Abstract
The gain-of-function variation p.I1157T in C3 was previously identified in 8 patients with atypical hemolytic uremic syndrome (aHUS) at Mie University Hospital. In the present study, we identified another 11 patients with aHUS with this variation, including 10 pediatric patients (onset age: 1-16 years). The variation seems to be geographically concentrated around Mie Prefecture in Japan. Fifteen of the 19 patients with aHUS experienced infection as probable triggering events. All 19 patients had renal dysfunction. Seven patients, including 2 from the previous study and 5 from the present study, were treated with eculizumab, with all showing a good response with hematological normalization. Among the 5 eculizumab-treated patients in the present study, 3 had an ambiguous diagnosis of aHUS due to low-grade hemolysis even with elevated levels of lactate dehydrogenase and bilirubin. In those cases, in-house targeted DNA sequencing identified the C3 p.I1157T variation carriers, which enabled the early initiation of treatment with eculizumab. The present study supports the early introduction of eculizumab in patients with aHUS, especially pediatric patients.
Keywords: aHUS, C3 p.I1157T, sC5b-9, eculizumab
Introduction
Thrombotic microangiopathy (TMA), characterized by the presence of microangiopathic hemolytic anemia, thrombocytopenia, and organ failure, includes hemolytic uremic syndrome (HUS) induced by Shiga toxin-producing Escherichia coli (STEC), thrombotic thrombocytopenic purpura (TTP), atypical HUS (aHUS), and other TMAs.1–5 Approximately 10% of TMA cases were classified as aHUS according to the criteria; the absence of STEC with no severe deficiency of a disintegrin-like and metalloproteinase with thrombospondin type 1 motifs 13 (ADAMTS13) activity and without underlying diseases for TMA as a trigger.4,5
During the activation of the complement system, the complement component C3 is cleaved to C3a and C3b and the latter directly binds to the microbe for opsonization or binds complement factor B (CFB) to yield the C3 convertase C3bBb for subsequent activation of the complement pathway.4,6 However, further activation of the C3 convertase is strictly limited by several endogenous complement regulatory proteins such as complement factor H (CFH), membrane cofactor protein (MCP), complement factor I (CFI), and thrombomodulin (THBD) present on the surface of vascular endothelial cells in the host.4 Complement factor H and MCP are cofactors for the proteolytic degradation of C3b by CFI. Thrombomodulin also functions as a cofactor for CFI-mediated C3b inactivation.7
The pathogenesis of aHUS is considered to be the uncontrolled activation of the C3 convertase of the alternative pathway of the complement system, leading to the formation of the anaphylatoxin C5a and the membrane attack complex C5b-9 on endothelial cells, which can promote cell lysis and thrombus formation. Thus, loss-of-function variations in the complement regulators (CFI, CFH, and MCP) of the C3 convertase or gain-of-function variations in the activators (C3 and CFB) of the C3 convertase can lead to unrestricted complement hyperactivity, which results in aHUS.7–12 Genetic variations in the genes involved in the alternative pathway of the complement system are identified in more than half of patients with aHUS.4,8,13,14
Eculizumab is a terminal complement inhibitor and has been approved for the treatment of aHUS.15 It binds with high affinity to human complement component C5 and blocks the generation of C5a and C5b-9. Prospective studies of adult and pediatric patients with aHUS have shown the efficacy and safety of eculizumab for treating aHUS.16,17 Since eculizumab has been associated with the time-dependent improvement in the renal function in patients with aHUS, it is recommended to initiate treatment with eculizumab as soon as possible.16,18
We have previously reported the clinical characteristics and genetic variations of patients with aHUS at the Mie University Hospital in Japan and identified a gain-of-function variation, C3 p.I1157T, in 8 Japanese patients with aHUS in 6 independent families.19,20 The p.I1157T variation is present in the thioester-containing domain in C3. Mutagenesis studies revealed that the p.I1157A variation in C3d attenuated the binding of short consensus repeat 19 to 20 in CFH by a factor of 4 to 6 when compared to wild-type C3d.21 Binding studies using intact C3 variant with p.I1157T showed lower affinity for CFH and MCP than wild-type C3.22,23 Structural analysis using electron microscopy showed that C3 showed a relatively compact conformation, but C3 with this variation showed a detached thioester-containing domain from the C3b body.23 These accumulating evidences suggest that, due to its lower affinity for complement regulators CFH and MCP, the C3 p.I1157T variant may be resistant to proteolytic inactivation by CFI, leading to unrestricted complement hyperactivity on host cell surfaces and the phenotypic expression of aHUS.
