Viral infections of neurons are often not cytopathic; thus, once-infected neurons survive, and viral RNAs can be detected long after apparent viral control. These RNAs are generally considered viral fossils, unlikely to contribute to central nervous system (CNS) disease. Using a mouse model of measles virus (MV) neuronal infection, we show that MV RNA is maintained in the CNS of infected mice long after acute control and in the absence of overt disease. Viral replication is suppressed by the adaptive immune response; when these immune cells are depleted, viral protein synthesis recurs, inducing a CNS disease that is distinct from that observed during acute infection. The studies presented here provide the basis for understanding how persistent RNA infections in the CNS are controlled by the host immune response, as well as the pathogenic consequences of noncytolytic viral control.
KEYWORDS: RNA virus, T resident memory cells, viral persistence, central nervous system, measles virus, neuron
ABSTRACT
Genomic material from many neurotropic RNA viruses (e.g., measles virus [MV], West Nile virus [WNV], Sindbis virus [SV], rabies virus [RV], and influenza A virus [IAV]) remains detectable in the mouse brain parenchyma long after resolution of the acute infection. The presence of these RNAs in the absence of overt central nervous system (CNS) disease has led to the suggestion that they are viral remnants, with little or no potential to reactivate. Here we show that MV RNA remains detectable in permissive mouse neurons long after challenge with MV and, moreover, that immunosuppression can cause RNA and protein synthesis to rebound, triggering neuropathogenesis months after acute viral control. Robust recrudescence of viral transcription and protein synthesis occurs after experimental depletion of cells of the adaptive immune response and is associated with a loss of T resident memory (Trm) lymphocytes within the brain. The disease associated with loss of immune control is distinct from that seen during the acute infection: immune cell-depleted, long-term-infected mice display severe gait and motor problems, in contrast to the wasting and lethal disease that occur during acute infection of immunodeficient hosts. These results illuminate the potential consequences of noncytolytic, immune-mediated viral control in the CNS and demonstrate that what were once considered “resolved” RNA viral infections may, in fact, induce diseases later in life that are distinct from those caused by acute infection.
IMPORTANCE Viral infections of neurons are often not cytopathic; thus, once-infected neurons survive, and viral RNAs can be detected long after apparent viral control. These RNAs are generally considered viral fossils, unlikely to contribute to central nervous system (CNS) disease. Using a mouse model of measles virus (MV) neuronal infection, we show that MV RNA is maintained in the CNS of infected mice long after acute control and in the absence of overt disease. Viral replication is suppressed by the adaptive immune response; when these immune cells are depleted, viral protein synthesis recurs, inducing a CNS disease that is distinct from that observed during acute infection. The studies presented here provide the basis for understanding how persistent RNA infections in the CNS are controlled by the host immune response, as well as the pathogenic consequences of noncytolytic viral control.
INTRODUCTION
Viruses have been proposed as triggers or cofactors in numerous central nervous system (CNS) disorders of unknown etiology, including multiple sclerosis (MS), Parkinson’s disease (PD), and amyotrophic lateral sclerosis (ALS) (1–3). However, the inability to recover infectious virus or consistently detect viral genomes in patient tissues has cast doubt on viral etiologies for these prevalent and devastating CNS diseases. The inability to recover infectious virus from affected brain tissues, however, does not preclude the possibility that earlier infection may have triggered neuronal dysfunction or autoimmune activation, ultimately resulting in disease symptoms in the absence of direct viral reproduction (4–6). Alternatively, while not considered an attribute of most RNA viral infections, if the viral genome is not fully eliminated, replication may rebound at some point later in life. In this report, we show that viral RNA remains detectable for months to years in CNS neurons of permissive mice; immunosuppression leads to recrudescence of viral translation and neuropathology that is phenotypically distinct from what is observed during the acute infection.
