Abstract
Pancreatic ductal adenocarcinoma (PDAC) is a deadly cancer often diagnosed late. Earlier detection is urgently needed. Pancreatic ductal adenocarcinoma is known to associate with increased coagulation activity. We studied whether preoperative coagulation biomarkers are useful in distinguishing PDAC from a benign tumor, intraductal papillary mucinous neoplasm (IPMN) in this observational study. We analyzed standard clinical and coagulation variables in patients operated during 2010 and 2015 at Helsinki University Hospital. Pancreatic ductal adenocarcinoma with preoperative coagulation variables available and no neoadjuvant treatment or other active cancer was observed in 80 patients (stage I-III in 67 and IV in 13) and IPMN in 18 patients. Fibrinogen, factor VIII (FVIII), carbohydrate antigen (CA) 19-9, albumin, alkaline phosphatase, and conjugated bilirubin were higher in both stages I to III and IV PDAC compared to IPMN (P < .05). Factor VIII was highest in stage IV (P < .05). Combining these variables in a panel increased sensitivity and specificity for PDAC. In receiver operating characteristic analysis, the area under the curve (95% confidence interval) was 0.95 (0.90-1.00) for the panel, compared to 0.80 (0.71-0.88) for CA 19-9 alone (P < .01). In conclusion, PDAC was associated with increased fibrinogen and FVIII. Combining these coagulation biomarkers with CA 19-9, albumin, and alkaline phosphatase improves diagnostic accuracy.
Keywords: pancreatic cancer, coagulation, CA 19-9, fibrinogen, FVIII, biomarker
Background
In recent years, intensive research has targeted cancer-associated coagulation. Cancer promotes coagulation activity and vice versa; increased coagulation promotes cancer growth.1 Cancer relates to increased risk of venous thromboembolic events (VTEs)2 and other coagulopathies.3 Venous thromboembolic events increase mortality by 3-fold.4 Chemotherapy also increases the risk of thrombosis. Khorana et al. introduced a risk scoring model to identify chemotherapy-associated VTE, which includes cancer site, hemoglobin level, platelet count, leukocyte count, and body mass index (BMI).5 This score predicts early mortality after pancreatic surgery.6 Pancreatic ductal adenocarcinoma (PDAC), representing 85% of all pancreatic cancers,7 is one of the leading causes of cancer-associated mortality worldwide.8 Malignancies of the pancreas, especially adenocarcinomas, present a high risk of thrombosis.9 Pancreatic ductal adenocarcinoma is reported to be associated with increased circulating microparticles, factor VIII (FVIII), and D-dimer.10,11 Non-overt coagulation activity acts beyond clinical detection, and its role in PDAC has not been widely studied.
Curative treatment for PDAC requires radical surgery.12,13 Because both surgery and malignancy are associated with enhanced risk of thrombosis, patients with PDAC require special attention. Owing to nonspecific symptoms, PDAC is often diagnosed at a late stage. Tools for early diagnosis are scarce; computed tomography (CT) or magnetic resonance imaging (MRI) is needed, and final diagnosis is confirmed by histopathological tissue analysis.
Serum carbohydrate antigen (CA) 19-9, a sialylated Lewis a antigen, is the most commonly used biomarker for PDAC.14 CA 19-9 is normally expressed by the pancreas biliary ducts, gastrointestinal tract, endometrium and salivary glands, but neoplastic cells increase this expression. Therein, the sensitivity and specificity for PDAC are only 70% to 90% and 68% to 91%,15 respectively, but CA 19-9 has been shown to increase as the disease progresses.16,17 CA 19-9 may thus be more optimal for follow-up than diagnostics.
Intraductal papillary mucinous neoplasms (IPMNs) are benign tumors originating from the pancreatic ducts that carry a risk of malignant transformation.18 Suspicion of IPMN can be raised at CT scan or MRI, but the final diagnosis can only be confirmed by histopathology. Because of similar preoperative psychophysiological stress known to affect coagulation activity and cause resistance to fibrinolysis,19 IPMN patients are appropriate controls for PDAC. The influence of IPMN on coagulation has not been reported. Because pancreatic tumor surgery is extensive, surgery for benign tumors should be avoided, unless there is high-grade dysplasia at biopsy or suspicion of malignant transformation. Therefore, clinical tools for distinguishing PDAC from benign tumors are needed.
At the Helsinki University Hospital, a coagulation panel is used to assess various coagulation disorders. The panel includes the following: clotting times—prothrombin time (PT) measuring vitamin K-dependent factors and activated partial thromboplastin time (APTT) for the intrinsic pathway; fibrinogen as the most abundant coagulation protein and the source of fibrin formation; thrombin time (TT) measuring thrombin functions on fibrinogen and circulating phospholipids and microvesicles3,20; FVIII as a marker of liver sinusoidal endothelium and risk of thrombosis21; antithrombin (AT) as the key regulator of coagulation; and finally, D-dimer, the sign of the balance between coagulation and fibrinolysis, evaluating fibrin turnover.
Current treatments for PDAC, including surgery, chemotherapy, and radiotherapy, increase tissue destruction, coagulation activity, and the risk of non-overt and overt thrombosis. The overall effects of cancer on coagulation, while excluding the effects of treatments, are worth studying in patient samples. To detect whether preoperative biomarkers could distinguish PDAC from IPMN, we evaluated the effect of PDAC and IPMN on the preoperative levels of the coagulation panel. In addition, we combined CA 19-9 with the coagulation biomarkers to assess the diagnostic accuracy.
Patients and Methods
Patients
Of 580 patients admitted to the Helsinki University Hospital for elective pancreatic surgery during 2010 to 2015, data on preoperative coagulation variables were available from 318 patients. Patients with PDAC and benign IPMN were included in this observational study. Patients who received preoperative neoadjuvant treatment (n = 43), those with another cancer within the previous 5 years (n = 21), those with tumors of different origin (e.g., duodenal or biliary tumors, n = 47), or those with another type of pancreatic tumor (e.g., papillary cancer) or chronic pancreatitis (n = 109) were excluded due to their possible confounding effects on coagulation activity. The diagnosis of PDAC was confirmed by histopathology of the surgical samples. Patients were divided into 3 groups: stage I to III PDAC, stage IV PDAC, and IPMN. Patient characteristics are shown in Table 1. In stage IV patients, distant metastasis was unknown preoperatively, but was verified from frozen sections early during the operation.
Table 1.