In the present study, to clarify renal dysfunction, triggering events for aHUS, the utility of the sC5b-9 level as a possible marker, the responsiveness to eculizumab, and the survival in patients with aHUS with this variation, we further collected the clinical features of 19 patients with aHUS with C3 p.I1157T in 14 families in total, including previously reported 8 patients,20 all diagnosed and treated in the Kinki area. In addition, we introduced in-house targeted DNA sequencing as a diagnostic means of identifying the C3 p.I1157T variation and rapidly initiating patient treatment with eculizumab. Our study revealed the phenotypic variations in patients with aHUS with this variation and suggested the rapid initiation of treatment with eculizumab for variation carriers with a familial history or multiple relapses of aHUS.
Materials and Methods
Patients
Nineteen patients with aHUS with C3 p.I1157T in 14 families, including 8 previous patients and 11 new patients, who had been all diagnosed in the Kinki region of Japan were investigated in this study. The diagnosis of TMA was made based on the simultaneous occurrence of microangiopathic hemolytic anemia (hemoglobin 100 g/L), thrombocytopenia (platelet count < 120 × 109/L), and organ failure including acute renal injury.5 Thrombotic microangiopathy without STEC, ADAMTS13 <10%, or underlying disease for TMA was diagnosed as aHUS. Thrombotic microangiopathy with STEC was diagnosed as STEC-HUS, and TMA with ADAMTS13 <10% was diagnosed as TTP.3 Thrombotic microangiopathies aside from aHUS, TTP, or STEC-HUS were diagnosed as “other TMAs.” “Acute” was defined as a state within 1 week from the onset and without treatment with eculizumab. “Chronic” was defined as a state without severe symptoms such as thrombocytopenia and marked hemolysis and without treatment such as plasma exchange. Systemic lupus erythematosus (SLE) was diagnosed according to the 1997 update of the 1982 American College of Rheumatology revised criteria for classification of SLE.24 The study protocol was approved by the Mie University Graduate School of Medicine and the National Cerebral and Cardiovascular Center, and written informed consent was obtained from all of the participants.
Measurements of Platelet Count and Plasma Levels of Hemoglobin, Complement Factors, CH50, ADAMTS13 activity, and sC5b-9
The hemoglobin levels and platelet count were measured using a multifield automatic hematology analyzer XN-3100 (Sysmex Co, Kobe, Japan). Lactate dehydrogenase (LDH), creatinine, total bilirubin (T-Bil), C3, C4, and CH50 were measured using an akua-auto kainos-LDH II kit (Kainos, Tokyo, Japan), L-type Wako CRE (Wako Pure Chemical Industries, Osaka, Japan), M Iatro LQ T-BIL II (LSI Medience, Tokyo, Japan), N-assay TIA C3-SH (Nittobo Medical, Tokyo, Japan), N-assay TIA C4-SH (Nittobo Medical), and CH50-HA test Wako (Wako Pure Chemical Industries), respectively.
Blood samples were drawn from the antecubital vein of patients through a needle into disposable, siliconized, evacuated plastic tubes containing 0.1 volume of 3.13% trisodium citrate. The plasma samples were obtained by centrifugation at 3430g for 10 minutes at room temperature, quick-frozen, and stored at −80°C until the analysis. The ADAMTS13 activity was measured using an FRETS-VWF73 peptide (Peptide Institute, Osaka, Japan).25,26
The sC5b-9 levels were measured by a neoantigen capture antibody method using the Human C5b-9 ELISA Set (BD Biosciences, San Diego, California). The sC5b-9 levels were measured using citrate plasma samples for 10 patients with aHUS, 11 patients with TTP, 3 patients with STEC-HUS, 20 patients with other TMA, 6 patients with SLE, and 96 healthy volunteers.
Genetic Variation Screening
The coding exons and the intronic flanking regions of CFH, C3, MCP, CFI, CFB, and THBD were sequenced by the chain termination method, as previously described.19,20 Targeted sequencing was performed on a variation-containing exon to identify the C3 p.I1157T variation in 6 patients (VI-2, VII, VIII, X-2, X-3, and XIII). The A of the ATG translation initiation start site was designated as the +1 position, and the initial Met was denoted as +1.
