Human immunodeficiency virus (HIV) disproportionally infects young women in sub-Saharan Africa. Current HIV-1 prevention options have had limited success among women, suggesting that alternative, female-controlled prevention options need to be developed. Microbicides that can be applied to the vaginal tract are a promising prevention option. In this study, we describe the testing of 15 potential candidates for inhibition of HIV-1 infection in a humanized mouse model of HIV-1 infection. Four of these candidates were able to provide significant protection from vaginal infection with HIV-1, with the most successful candidate protecting 75% of the mice from infection. This study describes the preclinical testing of a new strategy that could be a safe and effective option for HIV-1 prevention in women.
KEYWORDS: Caulobacter crescentus, HIV, microbicide, humanized mice
ABSTRACT
Over 2 million people are infected with HIV-1 annually. Approximately half of these new infections occur in women residing in low-income countries, where their access to and control over HIV-1 preventative measures are often limited, indicating that female-controlled prevention options for HIV-1 are urgently needed. Microbicides that can be topically applied to the vaginal tract in advance of sexual activity represent a promising female-controlled prevention option for HIV-1. We have previously described the development of an HIV-1-specific microbicide using the surface or S-layer recombinant protein display capabilities of the nonpathogenic, freshwater bacterium Caulobacter crescentus. Recombinant C. crescentus bacteria were created that displayed proteins that interfere with the HIV-1 attachment and entry process and that were able to provide significant protection of TZM-bl cells from infection with HIV-1 pseudovirus. These studies have been expanded to investigate if these recombinant C. crescentus bacteria are able to maintain efficacy with replication-competent HIV-1 and both TZM-bl cells and human peripheral blood mononuclear cells (PBMCs). In addition, we utilized the humanized bone marrow-liver-thymus (BLT) mouse model to determine if vaginal application of recombinant C. crescentus at the time of HIV-1JR-CSF infection could provide protection from HIV-1 infection. Recombinant C. crescentus bacteria expressing Griffithsin, GB virus C E2 protein, elafin, α-1-antitrypsin, indolicidin, and the fusion inhibitor T-1249 were able to protect 40 to 75% of the BLT mice from vaginal infection with HIV-1JR-CSF, with C. crescentus bacteria expressing Griffithsin being the most effective. Taken together, these data suggest that a C. crescentus-based microbicide could be a safe and effective method for HIV-1 prevention.
IMPORTANCE Human immunodeficiency virus (HIV) disproportionally infects young women in sub-Saharan Africa. Current HIV-1 prevention options have had limited success among women, suggesting that alternative, female-controlled prevention options need to be developed. Microbicides that can be applied to the vaginal tract are a promising prevention option. In this study, we describe the testing of 15 potential candidates for inhibition of HIV-1 infection in a humanized mouse model of HIV-1 infection. Four of these candidates were able to provide significant protection from vaginal infection with HIV-1, with the most successful candidate protecting 75% of the mice from infection. This study describes the preclinical testing of a new strategy that could be a safe and effective option for HIV-1 prevention in women.
INTRODUCTION
HIV-1 prevention for women is a global health priority. Approximately 1 million women are infected with HIV-1 each year (1). In sub-Saharan Africa, 75% of new HIV-1 infections in 15- to 19-year-olds are among women, making them twice as likely to acquire HIV-1 infection as men (1, 2). Existing HIV-1 prevention options, like condoms, male circumcision, and viral suppression, are not always feasible for women, as these rely on their partners for use. Thus, prevention options that women can use and control are urgently needed. With the difficulties in developing an effective vaccine and the inconsistent results of preexposure prophylaxis (PrEP) in women (1, 3–5), alternative female-controlled HIV-1 prevention options are urgently needed.
Recent microbicide clinical trials have focused on either a 1% tenofovir gel or a dapivirine vaginal ring for HIV-1 prevention (2, 6–8). Tenofovir gel demonstrated 39% efficacy in the CAPRISA 004 clinical trial (2). However, in both the FACTS 001 and VOICE trials, it had no protective effect on HIV-1 acquisition (2–4, 9). While adherence was a concern, follow-up studies have indicated that vaginal microbiota, particularly Gardnerella and Prevotella, can interfere with the effectiveness of tenofovir-based microbicides (10). In the ASPIRE and The Ring Study trials, the dapivirine vaginal ring reduced HIV-1 acquisition by 27% and 31%, respectively (7, 8). However, among women under the age of 21 years, the ring was 15% effective in The Ring Study and had no efficacy in the ASPIRE trial (7, 8). These clinical trial results indicate that the development of alternative microbicide strategies is urgently needed.
We have previously demonstrated up to 72% protection from HIV-1 infection in vitro using the surface or S-layer recombinant display capabilities of the nonpathogenic, freshwater bacterium Caulobacter crescentus (11–13). In these studies, 15 unique recombinant C. crescentus bacteria with the ability to prevent the attachment or entry of HIV-1 into a target cell were created (11, 12). The recombinant bacteria expressed a wide variety of anti-HIV proteins, including the carbohydrate binding agents cyanovirin-N (14), microvirin (15), and griffithsin (16, 17), ligands (macrophage inflammatory protein 1α [MIP-1α]) (18), decoy receptors (CD4, mimetic CD4M33F23) (18, 19), fusion inhibitors (Fuzeon [20], T-1249 [21], C52 variant [22]), and the antimicrobial peptides BmKn2 (23), α-1-antitrypsin (A1AT) (24), indolicidin (25), and elafin (26). The success of these recombinants for HIV-1 prevention in initial studies indicated that further studies using more physiologically relevant models are warranted.
While C. crescentus is a nonpathogenic bacterium, it is a Gram-negative bacterium that could stimulate an immune response in vivo. Previous work in our lab (13) and by collaborators (27) has demonstrated that C. crescentus appears to be safe for topical application to the vaginal tract. Importantly, there was no significant production of inflammatory cytokines, immune cell recruitment, or antibody production after vaginal application of C. crescentus in an immunocompetent mouse model (13). Furthermore, C. crescentus cannot be cultured from the peritoneal cavity of immunocompetent mice within 10 days following intraperitoneal injection (27). These data suggest that C. crescentus will likely be safe for use as a topical mucosal agent.
Herein, both in vitro and in vivo studies were undertaken to further test the ability of recombinant C. crescentus to prevent HIV-1 infection. In vitro studies using replication-competent HIV-1 isolates from several strains indicated that both TZM-bl cells and human peripheral blood mononuclear cells (PBMCs) were protected from HIV-1 infection. In addition, the recombinant C. crescentus was applied to the vaginal tract of humanized bone marrow-liver-thymus (BLT) mice (28–30), and HIV-1 infection was measured. We found that 40 to 75% of mice were protected from vaginal infection with HIV-1 using 6 different recombinants, with the C. crescentus recombinant expressing griffithsin (Cc-griffithsin) being the most effective at preventing HIV-1 acquisition. Taken together, these data suggest that a C. crescentus-based microbicide could be a worthwhile option for developing a safe and effective method for HIV-1 prevention.
RESULTS
Many recombinant C. crescentus bacteria provide protection from replication-competent HIV-1 infection.
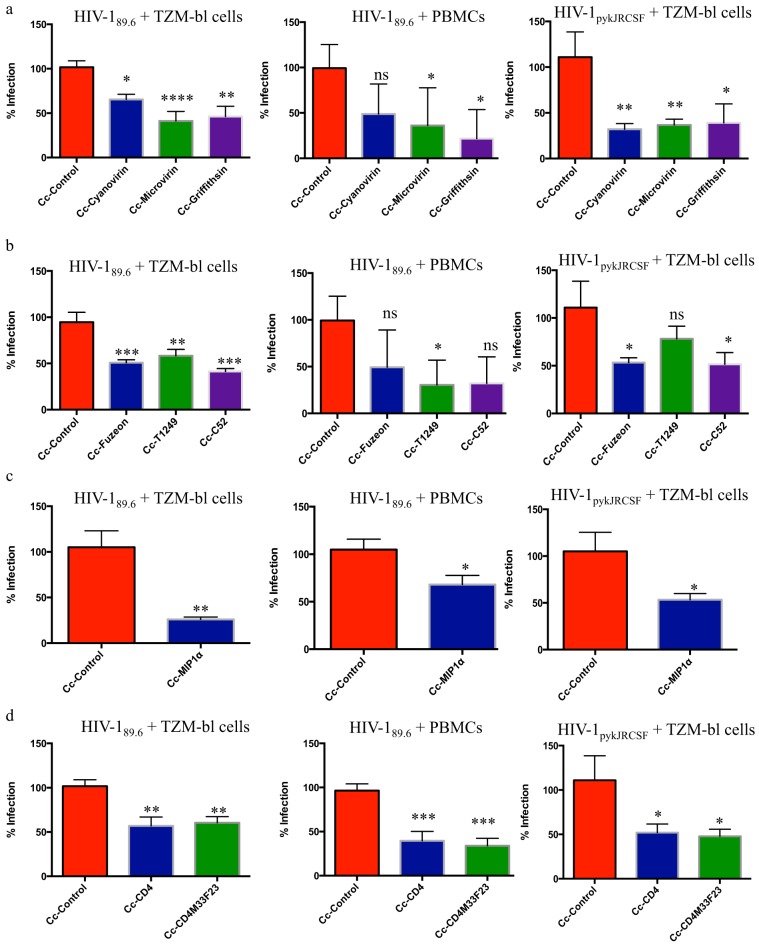
We have previously demonstrated a successful proof of concept for a recombinant C. crescentus-based microbicide expressing antiviral lectins, fusion inhibitors, decoys, and antiviral peptides (11–13), demonstrating inhibition of infection using HIV-1 pseudoviruses representing the two most common viral clades, B and C. These studies were repeated using replication-competent HIV-1 and both TZM-bl cells and human PBMCs. Previous studies have indicated that 1 × 108 recombinant bacteria provide the best protection from HIV-1 infection while minimizing nonspecific inhibition by control (no insert) C. crescentus bacteria (Cc-control) (13). HIV-1 infection rates were significantly decreased with all recombinant bacteria across various HIV-1 strains in both cell types. While the most effective recombinant varied depending on the virus/cell combination, several recombinants were able to provide a >90% decrease in HIV-1 infection in both TZM-bl cells and PBMCs with specific viral strains (Table 1; Fig. 1). With some of the recombinants, there was a wide range of effectiveness depending on the viral strain used, which is why we anticipate combining recombinants to improve efficacy in a final microbicide product. Notably, HIV-1 was suppressed, on average, by 50% with each recombinant, results that were consistent with those of our previous studies.
