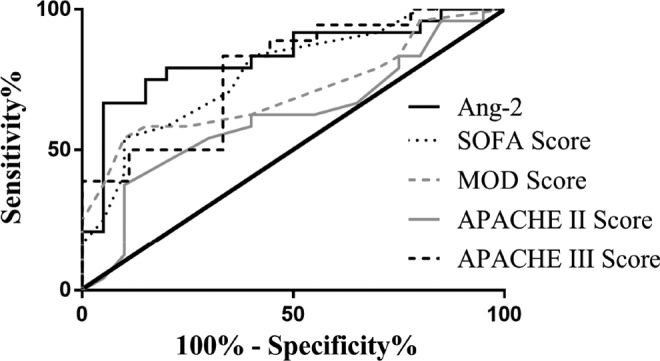
Receiver–operating characteristic (ROC) curves for DIC severity category prediction by baseline parameters. DIC indicates disseminated intravascular coagulation.
Results Summary
The results of this study show that Ang-2 levels are increased in patients with sepsis and suspected DIC compared to healthy controls, and median Ang-2 levels tend to decrease over time with standard of care. Additionally, baseline Ang-2 levels and clinical illness scores predicted 28-day mortality with comparable accuracy, while Ang-2 was a more accurate predictor of DIC severity.
Discussion
DIC is a systemic, secondarily acquired syndrome that affects multiple organ systems and has widespread consequences.1 An understanding of the role of the endothelium in sepsis and DIC is crucial. DIC originates from and causes damage to the endothelial-lined microvasculature, which if sufficiently severe can cause organ dysfunction.53 The pathophysiology of DIC involves three main mechanisms: (1) inflammatory cytokine-mediated activation of TF-dependent coagulation, (2) suppression or downregulation of anticoagulation pathways, and (3) inactivation or suppression of fibrinolysis.2 Combined, these processes result in thrombosis and endothelial dysfunction that can lead to organ failure.
The endothelium is a systemically distributed organ system that forms a barrier between the bloodstream and every organ of the body.44 ECs are highly heterogeneous and function in a site-specific manner and serve as a key connection between the immune system, endocrine and neural signaling, and coagulation.4,5 The endothelium is involved in a number of vital functions, including angiogenesis, balancing pro- and anti-inflammatory mediators, regulation of blood pressure, mediating leukocyte extravasation to sites of injury in immune responses, maintaining fluid balance, and providing oxygen and nutrient transport. ECs ultimately function to maintain homeostasis and respond appropriately to disturbances in homeostasis via their interaction with other organ systems.5,6,54–56
The (Ang)-Tie2 system and vascular endothelial growth factor (VEGF) are the two main regulators of the endothelium, and endothelial dysfunction is central in SAC pathophysiology.43–46 These two regulators have been studied in a variety of critical illnesses, but VEGF has been the subject of much more research and its role has been more firmly established than Ang-2. The literature is currently insufficient to recognize Ang-2 as a standard biomarker in DIC and SAC. Our results indicate the possible utility of Ang-2 in SAC diagnosis and treatment, prediction of DIC severity, and overall mortality prediction.