Statistical Analyses
The data are expressed as the medians with 25th to 75th percentiles. The differences between the groups were examined using the Mann-Whitney U test. A P value <.05 was considered to be statistically significant. All statistical analyses were performed using the Stat Flex, version 6, software package (Artec Co Ltd, Osaka, Japan).
Results
The clinical features of 19 patients with aHUS with the C3 p.I1157T variation in 14 families, including 8 previously reported patients and 11 new patients, are summarized in Table 1. None of the patients showed signs of infection with STEC. All 14 families were nonconsanguineous. All 19 patients had renal dysfunction. The first episode of aHUS occurred during pediatric age (≤16 years of age) in 15 patients, while 4 patients experienced their first episode at more than 20 years of age. Sixteen of the 19 patients experienced relapse, with a varying number of relapse events up to 10 times. Six of 14 families had an aHUS history in their family members. Fifteen of the 19 patients experienced infection as a probable triggering event, such as common cold, influenza, bacterial infection, or vaccination for influenza (Table 1).
Table 1.
The Clinical Manifestations, Efficacy of Treatments, and Genetic Variations for 19 Patients With aHUS in 14 Families.a
| Patient | Age of Onset (years) | Relapse | Family History | Inducement Factor | Plasma Exchange | Fresh Frozen Plasma | Hemodialysis | Eculizumab | C3 p.I1157T | Additional Genetic Variation |
|---|---|---|---|---|---|---|---|---|---|---|
| III | 1 | 2 | Negative | Common cold, bacterial infection | NA | NA | Withdrawal | Unused | Hetero | No |
| IV-1 | 2 | 7 | Positive | Common cold, bacterial infection | Slightly effective | Not effective | Maintenance dialysis | Unused | Hetero | No |
| II | 5 | 8 | Negative | Influenza, common cold | Effective | Effective | ND | Effective | Hetero | No |
| I-1 | 6 | 6 | Positive | Common cold | Effective | Effective | ND | Unused | Hetero | No |
| VI-1 | 9 | 2 | Positive | Bacterial infection | Effective | NA | ND | Effective | Hetero | No |
| I-2 | 20 | 2 | Positive | Unknown | NA | NA | ND | Unused | Homo | No |
| V | 21 | 4 | Negative | Common cold, vaccine | Effective | Effective | ND | Unused | Hetero | No |
| IV-2 | 70 | 0 | Positive | Unknown | NA | NA | Maintenance dialysis | Unused | Hetero | No |
| VII | 1 | 4 | Negative | Influenza | Effective | NA | ND | Effective | Hetero | No |
| VIII | 3 | 3 | Positive | Common cold | NA | Effective | ND | Effective | Hetero | No |
| IX | 3 | 10 | Negative | Unknown | Effective | Effective | ND | Unused | Hetero | No |
| X-2 | 6 | 3 | Positive | Influenza | Effective | NA | ND | Unused | Hetero | NA |
| XI | 6 | 0 | Negative | Mumps | Effective | NA | Withdrawal | Effective | Hetero | No |
| XII | 9 | 5 | Negative | Influenza | Effective | NA | Withdrawal | Unused | Hetero | CFB p.N331A |
| XIII | 10 | 1 | Positive | Influenza | NA | NA | ND | Effective | Hetero | No |
| XIV | 10 | 3 | Negative | Influenza | ND | NA | ND | Unused | Hetero | MCP p.P195S |
| X-1 | 14 | 1 | Positive | Influenza | NA | Not effective | ND | Effective | Hetero | No |
| X-3 | 16 | 1 | Positive | Common cold | Effective | NA | ND | Unused | Hetero | NA |
| VI-2 | 38 | 0 | Positive | Unknown | Effective | NA | ND | Unused | Hetero | NA |
Abbreviations: aHUS, atypical hemolytic uremic syndrome; CFB, complement factor B; MCP, membrane cofactor protein; NA, not available; ND, not done.
aPatients are ordered by the age of onset. The clinical presentations of 8 patients with aHUS (I-1 to VI-1) have been reported previously at least in part.16 Eleven patients (VI-2 to XIV) were newly identified. NA in the “additional genetic variations” column shows the targeted sequencing of only the C3 p.I1157T variation-containing exon. The patient number agreed with a previous report.20
Patients with C3 p.I1157T were treated with plasma exchange, transfusion of fresh-frozen plasma, steroid, infusion therapy, antiplatelet, antihypertensive, or antibiotic agents (Table 1). All 19 patients had kidney injury and 5 patients had been treated with hemodialysis (HD). Three of them were being weaned from HD, and 2 were maintenance dialysis.