TABLE 1.
Recombinant C. crescentus
| Protein | Name | Sequence | Reference in which construct was first described | Mean (range) % HIV-1 inhibition |
|
|---|---|---|---|---|---|
| Live virus-infected TZM-bl cellsa | Live virus-infected PBMCsb | ||||
| Blank control | Cc-control | NAc | 11 | 110.1 (80.8–137.7)d | 100 (99.9–100)d |
| Griffithsin | Cc-griffithsin | SLTHRKFGGSGGSPFSGLSSIAVRSGSYLDAIIIDGVHHGGSGGNLSPTFTFGSGEYISNMTIRSGDYIDNISFETNMGRRFGPYGGSGGSANTLSNVKVIQINGSAGDYLDSLDIYYEQY | 12 | 61 (19.6–85.2) | 56.7 (25.4–100) |
| Microvirin | Cc-microvirin | MPNFSHTCSSINYDPDSTILSAECQARDGEWLPTELRLSDHIGNIDGELQFGDQNFQETCQDCHLEFGDGEQSVWLVCTCQTMDGEWKSTQILLDSQIDNNDSQLEIG | 12 | 61.8 (11.9–82.4) | 56 (−7.9–100) |
| Cyanovirin | Cc-cyanovirin | LGKFSQTCYNSAIQGSVLTSTCERTNGGYNTSSIDLNSVIENVDGSLKWQGSNFIETCRNTQLAGSSELAAECKTRAQQFVSTKINLDDHIANIDGTLKYE | 12 | 51.6 (21.6–87.6) | 71.6 (15.9–100) |
| Fuzeon, T-20 | Cc-Fuzeon | MYTSLIHSLIEESQNQQEKNEQELLELDKWASLWNWFM | 12 | 58.8 (29.3–78.9) | 62 (8.3–100) |
| T-1249 | Cc-T1249 | WQEWEQKITALLEQAQIQQEKNEYELQKLDKWASLWEWF | 12 | 40 (4.9–54.2) | 27.6 (−30.9–100) |
| C52 | Cc-C52 | NHTTWMEWDREINNYTSLIHSLIEESQNQQEKNEQELLELDKWASLWNWFNI | 12 | 50.7 (12.2–81.3) | 86.8 (46.5–100) |
| MIP-1α | Cc-MIP1α | APLAADTPTACCFSYTSRQIPQNFIADYFETSSQCSKPSVIFLTKRGRQVCADPSEEWVQKYVSDLELSA | 11 | 60.3 (23–83) | 69.2 (11.2–97.2) |
| CD4 | Cc-CD4 | GDTVELTCTASQKKSIQFHWKNSNQIKILGNQGSFLTKGPSKLNDRADSRRSLWDQGNFPLIIKNLKIEDSDTYICEVEDQ | 11 | 55.5 (10.6–72.5) | 74.6 (18–97.5) |
| CD4 mimetic | Cc-CD4M33F23 | NLHFCQLRCKSLGLLGKCAGSFCACV | 12 | 36.2 (16–74.3) | 26.9 (36.5–93.2) |
| BmKn2 | Cc-BmKn2 | FIGAIARLLSKIF | —e | — | — |
| α-1-antitrypsin | Cc-A1AT | LEAIPCSIPPEFLFGKPFVFLMIEQNTKSPLFMG | — | — | — |
| Elafin | Cc-elafin | AQEPVKGPVSTKPGSCPIILIRCAMLNPPNRCLKDTDCPGIKKCCEGSCGMACFVPQ | — | — | — |
| Indolicidin | Cc-indolicidin | ILPWKWPWWPWRR | — | — | — |
| GB Virus C E2 protein | Cc-GBVCE2 | WDRGNVTLLCDCPNGPWVWVPAFCQAVG | Herein | 64.9 (19.5–86.4) | 75.7 (27.2–87.9) |
The mean and range presented are a summary of data from HIV-1 strains 89.6, BaL, JR-FL, and pykJR-CSF.
The mean and range presented are a summary of data from HIV-1 strains 89.6, JR-FL, and SF162.
cNA, not applicable.
Data represent the mean (range) percent infection.
—, data are presented elsewhere (13).
FIG 1.
Viral blocking assays. A total of 10,000 TZM-bl cells or PHA-stimulated PBMCs were incubated for 48 to 72 h with 200 TCID50 of HIV-189.6 or HIV-1pyk-JR-CSF and 108 recombinant C. crescentus bacteria. HIV-1 infection was measured using a β-galactosidase assay (TZM-bl cells) or p24 ELISA (PBMCs). To minimize assay-to-assay variability, the wells containing cells and HIV-1 were set as 100% infection, and the results for the other wells were normalized to the 100% infection. Each experiment was performed in quadruplicate and repeated three times. An unpaired two-tailed t test (for the Cc-control group and 1 group receiving recombinant C. crescentus bacteria) or ANOVA with Bonferroni’s correction for multiple comparisons (for the Cc-control group and >2 groups receiving recombinant C. crescentus bacteria, with each group being compared to the Cc-control group and each other) were used to evaluate the significance of differences between groups as appropriate. Results are reported as the mean + SEM. (a) Antiviral lectins. (b) Fusion inhibitors. (c) MIP-1α. (d) CD4-based inhibitors. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001; ns, not significant (P > 0.05).
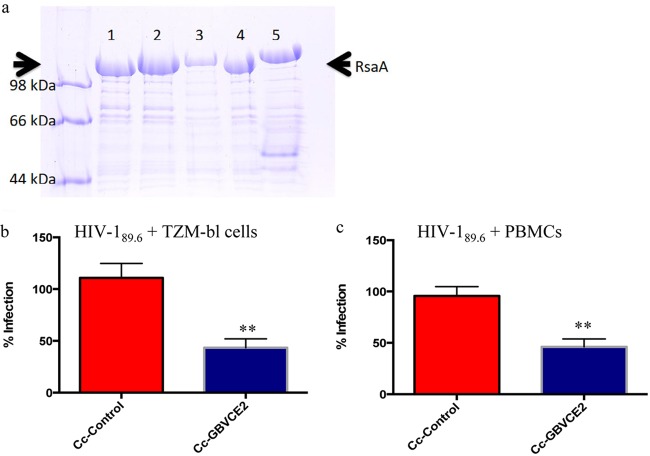
GB virus C E2 protein blocks HIV-1 infection in vitro.
GB virus C (GBVC; human pegivirus, formerly hepatitis G virus) causes a persistent viral infection that does not cause any known disease pathology and appears to improve the survival of HIV-positive individuals and delay progression to AIDS (31–33). While many factors have been linked to this favorable survival (34, 35), the E2 protein of GB virus C (GBVCE2) is a putative fusion peptide that can interfere with HIV binding or fusion (36, 37). The E2 protein of GB virus C was expressed in the S layer of C. crescentus (Fig. 2a) and used in in vitro viral blocking assays with both TZM-bl cells and PBMCs (Fig. 2b and c). The E2 protein was successfully expressed in the S layer of C. crescentus, and recombinant C. crescentus bacteria expressing GBVCE2 (Cc-GBVCE2) were able to provide 57% protection from HIV-189.6 in TZM-bl cells and 54% protection in PBMCs (Fig. 2). Furthermore, when Cc-GBVCE2 was tested for the ability to prevent HIV-1 infection with additional viral strains, it provided an average 65% reduction in HIV-1 infection in the TZM-bl cell line and 76% in PBMCs. These results suggest that Cc-GBVCE2 is an excellent candidate for additional microbicide testing.
FIG 2.
