Abstract
Several biosimilar versions of enoxaparin are already approved and in use globally. Analytical characterization can establish good quality control in manufacturing, but they may not assure similarity in clinical outcomes between biosimilar and branded enoxaparin. This study evaluated the efficacy and safety of biosimilar Cristália versus branded Sanofi enoxaparin in venous thromboembolism (VTE) prevention in patients undergoing major abdominal surgery at risk for VTE. In this randomized, prospective single-blind study, we compared Cristália enoxaparin (Ce), a biosimilar version, versus branded Sanofi enoxaparin (Se; at a dose of 40 mg subcutaneously per day postoperatively from 7 to 10 days) in 243 patients submitted to major abdominal surgery at risk for VTE for VTE prevention. The primary efficacy outcome was occurrence of VTE or death related to VTE. The principal safety outcomes were a combination of major bleeding and clinically relevant non-major bleeding. Bilateral duplex scanning of the legs was performed from days 10 to 14, and follow-ups were performed up to 60 days after surgery. The incidence of VTE was 4.9% in the Cristália group and 1.1% in the Sanofi group (absolute risk difference = 3.80%, 95% confidence interval [CI]: −1.4%-9.0%) yielding noninferiority since the 95% CI does not reach the prespecified value Δ = 20%. Clinically significant bleeding occurred in 9.9% in the Cristália group and in 5.5% in the Sanofi group (n.s. ). In conclusion, this study suggests that 40 mg once daily of Ce, a biosimilar enoxaparin, is as effective and safe as the branded Sanofi enoxaparin in the prophylaxis of VTE in patients submitted to major abdominal surgery at risk for VTE.
Keywords: enoxaparin, biosimilar low molecular weight heparins, randomized controlled trial, abdominal surgery, prophylaxis, venous thromboembolism
Introduction
Patients undergoing general surgery are at substantial risk of postoperative venous thromboembolism (VTE).1,2 It is known that abdominal surgery leads to a hypercoagulable state and an associated increased risk of deep vein thrombosis (DVT).3 The activation of the coagulation system persists for at least 14 days, and data suggest that the incidence of postoperative DVT to be as high as 25%4 and an incidence of pulmonary embolism (PE) ranging from 0.13% to 0.63% in 4 to 6 weeks after general surgery.5
Low-molecular-weight heparins (LMWHs) are grade 1A recommendations for VTE prophylaxis in patients undergoing major abdominal surgery at risk for VTE.2 The currently available brand-name LMWHs include dalteparin (Pfizer), enoxaparin (Sanofi), and nadroparin (Aspen Pharma). Other products available in Europe include certoparin (Novartis), reviparin (Abbott), and parnaparin (Alpha-Wasserman). Each of these LMWHs has a characteristic molecular weight profile and biological activity in terms of anti-FXa and anti-FIIa potency and clinical effect.6 It is now widely accepted that individual LMWHs are chemically unique agents and cannot be interchanged therapeutically. Each commercial LMWH has been individually developed for specific clinical indications, which are dose and product dependent. Recently, several generic LMWHs have become available worldwide, and companies have filed for regulatory approval of generic versions of enoxaparin, very few with pharmacodynamics (PD) data, based on compound biochemical characteristics, particularly anti-Xa and anti-IIa activity in healthy volunteers.7 As the primary aim of a generic drug is to reduce costs without compromising patient care, a biosimilar drug is required to be chemically and biologically equivalent to the pioneer drug.8 Biosimilar enoxaparins were previously tested in animal models to determine safety, efficacy, and PDs parameters.9 They were also tested for their comparative immunogenicity profile10 as well as in the prevention of VTE in major abdominal surgeries.11 Cristália enoxaparin was extensively characterized in comparison to Sanofi enoxaparin (physical, chemical, and biological characterizations). A phase I study was also conducted (a comparative study to evaluate PDs of anti-Xa and anti-IIa biomarkers) demonstrating no significant differences.12 In order to assure that the biosimilar LMWHs are clinically equivalent to branded LMWHs in humans, a randomized clinical trial comparing these 2 therapies as prophylaxis for VTE following major abdominal surgery in patients at risk for VTE was carried out. We have utilized a previously validated model of a clinical parallel prospective comparison of the branded versus biosimilar enoxaparin in the surgical VTE prevention setting.11
Patients and Methods
Patient Population
The study was conducted in 8 different sites in Brazil (Online Supplemental). The ethics committees of all sites involved in this research approved the study protocol. All patients signed a written informed consent before entry to the study, which was conducted according to the guidelines for good clinical practice (GCP).