Seven patients, including 2 from the previous study20 and 5 from the present study, were treated with eculizumab (Table 1), and all of them showed a good response with hematological normalization of platelet counts and LDH levels. Patients VII and VIII received only 1 dose of eculizumab, resulting in hematological normalization. Patient XIII received 4 successive doses, resulting in hematological normalization. Four patients (cases II, VI-1, X-1, and XI) who had relapse or renal failure continues treatment with eculizumab. As a result of their treatment, all 19 patients achieved remission and survived, and the renal function improved in 15 patients. Severe cases such as IV-1, VI-2, and X-1 had a family history of aHUS. Although 3 patients (cases I-1, IV-1, and V) had central nervous symptoms, these symptoms were improved after 1 or 2 plasma exchange sessions.
The C3 p.I1157T variation was identified in all 19 patients, comprising 18 heterozygotes and 1 homozygote (Table 1). In the case I family, the homozygote of this genetic variation (I-2) did not show more severe symptoms than the heterozygote (I-1). Two very rare potentially predisposing variations, CFB p.N331A (c.991A>G, rs 776825572) and MCP p.P195S (c.583C>T, rs773860894), were identified in patients XII and XIV, respectively. Being a double-variation carrier might be related with the earlier onset of aHUS, but these 2 carriers showed an onset at 9 and 10 years of age, respectively. In patients VII, VIII, and XIII, targeted DNA sequencing was performed to identify the C3 p.I1157T variation carriers before a conventional 6-gene analysis was performed, resulting in the early initiation of treatment with eculizumab. In some relatives with aHUS (VI-2, X-2, and X-3), only targeted DNA sequencing was performed, and the C3 p.I1157T variation was identified without sequencing of other genes.
The laboratory data of 15 patients with aHUS are summarized in Table 2. The platelet counts in all patients were markedly reduced to below the clinical diagnostic criterion (120 × 109/L), indicating thrombocytopenia. The hemoglobin levels in 6 of the previous patients (I-1 to VI-1) were below those in the clinical diagnostic criteria (100 g/L) with elevated LDH levels, indicating microangiopathic hemolysis. Among the 8 new patients with aHUS, the hemoglobin levels in 3 were below 100 g/L, and the remaining 5 showed mild hemolysis with hemoglobin levels of more than 100 g/L and elevated LDH levels. The creatinine levels were increased in most patients, while the total bilirubin levels were slightly increased.
Table 2.
The Laboratory Data of 15 Patients With aHUS in 14 Families.a
| Patient | PLT | Hb | ADAMTS13 | Creatinine | LDH | T-Bil | C3 (mg/L) | C4 (mg/L) | CH50 (U/mL) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (× 108/L) | (g/L) | (%) | (mg/L) | (U/L) | (mg/L) | Acute | Chronic | Acute | Chronic | Acute | Chronic | ||
| Normal Range | 130-370 | 13.7-16.8 | 68.5-153.5 | 4.6-10.7 | 124-222 | 4-15 | 720-1300 | 110-310 | 31.0-48.0 | ||||
| III | 26 | 59 | 53.8 | 47 | 4465 | 24 | 955 | 1116 | 299 | 451 | 58.6 | >60.0 | |
| IV-1 | 28 | 67 | 91.3 | 106 | 1280 | 11 | 791 | 585 | 328 | 408 | 46.3 | 51.7 | |
| II | 35 | 59 | 88.8 | 47 | 4485 | 35 | 710 | ND | 202 | ND | 48.7 | ND | |
| I-1 | 19 | 74 | 76.3 | 17 | 972 | 19 | 721 | ND | 137 | ND | 43.6 | ND | |
| VI-1 | 14 | 66 | 67.5 | 18 | 3160 | 14 | 1002 | ND | 166 | ND | >60.0 | ND | |
| V | 35 | 98 | 92.4 | 14 | 928 | 35 | ND | 85 | ND | 177 | ND | 55.3 | |
| VII | 19 | 135 | 58.0 | 39 | 3882 | 22 | ND | ND | ND | ND | ND | ND | |
| VIII | 10 | 59 | 60.0 | 14 | 2112 | 78 | 978 | 1187 | 122 | 236 | 9.6 | >60.0 | |
| IX | 22 | 60 | 61.2 | 9 | 1098 | 32 | 640 | 867 | 190 | 232 | 46 | 53.1 | |
| XI | 49 | 120 | 94.0 | 8 | 3232 | 22 | 820 | ND | 280 | ND | ND | ND | |
| XII | 25 | 149 | 58.3 | 22 | 2533 | 34 | ND | ND | ND | ND | ND | ND | |
| XIII | 3 | 78 | 45.3 | 15 | 1381 | 21 | 1030 | ND | 291 | ND | >60.0 | ND | |
| XIV | 35 | 119 | 73.5 | 8 | 911 | 23 | ND | ND | ND | ND | ND | ND | |
| X-1 | 18 | 127 | 100.0 | 34 | 1985 | 12 | 991 | ND | 341 | ND | 56.7 | ND | |
| VI-2 | 10 | 74 | 48.5 | 19 | 2850 | 18 | ND | ND | ND | ND | ND | ND | |
Abbreviations: aHUS, atypical hemolytic uremic syndrome; Hb, hemoglobin; LDH, lactate dehydrogenase; ND, not done; PLT, platelet; T-Bil, total bilirubin.
