Abstract
Venous thromboembolism (VTE) is a typical complication in patients with lung cancer. Khorana score is an established tool for thromboembolic risk stratification of ambulatory patients with cancer undergoing outpatient chemotherapy. The aim of this study was to evaluate the predictive value of the Khorana score for VTE and death in patients with lung adenocarcinoma during first-line or adjuvant chemotherapy. Medical records of 130 patients with lung adenocarcinoma receiving first-line or adjuvant chemotherapy were retrospectively studied during the time period June 2013 to May 2015. Venous thromboembolism occurred in 13 (10.0%) patients. Thromboembolic events were significantly correlated with reduced survival during treatment period (hazard ratio [HR]: 3.24; 95% confidence interval [CI]: 1.11-9.49; P = .032). The VTE rates did not present statistically significant difference between different Khorana score groups (P = .96). In univariate analysis, the risk of death during treatment period (median: 16 weeks) was 3.75 times higher in high-risk versus intermediate-risk patients (HR: 3.75, 95% CI: 1.36-10.36; P = .001) and had 2.25 times higher per point increase in the Khorana score (HR: 2.25, 95% CI: 1.36-3.73; P = .002); the above results were also reproduced in multivariate analysis. Khorana score represents a valuable tool for identifying patients with cancer in low thromboembolic risk but does not preserve its predictive value for higher risk individuals. Khorana score is an independent risk factor for death in patients with lung adenocarcinoma receiving first-line or adjuvant chemotherapy.
Keywords: Khorana score, lung cancer, venous thromboembolism, chemotherapy
Introduction
Venous thromboembolism (VTE), consisting of deep vein thrombosis (DVT) and pulmonary embolism (PE), represents one of the most common complications of lung cancer, with an incidence of 52 events per 1000 persons per year.1 Relative risk of VTE nearly doubles in patients with non-small cell lung cancer in comparison to patients with small-cell lung cancer.2 Among different histologic subtypes, individuals with lung adenocarcinoma have statistically significant increased rates of thromboembolic complications (hazard ratio [HR]: 2.0-3.1 vs squamous subtype).3 Despite its frequency, 32% to 63% of VTE events remain covert, resulting in worse overall prognosis due to failure to provide appropriate treatment.4–6 Risk of VTE also appears elevated during the first 3 months after cancer diagnosis; although VTE risk subsequently decreases, it lingers for up to 15 years after the diagnosis of malignancy.7
Current clinical practice guidelines recommend prophylactic anticoagulation in every patient with active malignancy who is either hospitalized or has reduced mobility. The VTE prophylaxis should also be initiated preoperatively in patients undergoing major cancer surgery and continued up to 28 days postsurgery, depending on the risk of thrombosis. However, recommendations are less explicit in the setting of ambulatory individuals with cancer receiving outpatient chemotherapy, in which most guidelines suggest personalization of VTE prophylaxis.8,9 Khorana et al invented and validated a predictive model for chemotherapy-associated VTE that takes into account 5 different variables, including primary cancer site, prechemotherapy platelet count, prechemotherapy leukocyte count, prechemotherapy hemoglobin levels or use of red cell growth factors, and patient body mass index. According to this score, individuals are divided into 3 distinct prognostic categories depending on the risk of VTE emergence.10
Given the high incidence of early VTE in patients with lung adenocarcinoma as well as its tendency for adverse outcomes, we conducted a single-center retrospective study to evaluate the predictive value of the Khorana score during first-line or adjuvant chemotherapy. We further investigated the score’s ability to predict early cancer mortality in the same group of patients.
Methods
Identification of Cohort
Medical records of sequential, nonselected patients with lung cancer at Oncology Unit, Sotiria General Hospital, Athens, Greece, between June 2014 and May 2016 were reviewed. Patients with confirmed non-small cell lung cancer and adenocarcinoma diagnosis were included. Diagnosis was made either in surgical resection specimens or in small biopsies. Individuals found positive for thyroid transcription factor 1 and negative for transformation-related protein 63 in immunohistochemical staining of cytology specimens were also included in the study cohort. The study protocol was approved by the hospital scientific committee. Written informed consent was obtained from all study participants.
