Abstract
Patient registry is a powerful tool for planning health care and setting groundwork for research. This survey reports a detailed registry of inherited bleeding disorders (IBD) and their management at a not-for-profit organization in a developing country to form the basis for planning development and research. We reviewed medical records of patients with IBD from 8 hemophilia treatment centers of Fatimid Foundation located in various cities. Information collected included sociodemographic data, diagnostic tests, severity of hemophilia A and B, number of bleeding episodes per year, site and frequency of hemarthrosis, and seropositivity for viral diseases. We analyzed 1497 patients from November 1, 2015, to April 30, 2016. There were 1296 (87%) males and 201 (13%) females with a mean age of 24.5 (11) years (range, 6 months to 65 years). Hemophilia A constituted the bulk of IBD (848, 57%) followed by von Willebrand disease (172, 11%), hemophilia B (144, 10%), platelet function defect (106, 7%), and rare bleeding disorders (70, 5%). Mucocutaneous bleeding (1144, 76%) and hemarthrosis (1035 patients, 69%) were the main complications. There were 1026 (69%) patients who received only blood components for treatment of any bleeding episode while the remaining 464 (31%) were on combination therapy (blood components and factor concentrate). Seroreactivity for hepatitis C was frequent (28%), while hepatitis B (1%) and human immunodeficiency virus (0.01%) were less commonly seen. This study was an important step toward a patient registry in a hemophilia treatment center in Pakistan. Hemophilia A is the most common bleeding disorder and hepatitis C is the most frequent treatment-related complication.
Keywords: registries, blood coagulation disorders, inherited, hemophilia A, hemophilia B, von Willebrand diseases
Introduction
Inherited bleeding disorders (IBD) include coagulation factor deficiencies and platelet function defects (PFDs). The treatment of coagulation factor deficiencies includes replacement therapy such as clotting factor concentrates, transfusion of fresh frozen plasma (FFP), cryoprecipitate (CP), and cryosupernatant (CS), while platelet transfusions are required in PFDs. Treatment complications such as inhibitors development and platelet refractoriness require infusion of prothrombin complex concentrates or factor eight-inhibitor-by pass agents (FEIBA).
The management of IBD is a challenging task in developing countries. Lack of diagnostic facilities, shortage of blood components, high cost of clotting factor concentrates, and poor infrastructure of hemophilia treatment centers are among the main reasons. In order to assess the burden of IBD and to plan health care, there is a need for a patient registry in organizations involved in managing patients with bleeding disorders.1 This was stressed upon during a joint meeting of World Health Organization and World Federation of Hemophilia (WFH) in 1997 in Geneva, Switzerland.2 Their report recommended that identification and diagnosis of patients and their families with hemophilia and related problems must be prioritized.
Pakistan is a developing country with an estimated population of 193 million people and a low gross domestic product per capita of US$4.3 The health budget is mainly spent on battling infectious disease and preventing maternal morbidity. Keeping this in view, the management of IBD on a governmental level becomes difficult, and this is where the role of nongovernmental organizations (NGO) becomes important. A disease registry in a rare disease is an important tool for advocacy. A major drawback of an NGO is a lack of patient registry, an online database system, and skilled staff to manage the registry. This results in a lack of data sharing from the NGOs to the national member organization responsible for managing and updating patient registry at a national level leading to underreporting with possible duplication of data.
Pakistan has submitted national data to the WFH annual global survey of 2015 published in 2016. According to this report from 113 countries, 1646 cases of IBD were reported from Pakistan.4
Patients receive on-demand replacement therapy with either blood components or plasma-derived clotting factor concentrates in Pakistan. As the country does not manufacture clotting factor concentrates, these are therefore imported and are expensive disallowing their prophylactic usage. There have been local sporadic data on the epidemiology and clinical characteristics of IBD from southern Pakistan.5,6 In this retrospective review of registry data of a single NGO, we describe the demographics, diagnosis, and treatment of IBD in 8 hemophilia treatment centers in Pakistan.
Materials and Methods
This was a cross-sectional study conducted by 8 centers of Fatimid Foundation (FF) from November 1, 2015, to June 1, 2016. Fatimid Foundation is a not-for-profit network of hemophilia treatment centers located in 4 provinces of Pakistan. The centers are located in Quetta (Balochistan Province), Peshawar (Khyber Pakhtoon Khwa province), Lahore and Multan (Punjab province), Karachi, Hyderabad, Khairpur, and Rashidabad (Sindh province).
A data collection form developed by staff at the Karachi center was used by staff at all of the hemophilia treatment centers to collect data on patients diagnosed or suspected with IBD. All patients either diagnosed with or suspected of having an IBD were eligible for inclusion in the study. Data collected included demographic information, laboratory data, diagnosis, severity of hemophilia A (HA) and hemophilia B (HB), presence of inhibitors, type of von Willebrand disease (vWD), infection status for human immunodeficiency virus (HIV), hepatitis C virus (HCV), hepatitis B virus (HBV), type of treatment received (either blood components and/or factor concentrates), and follow-up visits.