Inclusion and exclusion criteria are described in Table 1. To identify patients undergoing major abdominal surgery with formal indication of chemoprophylaxis for VTE, a Caprini score ≥5 was considered according to the eighth American College of Chest Physicians (ACCP) guidelines.2
Table 1.
Inclusion and Exclusion Criteria.
| Inclusion criteria |
|
| Exclusion criteria |
|
Abbreviations: ALT, alanine aminotransferase; ASA, acetylsalicylic acid; AST, aspartate aminotransferase; β-HCG, beta human chorionic gonadotropin; BMI, body mass index; LMWH, low-molecular weight heparin; UFH, unfractionated heparin; ULN, Upper Limit of Normal; VTE, venous thromboembolism.
Randomization
Randomization was done on a 1:1 basis, with the first patient randomly assigned one of the study drugs. The randomization sequence was created using SAS statistical software version 9.4 and was stratified by center with a 1:1 allocation using random block sizes of 4.
End Points
The primary efficacy outcome was occurrence of VTE or death related to VTE
The primary safety outcomes were a combination of major bleeding (MB) and clinically relevant nonmajor bleeding (CRNMB) according to International Society of Thrombosis and Hemostasis (ISTH) definitions. Adjudication of VTE events, death, and safety outcomes was performed by a central independent and blinded committee of experts.
Sample size calculation/statistical analysis
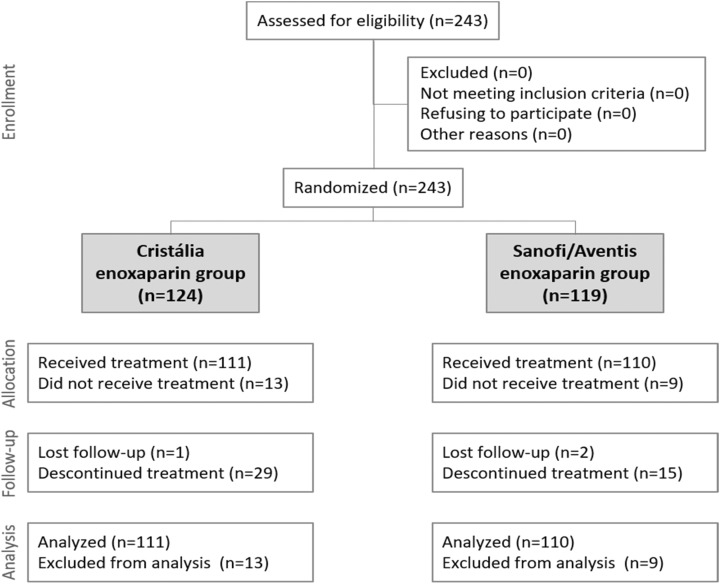
To verify the noninferiority of enoxaparin Cristália with a significance of 2.5%, power of 80%, and noninferiority margin of 20%, it would be necessary to analyze 80 participants in each group. Predicting discontinuations, we randomized 243 participants. The CONSORT diagram (https://swolpin.cirg.washington.edu/CSD/.) of the study enrollment and follow-up is shown in Figure 1.
Figure 1.
CONSORT diagram.
Primary effectiveness analysis
For efficacy analysis, the incidence by treatment group and the 95% confidence interval (CI) for the difference in the incidence of VTE between enoxaparin Cristália and enoxaparin Sanofi (incidence with enoxaparin Cristália vs incidence with enoxaparin Sanofi between visit 1[V1] and visit 5 [V5]) were calculated. If the upper threshold was less than 20%, the hypothesis that enoxaparin Cristália was not less efficacious than enoxaparin Sanofi was accepted. The Cochran-Mantel-Haenszel test was used to calculate this CI. The risk factor analyses of the primary efficacy variable were done by logistic regression using the SAS Proc NLMIXED procedure, in order to obtain the proportion of patients with VTE in each treatment group. The difference in proportions between the groups (Cristália − Sanofi) and the respective 95% CI were determined with this SAS procedure.