Neurons may be particularly hospitable hosts to harbor long-term viral infections. Most neurons are postmitotic and are thus generally nonrenewable. The host immune response uses strategies, including cytokines, such as interferon gamma (IFN-γ), to control some CNS infections in the absence of neuronal lysis. There are abundant and diverse neurotropic viruses that are controlled by such nonneurolytic immune mechanisms, including rabies virus (RV), Sindbis virus (SV), measles virus (MV), West Nile virus (WNV), herpes simplex virus (HSV), and varicella-zoster virus (VZV) (7–10). However, a consequence of nonneurolytic viral control may be that surviving neurons harbor noncytopathic viruses long after an acute infection; thus, infections may be controlled but not cleared from the CNS by antiviral immunity (7, 10–13).
The nonlytic cellular mechanisms that govern viral latency and reactivation of DNA viruses, such as HSV and VZV, are reasonably well understood (14–17). For example, studies with mice using VZV and HSV have shown that lymphocytes are crucial for suppressing viral reactivation from latency in the CNS and peripheral nervous system (PNS) (14, 15, 17–21). For HSV-1, CD8+ T cells are found in direct contact with latently infected trigeminal ganglionic neurons, and, interestingly, the same molecules as used for cytolysis in other cells (e.g., granzyme B and perforin) maintain viral quiescence in neurons in the absence of neuronal cell death (17–19, 22, 23). The CD8+ T cells implicated in this process possess markers of T resident memory (Trm) lymphocytes, which permanently reside, expand, and contract within the immediate vicinity of latent viral infections, distinct from memory T cells in the circulating lymph (20, 24, 25). Although studies with neurotropic DNA virus infections highlight how immune responses can be tailored based on the infected cell populations or tissues, little is known about the ability of RNA viruses to be maintained and reactivate in the CNS in a similar manner. This may be due to the presumption that few RNA viruses can establish long-term infections: most are cytoplasmic and cannot integrate into host DNA or form episomes to remain protected from cytoplasmic nucleases. Nevertheless, genomic RNA from several viruses has been found long after acute infection in the brain and in the absence of overt viral particle synthesis, suggesting that persistent RNA viral infections, especially within the CNS, may be more prevalent than once thought (10–13).
Here we show that MV RNA remains in neurons of permissive immunocompetent mice in the absence of clinical signs of CNS disease. Neuronal viral replication is controlled by the adaptive immune response, and loss of immune surveillance leads to reactivation of viral protein synthesis and resultant pathogenesis, including weight loss and gross motor dysfunction (spastic paraparesis with dyscoordination of the hind limbs). Together, our data highlight the ability of an RNA virus to establish long-term infection in CNS neurons and that following immune senescence or suppression (as in the elderly, chemotherapy patients, and those with immunosuppressive diseases), restoration of viral protein synthesis can lead to neuropathology distinct from what is seen during acute infection.
RESULTS
NSE-CD46+ transgenic mice survive neuronal MV challenge but fail to clear viral RNA.
Our laboratory developed and extensively characterized a mouse model of MV infection in the brain in which the MV vaccine strain receptor CD46 is restricted to CNS neurons by the neuron-specific enolase (NSE) promoter (NSE-CD46) (26). Because nontransgenic mice are nonpermissive for MV infection, the only susceptible cells in these mice are transgenic, CD46+ neurons. We previously showed that intracranial (i.c.) inoculation of MV into immunocompetent adult NSE-CD46+ mice results in 100% survival, with no signs of disease or weight loss at any time point postinfection (27). These mice mount an aggressive T cell response, which can be detected within the brain parenchyma starting at ∼7 days postinfection (dpi) and decline by ∼15 to 20 dpi. CD4+ and CD8+ T cells are found in approximately equal numbers (27).