Bleeding history was documented by reviewing the patient’s file and recording the various sites of any bleeding. For each patient, the number of bleeds was counted for each event, that is, the data were collected as number of bleeding episodes rather than number of patients with bleeding events. Data on joint disability, physiotherapy, and the use of any orthopedic appliance were also collected on the form.
All patients clinically diagnosed with IBD were screened through laboratory tests: bleeding time (BT; Ivy modified template method), activated partial thromboplastin time (APTT), prothrombin time (PT), and platelet count. Further testing included mixing studies with aged serum or adsorbed plasma for prolonged PT and APTT. A patient with a prolonged APTT correcting with adsorbed plasma/aged serum was clinically managed as HA and HB, respectively. Patients were clinically managed as vWD if they have prolonged BT and APTT while those with prolonged PT and APTT were managed as rare bleeding disorders (RBDs). Patients with a significant bleeding history and normal screening tests were further investigated with Factor XIII quantitative assay or urea clot lysis. Females with a low-factor VIII level without von Willebrand factor (vWF): Ag or vWF: ristocetin cofactor (RCoF) levels were managed as either carrier for HA or vWD. In case, no investigations or any one screening test was performed, the patient was considered as “undiagnosed.”
Platelet function defects were diagnosed when a patient had a prolonged BT with a defective platelet aggregometry. Factor assay (VIII, IX, I, II, V, X), vWF antigen (vWF:Ag) level, vWF:RCoF, and platelet aggregation studies were performed for confirmation, when available at a center. Patients with confirmed diagnosis were diagnosed as mild, moderate, or severe HA or HB if diagnostic factor level was more than 5% to 40%, 1% to 5%, and <1%, respectively. Factor assays were performed by one-stage coagulometric method on Sysmex CA-600 with a measuring range extending from 1% to 100% of the normal.
If FV level was found to be deficient, then FVIII level was also performed. Analysis of vWF:Ag was performed on Sysmex CA-600 by immunoturbidimetric method using Innovance reagent (Siemens, Marburg, Germany). To differentiate type 1 from type 2 vWD, vWF:RCoF to vWF:Ag ratio of <0.7 was used.7 Undetectable vWF:Ag and RCoF with very low FVIII level (1-9 IU/dL) led to the diagnosis of type 3 vWD.7 Multimer analysis, von Willebrand collagen binding activity, and ristocetin-induced platelet aggregation were not performed due to the nonavailability and cost constraints. Patients were labeled as “presumed vWD” in the presence of bleeding manifestation and inadequate laboratory data (low levels of vWFAg and FVIII).
Treatment details were recorded by reviewing the past medical record of the patient and recording any treatment (CP, CS, platelets, and/or clotting factor concentrates) that the patient had received at the hemophilia treatment center after registration. Patients were categorized into those who had been treated only with blood components or only clotting factor concentrates and those who had received combination therapy. Combination therapy is defined as those patients who had received treatment clotting factor concentrates and blood components to treat any bleeding episode.
The completed data collection forms from all the centers were sent to the hematologist (S.H.) in Karachi center who entered and analyzed the data using SPSS version 22 statistical software (IBM, Armonk, New York). The data bank was saved in the computer under lock and key to protect the confidentiality of information. Quantitative data were given as mean (standard deviation) when normally distributed and median (interquartile range) for nonparametric data. This study was conducted after ethical approval from institutional research ethics committee.
Results
The total number of patients with IBD registered at various FF centers is 1497, and the facilities provided to these patients by the respective centers are shown in Table 1. The majority of patients resided in Sindh province (800, 53%) followed by Punjab (348, 23%), Khyber Pakhtoon Khwa (283, 19%), Balochistan (59, 4%), Gilgit Baltistan (2, 0.1%), Azad Kashmir (2, 0.1%), and Afghanistan (3, 0.2%).
Table 1.
Summary of Diagnostic and Management Facilities Available at Each Center.a
| Center | No. of Registered Patients | Laboratory Facilities | Treatment Facilities | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Diagnosed | Clinical Diagnosis | Undiagnosed | Screening Tests (PT, APTT, Bleeding Time, and Platelet Count) | Diagnostic Tests (Mixing Studies, Factor Level, and Inhibitor Screening) | Screening for HBV, HCV, & HIV | CP, CS & Platelet | FFP | CFCb | Physiotherapy | |
| Karachi | 735 | 510 (70) | 201 (27) | 24 (3) | + | + | + | + | + | + | + |
| Lahore | 285 | 109 (38) | 154 (54) | 22 (8) | + | 0 | + | + | + | + | + |
| Peshawar | 289 | 44 (15) | 199 (69) | 46 (16) | + | 0 | + | + | + | + | 0 |
| Quetta | 44 | 4 (9) | 10 (23) | 30 (68) | + | 0 | + | 0 | + | + | + |
| Multan | 46 | 23 (50) | 9 (20) | 14 (30) | + | 0 | + | + | + | + | + |
| Hyderabad | 58 | 33 (57) | 21 (36) | 4 (7) | + | 0 | + | + | + | + | 0 |
| Khairpur | 11 | 8 (73) | 3 (27) | 0 | + | 0 | + | + | + | + | 0 |
| Rashidabad | 29 | 25 (86) | 4 (14) | 0 | 0 | 0 | + | + | + | + | 0 |
| Total | 1497 | 823 (55) | 539 (36) | 135 (9) | – | – | – | – | – | – | – |
Abbreviations: APTT, activated partial thromboplastin time; CFC, clotting factor concentrates; CP, cryoprecipitate; CS, cryosupernatant; FFP, fresh frozen plasma; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; PT, prothrombin time.