Patient Characteristics.
| Stage I-III PDAC (n = 67) | Stage IV PDAC (n = 13) | IPMN (n = 18) | |
|---|---|---|---|
| Male sex (n) | 31 (46%) | 9 (69%) | 11 (61%) |
| Age, years, median (range) | 68 (52-81) | 69 (52-78) | 66 (31-80) |
| Antithrombotic medicationa | |||
| Enoxaparin sodium (n) | 5 | 2 | 4 |
| Acetylsalicylic acid (n) | 6 | 2 | 6 |
Abbreviations: IPMN, intraductal papillary mucinous neoplasm; PDAC, pancreatic ductal adenocarcinoma.
a Used as thromboprophylaxis for conditions of atrial fibrillation, atherothrombosis, and previous venous thromboembolism.
The study was approved by the Surgical Ethics Committee of Helsinki University Hospital (Dnro HUS 226/E6/06, extension TMK02 §66 17.4.2013) and the National Supervisory Authority of Welfare and Health (Valvira Dnro 1041/06.01.03.01/2012) and was conducted according to the Helsinki Declaration. Written informed consent was obtained from the patients for their anonymized information to be published in this article.
Laboratory Analyses
Blood cell counts, ABO blood groups, and plasma concentrations of CA 19-9, C-reactive protein, creatinine, albumin, bilirubin, alkaline phosphatase (ALP), alanine amino transferase, and amylase were measured as part of routine preoperative laboratory analysis for all patients. In addition to the routine laboratory tests, we assessed the coagulation panel, including PT, APTT, fibrinogen, TT, FVIII, AT, and D-dimer. Blood samples were collected 1 to 10 days before surgery with 1 exception, at one month earlier. We compared the laboratory profiles of the patients having PDAC with those of IPMN, as well as PDAC at stage I to III with stage IV. All samples were analyzed by routine methods at the Helsinki University Hospital Laboratory, and data were gathered from laboratory records.
Coagulation Assays
Citrated 3.2% plasma was used for coagulation samples. Prothrombin time was analyzed with Nycotest PT (Axis-Shield PoC As, Oslo, Norway) and APTT with Actin FSL (Siemens Healthcare Diagnostics, Marburg, Germany, and all following reagents provided by Siemens are from the same source). Fibrinogen levels were determined using Multifibren U (Siemens and BC Thrombin Reagent (Siemens) was used to determine TT. Factor VIII: C (FVIII) was analyzed with a 1-stage clotting assay (Pathromtin SL and Coagulation Factor VIII Deficient Plasma; Siemens) and AT was measured with a chromogenic assay (Berichrom Antithrombin III; Siemens). D-dimer levels were measured with an immunoturbidimetric assay (Tina-quant D-dimer; Roche Diagnostics, Mannheim, Germany).
Biomarker Panel
As the sensitivity and specificity of CA 19-9 for PDAC are not uniform and known to vary and even miss metastatic disease, we studied whether its diagnostic value could be improved by combining it with other biomarkers. In a panel score combined the biomarkers that overall distinguished both stages I to III and IV PDAC from IPMN. The cutoff levels were chosen based on the sensitivity and specificity of each biomarker for PDAC (see Table 2). Each biomarker was stratified into 1 to 3 points. The cutoff value between points 1 and 2 was determined using the Youden J point, and the cutoff between points 2 and 3 was the value at which the specificity was 100% for PDAC.
Table 2.
Panel Score.
| Biomarker | Values | Panel Score | Patients (%) | ||
|---|---|---|---|---|---|
| Stage I-III PDAC | Stage IV PDAC | IPMN | |||
| CA 19-9 (kU/L) | <41 | 1 | 32 | 33 | 88 |
| 41-200 | 2 | 26 | 17 | 12 | |
| >200 | 3 | 42 | 50 | 0 | |
| FVIII (IU/dL) | <183 | 1 | 24 | 0 | 83 |
| 183-267 | 2 | 55 | 32 | 17 | |
| >267 | 3 | 21 | 68 | 0 | |
| Fibrinogen (g/L) | <3.6 | 1 | 13 | 23 | 72 |
| 3.6-6.3 | 2 | 81 | 77 | 28 | |
| >6.3 | 3 | 6 | 0 | 0 | |
| Albumin (g/L) | >40.1 | 1 | 24 | 15 | 94 |
| 40.1-34.7 | 2 | 58 | 46 | 6 | |
| <34.7 | 3 | 18 | 38 | 0 | |
| Alkaline phosphatase (U/L) | <88.5 | 1 | 37 | 38 | 83 |
| 88.5-106.5 | 2 | 15 | 8 | 17 | |
| >106.5 | 3 | 48 | 54 | 0 | |
| Conjugated bilirubin (µmol/L) | <4.5 | 1 | 42 | 31 | 83 |
| 4.5-12.5 | 2 | 42 | 23 | 17 | |
| >12.5 | 3 | 16 | 46 | 0 | |
Abbreviations: IPMN, intraductal pancreatic mucinous neoplasm; PDAC, pancreatic ductal adenocarcinoma.
Statistical Analyses
We performed statistical analysis using SPSS Statistics (v21-24; IBM Corp, Armonk, New York) and Medcalc (v18; Ostend, Belgium). We compared the variables between the groups using the independent-samples Kruskal-Wallis test and the post hoc Dunn test. The χ2 test was used to compare blood ABO-type distributions. The Spearman correlation coefficient (ρ) was used to identify correlations. Sensitivities and specificities were compared with receiver operating characteristic (ROC) curves. Confidence intervals (CIs) for the sensitivity and specificity were calculated using the Clopper-Pearson exact binomial test and for the area under the curves (AUCs) using binomial exact CIs. The statistical significance between the AUCs was determined using the DeLong method. Values presented as lower than a value (e.g., CRP <3 mg/L) were rounded to one decimal below the next highest value.
