Abstract
Patients with cancer have an increased risk of venous thromboembolism. Upper extremity venous system is a peculiar site, and little is known about the clinical course in patients with cancer. Electronic medical records were searched for patients with cancer with a diagnosis of upper extremity venous thrombosis. Individual patient data were reviewed. Eighty-seven patients were identified, and the median age was 52.4. The most common underlying malignancies were breast (23.0%), colorectal (18.4%), and gastroesophageal (18.4%). Median time from cancer diagnosis to upper extremity venous thromboembolism (UEDVT) was 3.44 months. Subclavian vein was the most common involved site (56.3%) and 54.0% patients had a central venous catheter; 50.6% of patients developed a complication; pulmonary embolism (PE) in 9.2%, superior vena cava (SVC) syndrome in 14.9%, and 26.4% had postthrombotic syndrome. In patients with isolated single vein thrombosis, complications were higher in the subset with internal jugular vein involvement compared to other sites (68.2% vs 52.2%) as were complications in patients with non-catheter-related thrombosis compared to patients with a central venous catheter in place (55% vs 27.7%). Median overall survival from time of cancer and UEDVT diagnoses was 29.6 and 13.25 months, respectively. In conclusion, UEDVT is an uncommon event. Around 50% developed a complication including PE, SVC or postthrombotic syndromes. Larger studies are needed to better identify risks associated with thrombosis and the best therapeutic approach and duration in this unique subset of patients with cancer.
Keywords: thrombosis, cancer, upper extremity, anticoagulation, central venous catheter
Introduction
The risk of venous thromboembolism (VTE) is increased in patients with a diagnosis of cancer compared to the general population. This is related to the underlying malignancy itself causing a constant prothrombotic state, the site of the tumor causing direct invasion or compression on the venous system, in addition to the risk associated with different cancer treatments such as chemotherapy, hormonal therapy, surgical interventions, and indwelling central venous catheters (CVCs). Patient-related factors such as immobility, age, and comorbidities also contribute to this population’s heightened risk{Falanga, 2013 #1}.1,2
Although most patients survive acute thrombotic events, these can be associated with serious complications. Postthrombotic syndrome is manifested by painful swelling and pigmentation and in severe cases can lead to skin ulcerations and debilitation.3 Pulmonary embolism (PE) can be associated with substantial morbidity and mortality with a potentially higher risk in patients with cancer who are more likely to have underlying pulmonary dysfunction due to metastasis, infection, and previous radiation among other factors. Although rare in this patient population, PE can be further complicated by pulmonary hypertension.4,5
In addition to the morbidity associated with thrombotic events, thrombosis has also been shown to have a negative impact on prognosis. One retrospective study used registry data to evaluate the survival of 668 patients with a diagnosis of cancer and VTE compared to survival of patients with cancer without a VTE and demonstrated significantly inferior survival rates (12% vs 36%, P < .001). This finding persisted on multivariate analysis after controlling for possible confounding factors such as site of primary cancer, age, and gender.6
Upper extremity deep vein thrombosis (UEDVT) is not commonly encountered in clinical practice and incidence varies according to patient population studied.7–10 The Malmö thrombophilia study included a total of 1203 patients diagnosed with VTE of whom only 5.2% had UEDVT.9 Another multicenter registry report identified 592 (10.9%) cases of UEDVT of a total of 5451 patients with ultrasound-confirmed VTE.10 This study suggested that risk factors for development of UEDVT differ from those for conventional deep vein thrombosis (DVT) and that several conventional risk factors for lower extremity DVT did not predispose to non–CVC-associated UEDVT. Reported incidence of UEDVT is increasing,11 and in patients with cancer, this might be related to the more frequent use of CVCs as cancer patient care, and chemotherapy administration is being increasingly shifted to the outpatient setting.12,13 Clinical evidence regarding long-term clinical outcomes of patients with UEDVT however is lacking.
UEDVT in patients having cancer with or without CVC is an even less well-characterized diagnosis in published literature. Complication rates, long-term outcomes, and effect on survival in patients with a diagnosis of cancer and UEDVT are not well described, and there are no standardized recommendations or guidelines on established standard management approaches. Our current report attempts to describe our single-center cohort of patients with a diagnosis of cancer and UEDVT.
