Rabies virus causes lethal encephalitis in mammals and poses a serious public health threat in many parts of the world. Numerous strategies have been explored to combat rabies; however, their efficacy has always been unsatisfactory. We previously reported a new drug, PGG, which possesses a potent inhibitory activity on RABV replication. Herein, we describe the underlying mechanisms by which PGG exerts its anti-RABV activity. Our results show that RABV induces overactivation of STAT3 in BHK-21 cells, which facilitates viral replication. Importantly, PGG effectively inhibits the activity of STAT3 by disrupting the expression of miR-455-5p and increases the level of SOCS3 by directly targeting the 3′ UTR of SOCS3. Furthermore, the downregulated STAT3 inhibits the production of IL-6, thereby contributing to a reduction in the inflammatory response in vivo. Our study indicates that PGG effectively inhibits the replication of RABV by the miR-455-5p/SOCS3/STAT3/IL-6-dependent pathway.
KEYWORDS: CVS-11, IL-6, PGG, SOCS3, STAT3, anti-RABV, miR-455-5p
ABSTRACT
Our previous study showed that pentagalloylglucose (PGG), a naturally occurring hydrolyzable phenolic tannin, possesses significant anti-rabies virus (RABV) activity. In BHK-21 cells, RABV induced the overactivation of signal transducer and activator of transcription 3 (STAT3) by suppressing the expression of suppressor of cytokine signaling 3 (SOCS3). Inhibition of STAT3 by niclosamide, small interfering RNA, or exogenous expression of SOCS3 all significantly suppressed the replication of RABV. Additionally, RABV-induced upregulation of microRNA 455-5p (miR-455-5p) downregulated SOCS3 by directly binding to the 3′ untranslated region (UTR) of SOCS3. Importantly, PGG effectively reversed the expression of miR-455-5p and its following SOCS3/STAT3 signaling pathway. Finally, activated STAT3 elicited the expression of interleukin-6 (IL-6), thereby contributing to RABV-associated encephalomyelitis; however, PGG restored the level of IL-6 in vitro and in vivo in a SOCS3/STAT3-dependent manner. Altogether, these data identify a new miR-455-5p/SOCS3/STAT3 signaling pathway that contributes to viral replication and IL-6 production in RABV-infected cells, with PGG exerting its antiviral effect by inhibiting the production of miR-455-5p and the activation of STAT3.
IMPORTANCE Rabies virus causes lethal encephalitis in mammals and poses a serious public health threat in many parts of the world. Numerous strategies have been explored to combat rabies; however, their efficacy has always been unsatisfactory. We previously reported a new drug, PGG, which possesses a potent inhibitory activity on RABV replication. Herein, we describe the underlying mechanisms by which PGG exerts its anti-RABV activity. Our results show that RABV induces overactivation of STAT3 in BHK-21 cells, which facilitates viral replication. Importantly, PGG effectively inhibits the activity of STAT3 by disrupting the expression of miR-455-5p and increases the level of SOCS3 by directly targeting the 3′ UTR of SOCS3. Furthermore, the downregulated STAT3 inhibits the production of IL-6, thereby contributing to a reduction in the inflammatory response in vivo. Our study indicates that PGG effectively inhibits the replication of RABV by the miR-455-5p/SOCS3/STAT3/IL-6-dependent pathway.
INTRODUCTION
Rabies virus (RABV) causes an acute progressive encephalitis that is almost always fatal (1). Despite the availability of effective vaccines and possible protection by postexposure treatment, more than 60,000 humans die of rabies worldwide every year, with more than 15 million receiving postexposure prophylaxis (2).
Pentagalloylglucose (PGG) is a hydrolyzable phenolic tannin which is found in many traditional medicinal herbs, such as Rhus chinensis Mill and Paeonia suffruticosa (3, 4). It has multiple bioactivities, including anti-inflammatory (5), anticancer (6, 7) and antioxidant (8) activities, and has been shown to be active against hepatitis B virus (HBV) (9), hepatitis C virus (HCV) (10), and influenza A virus (IAV) (11). Several studies have explored the mechanisms by which PGG exerts its biofunctions, including inhibition of viral enzymes, such as the integrase and reverse transcriptase of human immunodeficiency virus type 1 (HIV-1), inducing autophagy via the mTOR/p70S6K-dependent signaling pathway, and downregulating cofilin 1 and inhibiting herpes simplex virus 1 (HSV-1) infection (4). Our previous study revealed that PGG has potent anti-RABV efficacy (12), although the mechanism was not fully elucidated.
Signal transducer and activator of transcription 3 (STAT3) is an important transcription factor which contributes to viral replication, cytoprotection, and the inflammatory and immune responses (13, 14). It can be activated by phosphorylation of tyrosine kinases of the Janus kinase (JAK) families and deactivated by suppressor of cytokine signaling 3 (SOCS3) by shielding phosphotyrosine residues of upstream kinases, while specific inhibitors of activated STAT3 prevent binding of STAT3 dimers to DNA (15, 16). Diverse viruses stimulate or suppress the activation of STAT3, and STAT3 exerts opposing functions in diverse viral infections. HBV (17, 18), HCV (19), human cytomegalovirus (HCMV) (20), and Epstein-Barr virus (EBV) (21) stimulate the STAT3 pathway, thereby promoting viral replication. Conversely, some viruses have evolved to suppress the STAT3 pathway during viral infection and replication, including IAV (22, 23), measles virus (24), mumps virus (MuV) (25), and human metapneumovirus (26). Wang et al. (27) have reported that STAT3 was increased at both the mRNA and protein levels in RABV-infected mouse brain or primary neuronal cells. Additionally, Lieu et al. (28) have demonstrated that the RABV P protein can interact with STAT3 and inhibit STAT3 responses to cytokine activation (alpha interferon [IFN-α] or oncostatin M). In the present report, we clarify the interaction between RABV replication and STAT3 activation under conditions without external stimuli.
MicroRNAs (miRNAs) are noncoding single-stranded RNAs with 22 to 24 nucleotides that posttranscriptionally repress target gene expression by promoting mRNA degradation or inhibiting translation into protein (29). The aberrant production of miRNAs is widely recognized as a characteristic of certain diseases, including viral infections, by regulating host cellular signaling pathways and the innate immune response (30, 31). For example, miRNA 324-5p (miR-324-5p) suppresses H5N1 influenza viral replication by directly targeting the viral PB1 and host CUEDC2 genes, but H5N1 virus itself can downregulate the level of miR-324-5p (32). Two EBV-encoded genes (LMP1 and EBNA2) are known to promote the expression of miR-155, which is required for the growth of EBV-infected B cells and lymphoma development (33). Several miRNAs, such as miR-124, miR-20, and miR-29a, have been implicated in the RABV infection process by contributing to neuronal dysfunction, while miR-190, miR-203, and miR-145 regulate viral infection-associated JAK-STAT, the T-cell receptor signaling pathway, and natural killer cell-mediated cytotoxicity (34, 35). Recent evidence that miR-20a and miR-124 are responsible for neuroremodeling has been obtained (36, 37); however, whether the RABV-induced dysregulation of the SOCS3/STAT3 pathway is mediated by miRNAs remains unclear.
In this study, we demonstrate that RABV upregulates the expression of miR-455-5p, resulting in decreased SOCS3 expression by directly targeting its 3′ untranslated region (UTR) and promoting the overactivation of STAT3, thereby driving the production of interleukin-6 (IL-6) and the inflammatory response. Intriguingly, PGG was found to exert its antiviral effect by inhibiting the production of miR-455-5p and by activating STAT3.
RESULTS
PGG effectively reverses RABV-induced overactivation of the SOCS3/STAT3 signaling pathway.