Data Collection
A retrospective data review of eligible patients was conducted, including basic demographic and anthropometric data, stage at initial lung cancer diagnosis, line of treatment (first-line or adjuvant), Khorana score, occurrence of VTE, presence of symptoms, and overall survival. A VTE event was defined as development of DVT, PE, or both at any time between anticancer treatment initiation and completion of line of treatment or death, whichever occurred first. All cases of VTE were verified by assessment of both clinical records and radiographic reports.
Statistical Analysis
Normality of distributions was tested using the Kolmogorov-Smirnov test. Quantitative variables were expressed as mean values with standard deviation or medians and interquartile ranges, as appropriate. Absolute or relevant frequencies were used for the description of qualitative variables. The Pearson χ2 test was used in order to test whether the frequencies of qualitative variables were equal across subgroups. Receiver operating characteristic (ROC) curve analysis and area under the curve (AUC) were utilized for assessing the predictive value of the Khorana score in both VTE occurrence and overall survival until completion of line of treatment. Khorana score’s optimal cutoff for predicting adverse outcomes, as well as its sensitivity and specificity at this point, was also determined via ROC analysis. Cox regression models were used to explore the effects of the score as a predictor for death until treatment termination. The HRs and 95% confidence intervals (95% CIs) were also derived from the analysis. Survival curves were plotted using the Kaplan-Meier method. Analysis was performed using the Statistical Package for Social Sciences software (IBM Corp, 2012, IBM SPSS Statistics for Windows, version 21.0, Armonk, New York). A probability value P < .05 was considered statistically significant.
Results
Patient Characteristics
Three hundred twenty-four patients were reviewed. One hundred nineteen patients were excluded due to histopathological diagnosis other than lung adenocarcinoma, 53 were excluded due to administration of systemic therapy before study initiation, and 22 due to insufficient clinical data. A total of 130 patients were included in the study cohort. Overall, 110 patients received first-line chemotherapy during study period and 20 patients received adjuvant chemotherapy after complete surgical resection of primary tumor. Metastatic disease was diagnosed in 68.5% of participants at treatment initiation. One hundred fourteen (87.7%) patients survived until the end of treatment period. Median follow-up of all patients was 16 weeks. Exact patient characteristics are exhibited in Table 1.
Table 1.
Patient Characteristics.
| Characteristics | |
|---|---|
| Males (%) | 94 (72.3) |
| Age (years) | 64 (10.9) |
| βμΙ (kg/m2) | 25.7 (4.1) |
| History of smoking (%) | 114 (87.7) |
| Metastatic disease (%) | 89 (68.5) |
| First-line chemotherapy (%) | 110 (84.6) |
| Survived (%) | 114 (87.7) |
Abbreviation: BMI, body mass index.
The incidence and other characteristics of thromboembolic events among study patients are demonstrated in Table 2. Thirteen patients, representing 10% of the study population, developed VTE. Pulmonary embolism occurred in 10 patients, DVT of the lower limbs in 1, and 2 patients experienced concurrent DVT and PE. Supplementary Table 1 presents the frequency distribution of VTE in patients who died or survived during first-line or adjuvant chemotherapy. In addition, 4 individuals developed arterial thrombosis. Overall, 69.1% of VTE events were symptomatic, whereas 30.1% were incidental and discovered during computed tomography scans performed for staging purposes. In Cox regression analysis, the development of VTE was associated with an increased risk of death during first-line or adjuvant chemotherapy with an HR of 3.24 (95% CI: 1.11-9.49; P = .03).
Table 2.
Incidence and Characteristics of Venous Thromboembolism.
| VTE Event | n (%) |
|---|---|
| All | 13 (10.0) |
| DVT | 1 (7.7) |
| PE | 10 (76.9) |
| DVT and PE | 2 (15.4) |
| Presence of symptoms | 9 (69.1) |
Abbreviations: DVT, deep vein thrombosis; PE, pulmonary embolism; VTE, venous thromboembolism.