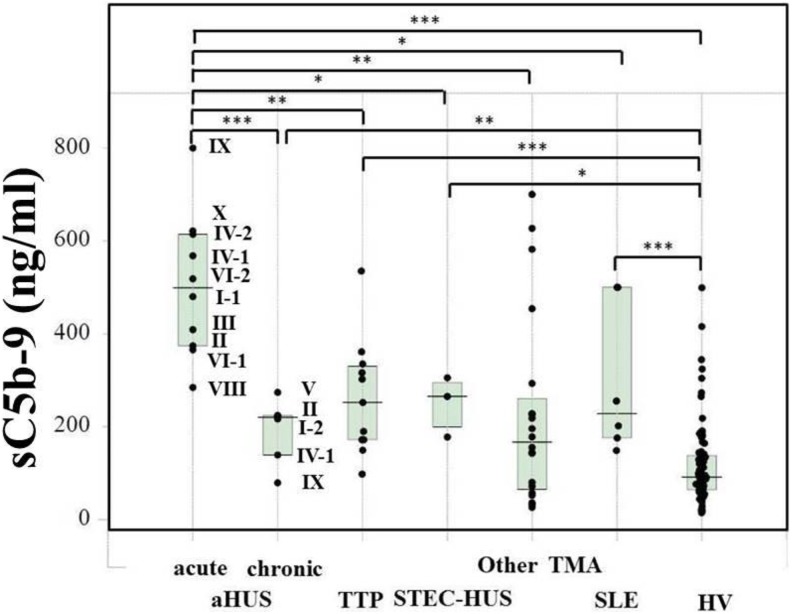
Figure 1 shows the plasma sC5b-9 levels in patients with aHUS, TTP, STEC-HUS, other TMAs and SLE as well as healthy individuals. Patients with aHUS with acute state (median, 499 ng/mL; 25th-75th percentile, 374-614 ng/mL, n = 10) showed significantly higher sC5b-9 levels than those with chronic state (median, 220 ng/mL; 25th-75th percentile, 139-224 ng/mL, n = 6), patients with TTP (median, 252 ng/mL; 25th-75th percentile, 172-330 ng/mL, n = 11), other TMA patients (median, 167 ng/mL; 25th-75th percentile, 64.5-261 ng/mL, n = 20), patients with SLE (median, 228 ng/mL; 25th-75th percentile, 176-500 ng/mL, n = 6), and healthy volunteers (median, 91.3 ng/mL; 25th-75th percentile, 63.9-138 ng/mL, n = 96). The levels of sC5b-9 were markedly reduced to less than 100 ng/mL after treatment with eculizumab (data not shown).
Figure 1.
Plasma sC5b-9 levels in patients with various thrombotic microangiopathy (TMA) conditions. Plasma samples obtained from patients with acute atypical hemolytic uremic syndrome (aHUS, n = 10), patients with chronic aHUS (n = 6), patients with thrombotic thrombocytopenic purpura (TTP, n = 11), patients with Shiga toxin-producing Escherichia coli–induced hemolytic uremic syndrome (STEC-HUS, n = 3), other TMA patients (n = 20), patients with systemic lupus erythematosus (SLE, n = 6), and healthy volunteers (HV, n = 96) were measured. *P < .05; **P < .01; ***P < .001.