Safety Analyses
Safety analysis was achieved by comparing the following parameters in the 2 groups:
Number, classification, and intensity of bleeding events from randomization up to 3 days after the last administration of the drug in the 2 treatment groups (between V1 and V5);
Incidence of bleeding events between V1 and V5;
Global incidence of adverse events attributable to the drug between V1 and visit 6 (V6);
Incidence of serious adverse events attributable to the drug between V1 and V6;
Incidence of adverse events attributable to the drug classified by type considered to be attributable to the drug, all events with a causal relationship considered to be certain/defined, likely or possible according to World Health Organization - Uppsala Monitoring Centre (WHO-UMC) classification between V1 and V6.
The difference in proportions between the groups (Cristália − Sanofi) and the respective 95% CI were also determined with the SAS procedure.
Study Design
Baselines characteristics are listed in Table 2. Two hundred forty-three consecutive patients were randomized in a 1:1 ratio to receive either 40 mg of Se subcutaneously (SC; 4000 IU) or 40 mg of Ce SC (4000 IU), in a single blinded manner, started 12 ± 2 hours preoperatively according to the physician discretion and then given once daily postoperatively from 7 to 10 days. An unblinded delegated team performed all the administration of study medication and collected patient diary at the end of treatment period for compliance measures.
Table 2.
Baseline Characteristics.
| Enoxaparin Cristália (n = 111) | Enoxaparin Sanofi (n = 110) | P Value | |
|---|---|---|---|
| Age (years) | |||
| Mean | 52.2 | 50.5 | MW: .4087 |
| Median | 51.0 | 50.0 | |
| SD | 15.4 | 13.0 | |
| Min-Max | 22.0-80.0 | 19.0-75.0 | |
| Gender n (%) | |||
| Male | 44 (39.6%) | 42 (38.2%) | CS: .8241 |
| Female | 67 (60.4%) | 68 (61.8%) | |
| Race n (%) | |||
| American Indian | 4 (3.6%) | 5 (4.5%) | FS: .9514 |
| Oriental | 1 (0.9%) | 1 (0.9%) | |
| Black | 12 (10.8%) | 9 (8.2%) | |
| Caucasian | 80 (72.1%) | 79 (71.8%) | |
| Multiracial | 14 (12.6%) | 16 (14.5%) | |
| BMI n (%) | |||
| ≤24.9 kg/m2 | 24 (21.6%) | 29 (26.4%) | CS: .0187 |
| ≥25 to ≤29.9 kg/m2 | 47 (42.3%) | 27 (24.5%) | |
| ≥30 kg/m2 | 40 (36.0%) | 54 (49.1%) |
Abbreviations: BMI, body mass index; CS, Chi-square test; FS, Fisher exact test; MW, Mann-Whitney test; SD, standard deviation.
Primary efficacy and safety end points were evaluated during all intention-to-treat (ITT) period . Compression ultrasound (CUS) was performed on day 10 to 14 (at the end of treatment period), or when DVT was clinically suspected, with a 12.5 MHz linear transducer, Phillips 11 or ATL-5000 or different devices according to the participant center standards, to detect the presence of DVT.
The ultrasound technique used in all patients was highly standardized as was the interpretation. Patients were examined in the supine position with the head of the bed raised 20°. The popliteal veins were examined with the knee slightly flexed (10°) to avoid collapse due to overextension. Veins below the knee were also assessed in the standing position. All vessels were scanned in the transverse and longitudinal axes and tested for the presence or absence of compressibility by applying direct pressure with the transducer. Doppler waveforms were recorded at all levels. Iliac vein patency was estimated indirectly by the presence or absence of short flow reversal in the common femoral vein while the patient performed a Valsalva maneuver.
The secondary end point was a composite of MB + CRNMB. This was defined as hemorrhage associated with a decrease in hemoglobin levels of >2 g/dL or an episode requiring transfusion of >2 units of blood. Bleeding was defined as minor if it was overt but did not meet the other criteria for MB. Retroperitoneal, intracranial, intraocular or bleeding that warranted the permanent cessation of treatment were also defined. Wounds, hematomas, need for surgical intervention, infection, and length of hospitalization were documented. Further end points included the occurrence of thrombocytopenia, defined as a platelet count below 100 000/mm3 and/or a minimum decrease of 40% compared to baseline. Allergic reactions are also usually included in adverse reactions to drugs.