Methods
After obtaining appropriate institutional review board (IRB) approval, all electronic medical records and radiology reports were searched for a diagnosis of UEDVT in patients with active malignancy, and all consecutive patients identified were included in our analysis. UEDVT was defined as thrombosis involving the internal jugular, subclavian, axillary, or brachial veins. The term “isolated” vein thrombosis will be used to refer to thrombosis involving a single vein and “extensive thrombosis” to refer to thrombosis extending to one or more other contagious veins.
Collected data included baseline characteristics such as age, gender, smoking status, complete blood count at the time of VTE diagnosis, primary cancer site and stage of disease at the time of VTE diagnosis, type of active antineoplastic treatment during the preceding 3 months, and the presence or absence of a CVC. Other known thrombotic risk factors needed to calculate Khorana risk score for patients on active treatment, including body mass index, were also reviewed and patients were stratified accordingly into the appropriate risk categories.14 Other collected variables included complications of VTE, management, and complications of treatment. Durations of follow-up and survival were also recorded.
Survival estimates were calculated using Kaplan-Meier curves, and univariate and multivariate analyses were based on log-rank testing using the statistical software JMP, Version 10, SAS Institute Inc. This is a low-risk retrospective analysis, and IRB granted exemption from individual patient consenting.
Results
Patient and Disease Characteristics
During the study period (December 2007 to November 2016), a total of 87 patients with a diagnosis of UEDVT were identified and included in the analysis. The median age at the time of cancer diagnosis was 50 years and at UEDVT diagnosis was 52.4 years (18.4-81.3). Forty-eight (55.2%) patients were females, and 56 (64.4%) were active smokers. All patients had a confirmed diagnosis of cancer and were treated and followed at our institution. The most common primary tumors were breast (23.0%), colorectal (18.4%), gastroesophageal (18.4%) and lymphoma (14.9%). Myeloma or leukemia was the underlying diagnosis in 7 patients and of all other patients with TNM-stageable disease, 81.3% had advanced-stage disease. At the time of UEDVT diagnosis, 58.6% of patients were on active chemotherapy, 9.2% were on hormonal therapy, and 5.7% had recent major surgery within the previous 30 days. Additionally, 54.0% of patients had a CVC inserted for chemotherapy infusion (Table 1). Khorana risk assessment model (RAM) was applicable for 51 patients who were on active chemotherapy (58.6%); risk scores were categorized as high, intermediate, or low in 25.5%, 51.0%, and 23.5%, respectively.
Table 1.
Patient Characteristics (N = 87).
| Age (Years) | Median | 52.4 | |
|---|---|---|---|
| Range | 18.4-81.3 | ||
| Number of Patients | Percentage | ||
| Gender | Male | 39 | 44.8 |
| Female | 48 | 55.5 | |
| Primary Tumor | Breast | 20 | 23.0 |
| Colorectal | 16 | 18.4 | |
| Gastroesophageal | 16 | 18.4 | |
| Genitourinary | 2 | 2.3 | |
| Head and Neck | 7 | 8.0 | |
| Leukemia | 5 | 5.7 | |
| Lung | 5 | 5.7 | |
| Lymphoma | 13 | 14.9 | |
| Multiple Myeloma | 2 | 2.3 | |
| Thyroid | 1 | 1.1 | |
| Khorana Risk Score | Low | 12 | 23.5 |
| Intermediate | 26 | 51.0 | |
| High | 13 | 25.5 | |
| Not applicable | 36 | 41.4 | |
| Disease Stage | Advanced | 65 | 74.7 |
| Early | 15 | 17.2 | |
| Treatment | Chemotherapy | 51 | 58.6 |
| Hormonal Therapy | 8 | 9.2 | |
| Surgery | 5 | 5.7 | |
| Radiation | 2 | 2.3 | |
| None | 21 | 24.1 | |
| Site of Thrombosisa | Internal Jugular | 38 | 43.7 |
| Subclavian | 49 | 56.3 | |
| Axillary | 40 | 46.0 | |
| Brachial | 21 | 24.1 | |
| Central Venous Catheter | Present | 47 | 54.0 |
| Absent | 40 | 46.0 | |
a More than one vein could be involved; total >87
Characteristics and Site of UEDVT
The median time between cancer diagnosis and UEDVT was 3.44 (0-90.3) months, with 21 (24.1%) patients diagnosed within the first 4 weeks of cancer diagnosis. Only 1 patient was on prophylactic anticoagulation at the time of UEDVT diagnosis. The subclavian vein, alone or in combination with other sites, was the most commonly involved and was identified in 49 (56.3%) patients. Axillary vein involvement was seen in 40 (46.0%) patients and internal jugular vein in 38 (43.7%). Brachial vein involvement was the least frequently involved vein and was identified in only 21 (24.1%) patients. Extensive thrombosis with more than 1 vein involvement was seen in 42 (48.3%) patients.