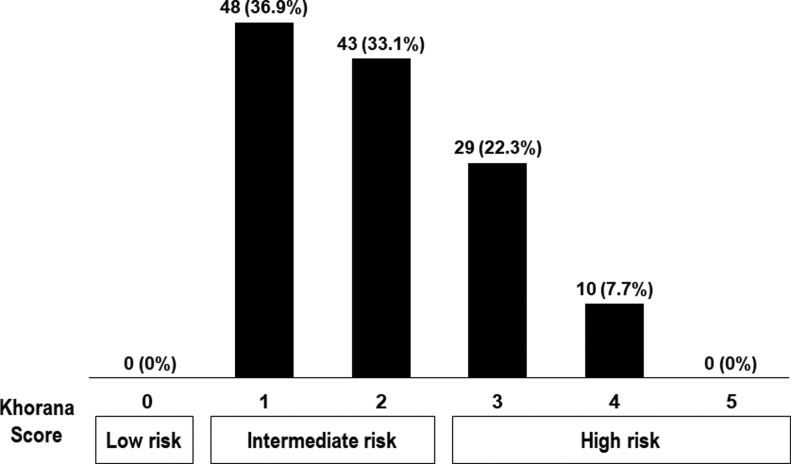
Due to the primary site of cancer in lung, all patients scored at least 1 point in the Khorana score. As a result, no patient was classified in low-risk group according to this score. The median value of Khorana score was 2 (interquartile range, 1-3). The majority of patients (70%) had an intermediate Khorana score of 1 or 2, and only 39 (30%) patients scored 3 points or more, required for allocation in high-risk group. Figure 1 summarizes Khorana score values and groups for study population with ratios of deaths for certain scores, whereas ratios of VTE are presented in Supplementary Figure 1.
Figure 1.
Khorana score values and groups for study population.
Khorana Score and VTE Prediction
Incidence of VTE did not present statistically significant difference for any single value of the Khorana score (P = .85). High-risk group individuals did not demonstrate statistically significant increased rates of VTE events as compared to patients categorized in intermediate-risk group (P = .96). Moreover, as illustrated in Supplementary Figure 2, ROC analysis showed that the score was not predictive for the development of VTE in patients with lung adenocarcinoma receiving first-line or adjuvant chemotherapy (AUC: 0.49; 95% CI: 0.33-0.64; P = .87).
Khorana Score and Early Mortality
Cox univariate analysis revealed that increase in the Khorana score by 1 point conferred a statistically significant rise in patient mortality during first-line or adjuvant chemotherapy (HR: 2.25; 95% CI: 1.36-3.73; P < .01), as shown by Kaplan-Meier analysis in Figure 2A. In addition, transition from intermediate- to high-risk group multiplied the risk of death by 3.75 times in the same period of time (95% CI, 1.36-10.36; P < .01), as shown by Kaplan-Meier analysis in Figure 2B. The above results were also replicated in multivariate analysis, after adjustment for confounders (age, sex, smoking history, metastatic disease, and occurrence of VTE), with an HR of 2.06 (95% CI: 1.25-3.38) and 3.62 (95% CI: 1.27-10.34), respectively (Ps < .05). The impact of each one of the Khorana components on mortality is presented in Supplementary Table 2. Furthermore, as shown in Figure 3, ROC analysis showed that the Khorana score was predictive of survival in patients with lung adenocarcinoma receiving first-line or adjuvant chemotherapy (AUC: 0.74; 95% CI: 0.60-0.88; P < .01).
Figure 2.
Kaplan-Meier plots of survival until the end of first-line or adjuvant chemotherapy in association with (A) Khorana score points and (B) Khorana risk subgroups.
Figure 3.