Outcome Assessment/Follow-Up
In the screening visit, participants were evaluated for eligibility (Caprini score is depicted in Table 3), signed the informed consent (IC), and performed safety examinations. At V1, the participants were randomized to enoxaparin Cristália or enoxaparin Sanofi and safety examinations were assessed, and if the participant was eligible, they would receive the first administration 12 ± 2 hours before the start of surgery, followed by an application between 6 to 8 hours after the end of surgery.
Table 3.
Caprini Score.
| Enoxaparin Cristália (n = 111) | Enoxaparin Sanofi (n = 110) | P Value | |
|---|---|---|---|
| Total risk, n (%) | |||
| 5 | 21 (18.92%) | 23 (20.91%) | |
| 6 | 33 (29.73%) | 32 (29.09%) | |
| 7 | 16 (14.41%) | 16 (14.55%) | |
| 8 | 13 (11.71%) | 11 (10.00%) | |
| 9 | 13 (11.71%) | 19 (17.27%) | |
| 10 | 8 (7.21%) | 6 (5.45%) | |
| 11 | 5 (4.50%) | 1 (0.91%) | |
| 12 | 1 (0.90%) | 1 (0.91%) | |
| 13 | 0 | 1 (0.91%) | |
| 14 | 1 (0.90%) | 0 | |
| Total risk | |||
| Mean | 7.2 | 7.0 | MW: .6627 |
| Median | 7.0 | 6.5 | |
| SD | 1.9 | 1.8 | |
| Min-Max | 5.0-14.0 | 5.0-13.0 | |
Abbreviations: MW: Mann-Whitney test; SD, Standard deviation.
Visits 2 and 3 were performed respectively on the first and second postoperative days. The participants received enoxaparin; the signs and symptoms of VTE and signs of hemorrhage were evaluated.
Visit 4 was discharge from hospital. The participants were evaluated clinically for signs and symptoms of VTE, received enoxaparin, and were instructed to complete the 10 days of home administration of the investigational medication and recognize VTE signs.
Visit 5 occurred up to 4 days after the end of administration. The investigator evaluated the participant and performed duplex scan with CUS of lower limbs. All images were adjudicated by an independent adjudication committee.
Visit 6 occurred 60 ± 4 days after randomization (visit 1). The participants were evaluated for VTE and completed their participation in the study. In accordance with GCP, participants were instructed to report to their sites any serious adverse event in the following 30 days of visit 6. At all visits, if the investigator considered necessary, the participants would perform CUS of lower limbs, helicoidal contrasted tomography of chest, or pulmonary scintigraphy.
Results
The groups were very homogeneous regarding demographics, gender, age race, weight, and height. There were no statistically significant differences between the groups compared to the median values of weight, height, and body mass index (BMI; P = .5664, P = 0.6574 and P = 0.3751, respectively). However, considering the BMI variable grouped into different classes, there were statistically significant differences (P = .0187); the majority of patients in the Sanofi group were obese (49.1%), while the majority of patients in the Cristália group were overweight (42.3%; Table 2).
The proportion of participants who completed the study was similar in both treatment groups, as well as the number of patients included in the ITT and per-protocol (PP) analyses. Considering the ITT population, 30 of the 111 patients in the Cristália group were excluded because they had technically inadequate CUS examinations or because they were discontinued before the visit. Thus, 81 patients were considered for analysis of the primary end point in this group, all with valid CUS. In the Sanofi group, 22 of the 110 patients were excluded because of technically inadequate CUS examinations or because they were discontinued prior to the visit. Thus, 88 patients were considered for analysis of the primary end point in this group, all with valid CUS. In both groups, the goal of 80 patients valid for the analysis of the primary end point of study efficacy was reached. Regarding deviations from the protocol, less than 2% of the events were considered as major and had an impact on the definition of the PP group. With these data, both groups had an excellent adhesion rate to the study treatment. The median number of syringes used was 10 in both groups and the rate of therapeutic adherence was greater than 90% (91.0% in the Cristália group and 99.1% in the Sanofi group). It is assumed that patients were adequately exposed to both the investigational product and the active comparator and with adequate quality control regarding the follow-up of the proposed protocol. Enrollment is depicted in the CONSORT diagram (Figure 1).