a “+” Indicates present and “0” indicates absent.
b Plasma derived, available free of cost only in emergency setting.
Clinical and Laboratory Diagnosis
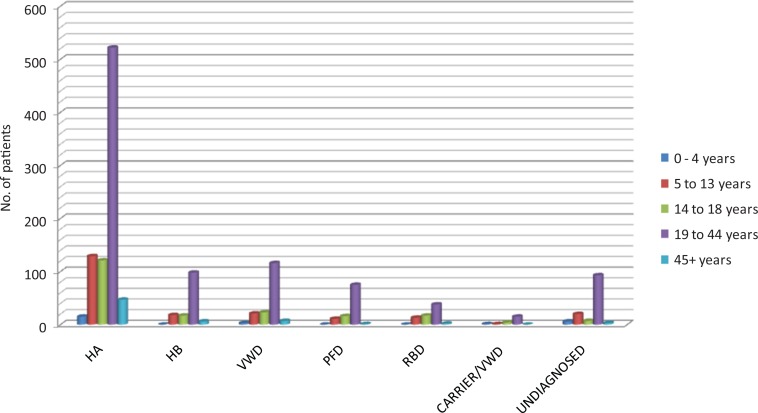
The distribution of patients according to different age groups is shown in Figure 1. Overall, inherited clotting factor deficiency was seen in 1256 (84%) patients while 106 (7%) were diagnosed as PFD and the remaining 135 (9%) patients were undiagnosed. As shown in Table 2, majority of registered patients had HA (848, 57%), and vWD constituted next common IBD (172, 11%) followed by HB (144, 10%), rare bleeding disorders (70, 5%), and suspected HA carrier/vWD (22, 1%) patients. Mild, moderate, and severe forms of HA and HB were seen in 19%, 34%, 47%, and 30%, 40%, 30% patients, respectively. Type 1 and 3 vWD was diagnosed in 13 (16%) and 70 (84%) patients, respectively.
Figure 1.
Patients in different age groups (n = 1497). HA indicates hemophilia A; HB, hemophilia B; vWD, von Willebrand disease; RBD, rare bleeding disorders; PFD, platelet function defect.
Table 2.
Demographic and Clinical Details in Various IBD.a
| Bleeding Disorder | N (M/F) | Age, Years, Mean (SD) | Mucocutaneous Bleeding, n (%) | Urinary, n (%) | Menorrhagia, n (%) | Circumcision,b n (%) | Gastrointestinal, n (%) | Muscle Hematomas, n (%) | Hemarthrosis, n (%) | Intracranial Bleeding, n (%) | Umbilical Cord, n (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| HA | 848 (848/0) | 25 (12) | 622 (73) | 32 (4) | – | 298 (35) | 30 (4) | 25 (3) | 699 (82) | 50 (6) | 4 (1) |
| Mild | 98 | 26 (11) | 70 (12) | 5 (15) | – | 32 (11) | 3 (10) | 1 (4) | 77 (11) | 2 (4) | 0 |
| Moderate | 172 | 23 (12) | 131 (21) | 7 (22) | – | 67 (22) | 9 (30) | 8 (8) | 150 (22) | 21 (42) | 2 (50) |
| Severe | 233 | 22 (11) | 195 (31) | 5 (16) | – | 86 (29) | 9 (30) | 6 (24) | 204 (29) | 12 (24) | 2 (50) |
| Presumed HAc | 226 | 27 (11) | 226 (36) | 15 (47) | – | 113 (38) | 9 (30) | 10 (40) | 268 (38) | 15 (30) | 0 |
| HB | 144 (144/0) | 25 (10) | 111 (77) | 3 (2) | – | 44 (31) | 6 (4) | 3 (2) | 122 (85) | 7 (5) | 1 (1) |
| Mild | 23 | 24 (9) | 22 (20) | 0 | – | 6 (14) | 1 (17) | 1 (33) | 21 (17) | 0 | 0 |
| Moderate | 31 | 23 (11) | 25 (23) | 0 | – | 11 (25) | 2 (33) | 2 (67) | 27 (22) | 2 (29) | 1 (100) |
| Severe | 23 | 25 (11) | 19 (17) | 1 (33) | – | 7 (16) | 1 (17) | 0 | 21 (17) | 1 (14) | 0 |
| Presumed HBc | 45 | 27 (10) | 45 (40) | 2 (67) | – | 20 (45) | 2 (33) | 0 | 53 (43) | 4 (57) | 0 |
| vWD | 172 (78/94) | 25 (11) | 148 (86) | 8 (5) | 28 (30) | 34 (43) | 14 (8) | 5 (3) | 78 (45) | 11 (6) | 3 (2) |
| Type 1 | 6 | 15 (7) | 6 (4) | 0 | 2 (7) | 0 | 0 | 0 | 3 (4) | 0 | 0 |
| Type 2 | 8 | 24 (10) | 8 (6) | 2 (25) | 3 (11) | 0 | 0 | 0 | 2 (3) | 0 | 0 |
| Type 3 | 2 | 25 (3) | 2 (1) | 0 | 0 | 0 | 0 | 0 | 2 (3) | 0 | 0 |
| Presumed vWDc | 156 | 26 (11) | 132 (89) | 6 (75) | 23 (82) | 34 (100) | 14 (100) | 5 (100) | 71 (90) | 11 (100) | 3 (100) |
| RBD | 70 (46/24) | 22 (10) | 57 (81) | 3 (4) | 2 (8) | 9 (20) | 3 (4) | 0 | 39 (56) | 3 (4) | 16 (23) |