Angiopoietin is a 70-kDa glycoprotein that binds to Tie2, an endothelial growth factor receptor tyrosine kinase (RTK). Four angiopoietins (Ang-1 through Ang-4) have been discovered. Tie2 is unique among growth factor RTKs in that it is constitutively activated to maintain quiescence in mature vasculature, whereas most growth factors would not be active in the adult.57–60
Angiopoietin-1, the Tie2 agonist, plays an important role during both vascular development and in the maintenance of healthy vasculature. During angiogenesis, Ang-1 signaling guides correct blood vessel patterning, such as vessel branching and vessel diameter.61 In healthy, developed vasculature, Ang-1 is constitutively excreted by perivascular cells, and to a lesser extent by platelets, to maintain quiescence.62,63 The Ang-1 tetramers and higher order oligomers induce autophosphorylation of the Tie2 RTK.57,64,65 Ultimately, phosphorylated Tie2 influences downstream signaling pathways (including the PI3K/AKT pathway, Rac1 and RhoA kinases, and vascular adhesion molecule 1 and intercellular adhesion molecule 1) in a defined and non-redundant manner to maintain vascular quiescence through anti-permeability, anti-inflammatory, and anti-apoptotic effects.5,6,62
Angiopoietin-2, the counterpart to Ang-1, is produced by ECs and macrophages. It is a much weaker agonist of Tie2 such that the receptor is effectively dephosphorylated and thus inactivated when Ang-2 binding displaces Ang-1 from receptor-binding sites. Therefore, Ang-2 is widely considered a Tie2 competitive antagonist. Decreased activation of Tie2 causes activation of the endothelium, resulting in the loss of barrier integrity and a pro-inflammatory, pro-apoptotic phenotype. Ultimately, Ang-2 causes the endothelium to transition from a quiescent to a pro-inflammatory state through inhibition of Ang-1-mediated signaling pathways as well as through cross-talk with various pro-inflammatory cytokines.5–15
Various strategies have demonstrated the benefits of Ang-1- and/or Ang-2-based therapies in critical illness. The Ang-1 treatment methods in animal models, such as adenoviral transfer of Ang-1 and mesenchymal stem cells transfected with Ang-1, have shown success in treating systemic inflammatory diseases such as endotoxic shock,28 lipopolysaccharide (LPS)-induced ALI,66 LPS-induced AKI,67 and others. Improved hemodynamic parameters, organ function, survival, and a decreased inflammatory response have also been achieved with Ang-2 inhibiting or depleting strategies in different critical illnesses.6,14,23,24,26
However, unlike the consistently increased admission Ang-2 levels in various disease states, Ang-1 levels have not shown consistent differences in comparison to control patients when assessing the severity of critical illnesses.4 Ang-1 levels showed no change in studies of atrial fibrillation,42 congestive heart failure,39 acute coronary syndrome,40 exudative pleural effusions,30 and acutely ill patients early after trauma.31 A study of hypertension leading to end-organ damage showed an increase in Ang-1,38 while another study of sepsis and septic shock in children19 and many similar studies in adults10,13,15,18,19,27 showed a decrease in Ang-1.
The results of our study were consistent with the existing literature in that patients with sepsis had significantly higher Ang-2 levels in comparison to normal healthy controls.10,12,13,23,43 Regardless of sepsis and suspected DIC severity, all patients in our study had elevated Ang-2 levels on day 0, day 4, and day 8 compared to normal controls. Higher DIC scores were also associated with higher Ang-2 levels. Jesmin and colleagues described a similar finding of elevated Ang-2 levels associated with a worse mortality outcome in SAC.43 A possible mechanism driving the increased plasma levels of Ang-2 in our patients is endothelial dysregulation leading to tissue hypoxia and increased thrombin formation which causes transcriptional upregulation and release of stored angiopoietin.44–46 Research into Ang-2 and other endothelial-regulating factors may provide a promising future for sepsis and DIC workup, as the endothelium is a highly dynamic, key organ system for maintaining homeostasis in the face of inflammatory insults.
When comparing baseline, day 4, and day 8 median Ang-2 levels, a downward trend appeared over time. Baseline Ang-2 levels were higher when compared separately to Ang-2 levels at day 4 and day 8, but day 4 and day 8 did not show significantly different Ang-2 levels. Other studies have found a similar downward trend in Ang-2 over time,43 with one study of morbidity and mortality in sepsis finding that survivors at twenty-eight days had significantly lower longitudinal Ang-2 levels compared to non-survivors.10 Clinicians could benefit from knowing a patient’s Ang-2 level in comparison to the normal level over the length of a hospital stay for two reasons. First, clinical suspicion of critical illness could be assisted by an admission Ang-2 level that is greater than a normal value. Second, if the patient is being adequately treated with proper standard of care, the clinician should expect the Ang-2 level to decrease over time.