Cc-GBVCE2 provides protection from HIV-1 infection. (a) Coomassie blue-stained 7.5% SDS-PAGE gel of normalized low-pH-extracted RsaA protein from C. crescentus strain JS 4038 containing RsaA plasmids. Arrows indicate the location of the S-layer protein. Lane 1, Cc-control; lane 2, Cc-GBVCE2; lanes 3 to 5, S-layer expression controls. (b and c) A total of 10,000 TZM-bl cells (b) or PHA-stimulated PBMCs (c) were incubated for 48 to 72 h with 200 TCID50 of HIV-189.6 and 108 recombinant C. crescentus bacteria expressing GBVCE2 (Cc-GBVCE2). HIV-1 infection was measured using a β-galactosidase assay (TZM-bl cells) or p24 ELISA (PBMCs). To minimize assay-to-assay variability, the wells containing cells plus HIV-1 were set as 100% infection, and the results for the other wells were normalized to the 100% infection. An unpaired two-tailed t test was used to evaluate the significance of differences between groups. Each experiment was performed in quadruplicate and repeated three times. Results are reported as the mean + SEM. **, P < 0.01.
Cc-griffithsin, Cc-GBVCE2, Cc-elafin, and Cc-A1AT provide significant protection from HIV-1 infection in BLT mice.
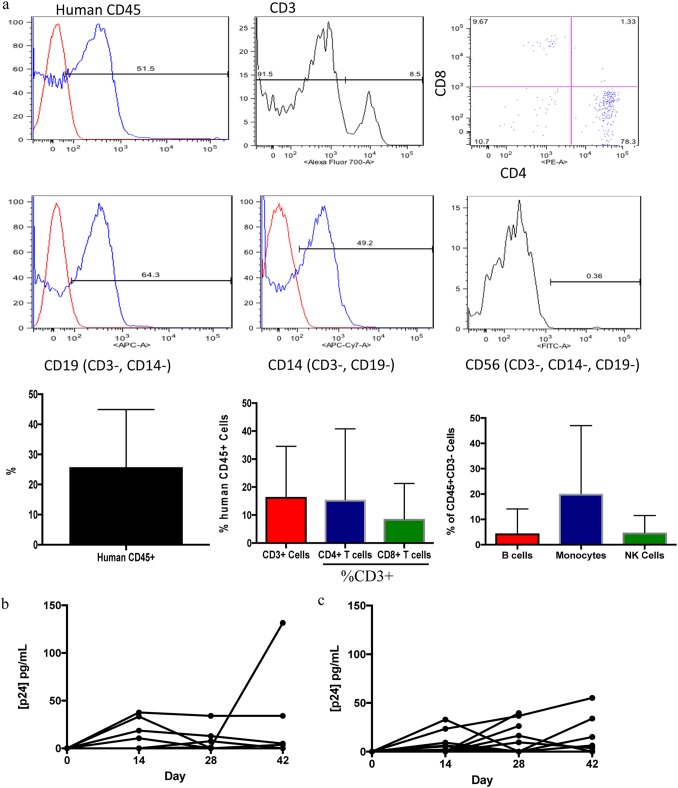
These in vitro studies suggested that recombinant C. crescentus could be an excellent option for an HIV-1-specific microbicide and that further preclinical testing was warranted. Therefore, humanized BLT mice (28–30) were used to test the ability of the recombinant C. crescentus bacteria to provide protection from vaginal infection with HIV-1. Human immune cell reconstitution of BLT mice was confirmed by flow cytometry (Fig. 3a; Table 2). Vaginal infection of the mice with HIV-1JR-CSF in the presence of 108 Cc-control bacteria indicated that C. crescentus did not impact susceptibility to HIV-1 infection (Fig. 3b and c). We have previously demonstrated by titration that 108 recombinant C. crescentus bacteria provide significant protection from infection in vitro without causing nonspecific inhibition of infection and have demonstrated that this dose can provide significant protection from vaginal infection with herpes simplex virus 2 (HSV-2) in C57BL/6 mice (13).
FIG 3.
Humanized BLT mice. (a) Flow cytometry analysis of human immune cells in BLT mice. Bar plots are the mean + SD. (b) BLT mice were infected intravaginally with 10,000 TCID50 of HIV-1JR-CSF, and blood was analyzed by p24 ELISA. Data are for 17 mice. (c) BLT mice were infected intravaginally with 10,000 TCID50 of HIV-1JR-CSF and 108 Cc-control bacteria, and blood was analyzed by p24 ELISA. Data are for 15 mice.
TABLE 2.
Summary of human immune cell reconstitution in BLT mouse cohorts
| Cohort identifiera | No. of mice | Avg % of CD45 cells (human) |
|---|---|---|
| 1 | 3 | 46.4 |
| 2 | 7 | 21.8 |
| 4 | 14 | 29.2 |
| 5 | 14 | 27.5 |
| 6 | 8 | 21.1 |
| 7 | 4 | 71.5 |
| 8 | 14 | 47.3 |
| 9 | 19 | 39.2 |
| 10 | 14 | 16.6 |
| 11 | 10 | 32.4 |
| 12 | 16 | 17.4 |
| 13 | 12 | 12.9 |
| 14 | 10 | —b |
| 15 | 7 | 2.2 |
| 16 | 14 | 6.0 |
| 17 | 15 | 3.8 |
Cohort 3 had 1 female mouse that was used as a control treated with PBS only.
—, cohort 14 had an antibody staining problem during flow cytometry, but control mice only infected with HIV-1JR-CSF had sustained vaginal infection, so microbicide experiments proceeded as normal for these mice.
While human immune cell reconstitution was variable across different cohorts of BLT mice, each cohort contained mice infected with HIV-1 only and mice infected with HIV-1 and treated with Cc-control, both groups of which were measured to be HIV-1 positive following infection, suggesting that each cohort was susceptible to vaginal infection with HIV-1. Furthermore, each recombinant C. crescentus bacterium was tested in multiple cohorts of BLT mice to verify that protection from HIV-1 infection was observed across more than one cohort.
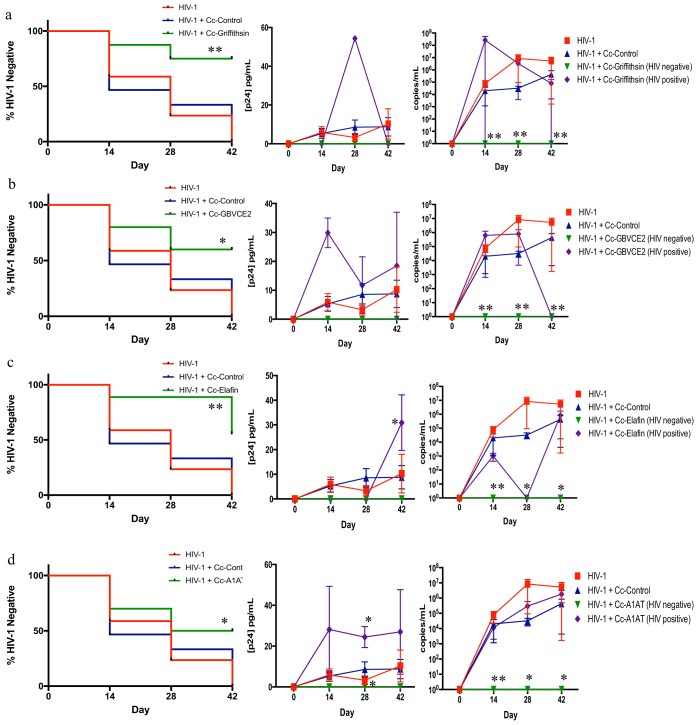
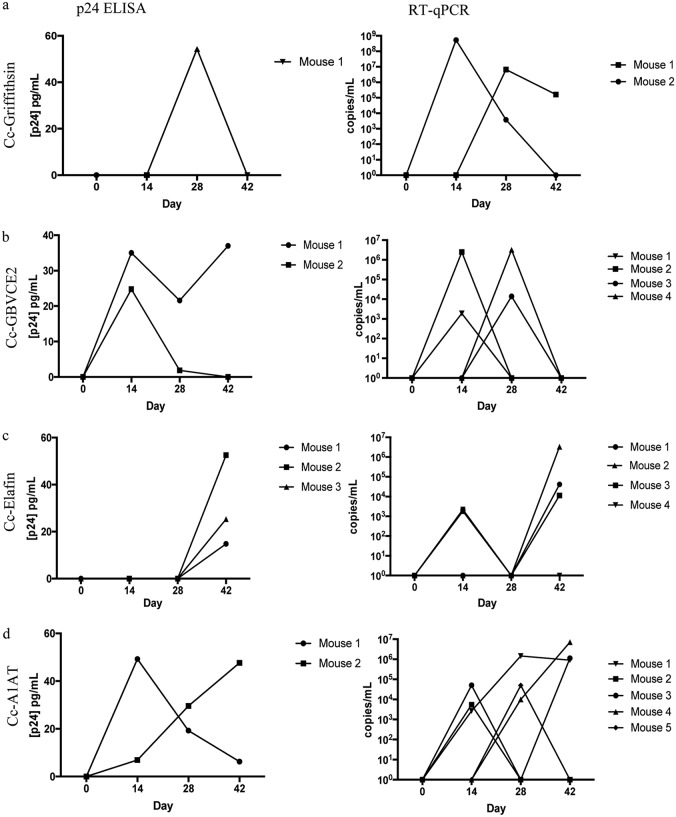
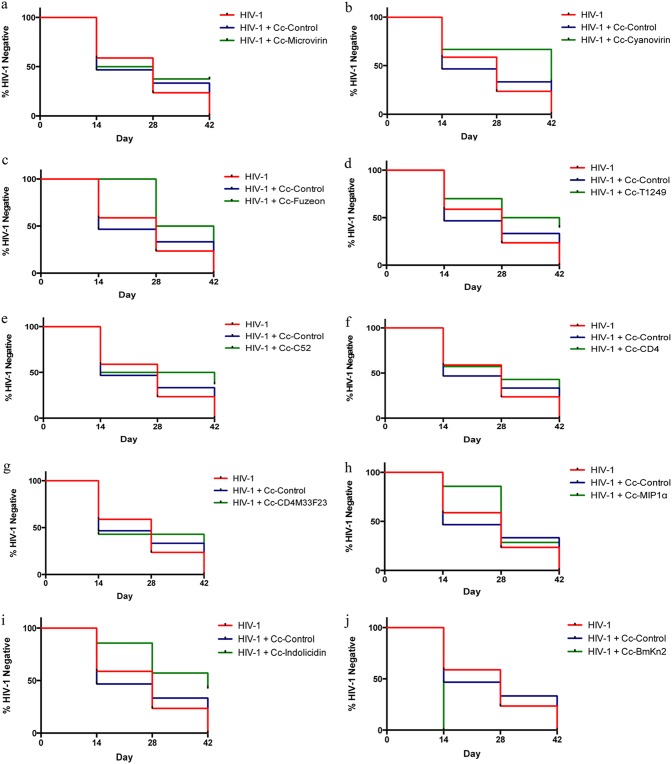
Cc-griffithsin was the most effective recombinant bacterium, protecting 6 of 8 mice (75%, P = 0.003) from vaginal infection with HIV-1, confirmed by both a p24 enzyme-linked immunosorbent assay (ELISA) and reverse transcription-quantitative PCR (RT-qPCR) (Fig. 4a and 5a). To ensure that all HIV-1-positive mice were detected, HIV-1 infection was measured by both p24 ELISA and RT-qPCR for each mouse, and a mouse was considered HIV-1 negative only if both assays had no virus detected. Interestingly, one mouse was HIV-1 negative by the p24 ELISA but had detectable HIV-1 RNA by RT-qPCR. Although the p24 levels in the blood of HIV-1-negative mice that received HIV-1 plus Cc-griffithsin (0 pg/ml p24) were not statistically significantly different from those of HIV-1-negative mice that received HIV-1 plus Cc-control, there was a significant reduction in HIV-1 RNA in the blood of mice that received HIV-1 plus Cc-griffithsin and that were HIV-1 negative compared to that in the blood of mice that received HIV-1 plus Cc-control. While the other antiviral lectins provided protection levels similar to those provided by Cc-griffithsin in vitro, recombinant C. crescentus bacteria expressing microvirin (Cc-microvirin) and cyanovirin (Cc-cyanovirin) were not effective at preventing infection in BLT mice. Cc-microvirin protected 37.5% (3 of 8, P > 0.05) of the BLT mice from HIV-1 infection (Fig. 6a). In a pilot experiment, Cc-cyanovirin protected 1 of 3 BLT mice from vaginal infection with HIV-1 (Fig. 6b). As this protection level was not statistically significant and at some times postinfection the HIV-1 levels were higher in mice that received HIV-1 plus Cc-cyanovirin, additional experiments were not undertaken.