Results
Routine PDAC-Associated Laboratory Variables
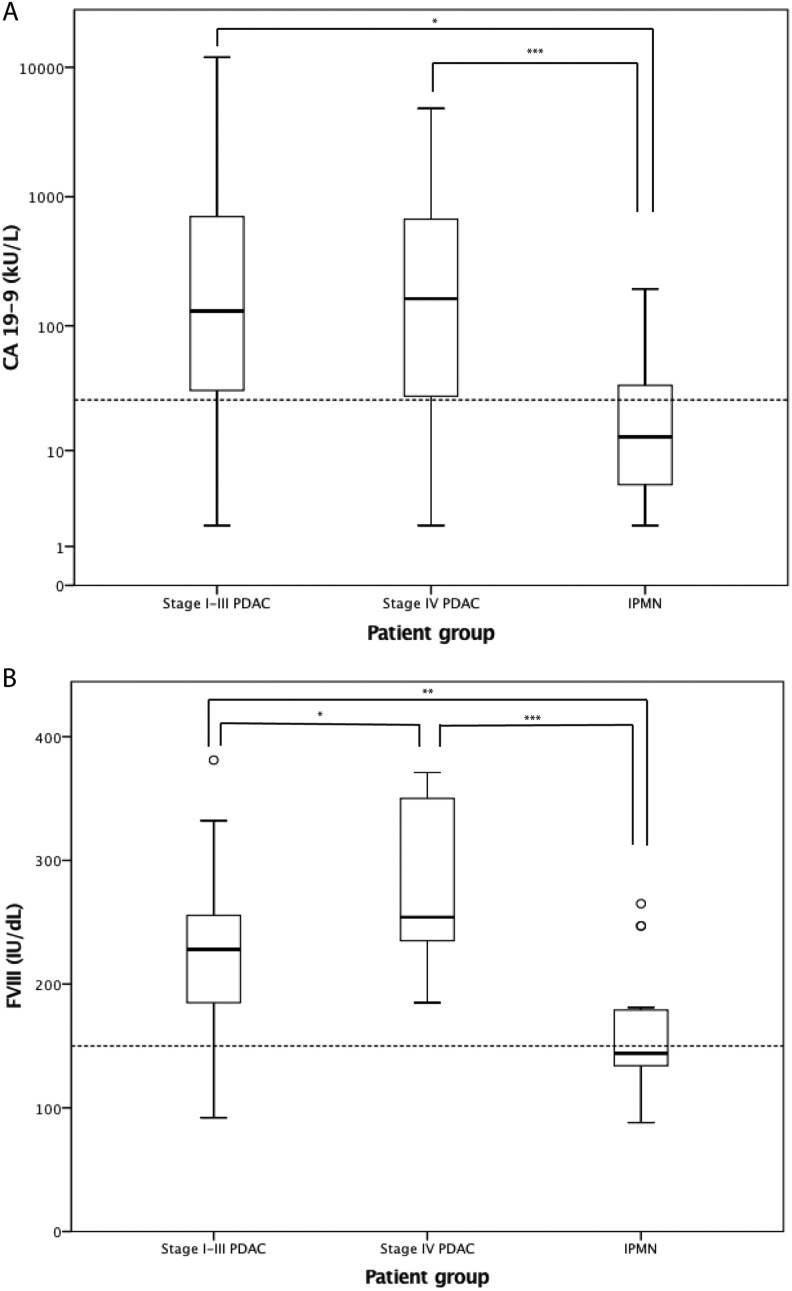
Before pancreatic surgery, CA 19-9 levels, blood cell counts, liver enzymes, and CRP were analyzed from each patient to determine surgical suitability, and we assessed these variables related to the nature of the tumor and the patient groups of stage I to III PDAC, stage IV PDAC, and IPMN. CA 19-9 was missing from 1 patient in each group. CA 19-9, ALP, and conjugated bilirubin were higher and albumin was lower (P < .05; Table 3) at all stages of PDAC compared with IPMN. Total bilirubin values were higher than in IPMN only at stage IV PDAC (P < .05; Table 3). Importantly, CA 19-9 was below the cutoff level in 16 (21%) patients with PDAC, of whom 3 had stage IV disease (Figure 1A). Then again, CA 19-9 was above the cutoff level in 30% of the IPMN patients. Patients with stage IV disease had higher leukocyte counts than IPMN patients (P < .05; Table 3), but hemoglobin, platelet counts, CRP, creatinine, alanine transaminase, and amylase did not differ between the groups. In the Khorana score, patients with PDAC receive 2 points based on tumor site, and we omitted the points for the cancer diagnosis and screened only the hematological laboratory values and BMI. This modified Khorana score did not distinguish PDAC from IPMN nor were there differences between stages (data not shown).
Table 3.
Laboratory Biomarkers Among the Patient Groups.a,b,c
| Normal Range | Stage I-III PDAC (n = 67) | Stage IV PDAC (n = 13) | IPMN (n = 18) | P Values | |
|---|---|---|---|---|---|
| Median (IQR) | |||||
| Routine biomarkers | |||||
| CA 19-9 (kU/L) | <26 | 130 (31-709) | 168 (25-711) | 13 (5-35) | P (IPMN vs stage IV) < .001; P (IPMN versus stage I-III) = .017 |
| Alkaline phosphatase (U/L) | 35-105 | 105 (74-141) | 108 (69-367) | 61 (53-83) | P (IPMN versus stage I-III) = .001; P (IPMN versus stage IV) = .004 |
| Bilirubin (µmol/L) | <20 | 13 (10-18) | 21 (9-29) | 10 (9-14) | P (IPMN versus stage IV) = .045 |
| Conjugated bilirubin (µmol/L) | 0-5 | 5 (3-9) | 11 (4-18) | 3 (2-4) | P (IPMN versus stage I-III) = .017; P (IPMN versus stage IV) = .003 |
| Albumin (g/L) | 36-45 (40-69 years); 34-65 (>69 years) | 37.7 (35.5-40.0) | 36.9 (32.3-39.0) | 40.9 (40.4-41.9) | P (IPMN versus stage I-III) < .001; P (IPMN versus stage IV) = .001 |
| Leukocyte count (E9/L) | 3.4-8.2 | 7.0 (5.6-8.7) | 7.3 (6.3-9.5) | 5.9 (5.9-7.3) | P (IPMN versus stage IV) = .046 |
| Coagulation biomarkers | |||||
| PT (%) | 70-130 | 93 (78-113) | 89 (79-102) | 83 (74-104) | NS |
| APTT (s) | 23-33 | 24 (23-26) | 24 (24-26) | 25 (25-28) | NS |
| Thrombin time (s) | 17-25 | 18 (17-19) | 18 (18-19) | 18 (17-20) | NS |
| Fibrinogen (g/L) | 1.7-4.0 | 4.6 (4.0-5.2) | 5.0 (3.8-5.4) | 3.3 (3.1-4.0) | P (IPMN versus stage I-III) = .001; P (IPMN versus stage IV) = .011 |
| FVIII (IU/dL) | 52-148 | 228 (184-256) | 254 (231-350) | 144 (133-180) | P (IPMN versus stage I-III) = .001; P (IPMN versus stage IV) ≤ .001; P (stage I-III vs IV) = .049 |
| Antithrombin (%) | 84-108 | 102 (94-119) | 104 (97-120) | 106 (98-116) | NS |
| D-dimer (mg/L) | <0.5 | 0.3 (<0.2-0.5) | 0.3 (<0.2-0.7) | <0.2 (<0.2-0.3) | NS |
Abbreviations: CA = carbohydrate antigen, NS = not significant; APTT, activated partial thromboplastin time; FVIII, factor VIII; IPMN, intraductal pancreatic mucinous neoplasm; PT, prothrombin time.
a P Values were obtained by Kruskal-Wallis tests with post hoc Dunn test.
b Prothrombin time (%) refers to PT in seconds as related to normal healthy laboratory controls. Seventeen IPMN patients had antithrombin levels available.
c P > .05
Figure 1.