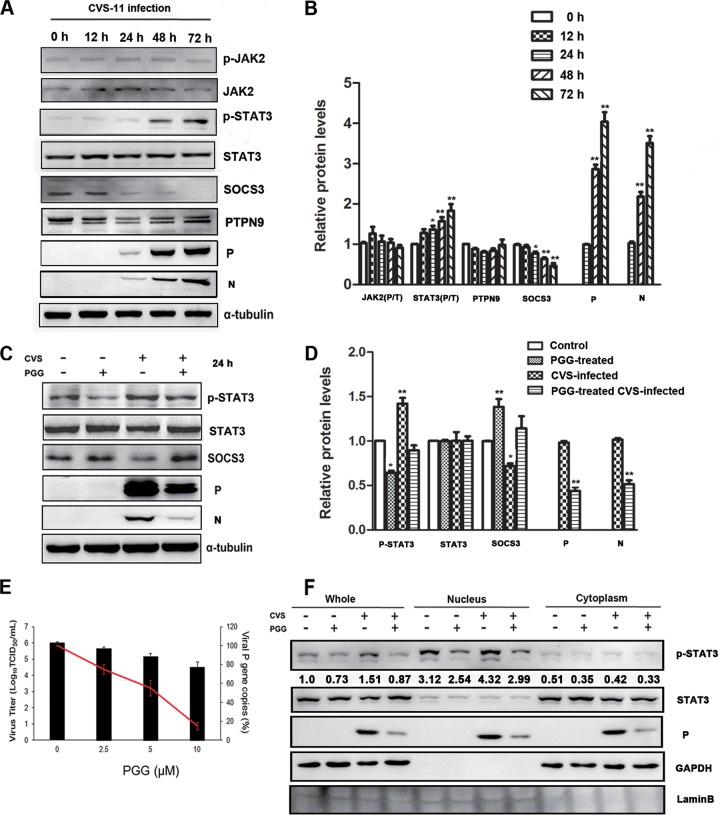
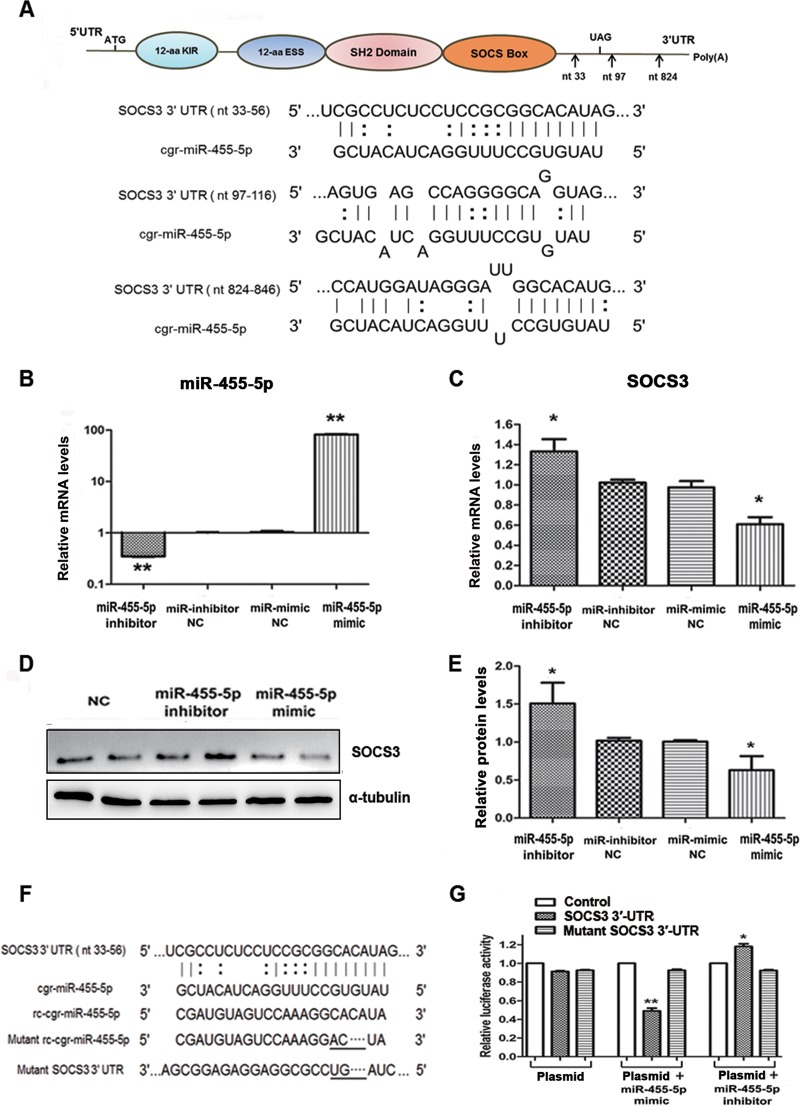
To clarify the interaction between RABV and STAT3, BHK-21 cells were infected with the challenge virus standard 11 (CVS-11) strain (multiplicity of infection [MOI] = 0.1) for various times (0, 12, 24, 48, 72 h postinfection [p.i.]), and the expression of STAT3 was analyzed by Western blotting. As shown in Fig. 1A and B, RABV infection significantly increased the phosphorylation level of STAT3 and JAK2 for over 72 h p.i. At the same time, the levels of SOCS3, as a negative mediator of STAT3, were decreased with increasing STAT3 activity. However, no significant change was observed with PTPN9, another STAT3 phosphatase that directly dephosphorylates STAT3 at the Tyr705 residue (38). These data indicate that RABV induced the activation of STAT3 in a JAK2-dependent fashion and was negatively regulated by SOCS3.
FIG 1.
RABV induced the activation of STAT3 via SOCS3, and PGG reversed it. BHK-21 cells were infected with CVS-11 for diverse times. (A) The expression levels of STAT3 pathway-associated proteins and the CVS P protein were detected by Western blotting. p-JAK2, phosphorylated JAK2. (B) The relative expression levels of the indicated proteins were analyzed by the use of ImageJ software and normalized to the α-tubulin expression level. BHK-21 cells were treated with CVS-11 or PGG alone or in combination. (C) The expression levels of SOCS3/STAT3 and the CVS P protein were detected by Western blotting. (D) The relative expression levels of the proteins were analyzed by the use of ImageJ software and normalized to the α-tubulin expression level. (E) The viral titer (bars) and P gene mRNA expression level (red line) of RABV were detected, respectively, by DFA and qRT-PCR. (F) BHK-21 cells were treated as described above for 48 h. Subcellular fractions of the cytosol (Cyt) and nucleus (Nuc) were isolated, and p-STAT3 and CVS P were analyzed by Western blotting. GAPDH and lamin B were used as purity controls for various fractions. The numbers indicate the ratio of p-STAT3/total STAT3. *, P < 0.05; **, P < 0.01. All data presented are representative of those from three independent experiments.
Our previous study uncovered that PGG effectively inhibits the replication of RABV. To explore whether the SOCS3/STAT3 pathway contributes to this anti-RABV function, the expression of SOCS3 and STAT3 (phosphorylated/total [P/T]) was examined after cells were treated with PGG (10 μM) for 24 h either with or without CVS-11 infection pretreatment. As shown in Fig. 1C and D, PGG significantly downregulated the level of phosphorylated STAT3 (p-STAT3) and upregulated SOCS3 whether PGG was used with or without CVS-11 infection, and, as expected, PGG induced significant inhibition of virus replication at the levels of all proteins and mRNAs and virus titers (Fig. 1C to E).
The RABV P protein has been reported to block the nuclear translocation of p-STAT3. To test whether CVS-11 infection affects the p-STAT3 subcellular localization, BHK-21 cells were incubated with CVS-11 and PGG (alone or in combination) for 48 h, and the subcellular fractions were prepared according to previously described methods (39), with their purity being confirmed by agarose gel electrophoresis (Fig. 1F). Lamin B1, a nuclear marker (40), was detected exclusively in the nuclear fraction (Nuc), and GAPDH (glyceraldehyde-3-phosphate dehydrogenase), a cytosol marker (41), was observed only in the cytosolic fraction. The results showed that, following incubation with CVS-11 alone, the distribution of p-STAT3 increased in the nucleus and decreased in the cytoplasm, but inclusion of PGG inhibited the phosphorylation of STAT3, thereby restoring its distribution to the base level (Fig. 1F). This indicates that CVS-11-mediated nuclear translocation of STAT3 occurs via its phosphorylation and that the effect of CVS-11 infection on STAT3 nuclear translocation is not the same as that produced by the viral P protein itself. These results indicate that RABV induces the activation of STAT3 and promotes its nuclear translocation and that PGG inhibits this action.
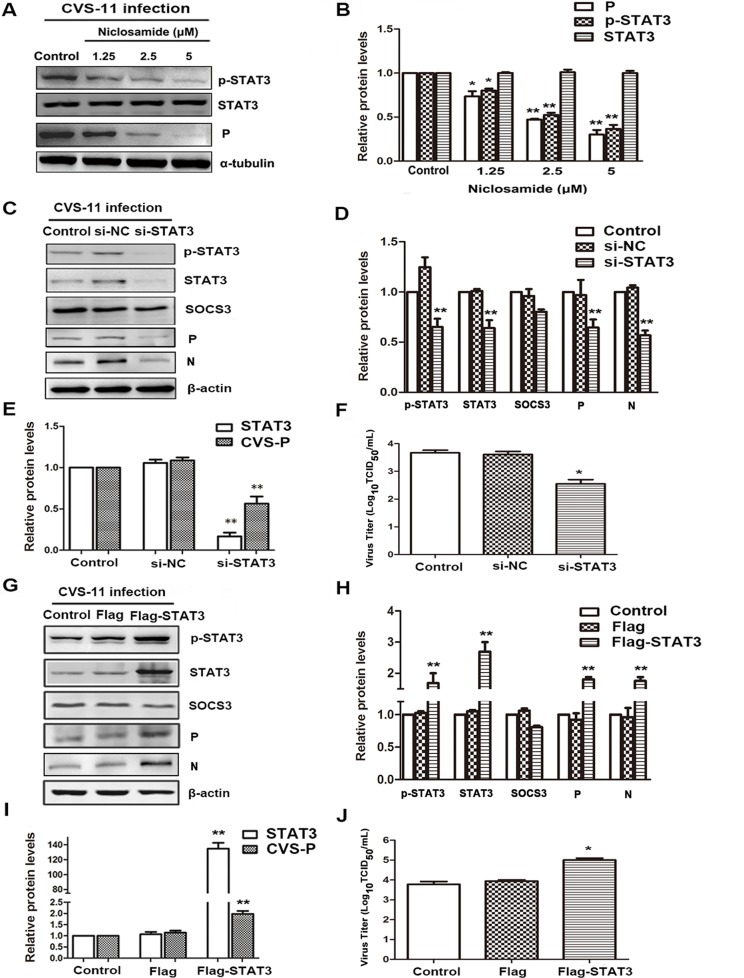
Activated STAT3 promotes the replication of RABV.
Numerous studies have shown that the STAT3 signaling pathway plays key roles in promoting or suppressing the process of virus infection and replication by diverse mechanisms (17–26). To confirm the role of STAT3 as a promoter of RABV replication, BHK-21 cells were infected with CVS-11 and with the STAT3-specific inhibitor niclosamide for 24 h. As shown in Fig. 2A and B, niclosamide potently inhibited the phosphorylation of STAT3, and, importantly, it also suppressed the expression of the CVS P protein. A similar inhibitory effect was observed in cells transfected with small interfering RNA (siRNA) targeting STAT3 (si-STAT3) (Fig. 2C to F). Silencing of STAT3 significantly inhibited not only CVS P mRNA and protein levels but also virus titers.
FIG 2.
Activated STAT3 promotes the replication of RABV. (A) CVS-treated BHK-21 cells were treated with niclosamide for 24 h, and STAT3 (P/T) and CVS P protein levels were detected by Western blotting. (B) The relative expression levels of the above-described proteins were analyzed by the use of ImageJ software and normalized to the α-tubulin expression level. (C to J) CVS-treated BHK-21 cells were transfected with STAT3-specific siRNA (C to F) or the Flag-STAT3 plasmid (G to J) for 48 h. (C, G) The expression of SOCS3/STAT3 (P/T) and CVS P was detected by Western blotting. (D, H) The relative expression levels of the above-described proteins were analyzed by the use of ImageJ software and normalized to the β-actin expression level. (E, F, I, J) CVS P mRNA levels and viral titers were analyzed by, respectively, qRT-PCR and DFA. *, P < 0.05; **, P < 0.01. Each experiment was independently repeated at least three times. si-NC, negative-control siRNA.