FIG 4.
Recombinant C. crescentus bacteria protect from vaginal infection with HIV-1JR-CSF in BLT mice. Humanized BLT mice were infected intravaginally with 10,000 TCID50 of HIV-1JR-CSF in the presence or absence of 108 recombinant C. crescentus bacteria. Blood was collected from the mice biweekly for 6 weeks and analyzed by p24 ELISA and RT-qPCR. Data are presented as a modified survival curve indicating when mice seroconverted, and statistics were performed as a log-rank test comparing HIV-1-infected mice receiving Cc-control and HIV-1-infected mice receiving a recombinant C. crescentus bacterium. p24 ELISA and RT-qPCR data are presented as the mean + SEM, and the presented statistics were performed by the Kruskal-Wallis test and represent the comparison between HIV-1-infected mice receiving Cc-control and HIV-1-infected mice receiving a recombinant C. crescentus bacterium. *, P < 0.05; **, P < 0.01. (a) Cc-griffithsin (n = 8); (b) Cc-GBVCE2 (n = 10); (c) Cc-elafin (n = 9); (d) Cc-A1AT (n = 10).
FIG 5.
Individual viral loads in HIV-1-positive BLT mice. BLT mice were infected intravaginally with HIV-1JR-CSF in the presence of 108 recombinant C. crescentus bacteria, and HIV-1 infection levels were measured biweekly by p24 ELISA and RT-qPCR. Results are shown for each individual mouse that was measured to be HIV-1 positive. Mouse identifiers for p24 ELISA and RT-qPCR results are matched for each recombinant C. crescentus bacterium when a mouse was measured to be HIV-1 positive by both techniques. (a) Cc-griffithsin; (b) Cc-GBVCE2; (c) Cc-elafin; (d) Cc-A1AT.
FIG 6.
Recombinant C. crescentus in BLT mice. BLT mice were infected intravaginally with 10,000 TCID50 of HIV-1JR-CSF in the presence or absence of 108 recombinant C. crescentus bacteria. Data are presented as a modified survival curve indicating when the mice were measured to be HIV positive, and statistics were performed as a log-rank test comparing HIV-1-infected mice receiving Cc-control and HIV-1-infected mice receiving a recombinant C. crescentus bacterium. (a) Cc-microvirin (n = 8); (b) Cc-cyanovirin (n = 3); (c) Cc-Fuzeon (n = 8); (d) Cc-T1249 (n = 10); (e) Cc-C52 (n = 8); (f) Cc-CD4 (n = 7); (g) Cc-CD4M33F23 (n = 7); (h) Cc-MIP1α (n = 7); (i) Cc-indolicidin (n = 7); (j) Cc-BmKn2 (n = 3).
Three additional recombinants were able to provide significant protection from vaginal infection in BLT mice: Cc-GBVCE2, recombinant C. crescentus bacteria expressing elafin (Cc-elafin), and recombinant C. crescentus bacteria expressing α-1-antitrypsin (Cc-A1AT). There were undetectable viral loads in 6 of 10 mice (60%, P = 0.0124) receiving Cc-GBVCE2 by both p24 ELISA and RT-qPCR following vaginal infection with HIV-1 (Fig. 4b and 5b). Cc-elafin provided 56% protection (P = 0.0036) from vaginal infection with HIV-1, with five of nine mice remaining HIV-1 negative, as determined by a lack of detection of p24 or viral RNA (Fig. 4c and 5c). Three mice that received Cc-elafin and that became HIV-1 positive had a delay in detectable levels of p24 in the blood until day 42 postinfection, although at this time p24 levels were significantly higher than the peak levels reached with HIV-1 alone. Cc-A1AT was able to protect 5 of 10 mice (50%, P = 0.0487) from vaginal infection with HIV-1, based on a lack of detection of p24 and viral RNA (Fig. 4d and 5d). Interestingly, those mice that received HIV-1 plus Cc-A1AT and that became HIV-1 positive appeared to have higher levels of virus in the blood, based on p24 levels.
Eight of the recombinant C. crescentus bacteria (those expressing Fuzeon [Cc-Fuzeon], T1249 [Cc-T1249], C52 [Cc-C52], CD4 [Cc-CD4], CD4M33F23 [Cc-CD4M33F23], MIP-1α [Cc-MIP1α], indolicidin [Cc-indolicidin], and BmKn2 [Cc-BmKn2]) provided some level of protection from vaginal infection with HIV-1, based on both p24 ELISA and RT-qPCR results, although this protection was not statistically significant (Fig. 6c to j).
DISCUSSION
In this preclinical microbicide study, recombinant bacteria expressing the antiviral lectin griffithsin (Cc-griffithsin) were able to protect 75% of BLT mice from vaginal infection with HIV-1. Based on these results and the promising safety profile of griffithsin (16, 38), Cc-griffithsin represents an excellent option for additional testing as a potential HIV-1 microbicide.
Cc-GBVCE2 is another promising microbicide candidate, as it was able to protect 60% of BLT mice from vaginal infection with HIV-1. While these studies focused on the use of Cc-GBVCE2 as a topical agent to prevent HIV-1 transmission, it is possible that Cc-GBVCE2 may also have utility as a therapeutic option for HIV-1 infection. It has been reported that people coinfected with GB virus C and HIV-1 have a better prognosis and slower progression to AIDS, suggesting that GB virus C has beneficial effects on HIV-1 disease through multiple mechanisms (34, 35, 39–42), including the E2 portion of GB virus C being a putative fusion peptide that interferes with HIV binding or fusion and that prevents cell-to-cell spread (42–44). As such, Cc-GBVCE2 may be able to limit the spread of HIV-1 in infected individuals, as well as lower viral shedding to prevent transmission.