Distribution of the biomarkers (A) carbohydrate antigen (CA) 19-9 and (B) factor VIII (FVIII) in different patient groups. The boxes show the median and the interquartile range, the whiskers represent the extremes, and outliers are presented as circles. The dotted line represents normal reference values. P values were obtained by Kruskal-Wallis test, after implementing post hoc Dunn test, *P < .05, **P < .01, ***P < .001 Abbreviations: PDAC, pancreatic ductal adenocarcinoma; IPMN intraductal papillary mucinous neoplasm
Coagulation Biomarkers
In addition to the routine laboratory variables, the patients were analyzed using the coagulation panel to screen whether any coagulation abnormality was associated with PDAC. Fibrinogen and FVIII were significantly higher in both stages I to III and IV PDAC compared to IPMN (P < .05; Table 3). Factor VIII was also higher in stage IV than stage I to III PDAC (P < .05; Table 3, Figure 1B). Coagulation times PT, APTT, and TT, as well as AT and D-dimer, were similar in PDAC and IPMN, and generally within normal ranges, but close to the reference limits (Table 3). The distribution of ABO blood groups between PDAC and IPMN patients accorded to that of the Finnish population (data by the Finnish Red Cross Blood Service, not shown), and it did not correlate with FVIII levels (data not shown).
Correlations Between the Biomarkers
Biomarkers showing a significant difference between PDAC irrespective of stage and IPMN were subjected to bivariate analysis. Spearman correlation coefficients (ρ) and P values are presented in Table 4. ALP correlated with both fibrinogen and conjugated bilirubin in both stages I to III and IV PDAC. In stage I to III PDAC, ALP also showed a correlation with FVIII, as did conjugated bilirubin. Albumin depicted negative correlations with ALP and conjugated bilirubin in both stages I to III and IV PDAC, and also with FVIII in stage I to III and fibrinogen in stage IV PDAC. CA 19-9 correlated with conjugated bilirubin in stage I to III PDAC and FVIII in stage IV PDAC. In stage I to III, FVIII and fibrinogen showed a correlation. In IPMN, there was only a correlation between CA 19-9 and ALP and a negative correlation between FVIII and albumin.
Table 4.
Spearman Correlation Between the Biomarkers Showing Significant Differences Between PDAC and IPMN.
| CA 19-9 | FVIII | Fibrinogen | Albumin | ALP | Conjugated Bilirubin | |||
|---|---|---|---|---|---|---|---|---|
| Stage I-III | CA 19-9 | ρ (SE) | 1.000 | |||||
| P | ||||||||
| FVIII | ρ (SE) | 0.205 (0.115) | 1.000 | |||||
| P | .098 | |||||||
| Fibrinogen | ρ (SE) | 0.096 (0.130) | 0.314a (0.115) | 1.000 | ||||
| P | .442 | .010 | ||||||
| Albumin | ρ (SE) | −0.215 (0.126) | −0.511a (0.090) | −0.160 (0.126) | 1.000 | |||
| P | .085 | .000 | .198 | |||||
| ALP | ρ (SE) | 0.010 (0.129) | 0.354a (0.107) | 0.372a (0.117) | −0.280b (0.118) | 1.000 | ||
| P | .939 | .003 | .002 | .023 | ||||
| Conjugated bilirubin | ρ (SE) | 0.328a (0.108) | 0.523a (0.094) | 0.069 (0.128) | −0.263b (0.123) | 0.365a (0.115) | 1.000 | |
| P | .007 | .000 | .582 | .033 | .002 | |||
| Stage IV | CA 19-9 | ρ (SE) | 1.000 | |||||
| P | ||||||||
| FVIII | ρ (SE) | 0.601b (0.211) | 1.000 | |||||
| P | .039 | |||||||
| Fibrinogen | ρ (SE) | 0.131 (0.354) | 0.393 (0.279) | 1.000 | ||||
| P | .685 | .185 | ||||||
| Albumin | ρ (SE) | 0.007 (0.266) | −0.413 (0.250) | −0.634b (0.172) | 1.000 | |||
| P | .983 | .161 | .020 | |||||
| ALP | ρ (SE) | −0.077 (0.339) | 0.374 (0.275) | 0.695a (0.205) | −0.703a (0.136) | 1.000 | ||
| P | .812 | .208 | .008 | .007 | ||||
| Conjugated bilirubin | ρ (SE) | 0.063 (0.253) | 0.351 (0.237) | 0.541 (0.216) | −0.863a (0.085) | 0.739a (0.098) | 1.000 | |
| P | .845 | .240 | .056 | .000 | .004 | |||
| IPMN | CA 19-9 | ρ (SE) | 1.000 | |||||
| P | ||||||||
| FVIII | ρ (SE) | 0.225 (0.226) | 1.000 | |||||
| P | .385 | |||||||
| Fibrinogen | ρ (SE) | 0.188 (0.245) | 0.124 (0.272) | 1.000 | ||||
| P | .469 | .623 | ||||||
| Albumin | ρ (SE) | −0.453 (0.241) | −0.507b (0.191) | −0.283 (0.283) | 1.000 | |||
| P | .068 | .032 | .255 | |||||
| ALP | ρ (SE) | 0.486b (0.198) | 0.247 (0.250) | −0.241 (0.270) | −0.270 (0.249) | 1.000 | ||
| P | .048 | .323 | .335 | .278 | ||||
| Conjugated bilirubin | ρ (SE) | 0.180 (0.244) | −0.253 (0.274) | −0.057 (0.249) | −0.161 (0.260) | 0.314 (0.220) | 1.000 | |
| P | .488 | .312 | .821 | .523 | .204 |
Abbreviations: ALP, alkaline phosphatase; CA, carbohydrate antigen; FVIII, factor VIII; IPMN, intraductal papillary mucinous neoplasm; PDAC, pancreatic ductal adenocarcinoma; ρ, Spearman correlation coefficient; SE, standard error.
a Correlation is significant at the P < .01 level.
b Correlation is significant at the P < .05 level.