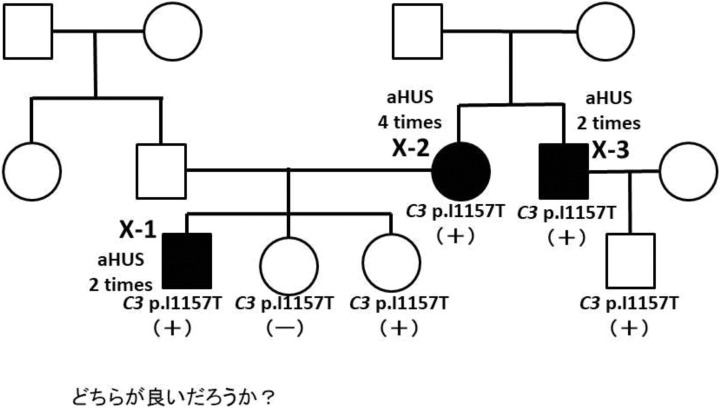
A family pedigree of case X is shown in Figure 2. Five members in the family were carriers of C3 p.I1157T. Three of them (X-1, X-2, X-3) had multiple aHUS episodes, but 2 did not have an aHUS episode. Thus, the phenotypic variation of aHUS episode was observed in this family. Some of the children in cases II and V were C3 p.I1157T carriers, but aHUS episodes did not appear in these carriers (data not shown).
Figure 2.
Family pedigree of case X. Five members shown with (+) carry the C3 p.I1157T variation, and one shown with (−) does not carry the variation. Closed symbols indicate carriers with atypical hemolytic uremic syndrome (aHUS). Three of 5 variant carriers (X-1, X-2, and X-3) had multiple aHUS episodes, and 2 of them did not have an HUS episode.
Discussion
In the present study, we identified the C3 p.I1157T variation as the predisposing genetic variation in 19 patients with aHUS (Table 1). According to a recent report, the genetic variations in CFH were the most prevalent, with a frequency of 19.8% in 795 patients with aHUS in the European Consortium (comprising France, Italy, the United Kingdom, and Spain14) but only 7% in Japan.27 In contrast to CFH, the frequency of genetic variations in C3 was 5.6% in the European Consortium but was relatively high (about 40%) in Japan. Most patients with aHUS with the C3 variations in Japan carry a single missense variation p.I1157T.19,20,27 Combined with previous 8 patients,19,20 the present study showed that 19 patients with aHUS with this variation in total have been treated at the hospitals in the Kinki area where Mie prefecture is located, suggesting that the C3 p.I1157T variation might be geographically concentrated in or around this particular area, suggesting a founder effect of the variation. The identification of a number of carriers prompted us to describe the aHUS phenotypes and the efficacies of several treatment strategies.
Eculizumab is an inhibitor for the terminal complement pathway and has been approved for the treatment of aHUS.15 In the present study, 7 patients with C3 p.I1157T were treated with eculizumab, and all had good response and survived. Among them, 4 had to continue eculizumab administration. The remaining 9 patients with C3 p.I1157T were treated with plasma exchange, plasma infusion, steroid, HD, or supportive therapy, and all survived. Only 2 patients were continuing HD. The frequency of CFH variation is high in patients with aHUS in Europe and the United States14 and their outcome was poor,8 resulting in the recommendation of eculizumab administration.
In a clinical trial of eculizumab, a shorter interval between the clinical manifestation of aHUS and initiation of treatment was associated with greater improvement in the estimated glomerular filtration rate.16 In the present study, we used targeted DNA sequencing to identify the variation carriers, as this method was easier to use than the conventional 6-gene analysis to identify the carriers. In the present study, 3 of 5 eculizumab-treated patients had an ambiguous diagnosis of aHUS due to low-grade hemolysis despite their elevated levels of LDH and bilirubin. In those cases and even in the aHUS cases, targeted DNA sequencing provides important information for the early initiation of treatment with eculizumab.