It is well established in existing literature that Ang-2 levels predict illness severity and 28-day mortality outcome in sepsis, and our findings are consistent with this as mentioned previously.10,12,13,23 However, there have not been any studies comparing Ang-2 and clinical illness severity scores in risk stratifying patients with sepsis, sepsis + overt DIC, or sepsis + non-overt DIC. As Tables 2 and 3 and Figure 5 show, median Ang-2 levels, SOFA score, and MOD score at baseline showed significant differences between the sepsis and sepsis + overt DIC groups. Additionally, Ang-2 showed significant differences between the sepsis + non-overt and sepsis + overt DIC groups. Importantly, Figure 6 reports an AUC value of 0.8292 for Ang-2, which outperforms SOFA, MOD, and APACHE II and III scores in predicting DIC severity in patients with sepsis at baseline. Ang-2 and other markers of endothelial activation show promise as novel tools for identifying patients with different severities of SAC that may otherwise be misdiagnosed by clinical illness severity scores alone.
When sepsis is suspected, most current practices implement a protocol in which blood is drawn for cultures and lactate measurement. Lactate has been used to help evaluate severity of disease and guide treatment, but it is a non-specific indicator of organ hypoxia.68–70 An endothelial biomarker such as Ang-2 could be incorporated into sepsis protocols to serve as a point-of-care marker of endothelial dysfunction. Ang-2, alone or in a panel of other biomarkers relevant to SAC, may be used as an indicator of sepsis and suspected DIC severity, negating the need for clinical scores of illness severity. Current clinical scores and ICU physicians’ prediction of survival have been shown to be unsatisfactory.71,72 Sinuff and colleagues showed that clinical prediction tools such as MOD and SOFA scores were not superior to a medical ICU physician’s overall prediction of survivors or non-survivors, especially in the first twenty-four hours after admission.72 In fact, their conclusions stated that physicians were better at predicting survival outcomes on ICU admission compared to clinical prediction tools; however, both physician assessment and clinical scores only had moderate accuracy.72 Therefore, there is a need for an objective laboratory measure that can improve survivor outcome predictions. Survival probabilities can not only aid physicians in treatment, triage, and delegation of limited medical resources in certain settings but could also give better answers to families of patients with sepsis and provide them with more accurate assessments of survival. Additionally, if physicians are better able to predict the severity of critical illnesses such as DIC, earlier, more aggressive interventions and treatments may be initiated to increase survival rates.
Acknowledgments
The authors gratefully acknowledge the staff of the Clinical Laboratories at the Loyola University Medical Center and the University of Utah Medical Center for the expert collection of the blood samples included in this study. We would also like to thank members of the Hemostasis and Thrombosis Research Laboratory for their continued guidance and collaboration during this study. Special thanks to Dr. Omar Iqbal for his helpful suggestions and facilitation of this study. Thanks to Dr. Eva Wojick, Chair of the Department of Pathology, and Dr. Keith Jones, Chair of the Department of Pharmacology, for their support during this study. A special thanks to Provost Margaret Calahan and Dean Goldstein for their leadership and continual support to foster medical student research programs.