We also compared these 6 biomarkers with the remaining coagulation panel markers PT, APTT, TT, AT, and D-dimer. In stage I to III PDAC, there was a correlation between ALP and AT (ρ = 0.31, P = .011), as well as negative correlations between FVIII and APTT (ρ = −0.31, P = .012), and fibrinogen and TT (ρ = −0.24, P = .048); the latter also clearly occurred in IPMN (ρ = −0.73, P = .001). In stage IV, fibrinogen correlated also with AT (ρ = 0.62, P = .023).
Biomarker Panel
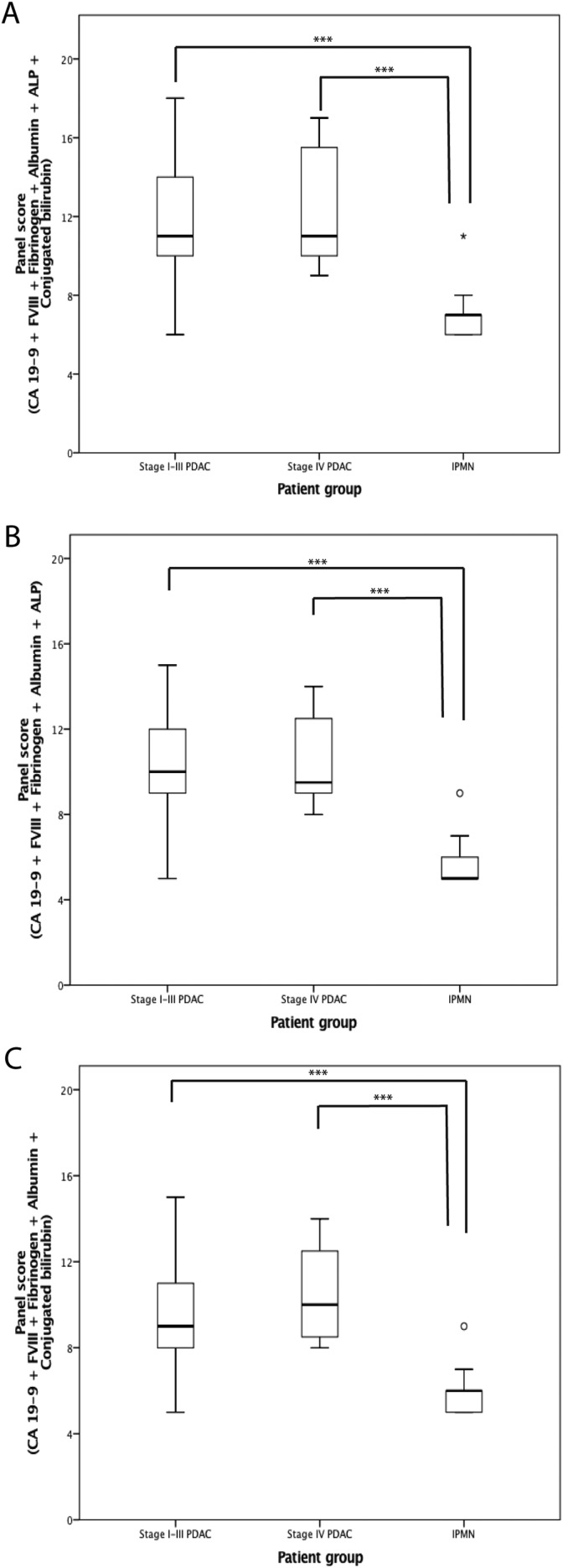
As CA 19-9 is used as a regular PDAC marker and we detected increased fibrinogen and FVIII in association with PDAC, we next aimed at forming panels to improve the sensitivity and specificity of these biomarkers. In addition to CA 19-9 FVIII, fibrinogen, albumin, ALP, and conjugated bilirubin distinguished PDAC from IPMN in both stages I to III and IV disease. We combined these biomarkers to form a panel score (Table 2). Using these scores, we formed 3 different panels, which all clearly distinguished PDAC from IPMN (P < .001; Figure 2). First, we combined all the biomarkers, and secondly formed targeted panels.
Figure 2.
Biomarker panel scores of the patient groups with carbohydrate antigen (CA) 19-9, fibrinogen, factor VIII (FVIII), albumin, alkaline phosphatase (ALP), and bilirubin conjugates (A); CA 19-9, fibrinogen, FVIII, albumin, and ALP (B); CA 19-9, fibrinogen, FVIII, albumin, and bilirubin conjugates (C). The boxes show the median and the interquartile range, the whiskers represent the extremes, and the circles the outliers. P values were obtained by Kruskal-Wallis test, using post hoc Dunn test, ***P < .001. Abbreviations: PDAC, pancreatic ductal adenocarcinoma; IPMN intraductal papillary mucinous neoplasm
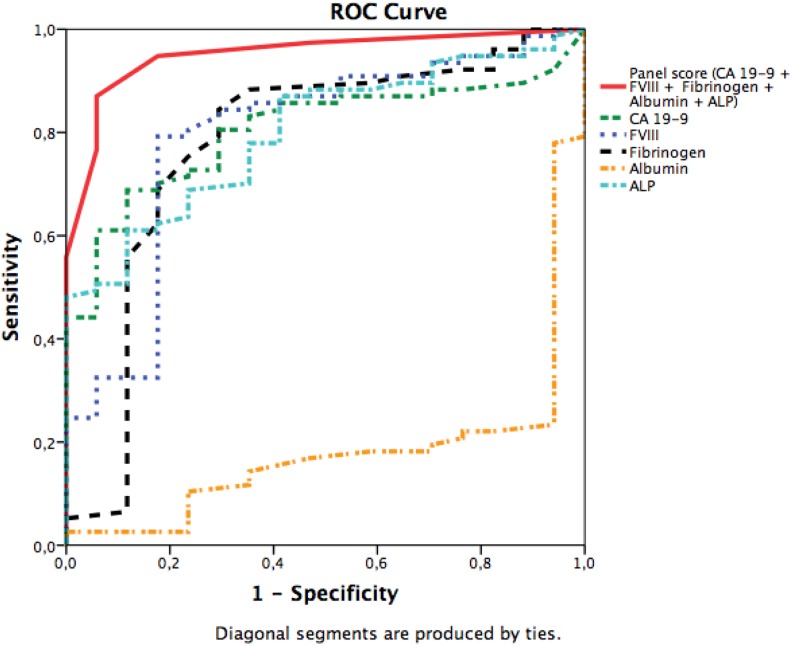
As ALP and conjugated bilirubin may both partially reflect the influence of bile obstruction and its patency after stent insertion, we evaluated the panel scores also without these markers of bile. The slope of the ROC curve was the steepest when conjugated bilirubin was excluded. Including CA 19-9, ALP, and albumin together with coagulation markers fibrinogen and FVIII into our final panel, the AUC improved to 0.95 (95% CI: 0.89-0.99) in comparison with 0.80 (0.71-0.88) for CA 19-9 alone (P = .002; Figure 3).