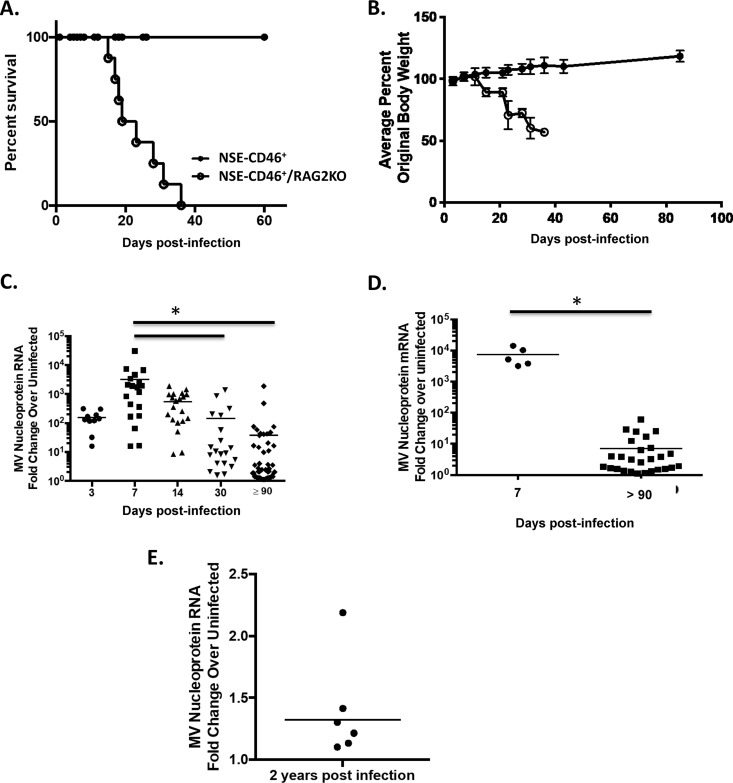
In contrast, immunodeficient NSE-CD46+ RAG2 knockout (KO) mice succumb to MV challenge within 3 to 4 weeks postinfection, likely due to unrestricted viral replication in neurons (Fig. 1A and B) (12, 27). To characterize the possible CNS consequences following prolonged infection of immunocompetent mice, we analyzed viral RNA and mRNA levels at various times postinfection (Fig. 1C and D). As expected, immunocompetent NSE-CD46+ mice controlled viral replication, reflected as statistically significant decreases (∼100-fold) in viral RNA from 7 to 90 dpi. Surprisingly, however, viral RNA and mRNA were still readily detected in brains of apparently healthy, immunocompetent mice, long after presumptive clearance (e.g., 90 dpi up to 2 years postinfection [Fig. 1C to E]).
FIG 1.
NSE-CD46+ mice survive MV challenge but fail to clear viral RNA. (A) NSE-CD46+ (n = 10) and NSE-CD46+ RAG2 KO (n = 8) mice were challenged i.c. with 1 × 104 PFU of MV-Ed and monitored daily for survival. (B) Weights of all mice from panel A were obtained throughout the infection time course and compared to baseline. Percent weight gain or loss was then calculated. (C) NSE-CD46+ mice were challenged i.c. with 1 × 104 PFU of MV-Ed and were sacrificed at the indicated day postinfection. RNA was purified from perfused brains and analyzed by RT-qPCR (using random hexamers to synthesize cDNA). (D) MV nucleoprotein mRNA in NSE-CD46+ mice challenged i.c. with MV-Ed was detected using the approach outlined above, substituting oligo(dT) primers to generate cDNA. Data are represented using the ΔΔCT method. Results are representative of at least 3 independent experiments with 5 to 10 mice per group. (E) NSE-CD46+ (n = 6) mice were challenged i.c. with 1 × 104 PFU of MV-Ed and were sacrificed at 2 years postinfection. RNA was purified from perfused brains and analyzed by RT-qPCR (using random hexamers to synthesize cDNA). Data are represented using the ΔΔCT method. *, P < 0.05, Mann-Whitney U test.
Immune-mediated control of a persistent viral infection.