Complications of UEDVT
The overall rate of complications due to UEDVT was 40.2%, with 9.2% of patients developing more than one complication. Pulmonary embolism was diagnosed in 9.2% of patients, while superior vena cava (SVC) syndrome developed in 14.9% and postthrombotic syndrome in 26.4%. Complication rates were more frequent in patients with isolated single-vein thrombosis compared to patients with extensive thrombosis, although this numeric difference was not statistically significant (48.9% vs 30.9%, P = .086). In particular, patients with isolated thrombosis had more frequent pulmonary embolic events (13.3% vs 4.8%, P = .27) in addition to more frequent SVC thrombosis (20.0% vs 9.5%, P = .23). Rates of postthrombotic syndrome were similar in both groups (Table 2). On reviewing complication rates in the subgroup of patients with isolated single vein thrombosis, patients with internal jugular vein involvement had a trend towards developing more complications. While 22.7% of patients with internal jugular thrombosis developed a PE, only 4.3% of patient in all other sites combined were diagnosed with a PE (P = .096). Similarly, SVC syndrome was reported in 36.4% of patients with isolated internal jugular thrombosis compared to only 4.3% among all other sites (P = .010). However, postthrombotic syndrome was less frequent in patients with internal jugular vein thrombosis (9.1% vs 43.5%, P = .017; Table 3).
Table 2.
Rates of Complications Following Extensive Versus Single Vein Thrombosis.
| Complication | All Patients N = 87 (100%) | Extensive UEDVT n = 42 (100%) | Single vein UEDVT n = 45 (100%) | P Value |
|---|---|---|---|---|
| Pulmonary Embolism | 8 (9.2) | 2 (4.8) | 6 (13.3) | .268 |
| Superior Vena Cava Syndrome | 13 (14.9) | 4 (9.5) | 9 (20.0) | .232 |
| Postthrombotic Syndrome | 23 (26.4) | 11 (26.2) | 12 (26.7) | .960 |
| Any complicationa | 35 (40.2) | 13 (30.9) | 22 (48.9) | .086 |
Abbreviation: UEDVT, upper extremity deep vein thrombosis
a 9.2% of all patients developed more than one complication
Table 3.
Rates of Complications Following UEDVT Involving an Isolated Vein; Internal Jugular Vein Versus Any Other Sitea.
| Complication | All Patients N = 45 (100%) | Internal Jugular Isolated UEDVT n = 22 (100%) | Other Sites a Isolated UEDVT n = 23 (100%) | P Value |
|---|---|---|---|---|
| Pulmonary Embolism | 6 (13.3) | 5 (22.7) | 1 (4.3) | .096 |
| Superior Vena Cava Syndrome | 9 (20.0) | 8 (36.4) | 1 (4.3) | .010 |
| Postthrombotic Syndrome | 12 (26.7) | 2 (9.1) | 10 (43.5) | .017 |
Abbreviation: UEDVT, upper extremity deep vein thrombosis
a Subclavian, axillary or brachial veins
Non-CVC Associated Thrombosis
As mentioned above, 40 (46.0%) patients did not have a CVC in place at the time of upper extremity thrombosis. Nineteen (47.5%) patients had a diagnosis of breast cancer, and 9 (22.5%) patients had lymphoma. The remaining patients had lung cancer (4), acute leukemia (3), genitourinary cancers (2), head and neck cancer (2), and thyroid cancer (1). Of the 37 patients with stageable disease, 30 (81.1%) patients had metastatic stage IV disease. In 19 (57.5%) patients, cancer was diagnosed within the preceding 3 months of UEDVT diagnosis, and only 50% of all patients were on active cancer-directed therapy at that time.