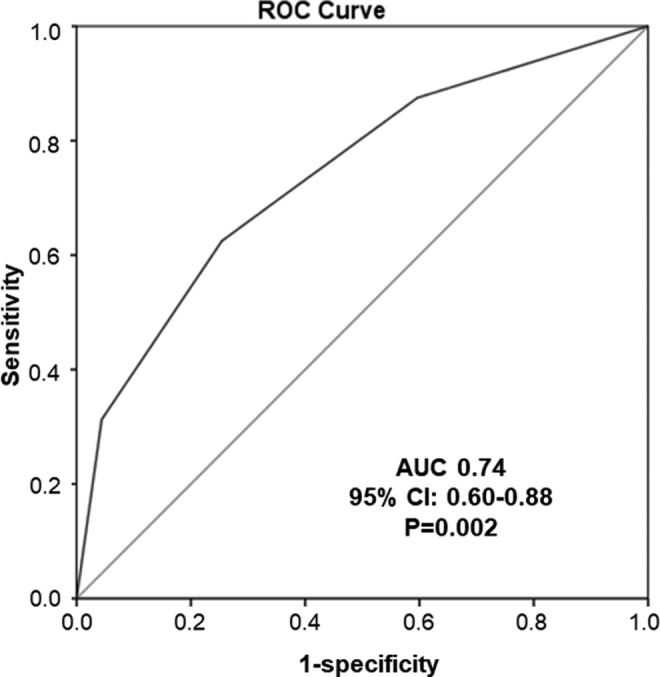
The ROC curve for the prediction of survival in patients with lung adenocarcinoma during first-line or adjuvant chemotherapy by the Khorana score. ROC indicates receiver operating characteristic curve.
Discussion
Identifying a clinical, readily available predictor of early mortality in patients with lung cancer could have a serious impact on patient care. Based on our analysis, we deduced that not previously treated patients with lung adenocarcinoma scoring 3 points or more in the Khorana score are at markedly increased risk of death during first-line or adjuvant chemotherapy. As far as VTE occurrence is concerned, nonetheless, risk stratification through Khorana score failed to predict early thromboembolic events in the same patient population.
Thirty percent of individuals in our study were classified in high-risk group, while the remaining 70% were allocated in intermediate-risk group, according to the Khorana score. However, VTE incidence did not present statistically significant differences between the 2 groups of patients with cancer. Likewise, thromboembolic event rates did not differ (P = .21) in accordance with the Khorana risk stratification model in a cohort of 719 patients with lung cancer prospectively followed up for a median of 15.2 months. Besides, 85% of VTE episodes occurred in patients categorized in intermediate-risk group.11 Khorana score represents a validated tool predicting chemotherapy-associated VTE among patients with cancer. In their original study, Khorana et al developed and established this score in a total of 4066 patients with various cancer types. In this population, 1407 (34.6%) patients had breast cancer, which, along with prostate cancer, is associated with the lowest VTE rates among all malignancies.7,12 Still, only 73 (1.8%) patients with the fairly thrombogenic gastric or pancreatic cancer types, rated with 2 points each in the Khorana score, were included. Furthermore, median follow-up was 73 days. Moderate VTE rates (2.2%) were, therefore, observed in this population.10 As a result, Khorana score appears as a valuable predictive tool for recognition of individuals at low risk for early VTE development but abolishes its predictive value among intermediate- to high-risk individuals.