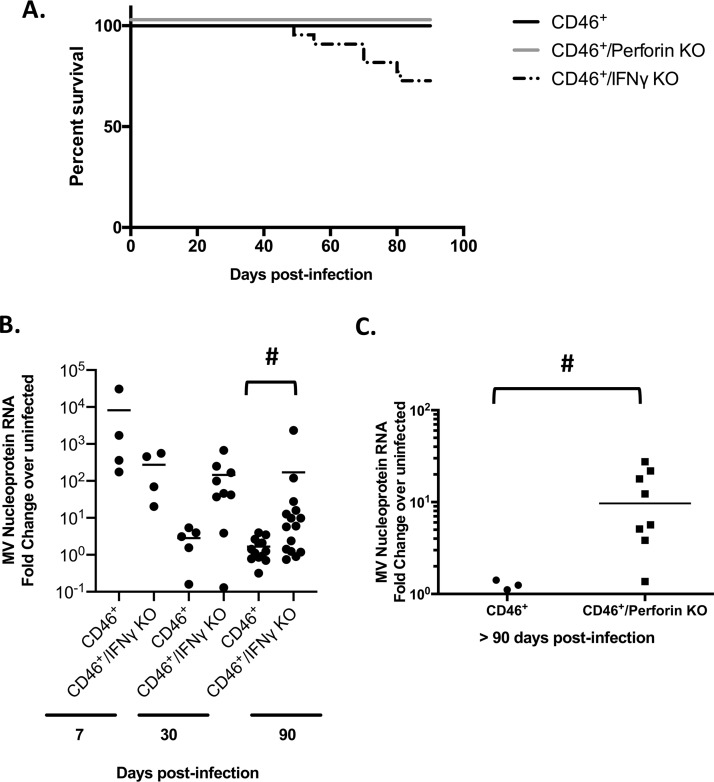
Considering the requirement of the adaptive immune system to control acute MV challenge (Fig. 1A and B), we next aimed to define how losses in specific components of this response might contribute to increased detection of viral RNA following acute clearance. Examination of NSE-CD46+ IFN-γ KO and NSE-CD46+ perforin KO mice 90 dpi showed a significant increase in viral RNA within the brain parenchyma compared to immunocompetent controls (Fig. 2). While these data imply an essential contribution of T cell effector proteins in controlling a long-lasting neurotropic infection, increases in detectable viral RNA could be the consequence of ineffective clearance of the initial acute infection.
FIG 2.
Immune-mediated control of a persistent viral infection. (A) NSE-CD46+, NSE-CD46+ perforin KO, and NSE-CD46+ IFN-γ KO mice were challenged i.c. with 1 × 104 PFU of MV Edmonston. Mice were monitored daily. Percent survival is indicated. Data represent at least 2 individual experiments using NSE-CD46+, NSE-CD46+ perforin KO, and NSE-CD46+ IFN-γ KO mice. (B and C) RNA was collected from whole-brain tissue of mice of the indicated genotypes. RT-qPCR data were generated using random-hexamer priming for cDNA generation, followed by qPCR using primers specific for the MV nucleoprotein and cyclophilin B as a standard. Data were analyzed using the ΔΔCT method. #, P < 0.05, Mann-Whitney U test of NSE-CD46+ controls compared to immunodeficient mice.
Resident memory T cells are the most abundant effector cells in the brain during persistent infection.
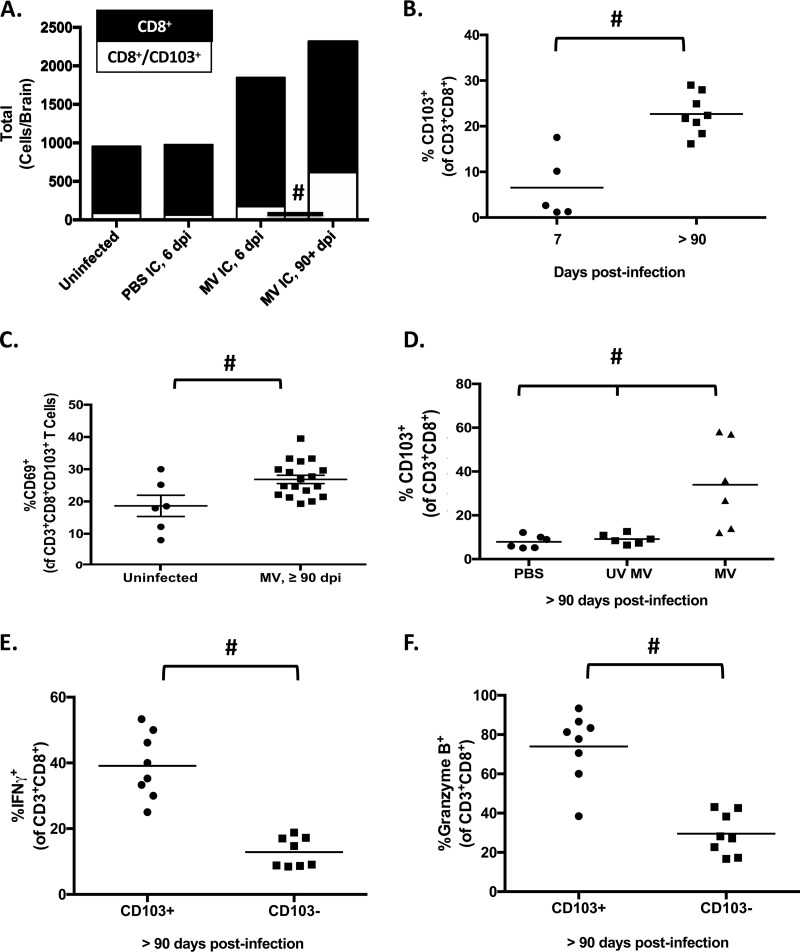
Studies using the herpesviruses VZV and HSV have shown that Trm lymphocytes are necessary to maintain viral latency (14–21, 23, 30). Considering that T cells are paramount for controlling acute neuronal MV infection in our model (31, 32), coupled with the knowledge that Trm lymphocytes prevent viral reactivation of neurotropic DNA viruses, we next characterized the immune cell populations present in brains of NSE-CD46+ mice long after viral challenge. Lymphocytes were purified from whole brains of mice infected for various time points and were analyzed by flow cytometry. No significant differences in the total number of intraparenchymal B cells, T cells, or NK cells were found between uninfected mice and those infected more than 90 days previously (data not shown). However, a significant increase was observed between these groups in the proportion of CD8+ T cells expressing resident memory markers CD103 and CD69, indicative of a Trm phenotype (Fig. 3A to C). We confirmed that the long-term presence of these lymphocytes in the brain was predicated on initial infection with replication-competent virus, as Trm lymphocytes were detected only after inoculation with replicating MV and were not found in either mock-infected controls (treated with phosphate-buffered saline [PBS]) or animals infected with a replication-incompetent MV (UV MV) (Fig. 3D).
FIG 3.
CD8+ T resident memory cells are abundant in the CNS during long-term infection and maintain an effector phenotype. (A) Lymphocytes were purified from whole-brain single-cell suspensions of perfused mice at the indicated days postinfection and subjected to flow cytometric analysis. Black bars, total numbers of CD3+ CD8+ T cells; white bars, total numbers of CD3+ CD8+ CD103+ T cells. #, P < 0.05, Mann-Whitney U test for the proportion of CD3+ CD8+ CD103+ T cells to CD3+ CD8+ T cells (n = 5 to 15/group). (B) Lymphocytes were purified from perfused whole brains of mice infected for 7 or ≥90 days and immunostained for CD103. The percentage of CD103+ cells of the CD3+ CD8+ population is shown. (C) Lymphocytes were purified from perfused whole brains of mice infected for ≥90 days; the percentage of CD69+ cells among CD3+ CD8+ CD103+ T cells in the brains of uninfected or persistently infected NSE-CD46+ mice is shown. (D) Lymphocytes purified from whole-brain tissue of mock-infected mice (PBS), mice inoculated with inactivated virus (UV MV), or mice infected with replication-competent MV, collected at 90 dpi, and subjected to flow cytometric analysis. CD103+ CD3+ CD8+ cells are shown as a percentage of total CD3+ CD8+ T cells (n ≥ 6/group). The percentage of CD3+ CD8+ CD103+ (Trm) cells among CD3+ CD8+ T cells in brains of mice infected for ≥90 days (n = 5 to 8/group) is also shown. (E and F) Lymphocytes purified from perfused whole brains of mice infected for ≥90 days. (E) Percentages of IFN-γ+ CD3+ CD8+ CD103+ and CD3+ CD8+ CD103− cells (n = 8/group). (F) Percentages of granzyme B+ CD3+ CD8+ CD103+ and CD3+ CD8+ CD103− cells (n = 8/group). #, P < 0.05, Mann-Whitney U test. Data are representative of those from at least 2 independent experiments.