To further assess the impact of STAT3 on RABV replication, a BHK-21 cell line overexpressing STAT3 was established by transfection with the p3Flag-STAT3 plasmid and G418 selection. As shown in Fig. 2G to I, the phosphorylation of STAT3 was significantly enhanced, and, importantly, RABV-P expression was increased at both the mRNA and protein levels. Furthermore, virus titers were enhanced about 10-fold in company with the overactivated STAT3 (Fig. 2J). These results demonstrate that phosphorylated STAT3 indeed contributes to promoting the replication of RABV.
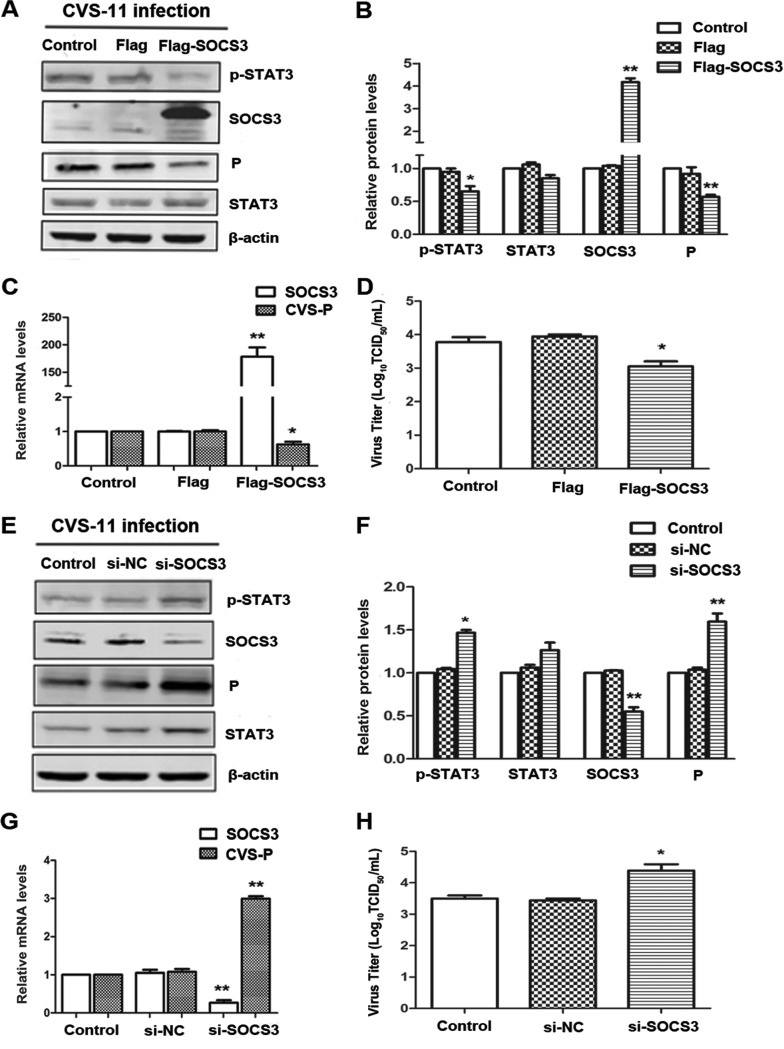
SOCS3 inhibits the replication of RABV.
SOCS proteins are negative regulators of cytokine-mediated JAK-STAT signaling (42). The results presented above imply that decreased SOCS3 might be responsible for the RABV-induced overactivation of STAT3. To examine further the role of SOCS3 in rabies viral replication, we analyzed the latter in BHK-21 cells transfected with the p3Flag-SOCS3 plasmid or an empty vector. Overexpression of SOCS3 was confirmed by Western blotting and quantitative real-time reverse transcription-PCR (qRT-PCR), followed by decreased phosphorylation of STAT3 (Fig. 3A and B). Importantly, overexpression of SOCS3 suppressed CVS P at both the mRNA and protein levels, and virus titers were also markedly decreased (Fig. 3A to D). Additionally, following silencing of SOCS3 by its specific small interfering RNA (siRNA), STAT3 was elevated in both its normal and phosphorylated states. CVS P was increased at both the mRNA and protein levels, and knockdown of SOCS3 enhanced the viral titer of RABV about 10-fold (Fig. 3E to H). These results provide strong evidence for the involvement of SOCS3/STAT3 as a viral promotion mechanism within the context of RABV infection.
FIG 3.
SOCS3 inhibits the replication of RABV. CVS-treated BHK-21 cells were transfected with Flag-SOCS3 plasmid (A to D) or SOCS3-specific siRNA (E to H) for 48 h. (A, E) The expression levels of SOCS3/STAT3 (P/T) and CVS P were detected by Western blotting. (B, F) The relative expression levels of the above-described proteins were analyzed by the use of ImageJ software and normalized to the β-actin expression level. (C, G) The CVS P mRNA levels were analyzed by qRT-PCR. (D, H) The viral titers of CVS-11 were detected by DFA. *, P < 0.05; **, P < 0.01. Each experiment was independently repeated at least three times.
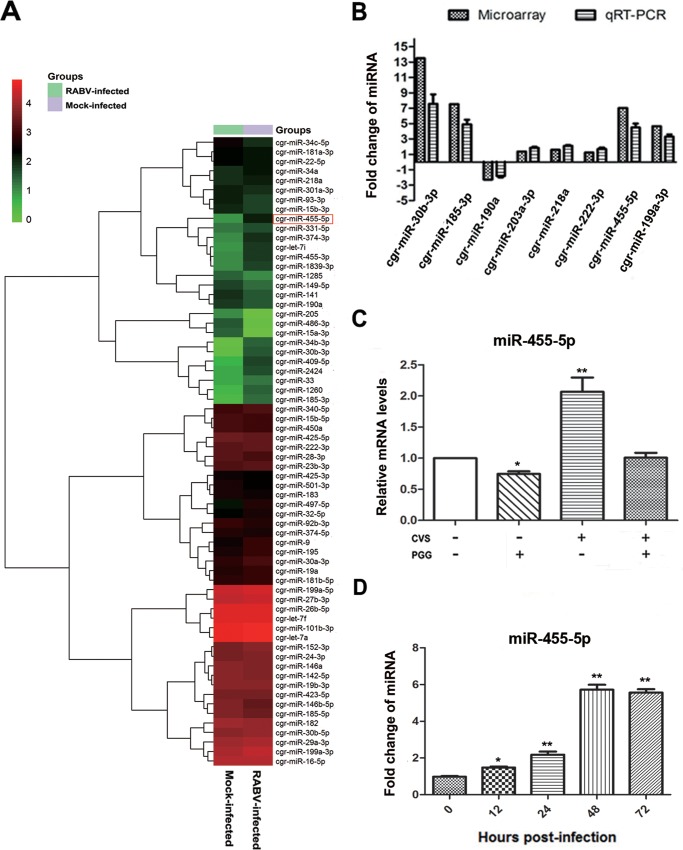
miR-455-5p expression is upregulated during RABV infection.
Increasingly, viruses are being found to employ host cellular miRNAs for promoting their replication and avoiding immunologic attack. It was therefore of interest to determine if host cellular miRNAs are involved in RABV infection, especially in regard to regulating the activity of SOCS3/STAT3. An miRNA microarray was therefore performed to analyze the expression of miRNAs in BHK-21 cells for 48 h in the presence or absence of CVS-11. A two-way hierarchical cluster heat map revealed that 37 miRNAs were upregulated and that 29 were downregulated (Fig. 4A). To determine if the RABV-induced downregulation of SOCS3 could attribute to the dysregulated miRNAs, the prediction programs TargetScan and miRDB (http://www.mirdb.org/) were used to predict candidate miRNAs that target SOCS3. Further quantitative PCR analysis of the 8 candidate miRNAs (miR-30b-3p, miR-185-3p, miR-190a, miR-203a-3p, miR-218a, miR-222-3p, miR-455-5p, miR-199b-3p) provided data almost consistent with the microarray results (Fig. 4B).
FIG 4.
miR-455-5p is upregulated in RABV-infected BHK-21 cells. (A) Two-way hierarchical cluster heat map showing all significantly expressed miRNAs. High-expression miRNAs are shown in red, and low-expression ones are shown in green. (B) The differentially expressed miRNAs which might directly target SOCS3 were detected by qRT-PCR. (C) BHK-21 cells were treated with CVS-11 or PGG, alone or in combination, and the expression level of miR-455-5p was detected by qRT-PCR. (D) The miR-455-5p level at different times postinfection was detected by qRT-PCR. *, P < 0.05; **, P < 0.01. Each experiment was independently repeated at least three times.
To determine if any of the 8 miRNAs could be suppressed by PGG, BHK-21 cells were incubated with CVS-11 (MOI = 0.1) with or without PGG (10 μM) for 48 h. Analysis by qRT-PCR showed that PGG effectively suppressed miR-455-5p in both the presence and the absence of RABV infection (Fig. 4C); however, no similar change was observed with the other 7 candidate miRNAs. Investigation of the expression of miR-455-5p at time points after RABV infection showed that the miR-455-5p level started to increase at about 12 h p.i. and maintained a high level from about 48 h p.i. onward (Fig. 4D). These results, showing that miR-455-5p is upregulated by RABV infection and blocked by PGG, imply that miR-455-5p is responsible for the RABV-induced reduction of SOCS3 and the PGG-induced antiviral effect.
SOCS3 is the direct target of miR-455-5p in BHK-21 cells.
To explore the direct binding between miR-455-5p and SOCS3, computational analysis was performed and identified 3 putative binding sites for miR-455-5p in the SOCS3 3′ UTR, with the target sequences being perfectly complementary to the seed sequence of miR-455-5p (Fig. 5A). Following confirmation that expression of miR-455-5p was functionally regulated by its specific mimic or inhibitor (Fig. 5B), it was confirmed that the miR-455-5p mimic strongly suppressed, whereas the miR-455-5p inhibitor increased, the expression of SOCS3 in BHK-21 cells at both the mRNA and protein levels (Fig. 5C to E).