Our analysis underscored Khorana score as an independent risk factor for mortality among patients with lung adenocarcinoma at a median of 16 weeks’ time. Our study was conducted in the most thrombogenic subgroup of patients with lung cancer, those having primary lung adenocarcinoma. Moreover, our study is the first to delineate the ability of this particular score to predict adverse outcomes as early as before first or adjuvant line of treatment completion. The above verdict was also replicated in the previously mentioned prospective study in 719 patients with lung cancer, where Mansfield et al came to the conclusion that patients in high-risk category according to the Khorana score are at increased risk of death (HR: 1.7; 95% CI: 1.4-2.2 vs intermediate-risk category).11 Khorana score has also been proclaimed as an independent predictor of mortality at 6 months in 334 patients with pancreatic adenocarcinoma after complete surgical excision of primary tumor (HR: 2.31; P = .039).13 Furthermore, in a prospective study involving 4405 patients with cancer, individuals at high risk according to this specific score were at significantly elevated risk for death as well as early disease progression at 4 months, as compared to both intermediate- and low-risk patients.14 Recent clinical data also suggested that higher Khorana scores are capable of predicting death but not VTE-related mortality in individuals with various malignancies.15
Patients with cancer exhibit impaired iron metabolism, resulting in increased prevalence of absolute as well as functional iron deficiency and anemia. Routine use of erythropoiesis-stimulating agents (ESAs) raises hemoglobin levels by depleting iron stores. Subsequent iron-restricted erythropoiesis with reactive thrombocytosis represents a well-known risk factor for cardiovascular events, including VTE and VTE recurrence.16 Current data suggest that repletion of stores using intravenous iron formulations is protective against thromboembolic complications by alleviating reactive platelet count elevation.17 Irrespective to VTE risk, the presence of anemia has been connected with a decrease in overall survival in patients with cancer; mortality data concerning the use of ESAs in patients with cancer rest conflicting. Cytokine-mediated paraneoplastic leukocytosis has also been established as an indicator of poor prognosis in patients with lung and bladder carcinoma.18,19 Ectopic granulocyte colony-stimulating factor, granulocyte macrophage colony-stimulating factor, and interleukin 6 (IL-6) secretion by tumor cells or their microenvironment have been linked to autocrine stimulation of malignant cell proliferation as well as enhanced matrix metalloproteinase production, potentially explaining increased tumor aggressiveness. The IL-6 production has also been associated with excess hepatic thrombopoietin synthesis, leading to paraneoplastic thrombocytosis in ovarian cancer models. High plasma IL-6 levels in conjunction with platelet counts of more than 450 000/mm3 have, in turn, been designated as independent predictors of reduced progression-free and overall survival after adjustment for stage, grade, and volume of residual disease after cytoreductive surgery in women with epithelial ovarian carcinoma.20
The retrospective study design, conferring the hazard of selection bias, and the small number of participants, confining study power, represent potential limitations of our study. The VTE events could also be underreported in our patient population. Duplex ultrasound of the upper and/or lower limbs was only performed in the presence of symptoms or clinical suspicion.
Conclusion
This study reveals that Khorana score is an independent predictor of early mortality, rather than VTE in patients with lung adenocarcinoma receiving first-line or adjuvant chemotherapy. If validated both prospectively and in other histologic subtypes, it could evolve into a basic component of risk assessment for newly diagnosed patients with lung cancer.
Supplemental Material
Supplemental Material, paper_validation_khorana_ONLINE_SUPPLEMENT for Khorana Score: Νew Predictor of Early Mortality in Patients With Lung Adenocarcinoma by Ioannis Vathiotis, Evangelos P. Dimakakos, Paraskevi Boura, Angeliki Ntineri, Andiani Charpidou, Grigoris Gerotziafas, and Konstantinos Syrigos in Clinical and Applied Thrombosis/Hemostasis
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Supplemental Material: Supplemental material for this article is available online
References
- 1. Horsted F, West J, Grainge MJ. Risk of venous thromboembolism in patients with cancer: a systematic review and meta-analysis. PLoS Med. 2012;9(7):e1001275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tesselaar ME, Osanto S. Risk of venous thromboembolism in lung cancer. Curr Opin Pulm Med. 2007;13(5):362–367. [DOI] [PubMed] [Google Scholar]
- 3. Walker AJ, Baldwin DR, Card TR, et al. Risk of venous thromboembolism in people with lung cancer: a cohort study using linked UK healthcare data. Br J Cancer. 2016;115(1):115–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sun JM, Kim TS, Lee J, et al. Unsuspected pulmonary emboli in lung cancer patients: the impact on survival and the significance of anticoagulation therapy. Lung Cancer. 2010;69(3):330–336. [DOI] [PubMed] [Google Scholar]
- 5. Shinagare AB, Okajima Y, Oxnard GR, et al. Unsuspected pulmonary embolism in lung cancer patients: comparison of clinical characteristics and outcome with suspected pulmonary embolism. Lung Cancer. 2012;78(2):161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Connolly GC, Menapace L, Safadjou S, et al. Prevalence and clinical significance of incidental and clinically suspected venous thromboembolism in lung cancer patients. Clin Lung Cancer. 2013;14(6):713–718. [DOI] [PubMed] [Google Scholar]
- 7. Blom JW, Doggen CJ, Osanto S, et al. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA. 2005;293(6):715–722. [DOI] [PubMed] [Google Scholar]
- 8. Lyman GH, Bohlke K, Falanga A; American Society of Clinical Oncology. Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology Clinical Practice Guideline update. J Oncol Pract. 2015;11(3):e442–e444. [DOI] [PubMed] [Google Scholar]
- 9. NCCN (National Comprehensive Cancer Network) Clinical Practice Guidelines in Oncology. Venous Thromboembolic Disease. Version 1 2016. http://NCCN.org.