| PFD | 106 (65/41) | 25 (9) | 97 (91) | 0 | 12 (29) | 18 (28) | 9 (8) | 0 | 26 (25) | 4 (4) | 1 (1) |
| Carrier/vWDd | 22 (0/22) | 24 (10) | 18 (82) | 0 | 6 (27) | 0 | 2 (9) | 0 | 5 (23) | 4 (18) | 0 |
| Undiagnosede | 135 (115/20) | 24 (11) | 91 (67) | 4 (3) | 5 (25) | 17 (15) | 2 (2) | 4 (3) | 66 (49) | 1 (1) | 3 (2) |
| Total | 1497 (1296/201) | 25 (11) | 1144 (76) | 50 (3) | 54 (27) | 420 (32) | 66 (4) | 37 (2) | 1035 (69) | 80 (5) | 28 (2) |
Abbreviations: F, female; HA, hemophilia A; HB, hemophilia B; IBD, inherited bleeding disorders; M, male; PFD, platelet function defect; RBD, rare bleeding disorders; SD, standard deviation; vWD, von Willebrand disease.
a n = 1497.
b Circumcision was performed in n = 487 patients.
c Presence of clinically significant bleeding and inadequate laboratory investigations responding to treatment with deficient clotting factor concentrates or blood component comprising the missing factor.
d Suspected carriers for HA or vWD; no confirmatory tests performed.
ePresence of clinically significant bleeding in the absence of laboratory investigations.
Factor V deficiency (15, 28%) was the most common RBD followed by hypofibrinogenemia (12, 22%), FXIII deficiency (8, 15%), and afibrinogenemia (6, 11%). Less frequent RBDs include FVII (4, 7%), FX (4, 7%), combined FV and VIII deficiency (4, 7%), and FII deficiency (1, 2%). There were 11 (73%) patients who had severe FV deficiency. Two patients had a FVII activity of less than 1% while the remaining 2 patients had moderate deficiency. Severe FX deficiency was present in 2 patients while the remaining 2 patients had moderate and mild deficiency, respectively.
Glanzmann thrombasthenia (GTT; 74, 86%) was the most common PFD followed by Bernard-Soulier syndrome (BSS; 10, 12%) and storage pool defect (2, 2%).
Demographics and Clinical Details
The demographics and clinical details of patients are summarized in Table 2. The mean age (known for 1466 patients) at the time of data collection was 24.5 (11) years (range, 6 months to 65 years), and there were 1296 (87%) males. The age at diagnosis of bleeding disorder was known for 1135 (76%) patients. The median age at diagnosis was 6 years (range, birth to 54 years). Majority of the individuals were either pediatrics (1172, 88%) or adolescent (93 or 7%) when first presented/diagnosed while 70 (5%) were adults. At the time of data collection, young adults (19-44 years) constituted 65% of registered patients.
Consanguinity was observed in 399/651 (61%) patients. Seven hundred patients (47%) had a family history of bleeding, while 84 (6%) family members of the registered patients died due to life-threatening bleeding. Hemoglobin level and red cell indices performed within 3 months of data collection were available for 1070 (71%) patients. Hypochromic and microcytic anemia was diagnosed in 699 (65%) patients, and169 (24%) patients were on iron replacement therapy. Laboratory tests for assessment of storage iron were not available.