No statistically significant differences between the 2 groups were detected in any of the measured outcomes: the incidence of VTE was 4.9% (4 out of 80 evaluable CUS, including 1 PE) in the Cristália group and 1.1% (1 out of 88 evaluable CUS) in the Sanofi group (absolute risk reduction = 3.80%, 95% CI: −1.4% to 9.0%; Table 4). No MB events occurred in either group (Table 5). The difference observed between the groups of patients in the incidence of VTE, according to the adjudication committee, from the randomization up to 12 days after surgery was 3.87% (95% CI: −1.5%-9.2%) in ITT and 3.87% (95% CI: −1.5%-9.2%) in PP and concluded by noninferiority to the Cristália group in both populations, once the CI does not reach the value Δ = 20%. Considering the incidence of VTE according to the adjudication committee until V6, the results of the difference between the groups were 3.84% (95% CI: −1.4% to 9.1%) in ITT and 3.94% (95% CI: −1.5% to 9.4%) in PP. As was reported for the primary analysis, noninferiority was observed for the Cristália group in both populations, since the CI did not reach Δ = 20%.
Table 4.
Primary Efficacy End Point.
| Enoxaparin Cristália (n = 111) | Enoxaparin Sanofi (n = 110) | |
|---|---|---|
| VTE incidence, n (%) | ||
| DVT | 0 | 0 |
| Pulmonary embolism | 1 (1.2%) | 0 (0.0%) |
| DVT + embolism | 0 | 0 |
| Asymptomatic DVT | 3 (3.7%) | 1 (1.1%) |
| Death related to VTE | 0 | 0 |
| Global VTE incidence, n (%) | ||
| Had VTE | 4 (4.9%) | 1 (1.1%) |
| Had no VTE | 77 (95.1%) | 87 (98.9%) |
| Unknowna | 30 | 22 |
| P Value | FS: 0.1952 | |
| 95% CI for VTE incidence | ||
| Cochran-Mantel-Haenszel | 3.80% (−1.4% to 9.0%) | |
| Logistic regression | 3.80% (−1.4% to 9.1%) | |
| OR/HR and 95% CI for incidence of bleeding episodes (enoxaparin Cristália versus enoxaparin Sanofi) | ||
| OR (logistic regression) | 4.519 (0.49 to 41.31) | |
| HR (Cox regression) | 5.578 (0.60 to 51.91) | |
Abbreviations: CI, confidence interval; DVT, deep vein thrombosis; FS, Fisher exact test; HR, hazard ratio; OR, odds ratio; VTE, venous thromboembolism.
aThis category should not be considered in the incidence calculation. It refers to patients with technically inadequate examinations and patients who discontinued prior to visit 5.
Table 5.
Safety End Points.
| Enoxaparin Cristália (n = 111) | Enoxaparin Sanofi (n = 110) | P Value | |
|---|---|---|---|
| Bleeding, n (%) | 11 (9.9%) | 6 (5.5%) | CS: .2139 |
| Bleeding, per patient | |||
| Mean | 1.09 | 1.17 | |
| Median | 1.00 | 1.00 | |
| SD | 0.30 | 0.41 | |
| Min | 1.00 | 1.00 | |
| Max | 2.00 | 2.00 | |
| Intensity of bleeding episodes, n (%) | |||
| Not serious, but clinically significant | 5 (45.5%) | 5 (83.3%) | |
| Serious | 6 (54.5%) | 1 (16.7%) | |
| Fatal | 0 | 0 | |
| OR and 95% CI for incidence of bleeding episodes | |||
| OR (logistic regression) | 1.907 (0.68-5.35) | ||
Abbreviations: CI, confidence interval; CS, Chi-square test; OR, odds ratio; SD, standard deviation.
Safety Results
There were no statistically significant differences between the 2 groups regarding the incidence of adverse events, serious adverse events, adverse events attributable to the medication, and serious adverse events attributable to the medication.