- 10. Khorana AA, Kuderer NM, Culakova E, et al. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111(10):4902–4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mansfield AS, Tafur AJ, Wang CE, Lyman GH, Francis CW. Predictors of active cancer thromboembolic outcomes: validation of the Khorana score among patients with lung cancer. J Thromb Haemost. 2016;14(9):1773–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Blom JW, Vanderschoot JP, Oostindier MJ, Osanto S, van der Meer FJ, Rosendaal FR. Incidence of venous thrombosis in a large cohort of 66,329 cancer patients: results of a record linkage study. J Thromb Haemost. 2006;4(3):529–535. [DOI] [PubMed] [Google Scholar]
- 13. Sohal DP, Shrotriya S, Glass KT, et al. Predicting early mortality in resectable pancreatic adenocarcinoma: a cohort study. Cancer. 2015;121(11):1779–1784. [DOI] [PubMed] [Google Scholar]
- 14. Kuderer NM, Culakova E, Lyman GH, et al. A validated risk score for venous thromboembolism is predictive of cancer progression and mortality. Oncologist. 2016;21(7):861–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Posch F, Riedl J, Reitter EM, et al. Hypercoagulabilty, venous thromboembolism, and death in patients with cancer. A multi-state model. Thromb Haemost. 2016;115(4):817–826. [DOI] [PubMed] [Google Scholar]
- 16. Potaczek DP, Jankowska EA, Wypasek E, Undas A. Iron deficiency: a novel risk factor of recurrence in patients after unprovoked venous thromboembolism. Pol Arch Med Wewn. 2016;126(3):159–65. [DOI] [PubMed] [Google Scholar]
- 17. Henry DH, Dahl NV, Auerbach MA. Thrombocytosis and venous thromboembolism in cancer patients with chemotherapy induced anemia may be related to ESA induced iron restricted erythropoiesis and reversed by administration of IV iron. Am J Hematol. 2012;87(3):308–310. [DOI] [PubMed] [Google Scholar]
- 18. Kasuga I, Makino S, Kiyokawa H, Katoh H, Ebihara Y, Ohyashiki K. Tumor-related leukocytosis is linked with poor prognosis in patients with lung carcinoma. Cancer. 2001;92(9):2399–2405. [DOI] [PubMed] [Google Scholar]
- 19. Izard JP, Gore JL, Mostaghel EA, Wright JL, Yu EY. Persistent, unexplained leukocytosis is a paraneoplastic syndrome associated with a poor prognosis in patients with urothelial carcinoma. Clin Genitourin Cancer. 2015;13(4):e253–e258. [DOI] [PubMed] [Google Scholar]
- 20. Stone RL, Nick AM, McNeish IA, et al. Paraneoplastic thrombocytosis in ovarian cancer. N Engl J Med. 2012;366(7):610–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, paper_validation_khorana_ONLINE_SUPPLEMENT for Khorana Score: Νew Predictor of Early Mortality in Patients With Lung Adenocarcinoma by Ioannis Vathiotis, Evangelos P. Dimakakos, Paraskevi Boura, Angeliki Ntineri, Andiani Charpidou, Grigoris Gerotziafas, and Konstantinos Syrigos in Clinical and Applied Thrombosis/Hemostasis