Interestingly, some mice appeared to be protected from HIV-1 infection initially but were measured to be HIV-1 positive at later time points. Further studies are necessary to investigate the potential mechanism responsible for this. Furthermore, some mice that became HIV-1 positive after receiving a recombinant C. crescentus bacterium that provided significant protection from HIV-1 infection in other mice appeared to have higher levels of HIV-1 infection than control mice, which may be a result of the variability of immune reconstitution inherent in this model.
Three of the microbicide candidates that were able to provide significant protection from vaginal infection with HIV-1 also target HSV-2: Cc-griffithsin, Cc-elafin, and Cc-A1AT (13, 45, 46). HSV-2 infection is a major risk factor for HIV-1 acquisition, increasing the risk of HIV acquisition by 2- to 4-fold (47–49). As HSV-2 can increase HIV-1 acquisition, this suggests that these recombinant C. crescentus bacteria could have a significant impact on HIV-1 infection rates, not only directly by preventing HIV-1 infection but also by preventing HSV-2 acquisition. In particular, Cc-A1AT provided 50% protection from HIV-1 infection in BLT mice and provided an 86% increase in survival following vaginal infection in C56BL/6 mice, whereas Cc-griffithsin provided 75% protection from HIV-1 infection and 57% protection from HSV-2 disease (13).
While these studies investigated using the recombinant C. crescentus individually, it is likely that combining the successful recombinant C. crescentus candidates will increase HIV-1 protection beyond those levels observed with each one individually. In addition, as all recombinant C. crescentus bacteria provided at least some low-level protection from HIV-1 infection, it is possible that combining multiple candidates that target different aspects of the HIV-1 attachment and entry process, including those that did not significantly reduce HIV-1 acquisition in BLT mice, may generate the most effective microbicide cocktail. In support of this, we have previously demonstrated that combining Cc-CD4 and Cc-MIP1α in vitro increases HIV-1 protection from 50 to 75% to 97% (11).
Our studies have focused on investigating a dosing strategy that would be coitally dependent, similar to a vaginal gel, as the first phase of testing. As a coitally dependent option may not be feasible in a real-world situation, we have begun to undertake additional studies to improve the utility of a C. crescentus-based microbicide. Preliminary data indicate that C. crescentus is able to prevent infection up to 8 h after application, and additional studies are ongoing to determine if this can be extended further. In addition, preliminary studies in an HSV-2 model suggest that C. crescentus may have some ability to prevent infection when applied after exposure. We have not investigated the use of recombinant C. crescentus as a long-term prevention option, such as on a vaginal ring, but it should be possible to assemble the recombinant S-layer proteins on a vaginal ring to provide long-term HIV-1 prevention. While C. crescentus is a nonpathogenic bacterium found in freshwater sources, including drinking water in the United States (50), the possibility does exist for immune responses to develop after prolonged usage. Our previous studies support a lack of immune response after the short-term use of C. crescentus in mouse models (13, 27, 51). In particular, the lipopolysaccharide (LPS) of C. crescentus has been found to produce greater than 100-fold less tumor necrosis factor than LPS isolated from Escherichia coli (51). To minimize the risk of an immune response, creating an abiotic delivery system containing just the recombinant S-layer proteins would greatly reduce the chance of an immune response.
HIV-1 remains a major global health priority, particularly for young women. A recombinant C. crescentus-based microbicide represents a safe and cost-effective option that should undergo further investigation for female-controlled prevention of HIV-1 as well as HSV-2. If a C. crescentus microbicide is developed, it could be combined with other prevention strategies for sexually transmitted infections and pregnancy as part of a comprehensive reproductive health package. In addition, adding a successful microbicide to the current HIV prevention tool kit will fulfill the need for a discreet, easy-to-use product that is targeted for women and that does not require male consent or continuous prophylactic antiretroviral use. Further preclinical studies are warranted to continue the investigation of a recombinant C. crescentus-based microbicide in a nonhuman primate model or clinical setting.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Recombinant C. crescentus strain JS4038 was grown in PYE medium (0.2% peptone, 0.1% yeast extract, 0.01% CaCl2, 0.02% MgSO4) with 2 μg/ml chloramphenicol at 30°C. Gene segments were synthesized by Integrated DNA Technologies, Inc. (Coralville, IA), with codon usage being adapted for C. crescentus. C. crescentus bacteria displaying chimeric S-layer proteins have been previously described (11–13). The amino acid sequence for Cc-GBVCE2 is WDRGNVTLLCDCPNGPWVWVPAFCQAVG. The synthesized DNA segment also specified BglII and SpeI restriction sites on the 5′ side and an NheI site on the 3′ end to facilitate directional cloning into p4BRsaA(723)/GSCC digested with BglII and NheI (52, 53).
Preparation of C. crescentus cells.
C. crescentus S-layer display constructs were grown in PYE medium to an optical density at 600 nm of approximately 1 (3.1 × 109 cells/ml). Cells were centrifuged and suspended in sterile water. This was repeated one time for in vitro experiments and three times for in vivo experiments.
Cell lines.
293T cells were a gift from Ninan Abraham (University of British Columbia) and were maintained in Dulbecco’s modified Eagle medium (DMEM) with 7.5% fetal bovine serum (FBS), 100 U/ml penicillin, and 0.1 mg/ml streptomycin. TZM-bl cells were obtained through the NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH, and maintained in DMEM with 7.5% FBS (Gibco), 100 U/ml penicillin, and 0.1 mg/ml streptomycin (Gibco) as previously described (13). 174xCEM cells were obtained through the NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH, and maintained in RPMI 1640 medium supplemented with 10% FBS as previously described (13).
Primary cells.
Whole blood was collected and processed using Lymphoprep medium (Stemcell Technologies Inc.). PBMCs were grown in RPMI 1640 supplemented with 20% FBS.
HIV-1.
The following reagents were obtained through the NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH: HIV-189.6 was from Ronald Collman (54); pYK-JRCSF (catalog number 2708) was from Irvin S. Y. Chen and Yoshio Koyanagi (55–57) and was a gift from Zabrina Brumme (Simon Fraser University); HIV-1SF162 was from Jay Levy (58); HIV-1BaL was from Suzanne Gartner, Mikulas Popovic, and Robert Gallo (59, 60); and HIV-1JR-FL was from Irvin S. Y. Chen (56, 61, 62).
HIV-1 propagation.
HIV-189.6 was propagated in 174xCEM cells with 7.5 μg/ml DEAE-dextran (Sigma) and was harvested at the peak cytopathic effect. HIV-1pykJR-CSF was propagated in 293T cells using the Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer’s instructions. HIV-1JR-FL and HIV-1SF162 were propagated in 5 × 106 phytohemagglutinin (PHA)-stimulated PBMCs using 10 μg/ml hexadimethrine bromide (Polybrene; Sigma). HIV-1BaL was propagated in PHA-stimulated PBMCs with 7.5 μg/ml DEAE-dextran.
Virus titration.
Serial dilutions of virus were prepared in 96-well plates using medium containing 7.5 μg/ml DEAE-dextran. TZM-bl cells (10,000) were added. The plates were maintained at 37°C in 5% CO2 for 48 h. Infection of cells was measured indirectly using a mammalian β-galactosidase assay kit (Pierce), followed by reading of the absorbance at 415 nm. An absorbance of greater than 0.2 was considered a positive infection. The 50% tissue culture infectious dose (TCID50) per milliliter was determined for each viral stock by identifying the dilution of virus in which 50% of the TZM-bl cells were infected.
Virus blocking experiments.
The C. crescentus constructs were grown and prepared as described above. Experiments were carried out in quadruplicate wells of 96-well plates. The volume of virus added was determined by calculating the 200 TCID50 value, and 1 × 108 C. crescentus cells were added to each well. The virus and C. crescentus constructs were incubated for 1 h at 37°C before adding 10,000 TZM-bl cells or PBMCs and 7.5μg/ml DEAE-dextran to each well. Cc-MIP1α was incubated with the TZM-bl cells or PBMCs for 1 h before virus addition. The level of infection was determined after 48 to 72 h by use of a β-galactosidase assay kit (TZM-bl cells) or a p24 ELISA (ZeptoMetrix Corporation and ProSci Incorporated) according to the manufacturers’ instructions (PMBCs). For the β-galactosidase assays, the data are presented and determined as a percentage of infection of the wells with TZM-bl plus HIV-1 with the background from uninfected TZM-bl cells subtracted. p24 ELISA data were normalized to infection of the untreated control wells with PBMCs and HIV-1, set as 100%.
Preparation of humanized bone marrow-liver-thymus (BLT) mice.
NSG mice were obtained from The Jackson Laboratory and maintained in the Modified Barrier Facility at the University of British Columbia. Six- to 12-week-old female mice were used in the experiments. Fetal liver was split, and 1-mm3 pieces of liver and thymus were implanted under the kidney capsule. Autologous fetal liver tissue was used for CD34+ cell isolation using a CD34+ cell positive selection kit from Stemcell Technology Inc. according to the manufacturer’s instructions, and cells were frozen in 90% human serum–10% dimethyl sulfoxide. At 3 weeks following the surgical implantation, mice received 225 cGy of irradiation from an X-ray source, followed by intravenous injection of 100,000 to 200,000 CD34+ cells within 30 h. The mice were incubated for 9 weeks before blood was characterized for human immune cell reconstitution.
Flow cytometry.