Eleven patients in the Cristália group recorded 12 episodes of bleeding (MB + CRNMB) and 6 patients of the Sanofi group recorded 7 episodes of bleeding. There were no statistically significant differences between the groups regarding the incidence of bleeding (9.9% in the Cristália group and 6.4% in the Sanofi group P = .21). Two non-VTE-related deaths were recorded, 1 in the Cristália group and 1 in the Sanofi group.
During the study, adverse events were recorded in a total of 185 participants, with a similar proportion between treatment groups presenting at least one adverse event: 83.8% in the Cristália group and 83.6% in the Sanofi group. In total, 594 adverse events were reported (291 in the Cristália group and 303 in the Sanofi group). In both groups, disorders of the gastrointestinal system and disorders of the general state were the most frequent adverse events. There were no statistically significant differences between the groups in relation to the incidence of adverse events. The intensity of adverse events did not differ between groups as well as the proportion of events attributable to the study drug.
Discussion
This noninferiority, national, multicenter, randomized, single-blind, active comparator-controlled phase III clinical study was designed to evaluate the efficacy and safety of enoxaparin Cristália in comparison to enoxaparin Sanofi for the prophylaxis of VTE in participants undergoing general abdominal surgeries with a high risk for the development of thromboembolic disease.
The study was adequately conducted, reaching the number of randomized participants (n = 243) predicted in the calculation of sample size (n = 200). To date, we have found only 1 clinical trial in the literature evaluating biosimilar versus branded enoxaparin, either in prophylaxis or in the treatment of VTE.
We decided to use major abdominal surgical patients, because lower doses of enoxaparin (40 mg/d) for a short period of time (7-10 days), according to the ACCP guidelines, are sufficient to provide acceptable protection against VTE, with low risk of hemorrhage. If any interim analysis during the trial showed any major problems, particularly regarding safety, the trial could be discontinued, avoiding patients to be exposed to unnecessary risk.
The treatment groups were comparable in their baseline characteristics regarding demographics, medical history, and Caprini risk classification. These findings corroborate an adequate randomization of study patients. There was a difference in BMI, which was significantly different, with Cristália group having more overweighed patients. We believe these findings have no clinical impact, given that there are no dose adjustments for VTE prophylaxis in major abdominal surgeries with enoxaparin. The recommended and studied doses were 40 mg once daily for both compounds, regardless of the weight.
The median value of the Caprini score was approximately 7 in both treatment groups. This finding corroborates the composition of a sample of patients at high risk of developing thromboembolic complications and with similar intensity. In addition, no significant differences were observed regarding the characteristics of the surgeries performed. Similar values of duration and type of surgery performed, as well as length of hospital stay, were similar between the study groups.
The efficacy assessment was based on the occurrence of VTE adjudicated by an independent committee. The absolute risk difference was 3.80%, favorable to the Sanofi group, with a CI varying between −1.4% and 9.0%. This result concludes noninferiority for the Cristália group, given the CI does not reach the prespecified value Δ = 20%. It is important to note that even for a more conservative noninferiority margin, for example 10%, noninferiority would continue in both ITT and PP populations, which reinforces noninferiority of the study drug compared to the branded compound. In addition, the estimate for the difference between the groups and the respective 95% CI were calculated by logistic regression analysis, confirming noninferiority for the Cristália group.
Despite a high incidence of adverse events throughout the study, it can be observed that less than 10% of the events were related to the research product. These data reinforce the safety of enoxaparin studied, even though the occurrence of adverse events as well as its intensity was comparable to the control group. It is important to emphasize that the study population consisted of patients with severe risk and medium-to-large surgery, justifying the occurrence of a large number of adverse events inherent to the therapeutic interventions of the underlying diseases.
The results of the present study argue for a more detailed appraisal, in larger, randomized controlled trials, of those new compounds. Both the products were equally safe and well tolerated. Considering the comparable physicochemical and biological attributes and similar pharmacokinetic (PK) and PD behavior to the branded Sanofi enoxaparin and based on current knowledge, the results of the present study suggest that the enoxaparin of Cristália bears high potential to be considered as a biosimilar therapeutic alternative.
Other head-to-head biochemical comparative evaluations like thrombin-activated fibrinolytic inhibitor (TAFI), tissue factor pathway inhibitor release (TFPI), D-dimers, anti-Xa/anti-IIa ratios in bench research and in new clinical trials are planned.