Authors’ Note: Paper was presented orally at International Society on Thrombosis and Hemostasis, Berlin, Germany, July 2017. Ethical approval to report this case was obtained from Loyola University Chicago IRB (LU number 207958). Written informed consent was obtained from the patient(s) for their anonymized information to be published in this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Singer M, Deutschman CS, Seymour C, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801–810. doi:10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gando S, Levi M, Toh C. Disseminated intravascular coagulation. Nat Rev Dis Primers. 2016;2:16037 doi:10.1038/nrdp.2016.37.2016;2:16037. [DOI] [PubMed] [Google Scholar]
- 3. Levi M. The coagulant response in sepsis. Clin Chest Med. 2008;29(4):627–642. [DOI] [PubMed] [Google Scholar]
- 4. Van Meurs M, Kümpers P, Ligtenberg JJ, Meertens JH, Molema G, Zijlstra JG. Bench-to-bedside review: angiopoietin signalling in critical illness – a future target? Crit Care. 2009;13(2):207 doi:10.1186/cc7153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lukasz A, Kümpers P, David S. Role of angiopoietin/Tie2 in critical illness: promising biomarker, disease mediator, and therapeutic target? Scientifica (Cairo). 2012;2012:160174 doi:10.6064/2012/160174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. David S, Kümpers P, van Slyke P, Parikh SM. Mending leaky blood vessels: the angiopoietin-Tie2 pathway in sepsis. J Pharmacol Exp Ther. 2013;345(1):2–6. [DOI] [PubMed] [Google Scholar]
- 7. Moss A. The angiopoietin: Tie2 interaction: a potential target for future therapies in human vascular disease. Cytokine Growth Factor Rev. 2013;24(6):579–592. doi:10.1111/nyas.12726. [DOI] [PubMed] [Google Scholar]
- 8. Scholz A, Plate KH, Reiss Y. Angiopoietin-2: a multifaceted cytokine that functions in both angiogenesis and inflammation. Ann N Y Acad Sci. 2015;1347:45–51. [DOI] [PubMed] [Google Scholar]
- 9. Siner JM. A tale of two ligands: angiopoietins, the endothelium, and outcomes. Crit Care. 2013;17(5):1007 doi:10.1186/cc13066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ricciuto DR, dos Santos CC, Hawkes M, et al. Angiopoietin-1 and angiopoietin-2 as clinically informative prognostic biomarkers of morbidity and mortality in severe sepsis. Crit Care Med. 2011;39(4):702–710. doi:10.1097/CCM.0b013e318206d285. [DOI] [PubMed] [Google Scholar]
- 11. Mussap M, Cibecchini F, Noto A, Fanos V. In search of biomarkers for diagnosing and managing neonatal sepsis: the role of angiopoietins. J Matern Fetal Neonatal Med. 2013;26(suppl 2):24–26. [DOI] [PubMed] [Google Scholar]
- 12. Kümpers P, Lukasz A, David S, et al. Excess circulating angiopoietin-2 is a strong predictor of mortality in critically ill medical patients. Crit Care. 2008;12(6):R147 doi:10.1186/cc7130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Siner JM, Bhandari V, Engle KM, Elias JA, Siegel MD. Elevated serum angiopoietin 2 levels are associated with increased morality in sepsis. Shock. 2009;31(4):348–353. doi:10.1097/SHK.0b013e318188bd06. [DOI] [PubMed] [Google Scholar]
- 14. Fiedler U, Reiss Y, Scharpfenecker M, et al. Angiopoietin-2 sensitizes endothelial cells to TNF-α and has a crucial role in the induction of inflammation. Nat Med. 2006;12(2):235–239. [DOI] [PubMed] [Google Scholar]
- 15. Parikh SM, Mammoto T, Schultz A, et al. Excess circulating angiopoietin-2 may contribute to pulmonary vascular leak in sepsis in humans. PLoS Med. 2006;3(3):e46 doi:10.1371/journal.pmed.0030046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Warren BL, Eid A, Singer P, et al. High-dose antithrombin iii in severe sepsis: a randomized controlled trial. JAMA. 2001;286:1869–1878. [DOI] [PubMed] [Google Scholar]
- 17. Ranieri VM, Thompson BT, Barie PS, et al. Drotrecogin alfa (activated) in adults with septic shock. N Engl J Med. 2012;366(22):2055–2064. [DOI] [PubMed] [Google Scholar]
- 18. Orfanos SE, Kotanidou A, Glynos C, et al. Angiopoietin-2 is increased in severe sepsis: correlation with inflammatory mediators. Crit Care Med. 2007;35(1):199–206. [DOI] [PubMed] [Google Scholar]
- 19. Giuliano JS, Lahni PM, Harmon K, et al. Admission angiopoietin levels in children with septic shock. Shock. 2007;28(6):650–654. [PMC free article] [PubMed] [Google Scholar]