One hundred microliters of blood was collected from BLT mice and treated with EDTA. Red blood cell lysis buffer was added, and samples were incubated for 15 min before washing with fluorescence-activated cells sorting buffer (phosphate-buffered saline [PBS] plus 2% FBS). Cells were stained with anti-mouse CD45 Pacific Blue (eBioscience), mouse anti-human CD45 phycoerythrin (PE)-Cy7 (BD Pharmingen), mouse anti-human CD3 Alexa Fluor 700 (eBioscience), mouse anti-human CD4 PE (BD Pharmingen), mouse anti-human CD8 PE-Cy5 (BD Pharmingen), mouse anti-human CD14 allophycocyanin (APC)-Cy7 (BD Pharmingen), mouse anti-human CD19 APC (BD Pharmingen), and mouse anti-human CD56 fluorescein isothiocyanate (FITC) (BD Pharmingen). Data were acquired on an LSR II flow cytometer and analyzed using FlowJo software (TreeStar). Mouse versus human CD45+ cells were examined to exclude any mouse CD45+ cells and include human CD45+ cells. The human CD45+ cells were gated for expression of CD3. CD3+ cells were examined for expression of CD4 and CD8. The CD3− cells were examined for expression of CD19 (B cells) versus CD56 (NK cells). Unstained and single-stained human PBMCs were used as controls.
HIV-1JR-CSF infection.
Mice were anesthetized before atraumatic vaginal infection with 10,000 TCID50 of HIV-1JR-CSF in the presence or absence of 108 C. crescentus bacteria. The C. crescentus bacteria were premixed with HIV-1JR-CSF less than 5 min before inoculation into the vaginal tract in a volume of 20 μl using a sterile p200 pipette tip, and then the entire volume was inoculated intravaginally. Sixteen independent cohorts of BLT mice were created for these studies. Each cohort contained at least one mouse infected with HIV-1JR-CSF only and one mouse infected with HIV-1JR-CSF and treated with Cc-control. Each recombinant C. crescentus bacterium was tested in mice from at least 2 different cohorts.
HIV-1JR-CSF infection analysis.
Blood was collected from the retro-orbital sinus on days 0, 14, 28, and 42 postinfection. Blood was centrifuged at 8,000 rpm for 13 min, and serum was aliquoted and frozen at −80°C before being analyzed by p24 ELISA (ZeptoMetrix) and RT-qPCR. The p24 ELISA was performed according to the manufacturer’s instructions and had a limit of detection of 3.9 pg/ml. Viral RNA was extracted from equal volumes of serum using a QIAamp viral RNA minikit (Qiagen). Equal volumes of RNA were converted to cDNA using an Applied Biosystems cDNA kit (Thermo Fisher Scientific). Equal volumes of cDNA were run in duplicate on an Mx3005p PCR multiplex quantitative PCR instrument (Stratagene) with Sybr green (Bio-Rad), forward primer ATCAAGCAGCTATGCAAATGCT, and reverse primer CTGAAGGGTACTAGTAGTCCCTGCTATGTC; the settings were 50°C for 2 min, 95°C for 10 min, and 40 cycles at 95°C for 15 s and 60°C for 1 min. The limit of detection was 500 copies/ml. Mice were considered HIV-1 negative if HIV-1 was not detected by both the p24 assay and the RT-qPCR at all time points.
Statistics.
Statistical analysis was performed with GraphPad Prism software. Viral blocking assay results are reported as the mean + standard error of the mean (SEM). Experiments were conducted in quadruplicate wells and repeated in a minimum of three independent experiments, unless indicated otherwise. An unpaired two-tailed t test (for comparison of the results for mice receiving the Cc-control and those receiving 1 recombinant C. crescentus bacterium) or analysis of variance (ANOVA) with Bonferroni’s correction for multiple comparisons (for comparison of the results for mice receiving the Cc-control and >2 groups receiving recombinant C. crescentus bacteria) was used as appropriate to evaluate the significance of differences between groups. Statistical analysis for BLT mice was performed using the log-rank (Mantel-Cox) test for survival curves and the Kruskal-Wallis test with Dunn’s multiple-comparison test for p24 ELISA and RT-qPCR analysis. The reported statistics represent the comparison between mice infected with HIV and receiving Cc-control and mice infected with HIV and receiving a recombinant C. crescentus bacterium. A P value of <0.05 was considered statistically significant.
Study approval.
All animal work was approved by the University of British Columbia Animal Care Committee (protocols A13-0055, A13-0234, and A12-0245). Human ethics approval was obtained from the University of British Columbia Clinical Ethics Board (H12-02480). Human PBMCs were obtained from healthy donors after they provided informed written consent. Second-trimester human fetal liver and thymus tissue was obtained after informed written consent from women undergoing elective abortion and procured by Advanced Biosciences Resources Inc. (Alameda, CA).
ACKNOWLEDGMENTS
We thank Iryna Shanina and Ana Citlali Marquez for technical assistance and Cheryl Stoddart for providing training on the development of humanized BLT mice.
This work was supported by a grant from CIHR to M.S.H., and J.S. C.F.Z. received a Ph.D. fellowship from the University of British Columbia.
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
C.F.Z., J.S., and M.S.H. conceived and designed the experiments; C.F.Z. and J.F.N. conducted the experiments; C.F.Z. and M.S.H. analyzed the results; C.F.Z. and J.F.N. wrote the manuscript and prepared figures. All authors reviewed the manuscript.
REFERENCES
- 1.UNAIDS. 2018. Global HIV & AIDS statistics—2018 fact sheet. http://www.unaids.org/en/resources/fact-sheet.
- 2.Abdool Karim Q, Abdool Karim SS, Frohlich JA, Grobler AC, Baxter C, Mansoor LE, Kharsany AB, Sibeko S, Mlisana KP, Omar Z, Gengiah TN, Maarschalk S, Arulappan N, Mlotshwa M, Morris L, Taylor D. 2010. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science 329:1168–1174. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marrazzo JM, Ramjee G, Richardson BA, Gomez K, Mgodi N, Nair G, Palanee T, Nakabiito C, van der Straten A, Noguchi L, Hendrix CW, Dai JY, Ganesh S, Mkhize B, Taljaard M, Parikh UM, Piper J, Masse B, Grossman C, Rooney J, Schwartz JL, Watts H, Marzinke MA, Hillier SL, McGowan IM, Chirenje ZM, VOICE Study Team. 2015. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med 372:509–518. doi: 10.1056/NEJMoa1402269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rossi L. 2011. Microbicide trials network statement on decision to discontinue use of tenofovir gel in VOICE, a major HIV prevention study in women. Microbicide Trials Network, Pittsburgh, PA. [Google Scholar]
- 5.Van Damme L, Corneli A, Ahmed K, Agot K, Lombaard J, Kapiga S, Malahleha M, Owino F, Manongi R, Onyango J, Temu L, Monedi MC, Mak'Oketch P, Makanda M, Reblin I, Makatu SE, Saylor L, Kiernan H, Kirkendale S, Wong C, Grant R, Kashuba A, Nanda K, Mandala J, Fransen K, Deese J, Crucitti T, Mastro TD, Taylor D. 2012. Preexposure prophylaxis for HIV infection among African women. N Engl J Med 367:411–422. doi: 10.1056/NEJMoa1202614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hladik F, Doncel GF. 2010. Preventing mucosal HIV transmission with topical microbicides: challenges and opportunities. Antiviral Res 88(Suppl 1):S3–S9. doi: 10.1016/j.antiviral.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baeten JM, Palanee-Phillips T, Brown ER, Schwartz K, Soto-Torres LE, Govender V, Mgodi NM, Matovu Kiweewa F, Nair G, Mhlanga F, Siva S, Bekker LG, Jeenarain N, Gaffoor Z, Martinson F, Makanani B, Pather A, Naidoo L, Husnik M, Richardson BA, Parikh UM, Mellors JW, Marzinke MA, Hendrix CW, van der Straten A, Ramjee G, Chirenje ZM, Nakabiito C, Taha TE, Jones J, Mayo A, Scheckter R, Berthiaume J, Livant E, Jacobson C, Ndase P, White R, Patterson K, Germuga D, Galaska B, Bunge K, Singh D, Szydlo DW, Montgomery ET, Mensch BS, Torjesen K, Grossman CI, Chakhtoura N, Nel A, Rosenberg Z, McGowan I, Hillier S, MTN-020–ASPIRE Study Team. 2016. Use of a vaginal ring containing dapivirine for HIV-1 prevention in women. N Engl J Med 375:2121–2132. doi: 10.1056/NEJMoa1506110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nel A, van Niekerk N, Kapiga S, Bekker LG, Gama C, Gill K, Kamali A, Kotze P, Louw C, Mabude Z, Miti N, Kusemererwa S, Tempelman H, Carstens H, Devlin B, Isaacs M, Malherbe M, Mans W, Nuttall J, Russell M, Ntshele S, Smit M, Solai L, Spence P, Steytler J, Windle K, Borremans M, Resseler S, Van Roey J, Parys W, Vangeneugden T, Van Baelen B, Rosenberg Z, Ring Study T. 2016. Safety and efficacy of a dapivirine vaginal ring for HIV prevention in women. N Engl J Med 375:2133–2143. doi: 10.1056/NEJMoa1602046. [DOI] [PubMed] [Google Scholar]