FIG 5.
miR-455-5p promoted RABV replication through direct targeting of SOCS3. (A) A sketch map of the SOCS3 mRNA structure and the predicted binding sites of miR-455-5p (marked by arrows). cgr, Cricetulus griseus. (B) The efficacies of the miR-455-5p mimic and inhibitor were examined by qRT-PCR. (C, D) Effects of the miR-455-5p mimic and inhibitor on SOCS3 mRNA and protein levels determined by qRT-PCR and Western blotting, respectively. (E) The relative expression levels of the SOCS3 protein were analyzed by the use of ImageJ software and normalized to the α-tubulin expression level. (F) Sequence alignment of miR-455-5p with reverse complementary (rc) miR-455-5p, the SOCS3 3′ UTR, mutant miR-455-5p, and a mutant SOCS3 3′ UTR. (G) The promoter activities of SOCS3 were measured using a dual-luciferase reporter assay after transfection of BHK-21 cells with the miR-455-5p mimic and inhibitor or a negative control (NC). *, P < 0.05; **, P < 0.01. aa, amino acids; nt, nucleotides.
Further confirmation of the specific interaction of miR-455-5p with SOCS3 was obtained following construction of endotoxin-free dual-luciferase reporter vectors containing the seed sequences within the 3′ UTR of SOCS3 (Fig. 5F). Along with their corresponding mutants, these vectors were used to transfect BHK-21 cells for 24 h, either alone or in combination with an miR-455-5p mimic or inhibitor. As shown in Fig. 5G, whereas the miR-455-5p mimic significantly inhibited and the miR-455-5p inhibitor enhanced the Renilla luciferase (Rluc) activity associated with the seed sequence in the SOCS3 3′ UTR, transfection with the mutant vectors had no effect on the activity. These data demonstrate that miR-455-5p specifically inhibits SOCS3 expression by directly targeting its 3′ UTR, thereby contributing to enhancement of the replication of RABV.
PGG induces upregulation of SOCS3 in an miR-455-5p-dependent manner.
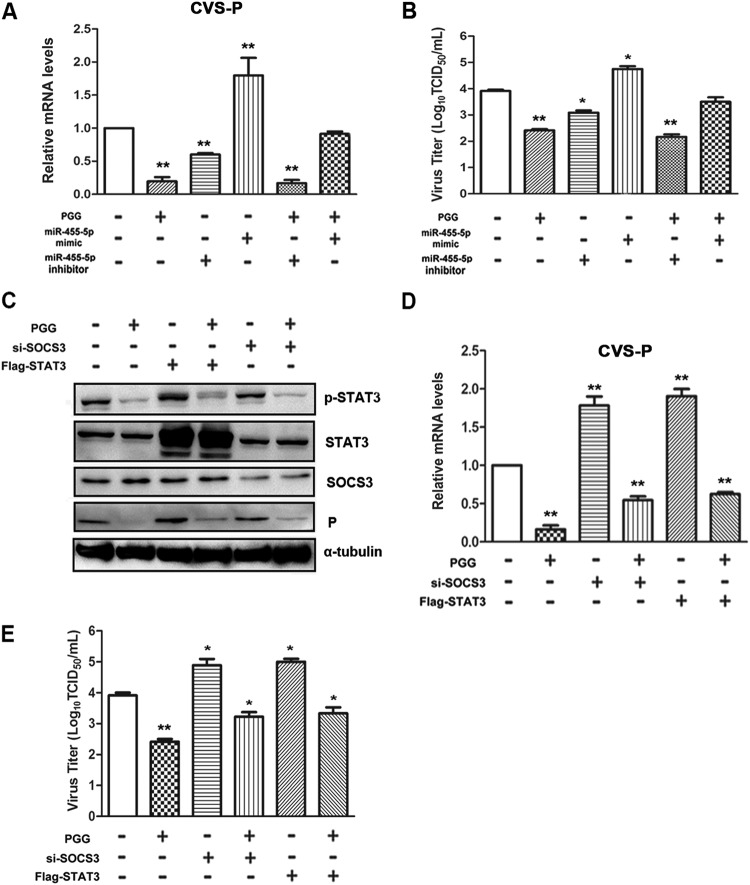
Having established that the PGG-induced upregulation of SOCS3 plays a key role in anti-RABV activity by depressing the activation of STAT3 and that SOCS3 is a direct target of miR-455-5p, it remained to be determined whether upregulation of SOCS3 and viral inhibition could be mediated by miR-455-5p in RABV-infected cells. In BHK-21 cells transfected with the miR-455-5p mimic, CVS P gene copy numbers were increased about 2-fold and viral titers were increased about 10-fold (Fig. 6A and B), while PGG treatment effectively reversed this activity. In contrast, the miR-455-5p inhibitor significantly decreased CVS P gene copy numbers and viral titers by approximately the same amounts (2- and 10-fold, respectively), while, intriguingly, PGG enhanced the inhibitory effect synergistically (Fig. 6A and B).
FIG 6.
PGG exerts an anti-RABV function in an miR-455-5p/SOCS3/STAT3-dependent fashion. BHK-21 cells were given different treatments, including PGG, CVS, or miR-455-5p mimic and inhibitor transfection, alone or in combination. (A) The CVS P mRNA level was detected by qRT-PCR. (B) Viral titers were examined by DFA. (C) BHK-21 cells were given different treatments, including PGG, CVS, or si-SOCS3 or Flag-STAT3 transfection, alone or in combination, and then SOCS3/STAT3 (P/T) protein and CVS P protein levels were detected by Western blotting. (D, E) The CVS P mRNA level and viral titers were detected, respectively, by qRT-PCR and DFA. *, P < 0.05; **, P < 0.01.
To further clarify the key role of the SOCS3/STAT3 pathway in PGG-induced inhibition of RABV replication, BHK-21 cells were transfected with siRNA targeting SOCS3 (si-SOCS3) or Flag-STAT3 before infection with CVS-11 and then treated with PGG for 24 h. As shown in Fig. 6C to E, silencing of SOCS3 or exogenous overexpression of STAT3 significantly attenuated the inhibitory action of PGG on CVS P protein expression and gene copy numbers and CVS-11 titers. These data therefore provide additional evidence that PGG exerts its anti-RABV function at least partially by decreasing the level of miR-455-5p, thereby increasing SOCS3 and decreasing the activity of STAT3.
PGG reversed the level of IL-6 through a SOCS3/STAT3-dependent pathway.
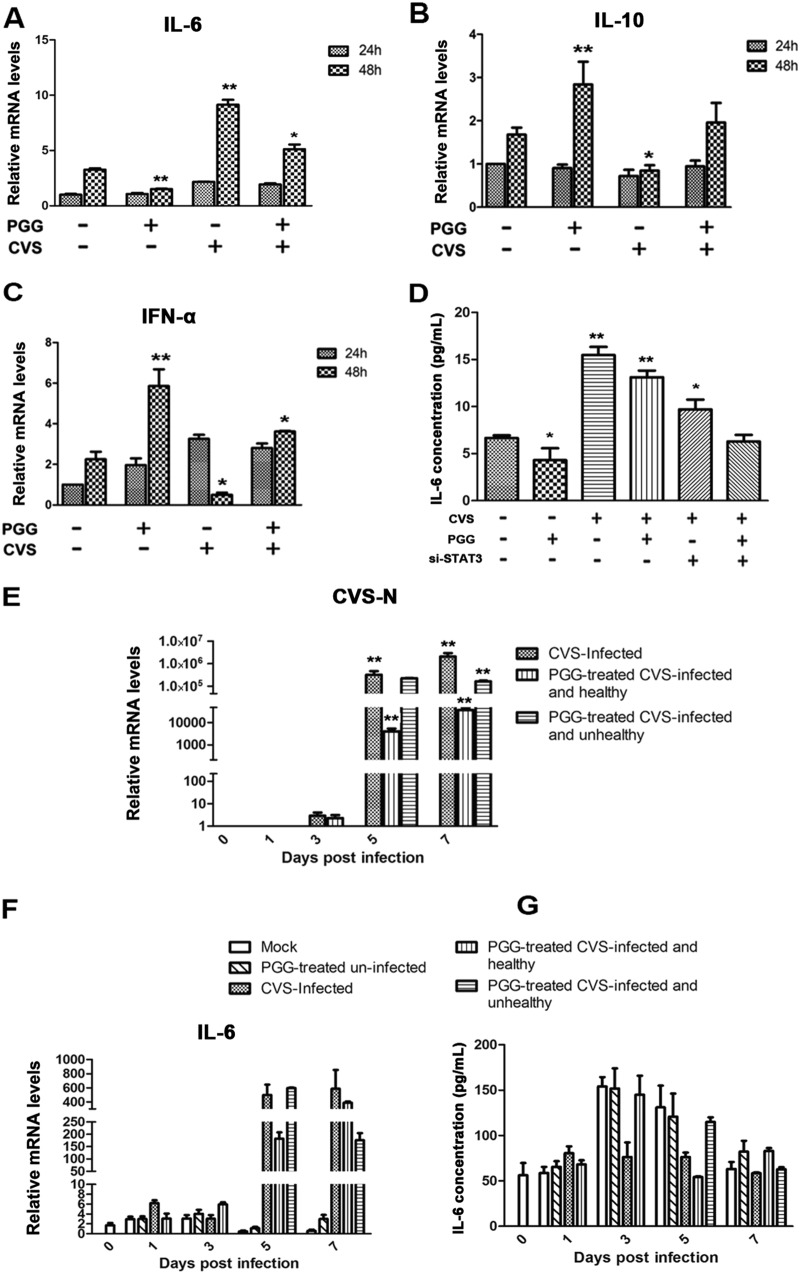
To determine the downstream effectors of SOCS3/STAT3, the activities of which could be reversed by PGG, thereby contributing to antiviral efficacy, several cytokines known to be induced by STAT3 were evaluated. In BHK-21 cells infected with CVS-11 for 48 h, IL-6 levels were increased but were significantly decreased by PGG (Fig. 7A). Moreover, we also detected other candidate cytokines, as demonstrated in Fig. 7B and C, in uninfected cells incubated with PGG, with cytokines IL-10 and IFN-α showing a moderate increase; however, these moderate effects were counteracted by RABV infection.