It can be assumed that, in addition to the main genetic variation C3 p.I1157T, environmental factors and/or other genetic variations are required for the manifestation of aHUS as a second hit. In the present study, 15 (79%) of 19 patients experienced infection as probable triggering events, suggesting the possibility of immune complex-initiated complement activation.28 More than 1 variation was identified in 12% of patients with aHUS with genetic variations.13 In the present study, 2 variations, CFB p.N331A and MCP p.P195S, have been identified in patients XII and XIV, respectively, indicating that 2 (11%) of 19 patients. These have not yet been identified in the Japanese general population consisting of 1208 individuals, deposited at the Human Genetic Variation Database,29 indicating that these variations are very rare and may have a possibility of functional effects on the complement activity regulation.
Few methods are available for measuring the anomalies of complement regulation. The C3, C4, and CH50 levels were in the normal range in most patients with aHUS in the present study. Only the plasma sC5b-9 level was useful for the differential diagnosis of aHUS from TTP and other TMAs. Elevated sC5b-9 levels with complement activation suggest that C3b is out of regulation, resulting in thrombocytopenia, hemolysis, and organ failure.
In conclusion, all patients with aHUS with C3 p.I1157T survived; however, their clinical courses varied and 2 patients that did not receive eculizumab progressed to end-stage renal disease. We used targeted DNA sequencing to rapidly identify the C3 p.I1157T carriers, which led to the early initiation of treatment with eculizumab. The administration of eculizumab is recommended for genetic variation carriers with a familial history or multiple relapse of aHUS.
Acknowledgments
The authors thank Y. Uchida for performing the DNA sequencing.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported in part by a Grant-in-Aid from the Ministry of Health, Labour and Welfare of Japan, Japan Society for the Promotion of Science, the Rare/Intractable Disease Project of Japan from Japan Agency for Medical Research and Development, AMED, and grant from Takeda Science Foundation.
References
- 1. Moake JL. Thrombotic microangiopathies. N Engl J Med. 2002;347(8):589–600. [DOI] [PubMed] [Google Scholar]
- 2. Boyce TG, Swerdlow DL, Griffin PM. Escherichia coli O157: H7 and the hemolytic-uremic syndrome. N Engl J Med. 1995;333(6):364–368. [DOI] [PubMed] [Google Scholar]
- 3. Matsumoto M, Fujimura Y, Wada H, et al. ; For TTP group of Blood Coagulation Abnormalities Research Team, Research on Rare and Intractable Disease supported by Health, Labour, and Welfare Sciences Research Grants. Diagnostic and treatment guidelines for thrombotic thrombocytopenic purpura (TTP) 2017 in Japan. Int J Hematol. 2017;106(1):3–15. [DOI] [PubMed] [Google Scholar]
- 4. Noris M, Remuzzi G. Atypical hemolytic-uremic syndrome. N Engl J Med. 2009;361(17):1676–1687. [DOI] [PubMed] [Google Scholar]
- 5. Wada H, Matsumoto T, Yamashita Y. Natural history of thrombotic thrombocytopenic purpura and hemolytic uremic syndrome. Semin Thromb Hemost. 2014;40(8):866–873. [DOI] [PubMed] [Google Scholar]
- 6. Roumenina LT, Loirat C, Dragon-Durey MA, Halbwachs-Mecarelli L, Sautes-Fridman C, Fremeaux-Bacchi V. Alternative complement pathway assessment in patients with atypical HUS. J Immunol Methods. 2011;365(1-2):8–26. [DOI] [PubMed] [Google Scholar]
- 7. Delvaeye M, Noris M, De Vriese A, et al. Thrombomodulin mutations in atypical hemolytic-uremic syndrome. N Engl J Med. 2009;361(4):345–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Noris M, Caprioli J, Bresin E, et al. Relative role of genetic complement abnormalities in sporadic and familial aHUS and their impact on clinical phenotype. Clin J Am Soc Nephrol. 2010;5(10):1844–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Richards A, Kemp EJ, Liszewski MK, et al. Mutations in human complement regulator, membrane cofactor protein (CD46), predispose to development of familial hemolytic uremic syndrome. Proc Natl Acad Sci U S A. 2003;100(22):12966–12971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sellier-Leclerc AL, Fremeaux-Bacchi V, Dragon-Durey MA, et al. Differential impact of complement mutations on clinical characteristics in atypical hemolytic uremic syndrome. J Am Soc Nephrol. 2007;18(8):2392–2400. [DOI] [PubMed] [Google Scholar]