- 9.Rees H, Delany-Moretlwe SA, Lombard C, Baron D, Panchia R, Myer L, Schwartz JL, Donce GF, Gray G. 2015. FACTS 001 phase III trial of pericoital tenofovir 1% gel for HIV prevention in women. Abstr 26LB, CROI 2015. [Google Scholar]
- 10.Klatt NR, Cheu R, Birse K, Zevin AS, Perner M, Noel-Romas L, Grobler A, Westmacott G, Xie IY, Butler J, Mansoor L, McKinnon LR, Passmore JS, Abdool Karim Q, Abdool Karim SS, Burgener AD. 2017. Vaginal bacteria modify HIV tenofovir microbicide efficacy in African women. Science 356:938–945. doi: 10.1126/science.aai9383. [DOI] [PubMed] [Google Scholar]
- 11.Nomellini JF, Li C, Lavallee D, Shanina I, Cavacini LA, Horwitz MS, Smit J. 2010. Development of an HIV-1 specific microbicide using Caulobacter crescentus S-layer mediated display of CD4 and MIP1alpha. PLoS One 5:e10366. doi: 10.1371/journal.pone.0010366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farr C, Nomellini JF, Ailon E, Shanina I, Sangsari S, Cavacini LA, Smit J, Horwitz MS. 2013. Development of an HIV-1 microbicide based on Caulobacter crescentus: blocking infection by high-density display of virus entry inhibitors. PLoS One 8:e65965. doi: 10.1371/journal.pone.0065965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farr Zuend C, Nomellini JF, Smit J, Horwitz MS. 2018. Generation of a dual-target, safe, inexpensive microbicide that protects against HIV-1 and HSV-2 disease. Sci Rep 8:2786. doi: 10.1038/s41598-018-21134-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boyd MR, Gustafson KR, McMahon JB, Shoemaker RH, O'Keefe BR, Mori T, Gulakowski RJ, Wu L, Rivera MI, Laurencot CM, Currens MJ, Cardellina JH, Buckheit RW, Nara PL, Pannell LK, Sowder RC, Henderson LE. 1997. Discovery of cyanovirin-N, a novel human immunodeficiency virus-inactivating protein that binds viral surface envelope glycoprotein gp120: potential applications to microbicide development. Antimicrob Agents Chemother 41:1521–1530. doi: 10.1128/AAC.41.7.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huskens D, Ferir G, Vermeire K, Kehr JC, Balzarini J, Dittmann E, Schols D. 2010. Microvirin, a novel alpha(1,2)-mannose-specific lectin isolated from Microcystis aeruginosa, has anti-HIV-1 activity comparable with that of cyanovirin-N but a much higher safety profile. J Biol Chem 285:24845–24854. doi: 10.1074/jbc.M110.128546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kouokam JC, Huskens D, Schols D, Johannemann A, Riedell SK, Walter W, Walker JM, Matoba N, O'Keefe BR, Palmer KE. 2011. Investigation of griffithsin's interactions with human cells confirms its outstanding safety and efficacy profile as a microbicide candidate. PLoS One 6:e22635. doi: 10.1371/journal.pone.0022635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mori T, O'Keefe BR, Sowder RC II, Bringans S, Gardella R, Berg S, Cochran P, Turpin JA, Buckheit RW Jr, McMahon JB, Boyd MR. 2005. Isolation and characterization of griffithsin, a novel HIV-inactivating protein, from the red alga Griffithsia sp. J Biol Chem 280:9345–9353. doi: 10.1074/jbc.M411122200. [DOI] [PubMed] [Google Scholar]
- 18.Gorry PR, Ancuta P. 2011. Coreceptors and HIV-1 pathogenesis. Curr HIV/AIDS Rep 8:45–53. doi: 10.1007/s11904-010-0069-x. [DOI] [PubMed] [Google Scholar]
- 19.Huang CC, Stricher F, Martin L, Decker JM, Majeed S, Barthe P, Hendrickson WA, Robinson J, Roumestand C, Sodroski J, Wyatt R, Shaw GM, Vita C, Kwong PD. 2005. Scorpion-toxin mimics of CD4 in complex with human immunodeficiency virus gp120 crystal structures, molecular mimicry, and neutralization breadth. Structure 13:755–768. doi: 10.1016/j.str.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 20.Matthews T, Salgo M, Greenberg M, Chung J, DeMasi R, Bolognesi D. 2004. Enfuvirtide: the first therapy to inhibit the entry of HIV-1 into host CD4 lymphocytes. Nat Rev Drug Discov 3:215–225. doi: 10.1038/nrd1331. [DOI] [PubMed] [Google Scholar]
- 21.Greenberg ML, Sista P, Miralles GD, Melby T, Davison D, Jin L, Mosier S, Mink M, Nelson E, Demasi R, Fang L, Cammack N, Salgo M, Duff F, the T1249-101 Study Group, Matthews TJ. 2001. Enfuvirtide (T-20) and T-1249 resistance: observations from phase II clinical trials of enfuvirtide in combination with oral antiretrovirals (ARVs) and a phase I/II dose-ranging monotherapy trial of T-1249. Antivir Ther 7:S140-S140. [Google Scholar]
- 22.Deng Y, Zheng Q, Ketas TJ, Moore JP, Lu M. 2007. Protein design of a bacterially expressed HIV-1 gp41 fusion inhibitor. Biochemistry 46:4360–4369. doi: 10.1021/bi7001289. [DOI] [PubMed] [Google Scholar]
- 23.Chen Y, Cao L, Zhong M, Zhang Y, Han C, Li Q, Yang J, Zhou D, Shi W, He B, Liu F, Yu J, Sun Y, Cao Y, Li Y, Li W, Guo D, Cao Z, Yan H. 2012. Anti-HIV-1 activity of a new scorpion venom peptide derivative Kn2-7. PLoS One 7:e34947. doi: 10.1371/journal.pone.0034947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jia Q, Jiang X, Yu F, Qiu J, Kang X, Cai L, Li L, Shi W, Liu S, Jiang S, Liu K. 2012. Short cyclic peptides derived from the C-terminal sequence of alpha1-antitrypsin exhibit significant anti-HIV-1 activity. Bioorg Med Chem Lett 22:2393–2395. doi: 10.1016/j.bmcl.2012.02.037. [DOI] [PubMed] [Google Scholar]
- 25.Robinson WE Jr, McDougall B, Tran D, Selsted ME. 1998. Anti-HIV-1 activity of indolicidin, an antimicrobial peptide from neutrophils. J Leukoc Biol 63:94–100. doi: 10.1002/jlb.63.1.94. [DOI] [PubMed] [Google Scholar]
- 26.Drannik AG, Nag K, Yao XD, Henrick BM, Jain S, Ball TB, Plummer FA, Wachihi C, Kimani J, Rosenthal KL. 2012. Anti-HIV-1 activity of elafin is more potent than its precursor's, trappin-2, in genital epithelial cells. J Virol 86:4599–4610. doi: 10.1128/JVI.06561-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhatnagar PK, Awasthi A, Nomellini JF, Smit J, Suresh MR. 2006. Anti-tumor effects of the bacterium Caulobacter crescentus in murine tumor models. Cancer Biol Ther 5:485–491. doi: 10.4161/cbt.5.5.2553. [DOI] [PubMed] [Google Scholar]
- 28.Lan P, Tonomura N, Shimizu A, Wang S, Yang YG. 2006. Reconstitution of a functional human immune system in immunodeficient mice through combined human fetal thymus/liver and CD34+ cell transplantation. Blood 108:487–492. doi: 10.1182/blood-2005-11-4388. [DOI] [PubMed] [Google Scholar]
- 29.Melkus MW, Estes JD, Padgett-Thomas A, Gatlin J, Denton PW, Othieno FA, Wege AK, Haase AT, Garcia JV. 2006. Humanized mice mount specific adaptive and innate immune responses to EBV and TSST-1. Nat Med 12:1316–1322. doi: 10.1038/nm1431. [DOI] [PubMed] [Google Scholar]
- 30.Olesen R, Wahl A, Denton PW, Garcia JV. 2011. Immune reconstitution of the female reproductive tract of humanized BLT mice and their susceptibility to human immunodeficiency virus infection. J Reprod Immunol 88:195–203. doi: 10.1016/j.jri.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stapleton JT. 2003. GB virus type C/hepatitis G virus. Semin Liver Dis 23:137–148. doi: 10.1055/s-2003-39943. [DOI] [PubMed] [Google Scholar]
- 32.Lefrere JJ, Roudot-Thoraval F, Morand-Joubert L, Petit JC, Lerable J, Thauvin M, Mariotti M. 1999. Carriage of GB virus C/hepatitis G virus RNA is associated with a slower immunologic, virologic, and clinical progression of human immunodeficiency virus disease in coinfected persons. J Infect Dis 179:783–789. doi: 10.1086/314671. [DOI] [PubMed] [Google Scholar]
- 33.Williams CF, Klinzman D, Yamashita TE, Xiang J, Polgreen PM, Rinaldo C, Liu C, Phair J, Margolick JB, Zdunek D, Hess G, Stapleton JT. 2004. Persistent GB virus C infection and survival in HIV-infected men. N Engl J Med 350:981–990. doi: 10.1056/NEJMoa030107. [DOI] [PubMed] [Google Scholar]