Complication rates were significantly lower in patients with catheter-related thrombosis; 27.7% of patients with CVCs had subsequent complications compared to 55.0% of patients with non-catheter-related thrombosis (P = .01). In particular, none of the patients with catheter-related thrombosis had a subsequent PE compared to 20.0% of patients without a CVC (P = .001). Postthrombotic syndrome was also lower at a rate of 17% compared to 37.5% in patients without a catheter (P = .031). No significant difference was observed in the rate of subsequent SVC thrombosis (Table 4).
Table 4.
Rates of Complications Following UEDVT; In Patients with Versus Without a Central Venous Catheter (CVC).
| Complication | CVC-associated n = 47 (100%) | No CVC n = 40 (100%) | P Value |
|---|---|---|---|
| Pulmonary Embolism | 0 (0) | 8 (20.0) | .001 |
| Superior Vena Cava Syndrome | 8 (17.0) | 5 (12.5) | .764 |
| Postthrombotic Syndrome | 8 (17.0) | 15 (37.5) | .031 |
| Any complicationa | 13 (27.7) | 22 (55.0) | .010 |
a 9.2% of all patients developed more than one complication
Treatment
Except for 8 (9.2%) patients, all were anticoagulated. After the initial acute treatment phase, 75 (86.2%) patients were anticoagulated with therapeutic low molecular weight heparin (LMWH) and the remaining 4 (4.6%) patients received warfarin with a target INR of 2 to 2.5 following a short course of bridging therapy with LMWH or unfractionated heparin. Duration of anticoagulation varied; 31.0% were anticoagulated for 12 months or longer, 20.7% were anticoagulated for 6 months, while all other patients were anticoagulated for 3 months or less. Anticoagulation was complicated by major bleeding in 3 (3.8%) patients with bleeding occurring at 3 days, 7 days, and 11 months after initiation of anticoagulation in these patients. During the duration of follow-up, 3 (3.4%) patients had a recurrence of thrombosis, two of whom were initially anticoagulated for less than three months at the time of first UEDVT diagnosis. We could not elucidate a statistically significant correlation between duration of anticoagulation and rate of complications, likely due to the small number of patients and complication events
Survival
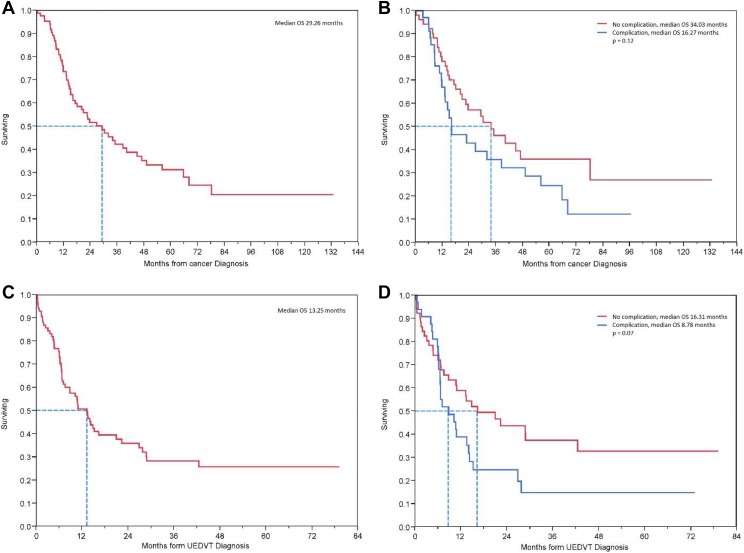
Finally, median overall survival (OS) from the time of cancer diagnosis was 29.26 months and the only factor with statistically significant effect on survival was the primary cancer site with longest OS seen in patients with lymphoma, breast, and colorectal cancers (68.1, 38.6, and 35.1 months, respectively, P = .0054). Median OS following the diagnosis of UEDVT was 13.25 months, and the site of primary cancer no longer significantly affected survival (P = .29). There was a trend toward worse OS in the subgroup of patients who developed a complication compared to those who did not; 16.27 versus 34.03 months (P = .12) and 8.78 versus 16.31 months (P = .07), following the diagnosis of cancer and UEDVT, respectively (Figure 1).