Bleeding Complications
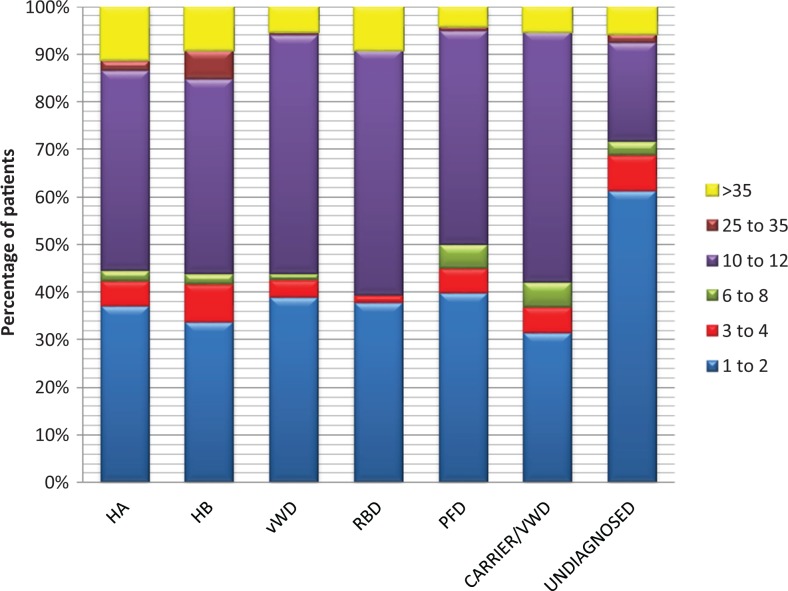
The number of bleeding episodes per year is shown in Figure 2. As shown in Table 2, mucocutaneous bleeding was the commonest symptom experienced by majority (76%) of the patients during their lifetime. Menorrhagia was common in females with vWD while bleeding during circumcision was predominant in vWD and HA.
Figure 2.
Annual bleeding episodes (n = 1413). HA indicates hemophilia A; HB, hemophilia B; vWD, von Willebrand disease; RBD, rare bleeding disorders; PFD, platelet function defect.
Acute hemarthrosis was another frequent bleeding complication experienced by 1035 (69%) patients, mainly HA and HB. However, it was also seen in patients with PFDs. Knee joint (823, 80%) was the most common site of hemarthrosis followed by elbow (474, 46%), ankle (412, 40%), shoulder (244, 24%), hip (211, 20%), and wrist (96, 9%) joints. Joint disability was present in 183 (18%) patients including 133 (73%) HA, 13 (7%) HB, 12 (7%) vWD, 5 (3%) RBD, 1 (0.5%) PFD, 2 (1%) HA carrier/vWD, and 17 (9%) undiagnosed patients. Knee joint (139, 76%) was the most commonly affected followed by elbow (21, 12%), hip (14, 8%), ankle (13, 7%), and shoulder joints (4, 2%). Flexion deformity and muscle contracture was present in 33(2%) and 5(0.3%) patients, respectively. Joint replacement surgery was performed in 11 patients while 2 underwent serial plaster casting. There were 73 (40%) patients with joint disability who were using orthopedic appliances of whom 15 (21%) were wheelchair bound, while 58 (79%) patients were using either walker/crutches or walking sticks/canes. Only 93 (6%) registered patients were on regular physiotherapy.
Treatment Details
There were 1026 (69%) patients who received only blood components for treatment of any bleeding episode while the remaining 464 (31%) were treated with blood components in combination with factor concentrates. The average dose of plasma-derived factor VIII or IX concentrate in combination therapy was 10 to 15 IU /kg within 5 to 7 days. Clotting factor concentrates in vWD were used on an average of 1 vial of 500 IU human coagulation factor VIII (FVIII) and 1200 IU human vWF per week. Clotting factor concentrates are usually given on the initial 1 to 2 days of bleed together with blood components. There were 349 (41%), 45 (31%), 66 (3.5%), and 4 (18%) patients with HA, HB, vWD, and suspected carrier females HA/vWD with on combination therapy respectively. Only 7 (0.5%) patients (5 patients with HA and 2 patients with vWD) have received treatment with clotting factor concentrates.
As shown in Table 3, FFP (47%) and CP (43%) were the most commonly transfused blood component and patients with HA were the highest consumers. Although CS is only indicated in HB, it was transfused in patients with other IBD if the respective center had exhausted its supply of required blood components such as FFP and CP. Screening for transfusion transmitted infections was performed on a yearly basis. Hepatitis C virus (28%) was the most prevalent transfusion transmitted infection as shown in Table 3. Patients with PFDs (38%) have the highest frequency of HCV followed by an equal prevalence of HCV in HA and HB (30%).
Table 3.