Limitations
Limitations included setting a noninferiority margin of 20%, which is a generous margin for absolute numbers. However, in this trial, no new indication for a new drug was tested, but the efficacy and safety of a biosimilar version of an already globally approved compound, enoxaparin. Enoxaparin has many biosimilar versions approved for clinical use, many of them tested only on PK/PD studies or bioequivalence tests. If more astringent margins of noninferiority were used, such as those applied for the Food and Drug Administration for new drug applications in cardiovascular diseases, we would need a trial with around 12 000 participants, nonrealistic for already mature preparations, such as enoxaparin.
Another limitation was the single-blinded design instead of a double-blinded study. Single blinded means the investigator was blinded to the study medications, but a designated nurse (nonblinded) was not. This technique is used to prevent bias of the investigator, whereas it is difficult to produce a real blinded injection for both the study drug and the reference enoxaparin.
Another drawback of this study was lack of evaluation of the immunogenicity potential of those compounds. In spite of not observing heparin–induced thrombocytopenia, an evaluation of Immunoglobulin A, Immunoglobulin M, and Immunoglobulin G would have been of great value and should be addressed in future. We also did not perform a cost-effectiveness analysis which would prove that it is an interesting option for a large group of hospitals and patients that cannot afford branded LMWHs, particularly in less developed countries.13–15
Conclusions
Treatment with biosimilar enoxaparin Cristália proved to be an effective and safe option in the prevention of VTE in adult participants undergoing general abdominal surgeries at high risk for the development of thromboembolic disease. Enoxaparin Cristália was as effective as branded enoxaparin and an attractive alternative for VTE prevention in patients undergoing general abdominal surgeries at high risk for VTE.
Supplemental Material
Supplemental_Material_Study_Sites for Efficacy and Safety of a Biosimilar Versus Branded Enoxaparin in the Prevention of Venous Thromboembolism Following Major Abdominal Surgery: A Randomized, Prospective, Single-Blinded, Multicenter Clinical Trial by Eduardo Ramacciotti, Ubirajara Ferreira, Agenor José Vasconcelos Costa, Selma Regina O. Raymundo, João Antônio Correa, Salvador Gullo Neto, Alessandro Bersch Osvaldt, Leandro Agati, Valéria Cristina Resende Aguiar, Ronaldo Davila, Tania Benevenuto Caltabiano, Flávia Magalhães Magella, Giuliano Giova Volpiani, Valter Castelli, Roberto Augusto Caffaro, Lucas Zeponi DalAcqua, Wagner Eduardo Matheus, Debora Yuri Sato, Gleison Juliano da Silva Russeff, Daniela Garcia de Souza, Lucas Eduardo Pazetto, Tiago Aparecido Maschio de Lima, Eloá Maria da Silva Colnago, Eliane Yumii Fugii, Juliana Sekeres Mussalem, Vanessa Therumi Assao, Odaly Toffoletto, Debora Garcia Rodrigues, Jorge Barros Afiune, and Gilson Roberto Araujo in Clinical and Applied Thrombosis/Hemostasis
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was sponsored by Cristália Produtos Químicos Farmacêuticos LTDA, São Paulo, Brazil.