Figure 1.
Overall survival: (A) from the time of cancer diagnosis in all patients, (B) from the time of cancer diagnosis in patients with or without a complication, (C) from the time of UEDVT diagnosis in all patients, (D) from the time of UEDVT diagnosis in patients with or without a complication.
Discussion
Much of the available data on UEDVT comes from large population-based registries and mostly in those with noncatheter-related thrombosis.15 Few studies, usually including small patient numbers, addressed upper extremity thrombosis in patients with cancer with or without CVCs.16 Except for cancer and CVC, other risk factors for UEDVT are not as well characterized or described as in patients with lower extremity thrombosis.17 In particular, Khorana RAM was unable to predict the occurrence of UEDVT in our cohort of patients, as only 25.5% of patients had a high-risk score.
Our study showed significant rates of subsequent complications following UEDVT such as PE, SVC thrombosis, and postthrombotic syndrome. In particular, the rate (9.2%) of PE was higher in our patient population than that was observed in some previously published reports. Rates of PE as a complication of UEDVT varied according to population studied with some studies reporting higher rates in patients with cancer and in those with CVC. In one review of prospective registry data, PE was reported in 3% (18 of 592) of patients with an UEDVT compared to 16% (767 of 4796) of patients with lower extremity DVT (P = .001). In this study, however, <40% of patients had a diagnosis of cancer.10 In another study, only 6 (2.0%) patients had PE among a total of 300 patients with UEDVT, 35% of who had documented malignancy, and 80% had associated or previous indwelling central venous lines or leads.18 In yet another study, the incidence of objectively confirmed PE was 9.0% among a group of 94 patients; 48% had a diagnosis of cancer and 93% had indwelling CVCs.19 All patients included in our cohort have an active malignancy and whether a diagnosis of PE in this population is a direct complication of UEDVT or a reflection of the generally heightened risk of thrombosis in association with a cancer diagnosis is difficult to determine.
The proportion of patients with non-CVC related thrombosis was high. Most of these patients had advanced-stage disease. The site of the primary tumor can explain some of these thrombotic events; 47.5% had breast cancer which can cause direct invasion or compression on the venous system, in addition to the risk associated with surgical intervention that can cause direct damage. It is important, however, to also note that the distribution of the diseases between the groups of patients with or without a CVC is confounded by the type of anticancer therapy given or planned to be given for different cancer types. Patients with diagnoses that require therapy with infusion chemotherapy are more likely to have a central line in place, and some of these cancers are known to be more inherently thrombogenic, for example, esophageal cancers.
Another peculiar observation was the lower rate of complications in our subset of patients with a CVC-associated thrombosis compared to those without a catheter. None of the 47 patients with CVC-associated thrombosis had PE compared to 8 (20.0%) of the 40 patients without a catheter. One possible explanation is that, given the more proximal site, catheter-associated thrombi are more readily identified on routine restaging imaging performed in majority of patients with cancer. This leads to a higher proportion of earlier diagnoses of incidental thrombi at an earlier stage before they become symptomatic and before complications develop. It is also possible, however, that other pathophysiologic factors contributing to the increased risk of thrombosis in this patient population with malignancies may play a more significant role in increasing risk for the development of PE than the mere presence of a catheter does.