FIG 7.
PGG decreased RABV-induced IL-6 expression in vitro and in vivo in a STAT3-dependent manner. BHK-21 cells were treated with CVS-11 or PGG alone or in combination. (A to C) IL-6, IL-10, and IFN-α mRNA levels were determined by qRT-PCR and normalized to the GAPDH level. (D) The IL-6 expression level was detected by ELISA after treated cells (treated as described above) received si-STAT3 transfection. (E and F) CVS N and IL-6 mRNA levels were determined in vivo by qRT-PCR. (G) The IL-6 expression level in vivo was detected by ELISA. *, P < 0.05; **, P < 0.01.
To investigate whether the PGG-induced downregulation of IL-6 could be ascribed to the activation of STAT3, STAT3 was silenced by its specific siRNA, and the concentration of IL-6 in cell supernatants was determined by enzyme-linked immunosorbent assay (ELISA). As shown in Fig. 7D, the level of IL-6 was significantly decreased by silencing of STAT3, although to a lesser extent than by PGG. PGG may therefore recruit multiple pathways to exert its antiviral activity, among which STAT3 is clearly an important factor.
To examine the level of IL-6 in vivo, a CVS-infected mouse model was established, and mice were treated with PGG as described in Materials and Methods. In the untreated challenged group, all infected mice (9/9) presented with incoordination by 4 to 6 days p.i. and rapidly developed overt clinical signs and body weight loss. However, in challenged mice receiving PGG treatment, clinical symptoms were observed in only 6/9 mice. Importantly, the level of CVS N in brain tissues was significantly decreased in the 3/9 PGG-treated mice remaining healthy compared with that in PGG-untreated mice or the 6/9 PGG-treated mice with clinical symptoms (Fig. 7E). As shown in Fig. 7F, RABV infection induced a significant increase in IL-6 levels, but this was downregulated by PGG in the brain tissues of infected mice with or without clinical symptoms, while neither viral infection nor PGG had any significant effect on IL-6 levels in the peripheral blood of the mice (Fig. 7G). Collectively, these results indicate that PGG downregulates IL-6 via an SOCS3/STAT3-dependent pathway, which, in CVS-11-infected mice, might contribute to relieve the RABV-induced inflammatory response.
DISCUSSION
Our previous study reported that PGG exhibits strong anti-RABV activity and multiple effects on the RABV life cycle (12). However, more studies were required to explore the underlying antiviral mechanisms of PGG, which would better promote its clinical application. The present work clearly implicates STAT3 as a pivotal player in the inhibitory activity.
STAT3 has two splice forms, STAT3α and STAT3β, both of which are activated by upstream JAKs, and the JAK-STAT3 pathway is under tight regulation by the induction of the SOCS3 protein. SOCS3 silences the pathway by acting as a pseudosubstrate that blocks JAK activity, binding to the receptor to prevent STAT3 interaction, and targeting proteins for proteasomal degradation (43). STAT3 plays diverse roles in different virus infections. HCV, EBV, and HCMV stimulate the activity of STAT3, thereby facilitating viral replication, but, conversely, some viruses, such as IAV, measles virus, and hepatitis E virus (HEV), suppress STAT3 to promote viral replication (16). Here, we present evidence that RABV promotes the activation of JAK2/STAT3. Then, by using SOCS3 siRNA and exogenous expression plasmids, we confirmed that the RABV-induced phosphorylation of STAT3 is negatively regulated by SOCS3. SOCS3 has emerged as arguably the most important SOCS family member, and, usually, its low expression is associated with autoimmunity and oncogenesis (44). However, in RABV-infected cells, low expression of SOCS3 appears to promote viral replication.
Multiple underlying mechanisms by which STAT3 contributes to viral replication in both SOCS3-dependent and -independent manners have been disclosed. HIV infection interferes with SOCS1/3 and activates STAT3 to drive a sustained immune activation, which disrupts the lymphatic system and favors HIV replication since HIV preferentially infects activated lymphoid cells (45). HSV-1 and HCV induce overexpression of SOCS3, which confers efficient viral replication via STAT3 by efficiently inhibiting IFN-α/γ signaling (46, 47). EBV infection creates a positive-feedback loop in HeLa cells, in which the viral protein LMP1 induces IL-6 expression and STAT3 phosphorylation, which in turn enhances LMP1 expression. In addition, mumps viral protein V induces STAT3 degradation by promoting STAT3-directed ubiquitin E3 ligase complexes (25), and the HEV ORF3 protein blocks the nuclear translocation of p-STAT3 (48). In RABV infection, viral protein P has been reported to be associated with p-STAT3 in the cytoplasm and impedes the latter's nuclear translocation. However, the effect of activation of STAT3 on RABV replication remained unclarified. Herein, by silencing or overexpressing SOCS3 and STAT3, we have confirmed that activated STAT3 strongly promotes RABV replication via a SOCS3-dependent pathway, while, conversely, inhibition of STAT3 or overexpression of SOCS3 effectively suppresses the replication of RABV.
Increasingly, dysregulated miRNAs are being found in virus-infected cells, which results in interference with several immune-related signaling pathways, and exert their functions in many pathophysiological processes, such as the JAK-STAT signaling pathway (SOCS3, STAT3, IFN), cytokine-cytokine receptor interaction (IL-6, IFN-γ), and T-cell receptor signaling pathways (IL-10) (34). miR-135a-5p is a negative regulator of STAT3 phosphatase PTPRD and is upregulated in HCV-infected hepatocytes, leading to enhanced STAT3 transcription (49). Some miRNAs, including miR-124, miR-20, and miR-29a, have been reported to be altered in RABV-infected mouse brain and neurons and have been predicted to contribute to immune modulation (34, 35, 50). These observations led to our hypothesis that the RABV-induced dysregulation of SOCS3/STAT3 was mediated by miRNAs. As demonstrated here, RABV induced the upregulation of multiple miRNAs in host cells, including miR-30b-3p, miR-203a-3p, miR-455-5p, miR-222-3p, and miR-185-3p, which were the candidate miRNAs targeting SOCS3, as determined by bioinformatics analysis. Then, we confirmed that miR-455-5p could directly target 3 sequences in the 3′ UTR of SOCS3 mRNA, thereby showing that the RABV-induced downregulation of SOCS3 in BHK-21 cells could be attributed, at least in part, to the upregulated expression of miR-455-5p. Importantly, the downregulation of miR-455-5p was found to significantly inhibit the replication of RABV, similar to the inhibition induced by the upregulation of SOCS3, which provides strong evidence that miR-455-5p plays a key role in the RABV-induced downregulation of SOCS3.
PGG, as a hydrolyzable phenolic compound, has been found to exert an antiviral effect on several viruses by diverse mechanisms, such as blocking the cell entry of hepatitis C virus (51) and inhibiting the replication of varicella-zoster virus (VZV) by suppression of JNK activation and the viral immediate early 62 protein (52). It has also been reported to inhibit herpes simplex virus 1 (HSV-1) infection by blocking nuclear transport and the process of nucleocapsid egress via inhibition of dynein and disruption of the cellular localization of UL31/UL34 (53). Additionally, some studies have revealed that PGG exerts anti-HSV activity by inducing mTOR-dependent autophagy (54) or by downregulating cofilin 1 (4). PGG also inhibits the productive replication of influenza A virus not only by inhibiting viral infection but also by interfering with viral budding and release (55).
Combined with our newfound anti-RABV drug PGG, we attempted to explore whether the SOCS3/STAT3 pathway was involved in PGG’s anti-RABV activity. Fortunately, our results indicated that PGG potently reverses the RABV-induced activation of STAT3, thereby inhibiting viral replication. Consistent with our finding, some studies have reported the inhibitory effect of PGG on STAT3 in breast or prostate cancers, which is closely associated with its antitumor activity (7, 56, 57). Moreover, PGG effectively upregulates SOCS3 through disruption of the RABV-induced increase of miR-455-5p, and an miR-455-5p mimic led to increased viral replication and reversed the inhibitory effect of PGG on RABV. These data provide sufficient evidence that PGG exerts its anti-RABV function at least partially by decreasing the level of miR-455-5p, thereby increasing SOCS3 and decreasing the activity of STAT3.
Noting that activated STAT3 plays a crucial role in selectively inducing and maintaining a proinflammatory microenvironment (16, 58) by eliciting the production of several cytokines and chemokines, such as IL-6, IL-8, and chemokine C-C motif ligand 2 (CCL2) (59–61), we propose that the antiviral function of PGG may be associated with the attenuation of inflammation. Based on our observation that activation of STAT3 and IL-6 was maintained following RABV infection, we further performed experiments to confirm the dependency between IL-6 and activated STAT3. As shown in Fig. 7D, knockdown of STAT3 potently suppressed the level of IL-6, and the efficacy was even much better than that of PGG, which positively supports our hypothesis that the production of IL-6 can be attributed at least partially to the sustained activation of STAT3 in RABV-infected BHK-21 cells. Certainly, noting that IL-6 could signal through STAT3 as part of an acute-phase response, we agree that the IL-6/STAT3 proinflammatory signal might coexist; however, undoubtedly, the miR-455-5p-mediated SOCS3/STAT3 pathway indeed plays vital roles in the production of IL-6 in RABV-infected cells. Additionally, noting previous reports that the RABV P protein blocks the nuclear translocation of p-STAT3, we examined the influence of RABV on p-STAT3 and showed that the effect is distinct from that induced by the CVS P protein alone, with CVS-11 inducing the phosphorylation of STAT3 and promoting its nuclear translocation. Altogether, we clarified that the RABV-induced activation of STAT3 promotes the production of IL-6, which is independent of the stimulation of IL-6 in BHK-21 cells.