Figure 3.
Receiver operating characteristic (ROC) curve of the panel scores. The state variable was pancreatic ductal adenocarcinoma (PDAC) diagnosis, irrespective of the stage. The area under the curve (AUC; 95% confidence interval [CI]) was 0.95 (0.90-1.00) for the panel including carbohydrate antigen (CA) 19-9, fibrinogen, factor VIII (FVIII), albumin, and alkaline phosphatase (ALP). For the biomarkers alone, the AUC (95% CI) were as follows: 0.80 (0.71-0.88) for CA 19-9, 0.78 (0.68-0.86) for fibrinogen, 0.79 (0.70-0.87) for FVIII: C, 0.18 (0.11-0.27) for albumin, and 0.79 (0.70-0-89) for ALP. The AUCs were calculated for the patients with all available panel biomarkers.
Discussion
The aim of our observational study was to assess whether PDAC, a highly thrombogenic cancer, is preoperatively distinguishable from benign IPMN by analyzing coagulation variables and whether the diagnostic value of CA 19-9 improves by combining it with coagulation variables. We found that, together with CA 19-9, the biomarkers of coagulation (fibrinogen and FVIII), ALP, and albumin best distinguished PDAC from benign IPMN, irrespective of stage. Combining them to form a panel score clearly improved the diagnostic accuracy. We also found that high circulating FVIII levels were linked to distant metastasis, while FVIII and fibrinogen overall were elevated in PDAC. These findings add to the evidence that coagulation activity integrates with pancreatic cancer and its progression.10 They even raise the question of whether CA 19-9 is directly linked to coagulation activity in pancreatic cancer.
CA 19-9 is the most commonly used tumor marker in PDAC diagnostics and monitoring of cancer progression and treatment.14 In our study, the sensitivity of CA 19-9 for PDAC was 80% and the specificity 71%, which lie within the previously reported margins of sensitivity 70% to 90% and specificity 68% to 91%.15 However, almost one-third of the patients with PDAC had normal CA 19-9 values. As CA 19-9 is a sialylated Lewis a blood group antigen, patients lacking the Lewis gene (approximately 6% of the Caucasians22) do not express CA 19-9,23 decreasing its sensitivity. CA 19-9 has been reported to correlate with tumor stage16,17; however, there were no differences in CA 19-9 levels between stage I to III and IV PDAC prior to surgery.
Cancer cells use sialylated structures, that is, sialyl Lewis a, to bind to the endothelium, one of the key mechanisms of metastasis.24 Endothelium cancer cell adhesion may activate coagulation. von Willebrand factor (VWF) is released from the endothelium, and it carries FVIII in the circulation. When not bound to VWF, FVIII disseminates quickly. The high levels of FVIII in patients with PDAC reflect endothelial activation and/or enhanced angiogenesis and perhaps also reduced clearance by the liver.25 Since CA 19-9 is a sialylated Lewis antigen, it may itself modify cellular adhesion and coagulation activity.
We found that FVIII activity was higher in PDAC than in IPMN. Factor VIII levels increase due to multiple stimuli, such as stress and acute phase response.21,26 However, the difference in FVIII levels seems to be linked to malignancy rather than inflammation or stress since emotional stress preoperatively is the same in both PDAC and IPMN. Many reports have referred to elevated FVIII in VTE and PDAC,27,28 but as our patients did not have symptomatic preoperative VTEs, a broader association between FVIII and PDAC can be suggested.
Fibrinogen is a crucial substrate of the coagulation cascade. High circulating fibrinogen levels have been shown to associate with malignancy and metastasis.29–31 In our study, fibrinogen was higher in PDAC than IPMN, supporting the link between malignancy and fibrinogen. However, fibrinogen was not higher in patients with distant metastasis in comparison with locoregional disease, and thus, our preoperative study did not confirm previous reports on its link with metastasis. Higher fibrinogen levels have been shown to distinguish high risk (high-grade and invasive IPMN) from low risk (low-grade and borderline) IPMN.32 In our study, we did not have high-risk IPMN patients, but we observed significant differences between fibrinogen levels in benign versus malignant disease.
Fibrin turnover is enhanced in cancer overall,33–37 including PDAC.11,33,38 Sun et al. concluded that higher pretreatment levels of D-dimer in PDAC predict advanced tumor stage and metastasis.11 In our study, however, D-dimer did not differ between PDAC and IPMN or between stage IV and stage I to III PDAC. D-dimer values may vary based on patient age,39 and also on the analysis method used; thus, direct comparison between laboratories is not valid.40 In addition, the regulation of fibrinolysis may vary among patients with cancer.
Albumin was lower in PDAC than in IPMN, reflecting increased permeability and possibly leakage of coagulation factors into tissue. Low albumin coincides with VTE and mortality in patients with cancer.41 Albumin showed a negative correlation with fibrinogen in stage IV PDAC and FVIII in stage I to III PDAC, supporting the link between low albumin and increased coagulation activity. Conjugated bilirubin and ALP were higher in PDAC than in IPMN. Most of the PDAC tumors reside in the head of pancreas, often obstructing the bile duct; preoperative bile duct stent insertion may therefore have confounded these results. However, high ALP may also be linked to coagulation. Alkaline phosphatase is an active exopolyphosphatase capable of degrading circulating polyphosphate.42 Because polyphosphates importantly modulate coagulation and fibrinolysis,43 they may provoke ALP generation in PDAC. We also noticed a positive correlation between ALP and FVIII as well as with fibrinogen, which supports the contribution of this exopolyphosphatase.
The modified Khorana score did not correlate with the biomarkers or cancer stage in our pre-diagnostic study. Non-blood type O has been reported as a risk factor for PDAC44 and of VTE.45 O blood types associate with low FVIII and VWF levels. However, the blood type did not contribute to our results.