We further analyzed Trm lymphocyte effector functions using intracellular staining and flow cytometric analysis. Coincident with the increase in Trm lymphocytes at late time points postinfection, protein levels of the effector molecules IFN-γ and granzyme B were also elevated, compared to baseline values in CD103-negative CD8+ T cells isolated from the same brains (Fig. 3E and F). Thus, Trm lymphocytes present during long-term infection maintain an effector phenotype for months after viral challenge.
Transient depletion of the adaptive immune response leads to viral reactivation.
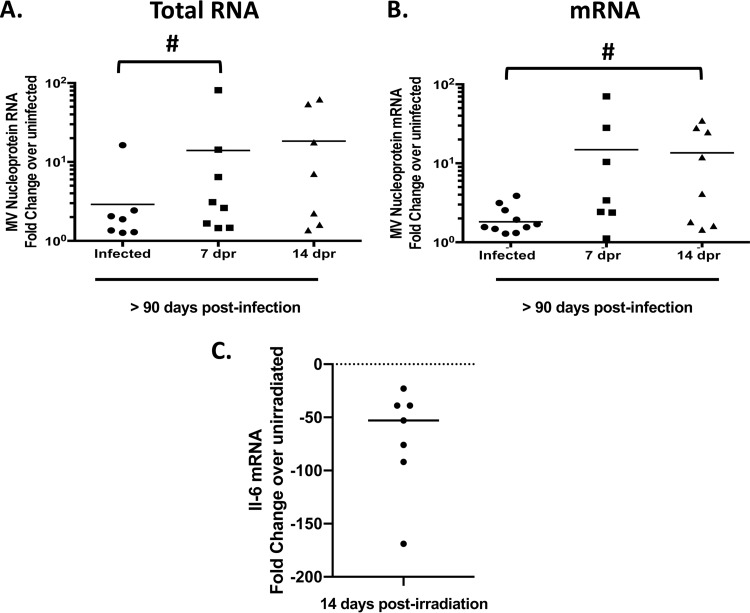
The presence of Trm lymphocytes in the brain during long-term MV infection led us to next ask whether depletion of Trm lymphocytes would lead to viral reactivation. We first used sublethal irradiation to transiently deplete adaptive immune cells in mice that had been infected >90 days previously and then assessed relative amounts of MV RNA and mRNA following irradiation. We observed significant increases in viral RNA and mRNA present within the brains of infected mice after immune depletion (Fig. 4A and B), coincident with a depletion of adaptive immune cells (data not shown). Furthermore, MV proteins were detected in the brains of immunosuppressed mice but not in unirradiated, control mice (Fig. 5). Because we were concerned that sublethal irradiation might induce inflammatory changes within the brain in addition to the anticipated immunodepletion, we assessed mRNA levels of the proinflammatory cytokine interleukin-6 (IL-6) at 14 days after sublethal irradiation. As shown in Fig. 4C, we did not detect an increase in IL-6 mRNA, indicating that irradiation does not induce a potent proinflammatory response. Rather, we found lower mRNA levels, though these are relative to the low basal levels in brains of unmanipulated mice. Thus, after transient immunosuppression, transcription and protein synthesis of viral genes rebounded in mice that had been infected months earlier.
FIG 4.
Sublethal irradiation leads to increased detection of MV RNA and mRNA. NSE-CD46+ mice were challenged i.c. with 1 × 104 PFU of MV Edmonston, and sublethally irradiated (6.5 gy) at least 90 days later. RNA expression levels from perfused brains were determined by RT-qPCR using random-hexamer or oligo(dT) priming for cDNA generation, followed by qPCR with primers specific for the MV nucleoprotein and cyclophilin B as a standard. Data were analyzed using the ΔΔCT method from at least 2 independent experiments (n = 8 to 12/group). #, P < 0.05, Mann-Whitney U Test. (A) cDNA generated using random-hexamer priming. (B) cDNA generated using oligo(dT) priming. (C) RNA was purified from perfused brains at 14 days post-sublethal irradiation, at the peak of viral RNA reemergence. Data are shown as percent decrease from mock-infected controls (dashed line). dpr, days postirradiation.