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Nicolaides AN, Breddin HK, Fareed J, et al. Prevention of venous thromboembolism. International Consensus Statement. Guidelines compiled in accordance with the scientific evidence. Int Angiol. 2001;20(1):1–37. [PubMed] [Google Scholar]
- 2. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149(2):315–352. [DOI] [PubMed] [Google Scholar]
- 3. Galster H, Kolb G, Kohsytorz A, Seidlmayer C, Paal V. The pre-, peri-, and postsurgical activation of coagulation and the thromboembolic risk for different risk groups. Thromb Res. 2000;100(5):381–388. [DOI] [PubMed] [Google Scholar]
- 4. Scurr JH, Coleridge-Smith PD, Hasty JH. Deep venous thrombosis: a continuing problem. BMJ. 1988;297(6640):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kiil J, Kiil J, Axelsen F, Andersen D. Prophylaxis against postoperative pulmonary embolism and deep-vein thrombosis by low-dose heparin. Lancet. 1978;1(8074):1115–1116. [DOI] [PubMed] [Google Scholar]
- 6. Fareed J, Leong W, Hoppensteadt DA, Jeske WP, Walenga J, Bick RL. Development of generic low molecular weight heparins: a perspective. Hematol Oncol Clin North Am. 2005;19(1):53–68, v–vi. [DOI] [PubMed] [Google Scholar]
- 7. Maddineni J, Walenga JM, Jeske WP, et al. Product individuality of commercially available low-molecular-weight heparins and their generic versions: therapeutic implications. Clin Appl Thromb Hemost. 2006;12(3):267–276. [DOI] [PubMed] [Google Scholar]
- 8. Guerrini M, Rudd TR, Mauri L, et al. Differentiation of generic enoxaparins marketed in the United States by employing NMR and multivariate analysis. Anal Chem. 2015;87(16):8275–8283. [DOI] [PubMed] [Google Scholar]
- 9. Fareed J, Hoppensteadt D, Schultz C, et al. Biochemical and pharmacologic heterogeneity in low molecular weight heparins. Impact on the therapeutic profile. Curr Pharm Des. 2004;10(9):983–999. [DOI] [PubMed] [Google Scholar]
- 10. Gomes M, Ramacciotti E, Hoppensteadt D, et al. An open label, non-randomized, prospective clinical trial evaluating the immunogenicity of branded enoxaparin versus biosimilars in healthy volunteers. Clin Appl Thromb Hemost. 2011;17(1):66–69. [DOI] [PubMed] [Google Scholar]
- 11. Gomes M, Ramacciotti E, Henriques AC, et al. Generic versus branded enoxaparin in the prevention of venous thromboembolism following major abdominal surgery: report of an exploratory clinical trial. Clin Appl Thromb Hemost. 2011;17(6):633–639. [DOI] [PubMed] [Google Scholar]
- 12. Inhixa. Assessment report. Committee for Medicinal Products for Human Use (CHMP). Procedure No. EMEA/H/C/004264/0000. European Medicines Agency 2016. http://21/jul/2016._Public_assessment_report/human/004264/WC500215211.pdf.
- 13. Merli GJ, Vanscoy GJ, Rihn TL, Groce JB, III, McCormick W. Applying scientific criteria to therapeutic interchange: a balanced analysis of low-molecular-weight heparins. J Thromb Thrombolysis. 2001;11(3):247–259. [DOI] [PubMed] [Google Scholar]
- 14. Kalodiki E, Leong W Sasat; Task Force on Generic LMWHs. SASAT (South Asian Society on Atherosclerosis & Thrombosis) proposal for regulatory guidelines for generic low-molecular weight heparins (LMWHs). Clin Appl Thromb Hemost. 2009;15(1):8–11. [DOI] [PubMed] [Google Scholar]
- 15. Liu X, St Ange K, Lin L, Zhang F, Chi L, Linhardt RJ. Top-down and bottom-up analysis of commercial enoxaparins. J Chromatogr A. 2017;1480:32–40. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental_Material_Study_Sites for Efficacy and Safety of a Biosimilar Versus Branded Enoxaparin in the Prevention of Venous Thromboembolism Following Major Abdominal Surgery: A Randomized, Prospective, Single-Blinded, Multicenter Clinical Trial by Eduardo Ramacciotti, Ubirajara Ferreira, Agenor José Vasconcelos Costa, Selma Regina O. Raymundo, João Antônio Correa, Salvador Gullo Neto, Alessandro Bersch Osvaldt, Leandro Agati, Valéria Cristina Resende Aguiar, Ronaldo Davila, Tania Benevenuto Caltabiano, Flávia Magalhães Magella, Giuliano Giova Volpiani, Valter Castelli, Roberto Augusto Caffaro, Lucas Zeponi DalAcqua, Wagner Eduardo Matheus, Debora Yuri Sato, Gleison Juliano da Silva Russeff, Daniela Garcia de Souza, Lucas Eduardo Pazetto, Tiago Aparecido Maschio de Lima, Eloá Maria da Silva Colnago, Eliane Yumii Fugii, Juliana Sekeres Mussalem, Vanessa Therumi Assao, Odaly Toffoletto, Debora Garcia Rodrigues, Jorge Barros Afiune, and Gilson Roberto Araujo in Clinical and Applied Thrombosis/Hemostasis