Utilization of Blood Components and Transfusion Transmitted Infections in Patients With IBD.
| Diagnosis | Blood Components, n = 1497 | TTI, n = 1288 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| FFP, n (%) | CP, n (%) | CS, n (%) | Platelets, n (%) | No. of Patients Screened, n | HBV, n (%) | HCV, n (%) | HIV, n (%) | Coinfection, n (%) | |
| HA | 397 (56) | 470 (74) | 2 (2) | – | 745 | 3 (0.4) | 223 (30) | 1 (0.1) | 5a (1) |
| HB | 54 (8) | – | 87 (92) | – | 129 | 1 (1) | 38 (30) | 0 | 0 |
| vWD | 90 (13) | 94 (15) | 1 (1) | 3 (3) | 146 | 1 (1) | 38 (26) | 0 | 0 |
| RBD | 70 (9) | 33 (5) | 66 (70) | – | 59 | 0 | 15 (25) | 0 | 0 |
| PFD | 9 (1) | – | 0 | 99 (87) | 84 | 2 (2) | 30 (36) | 0 | 0 |
| Carrier/vWD | 15 (2) | 16 (2) | 0 | 0 | 22 | 2 (9) | 3 (14) | 0 | 0 |
| Undiagnosed | 85 (12) | 15 (2) | 1 (1) | 10 (9) | 103 | 1 (1) | 8 (8) | 0 | 1a (1) |
| Total | 708 (47) | 639 (43) | 95 (13) | 114 (8) | 1288 | 10 (1) | 355 (28) | 1 (0.01) | 6 (0.5) |
Abbreviations: CP, cryoprecipitate; CS, cryosupernatant; FFP, fresh frozen plasma; HA, hemophilia A; HB, hemophilia B; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; IBD, inherited bleeding disorders; PFD, platelet function defect; RBD, rare bleeding disorders; TTI, transfusion transmitted infections; vWD, von Willebrand disease.
a In the HA group, n = 4 had coinfection with HCV and HBV, and 1 patient had coinfection with HIV and HCV, while in the 1 patient in the undiagnosed category had coinfection with HBV and HCV.
In HA, inhibitor screening was performed in 82 (10%) patients (37 severe, 19 moderate, 8 mild, and 18 patients with unknown severity), while in HB there were 17 (12%) patients (6 severe, 5 moderate, 5 mild, and 1 patient with unknown severity) screened for the presence of inhibitors. Inhibitors were detected in 22 (27%) patients with HA (10 patients with severe HA, 5 with moderate HA, and 7 patients with unknown severity) and 2 (12%) patients with moderate HB.
Discussion
Our survey identified that approximately 1500 patients with IBD were actively receiving care at 8 hemophilia treatment centers in Pakistan. With a high predominance of patients in early adulthood, it is apparent that very few survive past the age of 45 years. We report the frequencies of HA, vWD, HB, PFD, and RBDs as 57%, 12%, 10%, 7%, and 2%, respectively. Severe hemophilia (44%) was the most common. Literature review from southern Pakistan also revealed HA (37%) and vWD (18%) being the most frequent followed by PFDs (13%), HB (9%), and RBD (3%).5 A study from Iran8 reported frequencies of HA (52%), HB (17%), vWD (9%), and PFDs (7%) that was comparable to our population. In Brazil,9 the frequency of HA (63%), HB (12%), and vWD (21%) is reported to be higher in comparison to our population. In an Indian population,10 HA (42%) and PFDs (39%) was more common than HB (5%) and vWD (9%). In PFDs, GTT was more common than BSS, similar to that reported in our study.
We have shown an increased prevalence of HA, similar to the registries published by other developing countries. However, this is contrary to the data shown by the developed nations where diagnosis of vWD is almost equivalent to HA or showing a rising trend.11,12 There are significant limitations in the diagnosis of vWD in developing countries due to the high cost and lack of availability of diagnostic tests in majority of our centers. A similar diagnostic dilemma has also been reported from India.13
The frequency of RBD in our study was 5% and FV deficiency (28%) was the most common, while previous studies from southern Pakistan report FVII deficiency5,6 to be the most common. A study performed in Iran8 reported a higher frequency of RBD (16%) with combined FV and VIII deficiency being predominant. The most common RBD in India10 was FX deficiency while afibrinogenemia and factor VII deficiency is common in Egypt and Brazil, respectively.9,14
Screening and diagnostic facilities were available for 87% hemophilia treatment centers. However, specialized laboratory tests including inhibitor workup was only available in 1 hemophilia treatment center. There was a significant subset (45%) of patients who either remained undiagnosed or were managed according to clinical diagnosis. Diagnostic facilities were at its worst in the hemophilia treatment center located in Quetta where 68% remained undiagnosed compared to Karachi (3%) where diagnostic facilities are easily available. Reason behind barriers to diagnosis was lack of diagnostic facility at the hemophilia treatment center and surrounding area, expense of diagnostic tests, lack of awareness of physicians, and infrequent visits to the hemophilia treatment center in the off timings. These challenges have also been previously identified.15 The optimal solution would be setting up a diagnostic laboratory in each province with subsidized charges to cater its own treatment centers. This will be beneficial to the patients in terms of affordability. We aim to hold continuing medical education on bleeding disorders for increasing education of physicians as well as public awareness programs for patients and their families.