- 20. Kümpers P, van Meurs M, David S, et al. Time course of angiopoietin-2 release during experimental human endotoxemia and sepsis. Crit Care. 2009;13(3):R64 doi:10.1186/cc7866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Davis JS, Yeo TW, Piera KA, et al. Angiopoietin-2 is increased in sepsis and inversely associated with nitric oxide-dependent microvascular reactivity. Crit Care. 2010;14(3):R89 doi:10.1186/cc9020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Amerongen GP van N, Groeneveld AJ. A plethora of angiopoietin-2 effects during clinical sepsis. Crit Care. 2010;14(3):166 doi:10.1186/cc9053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. David S, Mukherjee A, Ghosh CC, et al. Angiopoietin-2 may contribute to multi-organ dysfunction and death in sepsis. Crit Care Med. 2012;40(11):3034–3041. doi:10.1097/CCM.0b013e31825fdc31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ziegler T, Horstkotte J, Schwab C, et al. Angiopoietin 2 mediates microvascular and hemodynamic alterations in sepsis. J Clin Invest. 2013;123(8):3436–3445. doi:10.1172/JCI66549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fisher J, Douglas JJ, Linder A, Boyd JH, Walley KR, Russel JA. Elevated plasma angiopoietin-2 levels are associated with fluid overload, organ dysfunction, and mortality in human septic shock. Crit Care Med. 2006;44(11):2018–2027. doi:0.1097/CCM.0000000000001853. [DOI] [PubMed] [Google Scholar]
- 26. Tzepi I, Giamarellos-Bourboulis E, Carrer D, et al. Angiopoietin-2 enhances survival in experimental sepsis induced by multidrug-resistant Pseudomonas aeruginosa. J Pharmacol Exp Ther. 2012;343(2):278–287. [DOI] [PubMed] [Google Scholar]
- 27. Lukasz A, Hellpap J, Horn R, et al. Circulating angiopoietin-1 and angiopoietin-2 in critically ill patients: development and clinical application of two new immunoassays. Critical Care. 2008;12(4):R94 doi:10.1186/cc6966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Witzenbichler B, Westermann D, Knueppel S, Schultheiss H, Tschope C. Protective role of angiopoietin-1 in endotoxic shock. Circulation. 2005;111(1):97–105. [DOI] [PubMed] [Google Scholar]
- 29. van der Heijden M, van Nieuw Amerongen GP, Chedamni S, van Hinsbergh VW, Johan Groeneveld AB. The angiopoietin-Tie2 system as a therapeutic target in sepsis and acute lung injury. Expert Opin Ther Targets. 2009;13(1):39–53. [DOI] [PubMed] [Google Scholar]
- 30. Kalomenidis I, Kollintza A, Sigala I, et al. Angiopoietin-2 levels are elevated in exudative pleural effusions. Chest. 2006;129(5):1259–1266. [DOI] [PubMed] [Google Scholar]
- 31. Ganter MT, Cohen MJ, Brohi K, et al. Angiopoietin-2, marker and mediator of endothelial activation with prognostic significant early after trauma? Ann Surg. 2008;247(2):320–326. doi:10.1097/SLA.0b013e318162d616. [DOI] [PubMed] [Google Scholar]
- 32. Parikh SM. Dysregulation of the angiopoietin–Tie-2 axis in sepsis and ARDS. Virulence. 2013;4(6):517–524. doi:10.4161/viru.24906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Aslan A, van Meurs M, Moser J, et al. Organ-specific differences in endothelial permeability-regulating molecular responses in mouse and human sepsis. Shock. 2017;48(1):69–77. doi:10.1097/SHK.0000000000000841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hadem J, Bockmeyer CL, Lukasz A, et al. Angiopoietin-2 in acute liver failure. Crit Care Med. 2012;40(5):1499–1505. doi:10.1097/CCM.0b013e318241e34e. [DOI] [PubMed] [Google Scholar]
- 35. Kümpers P, Hafer C, David S, et al. Angiopoietin-2 in patients requiring renal replacement therapy in the ICU: relation to acute kidney injury, multiple organ dysfunction syndrome and outcome. Intensive Care Med. 2010;36:462–470. [DOI] [PubMed] [Google Scholar]