We noticed correlations between the variables that distinguished PDAC from IPMN, suggesting that the variables could be linked to each other. As the diagnostic potential of CA 19-9 needs to be improved, and based on correlations with the other markers, we decided to combine them in a panel score. The panel score initially included CA 19-9, combined with coagulation variables fibrinogen and FVIII, as well as albumin, conjugated bilirubin, and ALP. High scores of this panel strongly coincided with PDAC compared with IPMN. In comparison with CA 19-9 alone, the sensitivity of the panel improved. As both conjugated bilirubin and ALP can be confounded by the bile obstruction or stent placement, we wanted to determine their role in the panel. Conjugated bilirubin in the panel without ALP seemed to have the highest AUC; however, the slope was the steepest at the beginning of the curve, when conjugated bilirubin was excluded, but ALP was included. Again, the proposed links between ALP and coagulation suggest that ALP could be preferred in the panel instead of conjugated bilirubin. Larger studies are needed to assess whether these panels will assist in clinical practice to distinguish metastasized from local cancer.
The limitations of our study include the inclusion of patients at different stages of their disease and the small numbers of patients in the subgroups. However, the larger stage I to III PDAC group (n = 67) provided representative data of links within coagulation and PDAC, and patients were in a treatment-naive state at the time of preoperative sample collection. A minority of the patients used anticoagulants or other drugs, which may have confounded the data. Another limitation of this study is the lack of VWF levels and microvesicle data analysis. In future studies, their roles in this predictive panel should be investigated.
Conclusions
In summary, our study supports the role of coagulation involvement in PDAC, FVIII, and fibrinogen in particular, among others. A combination panel including CA 19-9, FVIII, fibrinogen, albumin, and ALP deserves further clinical validation as a diagnostic tool and disease severity marker in pancreatic cancer. We have begun to gather data in a multicenter study to validate these results and examine the role of this panel and beyond in predicting early mortality after PDAC surgery.
Footnotes
Authors’ Note: Ethical approval to report this case was obtained from the Surgical Ethics Committee of Helsinki University Hospital (Dnro HUS 226/E6/06, extension TMK02 §66 17.4.2013) and the National Supervisory Authority of Welfare and Health (Valvira Dnro 1041/06.01.03.01/2012). Written informed consent was obtained from the patients for their anonymized information to be published in this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the researh, authorship and/or publication of this article: This study was supported by the Finnish Governmental Funding, the Mary and Georg C. Ehrnrooth Foundation, the Sigrid Jusélius Foundation, and the Finnish Society of Angiology. None of the foundations were involved in the study design.
ORCID iD: Nora Mattila  http://orcid.org/0000-0003-4007-6555
http://orcid.org/0000-0003-4007-6555
References
- 1. Falanga A, Marchetti M, Vignoli A. Coagulation and cancer: biological and clinical aspects. J Thromb Haemost. 2013;11(2):223–233. [DOI] [PubMed] [Google Scholar]
- 2. Heit JA, O’Fallon WM, Petterson TM, et al. Relative impact of risk factors for deep vein thrombosis and pulmonary embolism: a population-based study. Arch Intern Med. 2002;162(11):1245–1248. [DOI] [PubMed] [Google Scholar]
- 3. Falanga A, Panova-Noeva M, Russo L. Procoagulant mechanisms in tumour cells. Best Pract Res Clin Haematol. 2009;22(1):49–60. [DOI] [PubMed] [Google Scholar]
- 4. Posch F, Riedl J, Reitter EM, et al. Hypercoagulabilty, venous thromboembolism, and death in patients with cancer. A multi-state model. Thromb Haemost. 2016;115(4):817–826. [DOI] [PubMed] [Google Scholar]
- 5. Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111(10):4902–4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sohal DP, Shrotriya S, Glass KT, et al. Predicting early mortality in resectable pancreatic adenocarcinoma: a cohort study. Cancer. 2015;121(11):1779–1784. [DOI] [PubMed] [Google Scholar]
- 7. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. [DOI] [PubMed] [Google Scholar]
- 8. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. [DOI] [PubMed] [Google Scholar]
- 9. Petterson TM, Marks RS, Ashrani AA, Bailey KR, Heit JA. Risk of site-specific cancer in incident venous thromboembolism: a population-based study. Thromb Res. 2015;135(3):472–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Thaler J, Ay C, Mackman N, et al. Microparticle-associated tissue factor activity in patients with pancreatic cancer: correlation with clinicopathological features. Eur J Clin Invest. 2013;43(3):277–285. [DOI] [PubMed] [Google Scholar]
- 11. Sun W, Ren H, Gao CT, et al. Clinical and prognostic significance of coagulation assays in pancreatic cancer patients with absence of venous thromboembolism. Am J Clin Oncol. 2015;38(6):550–556. [DOI] [PubMed] [Google Scholar]
- 12. Tempero MA, Arnoletti JP, Behrman SW, et al. Pancreatic Adenocarcinoma, version 2.2012: featured updates to the NCCN Guidelines. J Natl Compr Canc Netw. 2012;10(6):703–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med. 2014;371(22):2140–2141. [DOI] [PubMed] [Google Scholar]
- 14. Ducreux M, Cuhna AS, Caramella C, et al. Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(suppl 5):v56–v68. [DOI] [PubMed] [Google Scholar]
- 15. Goonetilleke KS, Siriwardena AK. Systematic review of carbohydrate antigen (CA 19-9) as a biochemical marker in the diagnosis of pancreatic cancer. Eur J Surg Oncol. 2007;33(3):266–270. [DOI] [PubMed] [Google Scholar]
- 16. Karachristos A, Scarmeas N, Hoffman JP. CA 19-9 levels predict results of staging laparoscopy in pancreatic cancer. J Gastrointest Surg. 2005;9(9):1286–1292. [DOI] [PubMed] [Google Scholar]
- 17. Ferrone CR, Finkelstein DM, Thayer SP, Muzikansky A, Fernandez-delCastillo C, Warshaw AL. Perioperative CA19-9 levels can predict stage and survival in patients with resectable pancreatic adenocarcinoma. J Clin Oncol. 2006;24(18):2897–2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Del Chiaro M, Verbeke C, Salvia R, et al. European experts consensus statement on cystic tumours of the pancreas. Dig Liver Dis. 2013;45(9):703–711. [DOI] [PubMed] [Google Scholar]