In case of bleeding in undiagnosed patients, FFP is usually the first choice to resolve the bleed; if persistent, platelets are transfused as a second line therapy. Treatment facilities were variable with plasma therapy availability in 100% hemophilia treatment centers followed by platelets, CP, and CS in 87% hemophilia treatment centers. All hemophilia treatment centers had plasma-derived factors available all the time. However, it was provided to patients only in life-threatening bleeding. Pain management with acetaminophen, diclofenac sodium, and tramadol was offered at all hemophilia treatment centers while management of inhibitors was available at 2 hemophilia treatment centers with either FEIBA or recombinant factor VIIa, depending on its availability. Immune tolerance therapy was not available at any center.
Diagnostic facility for viral screening such as HIV, HBV, and HCV was available in 100% hemophilia treatment centers. Since 372 (29%) transfused patients acquired viral hepatitis, it is of utmost importance to transfuse patients with recombinant factors rather than blood or blood products. Transfusion transmitted infections were less common in neighboring country Iran, where HCV and HBV was found in 15% and 0.2% patients, respectively, while HIV was absent.16 This may be because of locally produced low-cost clotting factors in Iran. Similarly, Syria had lesser frequency of HBV (0.5%) and HCV (20%) compared to Pakistan.17
Inhibitor screening was performed only in those patients in whom bleeding failed to resolve 24 hours after beginning therapy with clotting factor concentrates and CP. Inhibitors were present in 27% patients with HA and 12% patients with HB. This is higher compared to that reported from Brazil (HA, 10% and HB, 2%),9 Turkey (HA, 10%),18 and China (HA, 4%)19 but lower than that reported from India (HA, 39%).20 We reported inhibitors in moderate form of HA. This is not surprising as this has been reported by Wight et al and Paschal et al.21,22
Physiotherapy was available in 50% of hemophilia treatment centers. Social, psychological, or genetic counseling services were not provided in any hemophilia treatment center. Emergency care for bleeds was only available for 24 hours in Karachi, while patients in the remaining localities went to the emergency services present at the tertiary care hospitals where the level of care depended on the level of awareness for IBD and availability of clotting factor concentrates or blood components. Prophylaxis and home treatment were not available to any patient.
There are several limitations in this study. Due to the manual collection of retrospective data, there is a possibility of errors that include missing information. Around 20% to 30% of data were not reported either because tests were not performed or because the reports were missing from patients’ medical record files. Since this is the initiation of a registry, mortality data were not available at the time of data collection. Treatment details of transfusion transmitted infections and factor concentrate utilization per annum was also missing. There is a propensity for the under representation of patients affected with vWD, since only patients with clinically significant bleeding seek referral for diagnosis and management. Lack of further sub typing of vWD due to the lack of diagnostic facilities in various treatment centers has made it difficult to ascertain the commonest subtype of vWD in our cohort. Flexion deformity, muscle contracture, and physiotherapy details were documented for few patients despite lack of regular prophylaxis. In treatment centers, there is a lack of electronic medical charts, and the data were entered manually in medical files. Hence, lack of documentation may be a possible reason.
Conclusion
This survey reports a detailed registry of IBD and their management at a not-for-profit organization in a developing country to form the basis for planning development and research. The next daunting task will be to diagnose undiagnosed patients or those being treated on the basis on clinical diagnosis. Also, it is important to acquire a centrally operated web-based registry in each hemophilia treatment center and to update the registry on a yearly basis. The regular, systematic collection of data will give a clearer picture of the burden of IBD in Pakistan and help us in planning care and evaluate effective use of resources in an already resource limited setting.
Acknowledgments
We gratefully acknowledge Dr. Mike Soucie (Associate Director for Science, Division of Blood Disorders, National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention) for critical review. We especially thank the staff from the following centers of Fatimid Foundation for data collection: Dr. Sumera Aqeel (Ultrasonologist, Karachi center), Dr. Farhat-ul-ain (Medical officer, Hyderabad center), Dr. Muhammad Bilal (Medical Officer, Lahore center), Dr.Ishrat Khurshid (Medical Officer, Lahore center), Mr. Ejaz Ahmad (Peshawar center), Mr. Sohail Jaffar (Phlebotomist, Peshawar center), Ms. Abida Rofan (Nurse, Quetta center), and Ms. Hafsah Tariq (Laboratory supervisor, Quetta center). We also acknowledge Dr. Zeya ur Rahman (Medical Director) for the support extended to us during data collection.
Footnotes
Author Contributions: S.H. analyzed the data and wrote the article. S.B. and A.P. contributed to research design and interpretation of data. A.N., F.M., M.J., R.N.M., F.B., and H.U. performed the research and provided data from their respective centers. B.M. critically reviewed the article. All authors approved the final manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Shabneez Hussain  http://orcid.org/0000-0001-8537-7322
http://orcid.org/0000-0001-8537-7322
Bushra Moiz  http://orcid.org/0000-0003-0777-3690
http://orcid.org/0000-0003-0777-3690
References
- 1. Evatt BL. World Federation of Haemophilia Guide to Developing a National Patient Registry; 2005. Published by the World Federation of Hemophilia. Montréal, Québec. Canada.