- 36. Lukasz A, Beneke J, Thamm K, et al. Involvement of angiopoietin-2 and Tie2 receptor phosphorylation in STEC-HUS mediated by Escherichia coli O104: H4. Mediators Inflamm. 2015;2015:670248 doi:10.1155/2015/670248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Safioleas K, Giamarellos-Bourboulis E, Carrer D, et al. Reverse kinetics of angiopoietin-2 and endotoxins in acute pyelonephritis: implications for anti-inflammatory treatment? Cytokine. 2016;81:28–34. [DOI] [PubMed] [Google Scholar]
- 38. Nadar SK, Blann A, Beevers DG, Lip GY. [Abnormal angiopoietins 1&2, angiopoietin receptor Tie-2 and vascular endothelial growth factor levels in hypertension: relationship to target organ damage a sub-study of the Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT)]. J Intern Med. 2005;258(4):336–343. [DOI] [PubMed] [Google Scholar]
- 39. Chong AY, Caine GJ, Freestone B, Blann AD, Lip GY. Plasma angiopoietin-1, angiopoietin-2, and angiopoietin receptor Tie-2 levels in congestive heart failure. J Am Coll Cardiol. 2004;43(3):423–428. [DOI] [PubMed] [Google Scholar]
- 40. Lee KW, Lip GY, Blann AD. Plasma angiopoietin-1, angiopoietin-2, angiopoietin receptor Tie-2, and vascular endothelial growth factor levels in acute coronary syndromes. Circulation. 2004;110(16):2355–2360. [DOI] [PubMed] [Google Scholar]
- 41. Wu H, Shou X, Liang L, Wang C, Yao X, Cheng G. Correlation between plasma angiopoietin-1, angiopoietin-2 and matrix metalloproteinase-2 in coronary heart disease. Arch Med Sci. 2016;12(6):1214–1219. doi:10.5114/aoms.2016.62909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Choudhury A, Freestone B, Patel J, Lip GYH. Relationship of soluble CD40 ligand to vascular endothelial growth factor, angiopoietins, and tissue factor in atrial fibrillation: a link among platelet activation, angiogenesis, and thrombosis? Chest. 2007;132(6):1913–1919. [DOI] [PubMed] [Google Scholar]
- 43. Jesmin S, Wada T, Gando S, Sultana SS, Zaedi S. The dynamics of angiogenic factors and their soluble receptors in relation to organ dysfunction in disseminated intravascular coagulation associated with sepsis. Inflammation. 2013;36(1):186–196. [DOI] [PubMed] [Google Scholar]
- 44. Fiedler U, Augustin HG. Angiopoietins: a link between angiogenesis and inflammation. Trends Immunol. 2006;27(12):552–558. [DOI] [PubMed] [Google Scholar]
- 45. Bae J-S, Rezaie AR. Thrombin up-regulates the angiopoietin/Tie2 axis: EPCR occupancy prevents the thrombin mobilization of angiopoietin2 and P-selectin from Weibel-Palade bodies. J Thromb Haemost. 2010;8(5):1107–1115. doi:10.1111/j.1538-7836.2010.03812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Oh H, Takagi H, Suzuma K, Otani A, Matsumura M, Honda Y. Hypoxia and vascular endothelial growth factor selectively up-regulate angiopoietin-2 in bovine microvascular endothelial cells. J Biol Chem. 1999;274(22):15732–15739. [DOI] [PubMed] [Google Scholar]
- 47. Rondina MT, Schwertz H, Harris ES, et al. The septic milieu triggers expression of spliced tissue factor mRNA in human platelets. J Thromb Haemost. 2011;9(4):748–758. doi:10.1111/j.1538-7836.2011.04208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rondina MT, Brewster B, Grissom CK, et al. In vivo platelet activation in critically ill patients with primary 2009 influenza A(H1N1). Chest. 2012;141(6):1490–1495. doi:10.1378/chest.11-2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rondina MT, Carlisle M, Fraughton T, et al. Platelet–monocyte aggregate formation and mortality risk in older patients with severe sepsis and septic shock. J Gerontol A Biol Sci Med Sci. 2015;70(2):225–231. doi:10.1093/gerona/glu082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Vincent J, Moreno R, Takala J, et al. The SOFA (Sepsis-Related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the working group on sepsis-related problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22(7):707–710. [DOI] [PubMed] [Google Scholar]
- 51. Knaus WA, Zimmerman JE, Wagner DP, Draper EA, Lawrence DE. APACHE – acute physiology and chronic health evaluation: a physiologically based classification system. Crit Care Med. 1981;9(8):591–597. [DOI] [PubMed] [Google Scholar]