- 34.George SL, Varmaz D, Tavis JE, Chowdhury A. 2012. The GB virus C (GBV-C) NS3 serine protease inhibits HIV-1 replication in a CD4+ T lymphocyte cell line without decreasing HIV receptor expression. PLoS One 7:e30653. doi: 10.1371/journal.pone.0030653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schwarze-Zander C, Neibecker M, Othman S, Tural C, Clotet B, Blackard JT, Kupfer B, Luechters G, Chung RT, Rockstroh JK, Spengler U. 2010. GB virus C coinfection in advanced HIV type-1 disease is associated with low CCR5 and CXCR4 surface expression on CD4(+) T-cells. Antivir Ther 15:745–752. doi: 10.3851/IMP1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mohr EL, Xiang J, McLinden JH, Kaufman TM, Chang Q, Montefiori DC, Klinzman D, Stapleton JT. 2010. GB virus type C envelope protein E2 elicits antibodies that react with a cellular antigen on HIV-1 particles and neutralize diverse HIV-1 isolates. J Immunol 185:4496–4505. doi: 10.4049/jimmunol.1001980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eissmann K, Mueller S, Sticht H, Jung S, Zou P, Jiang S, Gross A, Eichler J, Fleckenstein B, Reil H. 2013. HIV-1 fusion is blocked through binding of GB virus C E2D peptides to the HIV-1 gp41 disulfide loop. PLoS One 8:e54452. doi: 10.1371/journal.pone.0054452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kouokam JC, Lasnik AB, Palmer KE. 2016. Studies in a murine model confirm the safety of griffithsin and advocate its further development as a microbicide targeting HIV-1 and other enveloped viruses. Viruses 8:E311. doi: 10.3390/v8110311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jung S, Knauer O, Donhauser N, Eichenmuller M, Helm M, Fleckenstein B, Reil H. 2005. Inhibition of HIV strains by GB virus C in cell culture can be mediated by CD4 and CD8 T-lymphocyte derived soluble factors. AIDS 19:1267–1272. doi: 10.1097/01.aids.0000180097.50393.df. [DOI] [PubMed] [Google Scholar]
- 40.Xiang J, McLinden JH, Chang Q, Jordan EL, Stapleton JT. 2008. Characterization of a peptide domain within the GB virus C NS5A phosphoprotein that inhibits HIV replication. PLoS One 3:e2580. doi: 10.1371/journal.pone.0002580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McLinden JH, Kaufman TM, Xiang J, Chang Q, Klinzman D, Engel AM, Hess G, Schmidt U, Houghton M, Stapleton JT. 2006. Characterization of an immunodominant antigenic site on GB virus C glycoprotein E2 that is involved in cell binding. J Virol 80:12131–12140. doi: 10.1128/JVI.01206-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Herrera E, Tenckhoff S, Gomara MJ, Galatola R, Bleda MJ, Gil C, Ercilla G, Gatell JM, Tillmann HL, Haro I. 2010. Effect of synthetic peptides belonging to E2 envelope protein of GB virus C on human immunodeficiency virus type 1 infection. J Med Chem 53:6054–6063. doi: 10.1021/jm100452c. [DOI] [PubMed] [Google Scholar]
- 43.Mohr EL, Stapleton JT. 2009. GB virus type C interactions with HIV: the role of envelope glycoproteins. J Viral Hepat 16:757–768. doi: 10.1111/j.1365-2893.2009.01194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koedel Y, Eissmann K, Wend H, Fleckenstein B, Reil H. 2011. Peptides derived from a distinct region of GB virus C glycoprotein E2 mediate strain-specific HIV-1 entry inhibition. J Virol 85:7037–7047. doi: 10.1128/JVI.02366-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nixon B, Stefanidou M, Mesquita PM, Fakioglu E, Segarra T, Rohan L, Halford W, Palmer KE, Herold BC. 2013. Griffithsin protects mice from genital herpes by preventing cell-to-cell spread. J Virol 87:6257–6269. doi: 10.1128/JVI.00012-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Drannik AG, Nag K, Sallenave JM, Rosenthal KL. 2013. Antiviral activity of trappin-2 and elafin in vitro and in vivo against genital herpes. J Virol 87:7526–7538. doi: 10.1128/JVI.02243-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Suazo PA, Tognarelli EI, Kalergis AM, Gonzalez PA. 2015. Herpes simplex virus 2 infection: molecular association with HIV and novel microbicides to prevent disease. Med Microbiol Immunol 204:161–176. doi: 10.1007/s00430-014-0358-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barnabas RV, Wasserheit JN, Huang Y, Janes H, Morrow R, Fuchs J, Mark KE, Casapia M, Mehrotra DV, Buchbinder SP, Corey L, NIAID HIV Vaccine Trials Network. 2011. Impact of herpes simplex virus type 2 on HIV-1 acquisition and progression in an HIV vaccine trial (the Step study). J Acquir Immune Defic Syndr 57:238–244. doi: 10.1097/QAI.0b013e31821acb5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA, Hayes RJ. 2006. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS 20:73–83. doi: 10.1097/01.aids.0000198081.09337.a7. [DOI] [PubMed] [Google Scholar]
- 50.Bingle WH, Nomellini JF, Smit J. 1997. Cell-surface display of a Pseudomonas aeruginosa strain K pilin peptide within the paracrystalline S-layer of Caulobacter crescentus. Mol Microbiol 26:277–288. doi: 10.1046/j.1365-2958.1997.5711932.x. [DOI] [PubMed] [Google Scholar]
- 51.Smit J, Kaltashov IA, Kaltoshov IA, Cotter RJ, Vinogradov E, Perry MB, Haider H, Qureshi N. 2008. Structure of a novel lipid A obtained from the lipopolysaccharide of Caulobacter crescentus. Innate Immun 14:25–37. doi: 10.1177/1753425907087588. [DOI] [PubMed] [Google Scholar]
- 52.Nomellini JF, Duncan G, Dorocicz IR, Smit J. 2007. S-layer-mediated display of the immunoglobulin G-binding domain of streptococcal protein G on the surface of Caulobacter crescentus: development of an immunoactive reagent. Appl Environ Microbiol 73:3245–3253. doi: 10.1128/AEM.02900-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lau JH, Nomellini JF, Smit J. 2010. Analysis of high-level S-layer protein secretion in Caulobacter crescentus. Can J Microbiol 56:501–514. doi: 10.1139/w10-036. [DOI] [PubMed] [Google Scholar]
- 54.Collman R, Balliet JW, Gregory SA, Friedman H, Kolson DL, Nathanson N, Srinivasan A. 1992. An infectious molecular clone of an unusual macrophage-tropic and highly cytopathic strain of human immunodeficiency virus type 1. J Virol 66:7517–7521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Haltiner M, Kempe T, Tjian R. 1985. A novel strategy for constructing clustered point mutations. Nucleic Acids Res 13:1015–1025. doi: 10.1093/nar/13.3.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Koyanagi Y, Miles S, Mitsuyasu RT, Merrill JE, Vinters HV, Chen IS. 1987. Dual infection of the central nervous system by AIDS viruses with distinct cellular tropisms. Science 236:819–822. doi: 10.1126/science.3646751. [DOI] [PubMed] [Google Scholar]
- 57.Cann AJ, Zack JA, Go AS, Arrigo SJ, Koyanagi Y, Green PL, Koyanagi Y, Pang S, Chen IS. 1990. Human immunodeficiency virus type 1 T-cell tropism is determined by events prior to provirus formation. J Virol 64:4735–4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cheng-Mayer C, Levy JA. 1988. Distinct biological and serological properties of human immunodeficiency viruses from the brain. Ann Neurol 23(Suppl):S58–S61. doi: 10.1002/ana.410230716. [DOI] [PubMed] [Google Scholar]
- 59.Gartner S, Markovits P, Markovitz DM, Kaplan MH, Gallo RC, Popovic M. 1986. The role of mononuclear phagocytes in HTLV-III/LAV infection. Science 233:215–219. doi: 10.1126/science.3014648. [DOI] [PubMed] [Google Scholar]
- 60.Popovic M, Gartner S, Read-Connole E, Beaver B, Reitz M. 1989. Cell tropism and expression of HIV-1 isolates in natural targets, p 21–27. In Girard M, Valette L (ed), Retroviruses of human AIDS and related animal diseases. Colloque Des Cent Gardes, Paris and Marnes-La-Coquette, France. [Google Scholar]
- 61.O'Brien WA, Koyanagi Y, Namazie A, Zhao JQ, Diagne A, Idler K, Zack JA, Chen IS. 1990. HIV-1 tropism for mononuclear phagocytes can be determined by regions of gp120 outside the CD4-binding domain. Nature 348:69–73. doi: 10.1038/348069a0. [DOI] [PubMed] [Google Scholar]
- 62.Koyanagi Y, O'Brien WA, Zhao JQ, Golde DW, Gasson JC, Chen IS. 1988. Cytokines alter production of HIV-1 from primary mononuclear phagocytes. Science 241:1673–1675. doi: 10.1126/science.3047875. [DOI] [PubMed] [Google Scholar]