- 19. Jiang Q, Gingles NA, Olivier MA, Miles LA, Parmer RJ. The anti-fibrinolytic xRPIN, plasminogen activator inhibitor 1 (PAI-1), is targeted to and released from catecholamine storage vesicles. Blood. 2011;117(26):7155–7163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zwicker JI, Liebman HA, Neuberg D, et al. Tumor-derived tissue factor-bearing microparticles are associated with venous thromboembolic events in malignancy. Clin Cancer Res. 2009;15(22):6830–6840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jenkins PV, Rawley O, Smith OP, O’Donnell JS. Elevated factor VIII levels and risk of venous thrombosis. Br J Haematol. 2012;157(6):653–663. [DOI] [PubMed] [Google Scholar]
- 22. Scara S, Bottoni P, Scatena R. CA 19-9: biochemical and clinical aspects. Adv Exp Med Biol. 2015;867:247–260. [DOI] [PubMed] [Google Scholar]
- 23. Tempero MA, Uchida E, Takasaki H, Burnett DA, Steplewski Z, Pour PM. Relationship of carbohydrate antigen 19-9 and Lewis antigens in pancreatic cancer. Cancer Res. 1987;47(20):5501–5503. [PubMed] [Google Scholar]
- 24. Renkonen R, Mattila P, Majuri ML, et al. In vitro experimental studies of sialyl Lewis x and sialyl Lewis a on endothelial and carcinoma cells: crucial glycans on selectin ligands. Glycoconj J. 1997;14(5):593–600. [DOI] [PubMed] [Google Scholar]
- 25. Shahani T, Lavend’homme R, Luttun A, Saint-Remy JM, Peerlinck K, Jacquemin M. Activation of human endothelial cells from specific vascular beds induces the release of a FVIII storage pool. Blood. 2010;115(23):4902–4909. [DOI] [PubMed] [Google Scholar]
- 26. Austin AW, Wirtz PH, Patterson SM, Stutz M, von Kanel R. Stress-induced alterations in coagulation: assessment of a new hemoconcentration correction technique. Psychosom Med. 2012;74(3):288–295. [DOI] [PubMed] [Google Scholar]
- 27. Vormittag R, Simanek R, Ay C, et al. High factor VIII levels independently predict venous thromboembolism in cancer patients: the cancer and thrombosis study. Arterioscler Thromb Vasc Biol. 2009;29(12):2176–2181. [DOI] [PubMed] [Google Scholar]
- 28. Reitter EM, Kaider A, Ay C, et al. Longitudinal analysis of hemostasis biomarkers in cancer patients during antitumor treatment. J Thromb Haemost. 2016;14(2):294–305. [DOI] [PubMed] [Google Scholar]
- 29. Guo Q, Zhang B, Dong X, et al. Elevated levels of plasma fibrinogen in patients with pancreatic cancer: possible role of a distant metastasis predictor. Pancreas. 2009;38(3):e75–e79. [DOI] [PubMed] [Google Scholar]
- 30. Wang H, Gao J, Bai M, et al. The pretreatment platelet and plasma fibrinogen level correlate with tumor progression and metastasis in patients with pancreatic cancer. Platelets. 2014;25(5):382–387. [DOI] [PubMed] [Google Scholar]
- 31. Palumbo JS, Talmage KE, Massari JV, et al. Platelets and fibrin(ogen) increase metastatic potential by impeding natural killer cell-mediated elimination of tumor cells. Blood. 2005;105(1):178–185. [DOI] [PubMed] [Google Scholar]
- 32. Nentwich MF, Menzel K, Reeh M, et al. Blood fibrinogen levels discriminate low- and high-risk intraductal papillary mucinous neoplasms (IPMNs). Eur J Surg Oncol. 2017;43(4):758–762. [DOI] [PubMed] [Google Scholar]
- 33. Ay C, Dunkler D, Pirker R, et al. High D-dimer levels are associated with poor prognosis in cancer patients. Haematologica. 2012;97(8):1158–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dirix LY, Salgado R, Weytjens R, et al. Plasma fibrin D-dimer levels correlate with tumour volume, progression rate, and survival in patients with metastatic breast cancer. Br J Cancer. 2002;86(3):389–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Oya M, Akiyama Y, Okuyama T, Ishikawa H. High preoperative plasma D-dimer level is associated with advanced tumor stage and short survival after curative resection in patients with colorectal cancer. Jpn J Clin Oncol. 2001;31(8):388–394. [DOI] [PubMed] [Google Scholar]
- 36. Blackwell K, Hurwitz H, Lieberman G, et al. Circulating D-dimer levels are better predictors of overall survival and disease progression than carcinoembryonic antigen levels in patients with metastatic colorectal carcinoma. Cancer. 2004;101(1):77–82. [DOI] [PubMed] [Google Scholar]
- 37. Altiay G, Ciftci A, Demir M, et al. High plasma D-dimer level is associated with decreased survival in patients with lung cancer. Clin Oncol (R Coll Radiol). 2007;19(7):494–498. [DOI] [PubMed] [Google Scholar]
- 38. Durczynski A, Kumor A, Hogendorf P, Szymanski D, Grzelak P, Strzelczyk J. Preoperative high level of D-dimers predicts unresectability of pancreatic head cancer. World J Gastroenterol. 2014;20(36):13167–13171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schouten HJ, Geersing GJ, Koek HL, et al. Diagnostic accuracy of conventional or age adjusted D-dimer cutoff values in older patients with suspected venous thromboembolism: systematic review and meta-analysis. BMJ. 2013;346(5):f2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Di Nisio M, Squizzato A, Rutjes AW, Buller HR, Zwinderman AH, Bossuyt PM. Diagnostic accuracy of D-dimer test for exclusion of venous thromboembolism: a systematic review. J Thromb Haemost. 2007;5(2):296–304. [DOI] [PubMed] [Google Scholar]
- 41. Konigsbrugge O, Posch F, Riedl J, et al. Association between decreased serum albumin with risk of venous thromboembolism and mortality in cancer patients. Oncologist. 2016;21(2):252–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lorenz B, Schroder HC. Mammalian intestinal alkaline phosphatase acts as highly active exopolyphosphatase. Biochim Biophys Acta. 2001;1547(2):254–261. [DOI] [PubMed] [Google Scholar]
- 43. Smith SA, Mutch NJ, Baskar D, Rohloff P, Docampo R, Morrissey JH. Polyphosphate modulates blood coagulation and fibrinolysis. Proc Natl Acad Sci U S A. 2006;103(4):903–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wolpin BM, Chan AT, Hartge P, et al. ABO blood group and the risk of pancreatic cancer. J Natl Cancer Inst. 2009;101(6):424–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Li D, Pise MN, Overman MJ, et al. ABO non-O type as a risk factor for thrombosis in patients with pancreatic cancer. Cancer Med. 2015;4(11):1651–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]