- 52. Bouch DC, Thompson JP. Severity scoring systems in the critically ill. Cont Edu Anaesthesia Criti Care Pain. 2008;8(5):181–185. [Google Scholar]
- 53. Taylor FB, Jr, Toh CH, Hoots WK, et al. Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost. 2001;86(5):1327–1330. [PubMed] [Google Scholar]
- 54. Aird WC. Endothelium as an organ system. Crit Care Med. 2004;32(suppl 5):S271–S279. [DOI] [PubMed] [Google Scholar]
- 55. Aird WC. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ Res. 2007;100(2):158–173. [DOI] [PubMed] [Google Scholar]
- 56. Aird WC. Phenotypic heterogeneity of the endothelium: II. Representative vascular beds. Circ Res. 2007;100(2):174–190. [DOI] [PubMed] [Google Scholar]
- 57. Singh H, Tahir TA, Alawo DO, Issa E, Brindle NPJ. Molecular control of angiopoietin signalling. Biochem Soc Trans. 2011;39(6):1592–1596. [DOI] [PubMed] [Google Scholar]
- 58. Davis S, Aldrich TH, Jones PF, et al. Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell. 1996;87(7):1161–1169. [DOI] [PubMed] [Google Scholar]
- 59. Maisonpierre PC, Suri C, Jones PF, et al. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277(5322):55–60. [DOI] [PubMed] [Google Scholar]
- 60. Valenzuela DM, Griffiths JA, Rojas J, et al. Angiopoietins 3 and 4: diverging gene counterparts in mice and humans. Proc Natl Acad Sci U S A. 1999;96(5):1904–1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Suri C, Jones PF, Patan S, et al. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87(7):1171–1180. [DOI] [PubMed] [Google Scholar]
- 62. Milam KE, Parikh SM. The angiopoietin-Tie2 signaling axis in the vascular leakage of systemic inflammation. Tissue Barr. 2015;3(1-2):e957508 doi:10.4161/21688362.2014.957508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Yu X, Seegar TCM, Dalton AC, et al. Structural basis for angiopoietin-1–mediated signaling initiation. Proc Natl Acad Sci U S A. 2013;110(18):7205–7210. doi:10.1073/pnas.1216890110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Davis S, Papadopoulos N, Aldrich TH, et al. Angiopoietins have distinct modular domains essential for receptor binding, dimerization and superclustering. Nat Struct Biol. 2003;10(1):38–44. [DOI] [PubMed] [Google Scholar]
- 65. Kim K, Choi H, Steinmetz MO, et al. Oligomerization and multimerization are critical for angiopoietin-1 to bind and phosphorylate Tie2. J Biol Chem. 2005;280(20):20126–20131. [DOI] [PubMed] [Google Scholar]
- 66. Mei SHJ, McCarter SD, Deng Y, Parker CH, Liles WC, Stewart DJ. Prevention of LPS-induced acute lung injury in mice by mesenchymal stem cells overexpressing angiopoietin 1. PLoS Med. 2007;4(9):e269 doi:10.1371/journal.pmed.0040269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kim DH, Jung YJ, Lee AS, et al. COMP-sngiopoietin-1 decreases lipopolysaccharide-induced acute kidney injury. Kidney Int. 2009;76(11):1180–1191. [DOI] [PubMed] [Google Scholar]
- 68. Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43(3):304–377. [DOI] [PubMed] [Google Scholar]
- 69. Jansen TC, van Bommel J, Schoonderbeek FJ, et al. Early lactate-guided therapy in intensive care unit patients: a multicenter, open-label, randomized controlled trial. Am J Respir Crit Care Med. 2010;182(6):752–761. doi:10.1164/rccm.200912-1918OC. [DOI] [PubMed] [Google Scholar]
- 70. Jones AE, Shapiro NI, Trzeciak S, Arnold RC, Claremont HA, Kline JA. Lactate clearance vs central venous oxygen saturation as goals of early sepsis therapy: a randomized clinical trial. JAMA. 2010;303(8):739–746. doi:10.1001/jama.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Vincent JL, Moreno R. Clinical review: scoring systems in the critically ill. Crit Care. 2010;14(2):207 doi:10.1186/cc8204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sinuff T, Adhikari NK, Cook DJ, et al. Mortality predictions in the intensive care unit: comparing physicians with scoring systems. Crit Care Med. 2006;34(3):878–885. [DOI] [PubMed] [Google Scholar]