- 11. Fremeaux-Bacchi V, Moulton EA, Kavanagh D, et al. Genetic and functional analyses of membrane cofactor protein (CD46) mutations in atypical hemolytic uremic syndrome. J Am Soc Nephrol. 2006;17(7):2017–2025. [DOI] [PubMed] [Google Scholar]
- 12. Goicoechea de Jorge E, Harris CL, Esparza-Gordillo J, et al. Gain-of-function mutations in complement factor B are associated with atypical hemolytic uremic syndrome. Proc Natl Acad Sci U S A. 2007;104(1):240–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Maga TK, Nishimura CJ, Weaver AE, Frees KL, Smith RJ. Mutations in alternative pathway complement proteins in American patients with atypical hemolytic uremic syndrome. Hum Mutat. 2010;31(6): E1445–E1460. [DOI] [PubMed] [Google Scholar]
- 14. Vieira-Martins P, El Sissy C, Bordereau P, Gruber A, Rosain J, Fremeaux-Bacchi V. Defining the genetics of thrombotic microangiopathies. Transfus Apher Sci. 2016;54(2):212–219. [DOI] [PubMed] [Google Scholar]
- 15. Zimmerhackl LB, Hofer J, Cortina G, et al. Prophylactic eculizumab after renal transplantation in atypical hemolytic-uremic syndrome. N Engl J Med. 2010;362(18):1746–1748. [DOI] [PubMed] [Google Scholar]
- 16. Legendre CM, Licht C, Muus P, et al. Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N Engl J Med. 2013;368(23):2169–2181. [DOI] [PubMed] [Google Scholar]
- 17. Greenbaum LA, Fila M, Ardissino G, et al. Eculizumab is a safe and effective treatment in pediatric patients with atypical hemolytic uremic syndrome. Kidney Int. 2016;89(3):701–711. [DOI] [PubMed] [Google Scholar]
- 18. Scully M, Goodship T. How I treat thrombotic thrombocytopenic purpura and atypical haemolytic uraemic syndrome. Br J Haematol. 2014;164(6):759–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fan X, Yoshida Y, Honda S, et al. Analysis of genetic and predisposing factors in Japanese patients with atypical hemolytic uremic syndrome. Mol Immunol. 2013;54(2):238–246. [DOI] [PubMed] [Google Scholar]
- 20. Matsumoto T, Fan X, Ishikawa E, et al. Analysis of patients with atypical hemolytic uremic syndrome treated at the Mie University Hospital: concentration of C3 p.I1157T mutation. Int J Hematol. 2014;100(5):437–442. [DOI] [PubMed] [Google Scholar]
- 21. Morgan HP, Schmidt CQ, Guariento M, et al. Structural basis for engagement by complement factor H of C3b on a self surface. Nat Struct Mol Biol. 2011;18(4):463–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schramm EC, Roumenina LT, Rybkine T, et al. Mapping interactions between complement C3 and regulators using mutations in atypical hemolytic uremic syndrome. Blood. 2015;125(15):2359–2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Martinez-Barricarte R, Heurich M, Lopez-Perrote A, et al. The molecular and structural bases for the association of complement C3 mutations with atypical hemolytic uremic syndrome. Mol Immunol. 2015;66(2):263–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.2012 SLICC SLE Criteria: https://www.rheumtutor.com/2012-slicc-sle-criteria/.
- 25. Kokame K, Nobe Y, Kokubo Y, Okayama A, Miyata T. FRETS-VWF73, a first fluorogenic substrate for ADAMTS13 assay. Br J Haematol. 2005;129(1):93–100. [DOI] [PubMed] [Google Scholar]
- 26. Kobayashi T, Wada H, Kamikura Y, et al. Decreased ADAMTS13 activity in plasma from patients with thrombotic thrombocytopenic purpura. Thromb Res. 2007;119(4):447–452. [DOI] [PubMed] [Google Scholar]
- 27. Yoshida Y, Miyata T, Matsumoto M, et al. A novel quantitative hemolytic assay coupled with restriction fragment length polymorphisms analysis enabled early diagnosis of atypical hemolytic uremic syndrome and identified unique predisposing mutations in Japan. PLoS One. 2015;10(5):e0124655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bomback AS, Markowitz GS, Appel GB. Complement-mediated glomerular diseases: a tale of 3 pathways. Kidney Int Rep. 2016;1(3):148–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Human Genetic Variation Database: http://www.hgvd.genome.med.kyoto-u.ac.jp/index.html, 2012.