In summary, our studies have disclosed the underlying cellular mechanisms which facilitate the replication of RABV and which can be disrupted by PGG. RABV infection promotes the activation of STAT3 and the production of IL-6 via elevation of the expression of miR-455-5p and the suppression of SOCS3, while PGG effectively reverses the expression of miR-455-5p and the following pathways, thereby exerting its anti-RABV function. These findings indicate that STAT3 might be used as a primary anti-RABV target and suggest that PGG holds promise for development as a therapeutic agent for RABV infection.
MATERIALS AND METHODS
Reagents and plasmids.
1,2,3,4,6-Pentagalloylglucose (PGG; >98% purity) was purchased from MedChemExpress (NJ, USA). It was stored as a 10 mM solution in dimethyl sulfoxide (DMSO) before dilution to working concentrations.
The chemically synthesized miR-455-5p mimic and inhibitor were purchased from Ribobio (Guangzhou, China). SOCS3- and STAT3-specific small interfering RNAs (siRNA) were purchased from GenePharm (Shanghai, China). The p3Flag-CMV-10 plasmids were constructed in our laboratory (Institute of Military Veterinary Medicine). The p3Flag-SOCS3 and p3Flag-STAT3 plasmids were generated by amplifying the SOCS3 (GenBank accession number XM_021227047.1) and STAT3 (GenBank accession number XM_021227207.1) genes and then cloning them into the p3Flag-CMV-10 vector. The plasmids were confirmed by restriction enzyme digestion and sequencing.
Cell culture, transfection, and viruses.
Baby hamster kidney (BHK-21) cells were propagated in minimum essential medium (MEM; Corning, USA) supplemented with 5% fetal bovine serum (Corning, USA), 100 U/ml penicillin G, and 100 μg/ml streptomycin and maintained at 37°C in 5% CO2.
For transfection experiments, BHK-21 cells were cultured in 12-well plates to 70 to 80% confluence and then transfected with SOCS3/STAT3-specific siRNA (20 pM), the p3Flag-SOCS3/STAT3 plasmids (1.2 μg), or the miR-455-5p mimic and inhibitor (60 pM) alone or in combination using the Lipofectamine 2000 reagent (Invitrogen, USA).
The challenge virus standard 11 (CVS-11) strain of RABV was propagated in BHK-21 cells and stored at −80°C. Virus titers were determined as the 50% tissue culture infective doses (TCID50) per milliliter by direct immunofluorescence assay (DFA).
miRNA microarray analysis and miRNA target prediction.
RNAs were extracted from BHK-21 cells infected with CVS-11 at 48 h postinfection (p.i.) using TRIzol reagent (Invitrogen, USA). A miRNA microarray assay was performed by BioClouds (Shanghai, China). The miRNA array data can be accessed on the NCBI’s Gene Expression Omnibus (GEO) database (GSE134156). The miRNA targets in host cells were predicted by the use of the programs TargetScan (version 3.1; Whitehead Institute for Biomedical Research [http://www.targetscan.org/mamm_31/]) and miRDB (http://www.mirdb.org/).
qRT-PCR.
RNA was isolated using TRIzol reagents, and cDNA was synthesized using Moloney murine leukemia virus reverse transcriptase (TaKaRa, Japan). The genome copies were quantified by quantitative real-time reverse transcription-PCR (qRT-PCR) using SYBR PrimeScript Ex Taq II polymerase (TaKaRa, Japan) and an Agilent Stratagene Mx3000P Q-PCR system. Fold variations between RNA samples were calculated by the 2−ΔΔCT threshold cycle (CT) method after normalization to the amount of GAPDH mRNA or U6 RNA. The primers were synthesized according to the following sequences: for miR-218a, UUGUG CUUGA UCUAA CCAUG UG; for miR-222-3p, AGCUA CAUCU GGCUA CU-GGG UCUCU; for miR-190a, UGAUA UGUUU GAUAU UAGGU UG; for miR-203a-3p, UGAAA UGUUU AGGAC CACUA GU; for miR-455-5p, UGUGC CUUUG GACUA CAUCG UG; for miR-30b-3p, CUGGG AGGUG GAUGU UUACU UC; for miR-199a-3p, ACAGU AGUCU GCACA UUGGU U; and for miR-185-3p, CAGGG GCUGG CUUUC CUCUG GA. The primer sequences for genome amplification were as follows: for RABV P, 5′-CCTCC TTTCA AACCA TCCCA-3′ for the forward primer and 5′-ACTTG CCTTC TCCCA CCC-TA-3′ for the reverse primer; for IL-6, 5′-GACAC TACTC CCAAC AGACC-3′ for the forward primer and 5′-CTCAA TTCGT AGATT TCCCT-3′ for the reverse primer; and for GAPDH, 5′-GTTCA AAGGC ACAGT CAAGG-3′ for the forward primer and 5′-ACGCC AGTAG ACTCC ACAAC-3′ for the reverse primer. PCR amplification conditions were as follows: denaturing at 95°C for 30 s, followed by 40 cycles of 95°C for 5 s and 60°C for 1 min. Melting curves were performed to verify the specificity of the products. All PCR experiments were performed in triplicate.
Western blotting assay.
BHK-21 cells were thoroughly lysed in ice-cold Pierce radioimmunoprecipitation assay buffer containing Halt protease inhibitor cocktail (Thermo, USA). Nuclear and cytoplasmic proteins were prepared using a nuclear and cytoplasmic protein extraction kit (Beyotime Biotech Inc., China) according to the manufacturer's instructions. Forty-microgram aliquots of protein were subjected to SDS-PAGE before being transferred to polyvinylidene difluoride membranes. After blocking with skimmed milk for 1 h, the membranes were subsequently incubated with primary antibodies, including antibodies to CVS P (prepared by our laboratory [Institute of Military Veterinary Medicine]), SOCS3 (Abcam, USA), STAT3 (P/T) (Cell Signaling Technology, USA), and α-tubulin (Beyotime, China), and Alexa Fluor 680-conjugated secondary antibodies. Specific protein bands were visualized in an Odyssey infrared imaging system (LI-COR, USA) and quantified by ImageJ software (version 1.36b; NIH, MD, USA).
Vector construction and luciferase reporter assay.
The dual-luciferase vectors psiCHECK-SOCS3-3′UTR-WT and psiCHECK-rcmiR-455-5p-WT were constructed by amplifying the seed sequence in the 3′ UTR of SOCS3 or the reverse complementary sequence of miR-455-5p and inserting them into the psiCHECK-2 vector. For mutant vectors, 3- to 4-bp mutations were introduced into the seed sequence. All plasmids were confirmed by DNA sequencing. For reporter assays, BHK-21 cells were seeded in 12-well plates and transfected with recombinant vector alone or with the vector plus the miR-455-5p mimic and inhibitor. Firefly and Renilla luciferase activities in cell lysates were measured 48 h later using a Dual-Glo reporter assay system (Promega, USA).
Mouse experimental protocol.
Kunming mice (female; weight, 18 to 20 g) were purchased from the Changchun Institute of Biological Products. Sample collection and mouse experiments were approved by the Administrative Committee on Animal Welfare of the Institute of Military Veterinary, China (Laboratory Animal Care and Use Committee Authorization, permit number JSY-DW-2016-02). Mice were randomly divided into 4 groups (n = 51), with mouse infection and administration being conducted as described previously (12), Briefly, the mice were injected intramuscularly (i.m.) in both hind legs with a lethal dose of CVS-11 (10 50% lethal doses, 106.5 TCID50) and 1 h later with intraperitoneal (i.p.) injections of saline or various concentrations of PGG daily for three consecutive days. Serum and brain tissue samples were taken on days 0, 1, 3, 5, and 7 p.i. The production of cytokines (IL-6) in the culture supernatant and serum from the mice was specifically assayed by an ELISA for mouse IL-6 (Cusabio, China) according to the manufacturer’s instructions (62, 63).
Statistical analysis.
Data are expressed as the mean ± standard error of the mean, and all statistical analyses were performed using GraphPad Prism software (version 5.0; La Jolla, CA, USA). For two data sets, Student's t test was used. P values of <0.05 were considered statistically significant.
Data availability.
The miRNA array data are available on the NCBI’s Gene Expression Omnibus (GEO) database under accession number GSE134156.
ACKNOWLEDGMENTS
This work was supported by grants from the National Key Research and Development Program of China (grant 2016YFD0501002 to Y.L. and grant 2016YFD0500401 to Y.F.) and a China Postdoctoral Science Foundation grant (grant 2018T110253).
Zhongzhong Tu, Mengxian Xu, Jian Zhang, Ye Feng, and Zhuo Hao conducted the experiments; Zhongzhong Tu, Yan Liu, and Changchun Tu designed the experiments and wrote the paper.
We declare that we have no competing interests.