The site of thrombosis was an important determinant of subsequent complications. In our study, 6 patients with single vein thrombosis developed a PE; only one had a thrombus involving the subclavian, axillary or brachial veins, while all the other 5 patients had thrombosis in the internal jugular vein. Interestingly, the 2 other cases of PE encountered in patients with extensive UEDVT also had internal jugular vein thrombosis among the multiple involved veins. Similarly, among the 13 patients who developed SVC syndrome, 77% had internal jugular vein involvement. Whether the anatomic course of the internal jugular vein contributes to these higher rates of both PE and SVC syndrome is not clear and our small number of patients is insufficient to make definite conclusions. This observation might weigh in decision-making regarding aggressiveness and duration of anticoagulation if validated in other studies.
Our observed thrombosis recurrence rate (3.4%) was notably low compared to published literature. This can be explained by the short duration of median follow-up of our patient cohort. Recurrence rates have been reported to be as high as 18% among patients with cancer with UEDVT after a median follow-up of 3.5 years in 1 study20 and 13% at a median follow-up of around 5 years in another study.9 The 3.8% rate of major bleeding as a complication of anticoagulation among our patients was similar to what has been previously reported. In one study, 5.0% of patients anticoagulated for UEDVT experienced major bleeding.20 Analysis of patients from the RIETE registry reported a higher rate of bleeding among patients with cancer; observed in 9.7% of patients with DVT and cancer (n = 319) compared to 2.4% in non-cancer patients (n = 781), all with non-catheter related thrombosis.15
Limitations
There are multiple limitations to our study, most importantly the small number of patients. Although all patients have a diagnosis of active cancer, there is also considerable heterogeneity related to different primary sites, different stages of the disease, and different treatments administered. The lack of a comparator group without an UEDVT and otherwise similar disease characteristics limits the interpretation of survival data. UEDVT could be a maker of more advanced diseases as opposed to a marker of worse prognosis in and by itself.
Conclusions
Upper extremity DVT is an uncommon event in patients with cancer. Subsequent complications including PE, SVS, and postthrombotic syndrome are frequent and were more commonly diagnosed among patients with non-catheter-related thrombosis and in those with internal jugular vein involvement. Although definite conclusions could not be made based on this small cohort of patients, it seems likely based on our findings that patients with internal jugular thrombosis are more prone to complications and may benefit form more aggressive treatment compare to those with thrombosis in other sites. This is supported by the low rate of treatment-related complications and bleeding. Larger studies addressing these findings can improve identification of factors associated with an increased risk for the development of UEDVT and identify patients at higher risk for development of complications. This in turn, can help inform treatment decisions as some patient may require more aggressive or prolonged treatment, which might not be indicated in others. The role of prophylactic anticoagulation in specific subsets of patients at higher risk to develop UEDVT and its complications is also unclear. In the absence of predictive risk stratification scoring tools, this is an area in need of more investigation.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Asem Mansour  http://orcid.org/0000-0003-3443-5873
http://orcid.org/0000-0003-3443-5873
Salwa S. Saadeh  http://orcid.org/0000-0002-1052-9093
http://orcid.org/0000-0002-1052-9093
Ethics Approval: Ethical approval to report this case series was obtained from the institutional review board at King Hussein Cancer Center (16 KHCC 58).
Informed consent: Informed consent for patient information to be published in this article was not obtained as a weiver was granted by the institutional review board committee since it is work associated with minimal patient risk.