- 2. Haemophilia care in developing countries. A report of a joint WHO/World Federation of Hemophilia meeting. Geneva, Switzerland, 16-17 June 1997. Haemophilia. 1998;4(suppl 2):v,1–66. [PubMed] [Google Scholar]
- 3. Ardakani MA. Pakistan World Health Organization: World Health Organization; 2016 [cited 29 May 2018]. Available from: http://www.who.int/countries/pak/en/ [Google Scholar]
- 4. World Federation of Hemophilia report on the annual global survey 2016. Published by the World Federation of Hemophilia Montréal, Québec; Canada. [Google Scholar]
- 5. Borhany M, Shamsi T, Naz A, et al. Congenital bleeding disorders in Karachi, Pakistan. Clin Appl Thromb Hemost. 2011;17(6):E131–E137. [DOI] [PubMed] [Google Scholar]
- 6. Sajid R, Khalid S, Mazari N, Azhar WB, Khurshid M. Clinical audit of inherited bleeding disorders in a developing country. Indian J Pathol Microbiol. 2010;53(1):50–53. [DOI] [PubMed] [Google Scholar]
- 7. Nummi V, Lassila R, Joutsi-Korhonen L, Armstrong E, Szanto T. Comprehensive re-evaluation of historical von Willebrand disease diagnosis in association with whole blood platelet aggregation and function. Int J Lab Hematol. 2018;40(3):304–311. [DOI] [PubMed] [Google Scholar]
- 8. Mansouritorghabeh H, Manavifar L, Banihashem A, et al. An investigation of the spectrum of common and rare inherited coagulation disorders in north-eastern Iran. Blood Transfus. 2013;11(2):233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rezende SM, Pinheiro K, Caram C, Genovez G, Barca D. Registry of inherited coagulopathies in Brazil: first report. Haemophilia. 2009;15(1):142–149. [DOI] [PubMed] [Google Scholar]
- 10. Gupta M, Bhattacharyya M, Choudhry VP, Saxena R. Spectrum of inherited bleeding disorders in Indians. Clin Appl Thromb Hemost. 2005;11(3):325–330. [DOI] [PubMed] [Google Scholar]
- 11. Baker JR, Riske B, Drake JH, et al. US hemophilia treatment center population trends 1990-2010: patient diagnoses, demographics, health services utilization. Haemophilia. 2013;19(1):21–26. [DOI] [PubMed] [Google Scholar]
- 12. Hassan HJ, Morfini M, Taruscio D, et al. Current status of Italian registries on inherited bleeding disorders. Blood Transfus. 2014;12(suppl 3):s576–s581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ghosh K, Shetty S. Epidemiology, diagnosis, and management of von Willebrand disease in India. Semin Thromb Hemost. 2011;37(5):595–601. [DOI] [PubMed] [Google Scholar]
- 14. Abdelwahab M, Khaddah N. Rare coagulation disorders: a study of 70 cases in the Egyptian population. Haemophilia. 2012;18:e386–e388. [DOI] [PubMed] [Google Scholar]
- 15. O’Mahony B, Black C. Expanding hemophilia care in developing countries. Semin Thromb Hemost. 2005;31(5):561–568. [DOI] [PubMed] [Google Scholar]
- 16. Karimi M, Yarmohammadi H, Ardeshiri R, Yarmohammadi H. Inherited coagulation disorders in southern Iran. Haemophilia. 2002;8(6):740–744. [DOI] [PubMed] [Google Scholar]
- 17. Ali T, Schved JF. Registry of hemophilia and other bleeding disorders in Syria. Haemophilia. 2012;18:851–854. [DOI] [PubMed] [Google Scholar]
- 18. Oren H, Yaprak I, Irken G. Factor VIII inhibitors in patients with hemophilia A. Acta Haematol. 1999;102(1):42–46. [DOI] [PubMed] [Google Scholar]
- 19. Wang XF, Zhao YQ, Yang RC, et al. The prevalence of factor VIII inhibitors and genetic aspects of inhibitor development in Chinese patients with hemophilia A. Haemophilia. 2010;16(4):632–639. [DOI] [PubMed] [Google Scholar]
- 20. Shetty S, Ghosh K, Pathare A, Mohanty D. Clinically significant inhibitors in hemophilia A patients from India tend to persist. Acta Haematol. 2000;103(3):175–176. [DOI] [PubMed] [Google Scholar]
- 21. Wight J, Paisley S. The epidemiology of inhibitors in haemophilia A: a systematic review. Haemophilia. 2003;9(4):418–435. [DOI] [PubMed] [Google Scholar]
- 22. Paschal RD, Meeks SL, Neff AT. Development of factor VIII inhibitors in two patients with moderate haemophilia A. Haemophilia. 2013;19(1):e55–e57. [DOI] [PubMed] [Google Scholar]