REFERENCES
- 1.Hemachudha T, Laothamatas J, Rupprecht CE. 2002. Human rabies: a disease of complex neuropathogenetic mechanisms and diagnostic challenges. Lancet Neurol 1:101–109. doi: 10.1016/S1474-4422(02)00041-8. [DOI] [PubMed] [Google Scholar]
- 2.Singh R, Singh KP, Cherian S, Saminathan M, Kapoor S, Manjunatha Reddy GB, Panda S, Dhama K. 2017. Rabies—epidemiology, pathogenesis, public health concerns and advances in diagnosis and control: a comprehensive review. Vet Q 37:212–251. doi: 10.1080/01652176.2017.1343516. [DOI] [PubMed] [Google Scholar]
- 3.Kim YH, Yang X, Yamashita S, Kumazoe M, Huang Y, Nakahara K, Won YS, Murata M, Lin I-C, Tachibana H. 2015. 1,2,3,4,6-Penta-O-galloyl-β-d-glucopyranose increases a population of T regulatory cells and inhibits IgE production in ovalbumin-sensitized mice. Int Immunopharmacol 26:30–36. doi: 10.1016/j.intimp.2015.02.025. [DOI] [PubMed] [Google Scholar]
- 4.Pei Y, Xiang YF, Chen JN, Lu CH, Hao J, Du Q, Lai CC, Qu C, Li S, Ju HQ, Ren Z, Liu QY, Xiong S, Qian CW, Zeng FL, Zhang PZ, Yang CR, Zhang YJ, Xu J, Kitazato K, Wang YF. 2011. Pentagalloylglucose downregulates cofilin1 and inhibits HSV-1 infection. Antiviral Res 89:98–108. doi: 10.1016/j.antiviral.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 5.Kang DG, Moon MK, Choi DH, Lee JK, Kwon TO, Lee HS. 2005. Vasodilatory and anti-inflammatory effects of the 1,2,3,4,6-penta-O-galloyl-beta-d-glucose (PGG) via a nitric oxide-cGMP pathway. Eur J Pharmacol 524:111–119. doi: 10.1016/j.ejphar.2005.08.061. [DOI] [PubMed] [Google Scholar]
- 6.Mizushina Y, Zhang J, Pugliese A, Kim SH, Lu J. 2010. Anti-cancer gallotannin penta-O-galloyl-beta-d-glucose is a nanomolar inhibitor of select mammalian DNA polymerases. Biochem Pharmacol 80:1125–1132. doi: 10.1016/j.bcp.2010.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang J, Li L, Kim SH, Hagerman AE, Lu J. 2009. Anti-cancer, anti-diabetic and other pharmacologic and biological activities of penta-galloyl-glucose. Pharm Res 26:2066–2080. doi: 10.1007/s11095-009-9932-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abdelwahed A, Bouhlel I, Skandrani I, Valenti K, Kadri M, Guiraud P, Steiman R, Mariotte AM, Ghedira K, Laporte F, Dijoux-Franca MG, Chekir-Ghedira L. 2007. Study of antimutagenic and antioxidant activities of gallic acid and 1,2,3,4,6-pentagalloylglucose from Pistacia lentiscus. Confirmation by microarray expression profiling. Chem Biol Interact 165:1–13. doi: 10.1016/j.cbi.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 9.Lee SJ, Lee HK, Jung MK, Mar W. 2006. In vitro antiviral activity of 1,2,3,4,6-penta-O-galloyl-beta-d-glucose against hepatitis B virus. Biol Pharm Bull 29:2131–2134. doi: 10.1248/bpb.29.2131. [DOI] [PubMed] [Google Scholar]
- 10.Duan D, Li Z, Luo H, Zhang W, Chen L, Xu X. 2004. Antiviral compounds from traditional Chinese medicines Galla Chinese as inhibitors of HCV NS3 protease. Bioorg Med Chem Lett 14:6041–6044. doi: 10.1016/j.bmcl.2004.09.067. [DOI] [PubMed] [Google Scholar]
- 11.Liu G, Zhong M, Guo C, Komatsu M, Xu J, Wang Y, Kitazato K. 2016. Autophagy is involved in regulating influenza A virus RNA and protein synthesis associated with both modulation of Hsp90 induction and mTOR/p70S6K signaling pathway. Int J Biochem Cell Biol 72:100–108. doi: 10.1016/j.biocel.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 12.Tu Z, Gong W, Zhang Y, Feng Y, Liu Y, Tu C. 2018. Inhibition of rabies virus by 1,2,3,4,6-penta-O-galloyl-beta-d-glucose involves mTOR-dependent autophagy. Viruses 10:E201. doi: 10.3390/v10040201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Larrea E, Aldabe R, Molano E, Fernandez-Rodriguez CM, Ametzazurra A, Civeira MP, Prieto J. 2006. Altered expression and activation of signal transducers and activators of transcription (STATs) in hepatitis C virus infection: in vivo and in vitro studies. Gut 55:1188–1196. doi: 10.1136/gut.2005.070060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao LJ, He SF, Wang W, Ren H, Qi ZT. 2016. Interferon alpha antagonizes STAT3 and SOCS3 signaling triggered by hepatitis C virus. Cytokine 80:48–55. doi: 10.1016/j.cyto.2015.08.264. [DOI] [PubMed] [Google Scholar]
- 15.Nicholson SE, De Souza D, Fabri LJ, Corbin J, Willson TA, Zhang JG, Silva A, Asimakis M, Farley A, Nash AD, Metcalf D, Hilton DJ, Nicola NA, Baca M. 2000. Suppressor of cytokine signaling-3 preferentially binds to the SHP-2-binding site on the shared cytokine receptor subunit gp130. Proc Natl Acad Sci U S A 97:6493–6498. doi: 10.1073/pnas.100135197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roca Suarez AA, Van Renne N, Baumert TF, Lupberger J. 2018. Viral manipulation of STAT3: evade, exploit, and injure. PLoS Pathog 14:e1006839. doi: 10.1371/journal.ppat.1006839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yuan K, Lei Y, Chen HN, Chen Y, Zhang T, Li K, Xie N, Wang K, Feng X, Pu Q, Yang W, Wu M, Xiang R, Nice EC, Wei Y, Huang C. 2016. HBV-induced ROS accumulation promotes hepatocarcinogenesis through Snail-mediated epigenetic silencing of SOCS3. Cell Death Differ 23:616–627. doi: 10.1038/cdd.2015.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee YH, Yun Y. 1998. HBx protein of hepatitis B virus activates Jak1-STAT signaling. J Biol Chem 273:25510–25515. doi: 10.1074/jbc.273.39.25510. [DOI] [PubMed] [Google Scholar]
- 19.McCartney EM, Helbig KJ, Narayana SK, Eyre NS, Aloia AL, Beard MR. 2013. Signal transducer and activator of transcription 3 is a proviral host factor for hepatitis C virus. Hepatology 58:1558–1568. doi: 10.1002/hep.26496. [DOI] [PubMed] [Google Scholar]
- 20.Lepiller Q, Abbas W, Kumar A, Tripathy MK, Herbein G. 2013. HCMV activates the IL-6-JAK-STAT3 axis in HepG2 cells and primary human hepatocytes. PLoS One 8:e59591. doi: 10.1371/journal.pone.0059591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kung CP, Meckes DG Jr, Raab-Traub N. 2011. Epstein-Barr virus LMP1 activates EGFR, STAT3, and ERK through effects on PKCdelta. J Virol 85:4399–4408. doi: 10.1128/JVI.01703-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hui KP, Li HS, Cheung MC, Chan RW, Yuen KM, Mok CK, Nicholls JM, Peiris JS, Chan MC. 2016. Highly pathogenic avian influenza H5N1 virus delays apoptotic responses via activation of STAT3. Sci Rep 6:28593. doi: 10.1038/srep28593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li L, Chong HC, Ng SY, Kwok KW, Teo Z, Tan EH, Choo CC, Seet JE, Choi HW, Buist ML, Chow VT, Tan NS. 2015. Angiopoietin-like 4 increases pulmonary tissue leakiness and damage during influenza pneumonia. Cell Rep 10:654–663. doi: 10.1016/j.celrep.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palosaari H, Parisien JP, Rodriguez JJ, Ulane CM, Horvath CM. 2003. STAT protein interference and suppression of cytokine signal transduction by measles virus V protein. J Virol 77:7635–7644. doi: 10.1128/JVI.77.13.7635-7644.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ulane CM, Rodriguez JJ, Parisien JP, Horvath CM. 2003. STAT3 ubiquitylation and degradation by mumps virus suppress cytokine and oncogene signaling. J Virol 77:6385–6393. doi: 10.1128/jvi.77.11.6385-6393.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mitzel DN, Jaramillo RJ, Stout-Delgado H, Senft AP, Harrod KS. 2014. Human metapneumovirus inhibits the IL-6-induced JAK/STAT3 signalling cascade in airway epithelium. J Gen Virol 95:26–37. doi: 10.1099/vir.0.055632-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang ZW, Sarmento L, Wang Y, Li XQ, Dhingra V, Tseggai T, Jiang B, Fu ZF. 2005. Attenuated rabies virus activates, while pathogenic rabies virus evades, the host innate immune responses in the central nervous system. J Virol 79:12554–12565. doi: 10.1128/JVI.79.19.12554-12565.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lieu KG, Brice A, Wiltzer L, Hirst B, Jans DA, Blondel D, Moseley GW. 2013. The rabies virus interferon antagonist P protein interacts with activated STAT3 and inhibits Gp130 receptor signaling. J Virol 87:8261–8265. doi: 10.1128/JVI.00989-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang Q, Hou C, Huang D, Zhuang C, Jiang W, Geng Z, Wang X, Hu L. 2017. miR-455-5p functions as a potential oncogene by targeting galectin-9 in colon cancer. Oncol Lett 13:1958–1964. doi: 10.3892/ol.2017.5608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao D, Zhai A, Qian J, Li A, Li Y, Song W, Zhao H, Yu X, Wu J, Zhang Q, Kao W, Wei L, Zhang F, Zhong Z. 2015. Down-regulation of suppressor of cytokine signaling 3 by miR-122 enhances interferon-mediated suppression of hepatitis B virus. Antiviral Res 118:20–28. doi: 10.1016/j.antiviral.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 31.Liu WH, Yeh SH, Chen PJ. 2011. Role of microRNAs in hepatitis B virus replication and pathogenesis. Biochim Biophys Acta 1809:678–685. doi: 10.1016/j.bbagrm.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 32.Kumar A, Kumar A, Ingle H, Kumar S, Mishra R, Verma MK, Biswas D, Kumar NS, Mishra A, Raut AA, Takaoka A, Kumar H. 2018. MicroRNA hsa-miR-324-5p suppresses H5N1 virus replication by targeting the viral PB1 and host CUEDC2. J Virol 92:e01057-18. doi: 10.1128/JVI.01057-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wood CD, Carvell T, Gunnell A, Ojeniyi OO, Osborne C, West MJ. 2018. Enhancer control of miR-155 expression in Epstein-Barr virus infected B cells. J Virol 92:e00716-18. doi: 10.1128/JVI.00716-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao P, Zhao L, Zhang T, Wang H, Qin C, Yang S, Xia X. 2012. Changes in microRNA expression induced by rabies virus infection in mouse brains. Microb Pathog 52:47–54. doi: 10.1016/j.micpath.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 35.Shi N, Zhang XY, Dong CY, Hou JL, Zhang ML, Guan ZH, Li ZY, Duan M. 2014. Alterations in microRNA expression profile in rabies virus-infected mouse neurons. Acta Virol 58:120–127. doi: 10.4149/av_2014_02_120. [DOI] [PubMed] [Google Scholar]