References
- 1. Falanga A, Marchetti M, Vignoli A. Coagulation and cancer: biological and clinical aspects. J Thromb Haemost. 2013;11(2):223–233. [DOI] [PubMed] [Google Scholar]
- 2. Khorana AA. Risk assessment and prophylaxis for VTE in cancer patients. J Natl Compr Canc Netw. 2011;9(7):789–797. [DOI] [PubMed] [Google Scholar]
- 3. Prandoni P, Lensing AW, Cogo A, et al. The long-term clinical course of acute deep venous thrombosis. Ann Intern Med. 1996;125(1):1–7. [DOI] [PubMed] [Google Scholar]
- 4. Carson JL, Kelley MA, Duff A, et al. The clinical course of pulmonary embolism. N Engl J Med. 1992;326(19):1240–1245. [DOI] [PubMed] [Google Scholar]
- 5. Pengo V, Lensing AW, Prins MH, et al. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med. 2004;350(22):2257–2264. [DOI] [PubMed] [Google Scholar]
- 6. Sorensen HT, Mellemkjaer L, Olsen JH, Baron JA. Prognosis of cancers associated with venous thromboembolism. N Engl J Med. 2000;343(25):1846–1850. [DOI] [PubMed] [Google Scholar]
- 7. Kucher N. Clinical practice. Deep-vein thrombosis of the upper extremities. N Engl J Med. 2011;364(9):861–869. [DOI] [PubMed] [Google Scholar]
- 8. Munoz FJ, Mismetti P, Poggio R, et al. Clinical outcome of patients with upper-extremity deep vein thrombosis: results from the RIETE Registry. Chest. 2008;133(1):143–148. [DOI] [PubMed] [Google Scholar]
- 9. Isma N, Svensson PJ, Gottsater A, Lindblad B. Upper extremity deep venous thrombosis in the population-based Malmo thrombophilia study (MATS). Epidemiology, risk factors, recurrence risk, and mortality. Thromb Res. 2010;125(6):e335–e338. [DOI] [PubMed] [Google Scholar]
- 10. Joffe HV, Kucher N, Tapson VF, Goldhaber SZ, Deep Vein Thrombosis FSC. Upper-extremity deep vein thrombosis: a prospective registry of 592 patients. Circulation. 2004;110(12):1605–1611. [DOI] [PubMed] [Google Scholar]
- 11. Bleker SM, van Es N, van Gils L, et al. Clinical course of upper extremity deep vein thrombosis in patients with or without cancer: a systematic review. Thromb Res. 2016;140(suppl 1):S81–S88. [DOI] [PubMed] [Google Scholar]
- 12. Kraaijpoel N, van Es N, Porreca E, Buller HR, Di Nisio M. The diagnostic management of upper extremity deep vein thrombosis: a review of the literature. Thromb Res. 2017;156:54–59. [DOI] [PubMed] [Google Scholar]
- 13. Cohen AT, Katholing A, Rietbrock S, Bamber L, Martinez C. Epidemiology of first and recurrent venous thromboembolism in patients with active cancer. A population-based cohort study. Thromb Haemost. 2017;117(1):57–65. [DOI] [PubMed] [Google Scholar]
- 14. Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111(10):4902–4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Newton DH, Monreal Bosch M, Amendola M, et al. Analysis of noncatheter-associated upper extremity deep venous thrombosis from the RIETE registry. J Vasc Surg Venous Lymphat Disord. 2017;5(1):18–24 e1. [DOI] [PubMed] [Google Scholar]
- 16. Delluc A, Le Gal G, Scarvelis D, Carrier M. Outcome of central venous catheter associated upper extremity deep vein thrombosis in cancer patients. Thromb Res. 2015;135(2):298–302. [DOI] [PubMed] [Google Scholar]
- 17. Cote LP, Greenberg S, Caprini JA, et al. Comparisons between upper and lower extremity deep vein thrombosis: a review of the RIETE registry. Clin Appl Thromb Hemost. 2017;23(7):748–754. [DOI] [PubMed] [Google Scholar]
- 18. Levy MM, Albuquerque F, Pfeifer JD. Low incidence of pulmonary embolism associated with upper-extremity deep venous thrombosis. Ann Vasc Surg. 2012;26(7):964–972. [DOI] [PubMed] [Google Scholar]
- 19. Lee JA, Zierler BK, Zierler RE. The risk factors and clinical outcomes of upper extremity deep vein thrombosis. Vasc Endovascular Surg. 2012;46(2):139–144. [DOI] [PubMed] [Google Scholar]
- 20. Bleker SM, van Es N, Kleinjan A, et al. Current management strategies and long-term clinical outcomes of upper extremity venous thrombosis. J Thromb Haemost. 2016;14(5):973–981. [DOI] [PubMed] [Google Scholar]