- 36.Roshan R, Shridhar S, Sarangdhar MA, Banik A, Chawla M, Garg M, Singh VP, Pillai B. 2014. Brain-specific knockdown of miR-29 results in neuronal cell death and ataxia in mice. RNA 20:1287–1297. doi: 10.1261/rna.044008.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang J, Zhang X, Chen X, Wang L, Yang G. 2017. Exosome mediated delivery of miR-124 promotes neurogenesis after ischemia. Mol Ther Nucleic Acids 7:278–287. doi: 10.1016/j.omtn.2017.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Su F, Ren F, Rong Y, Wang Y, Geng Y, Wang Y, Feng M, Ju Y, Li Y, Zhao ZJ, Meng K, Chang Z. 2012. Protein tyrosine phosphatase Meg2 dephosphorylates signal transducer and activator of transcription 3 and suppresses tumor growth in breast cancer. Breast Cancer Res 14:R38. doi: 10.1186/bcr3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu Y, Sun SY, Owonikoko TK, Sica GL, Curran WJ, Khuri FR, Deng X. 2012. Rapamycin induces Bad phosphorylation in association with its resistance to human lung cancer cells. Mol Cancer Ther 11:45–56. doi: 10.1158/1535-7163.MCT-11-0578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moir RD, Montag-Lowy M, Goldman RD. 1994. Dynamic properties of nuclear lamins: lamin B is associated with sites of DNA replication. J Cell Biol 125:1201–1212. doi: 10.1083/jcb.125.6.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Timmins JM, Ozcan L, Seimon TA, Li G, Malagelada C, Backs J, Backs T, Bassel-Duby R, Olson EN, Anderson ME, Tabas I. 2009. Calcium/calmodulin-dependent protein kinase II links ER stress with Fas and mitochondrial apoptosis pathways. J Clin Invest 119:2925–2941. doi: 10.1172/JCI38857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Inagaki-Ohara K, Kondo T, Ito M, Yoshimura A. 2013. SOCS, inflammation, and cancer. JAKSTAT 2:e24053. doi: 10.4161/jkst.24053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Croker BA, Kiu H, Nicholson SE. 2008. SOCS regulation of the JAK/STAT signalling pathway. Semin Cell Dev Biol 19:414–422. doi: 10.1016/j.semcdb.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mahony R, Ahmed S, Diskin C, Stevenson NJ. 2016. SOCS3 revisited: a broad regulator of disease, now ready for therapeutic use? Cell Mol Life Sci 73:3323–3336. doi: 10.1007/s00018-016-2234-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miller RC, Schlaepfer E, Baenziger S, Crameri R, Zeller S, Byland R, Audige A, Nadal D, Speck RF. 2011. HIV interferes with SOCS-1 and -3 expression levels driving immune activation. Eur J Immunol 41:1058–1069. doi: 10.1002/eji.201041198. [DOI] [PubMed] [Google Scholar]
- 46.Yokota S, Yokosawa N, Okabayashi T, Suzutani T, Fujii N. 2005. Induction of suppressor of cytokine signaling-3 by herpes simplex virus type 1 confers efficient viral replication. Virology 338:173–181. doi: 10.1016/j.virol.2005.04.028. [DOI] [PubMed] [Google Scholar]
- 47.Bode JG, Ludwig S, Ehrhardt C, Albrecht U, Erhardt A, Schaper F, Heinrich PC, Haussinger D. 2003. IFN-alpha antagonistic activity of HCV core protein involves induction of suppressor of cytokine signaling-3. FASEB J 17:488–490. doi: 10.1096/fj.02-0664fje. [DOI] [PubMed] [Google Scholar]
- 48.Chandra V, Kar-Roy A, Kumari S, Mayor S, Jameel S. 2008. The hepatitis E virus ORF3 protein modulates epidermal growth factor receptor trafficking, STAT3 translocation, and the acute-phase response. J Virol 82:7100–7110. doi: 10.1128/JVI.00403-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Van Renne N, Roca Suarez AA, Duong FHT, Gondeau C, Calabrese D, Fontaine N, Ababsa A, Bandiera S, Croonenborghs T, Pochet N, De Blasi V, Pessaux P, Piardi T, Sommacale D, Ono A, Chayama K, Fujita M, Nakagawa H, Hoshida Y, Zeisel MB, Heim MH, Baumert TF, Lupberger J. 2018. miR-135a-5p-mediated downregulation of protein tyrosine phosphatase receptor delta is a candidate driver of HCV-associated hepatocarcinogenesis. Gut 67:953–962. doi: 10.1136/gutjnl-2016-312270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao P, Zhao L, Zhang K, Feng H, Wang H, Wang T, Xu T, Feng N, Wang C, Gao Y, Huang G, Qin C, Yang S, Xia X. 2012. Infection with street strain rabies virus induces modulation of the microRNA profile of the mouse brain. Virol J 9:159. doi: 10.1186/1743-422X-9-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Behrendt P, Perin P, Menzel N, Banda D, Pfaender S, Alves MP, Thiel V, Meuleman P, Colpitts CC, Schang LM, Vondran FWR, Anggakusuma, Manns MP, Steinmann E, Pietschmann T. 2017. Pentagalloylglucose, a highly bioavailable polyphenolic compound present in Cortex moutan, efficiently blocks hepatitis C virus entry. Antiviral Res 147:19–28. doi: 10.1016/j.antiviral.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 52.Bae S, Kim SY, Do MH, Lee CH, Song YJ. 2017. 1,2,3,4,6-Penta-O-galloyl-ss-d-glucose, a bioactive compound in Elaeocarpus sylvestris extract, inhibits varicella-zoster virus replication. Antiviral Res 144:266–272. doi: 10.1016/j.antiviral.2017.06.018. [DOI] [PubMed] [Google Scholar]
- 53.Jin F, Ma K, Chen M, Zou M, Wu Y, Li F, Wang Y. 2016. Pentagalloylglucose blocks the nuclear transport and the process of nucleocapsid egress to inhibit HSV-1 infection. Jpn J Infect Dis 69:135–142. doi: 10.7883/yoken.JJID.2015.137. [DOI] [PubMed] [Google Scholar]
- 54.Pei Y, Chen ZP, Ju HQ, Komatsu M, Ji YH, Liu G, Guo CW, Zhang YJ, Yang CR, Wang YF, Kitazato K. 2011. Autophagy is involved in anti-viral activity of pentagalloylglucose (PGG) against herpes simplex virus type 1 infection in vitro. Biochem Biophys Res Commun 405:186–191. doi: 10.1016/j.bbrc.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 55.Liu G, Xiong S, Xiang YF, Guo CW, Ge F, Yang CR, Zhang YJ, Wang YF, Kitazato K. 2011. Antiviral activity and possible mechanisms of action of pentagalloylglucose (PGG) against influenza A virus. Arch Virol 156:1359–1369. doi: 10.1007/s00705-011-0989-9. [DOI] [PubMed] [Google Scholar]
- 56.Hu H, Lee HJ, Jiang C, Zhang J, Wang L, Zhao Y, Xiang Q, Lee EO, Kim SH, Lu J. 2008. Penta-1,2,3,4,6-O-galloyl-beta-d-glucose induces p53 and inhibits STAT3 in prostate cancer cells in vitro and suppresses prostate xenograft tumor growth in vivo. Mol Cancer Ther 7:2681–2691. doi: 10.1158/1535-7163.MCT-08-0456. [DOI] [PubMed] [Google Scholar]
- 57.Lee HJ, Seo NJ, Jeong SJ, Park Y, Jung DB, Koh W, Lee HJ, Lee EO, Ahn KS, Ahn KS, Lu J, Kim SH. 2011. Oral administration of penta-O-galloyl-beta-d-glucose suppresses triple-negative breast cancer xenograft growth and metastasis in strong association with JAK1-STAT3 inhibition. Carcinogenesis 32:804–811. doi: 10.1093/carcin/bgr015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yu H, Pardoll D, Jove R. 2009. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer 9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang K, Kaufman RJ. 2008. From endoplasmic-reticulum stress to the inflammatory response. Nature 454:455–462. doi: 10.1038/nature07203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yuan Y, Lin D, Feng L, Huang M, Yan H, Li Y, Chen Y, Lin B, Ma Y, Ye Z, Mei Y, Yu X, Zhou K, Zhang Q, Chen T, Zeng J. 2018. Upregulation of miR-196b-5p attenuates BCG uptake via targeting SOCS3 and activating STAT3 in macrophages from patients with long-term cigarette smoking-related active pulmonary tuberculosis. J Transl Med 16:284. doi: 10.1186/s12967-018-1654-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Meares GP, Liu Y, Rajbhandari R, Qin H, Nozell SE, Mobley JA, Corbett JA, Benveniste EN. 2014. PERK-dependent activation of JAK1 and STAT3 contributes to endoplasmic reticulum stress-induced inflammation. Mol Cell Biol 34:3911–3925. doi: 10.1128/MCB.00980-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhao L, Zhong S, Qu H, Xie Y, Cao Z, Li Q, Yang P, Varghese Z, Moorhead JF, Chen Y, Ruan XZ. 2015. Chronic inflammation aggravates metabolic disorders of hepatic fatty acids in high-fat diet-induced obese mice. Sci Rep 5:10222. doi: 10.1038/srep10222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu Y, Xu M, Min X, Wu K, Zhang T, Li K, Xiao S, Xia Y. 2017. TWEAK/Fn14 activation participates in Ro52-mediated photosensitization in cutaneous lupus erythematosus. Front Immunol 8:651. doi: 10.3389/fimmu.2017.00651. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The miRNA array data are available on the NCBI’s Gene Expression Omnibus (GEO) database under accession number GSE134156.