FIG 5.
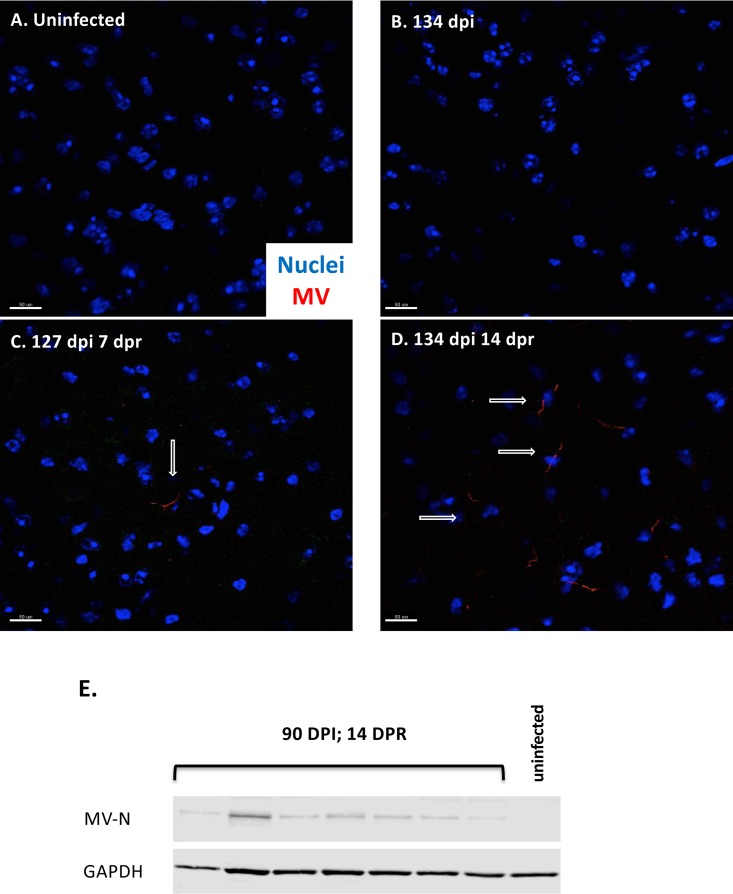
Sublethal irradiation leads to increased expression of MV protein. (A to D) Immunofluorescence of whole-brain tissue obtained from uninfected mice (A), immunocompetent persistently infected (134 days postinfection) mice (B), persistently infected mice sublethally irradiated for 7 days (C), and persistently infected mice sublethally irradiated for 14 days (D). Nuclear staining (Hoechst [blue]) and MV staining (human polyclonal MV antibody [red]) were used. Arrows indicate MV-positive cells. Scale bar = 50 μm. (E) NSE-CD46+ mice were challenged i.c. with 1 × 104 PFU of MV-Ed for at least 90 dpi, followed by 14 days of sublethal irradiation. Shown is Western blot analysis of protein purified from whole-brain tissue of individual mice probed for MV nucleoprotein and GAPDH.
Complete ablation of the adaptive immune response leads to viral reactivation and pathogenesis.
Sublethal irradiation only transiently depletes dividing cells; thus, reactivated virus may be controlled as immune cells are repopulated in transiently immunosuppressed mice. Therefore, to further investigate the possible pathogenic outcomes of viral reactivation under conditions of sustained immune cell loss, we generated bone marrow chimeras (BMCs) to permanently ablate adaptive immunity in long-term-infected mice. BMCs were established using bone marrow from donor immunocompetent (WT) or immunodeficient (RAG2 KO) mice transferred into lethally irradiated recipients that had been infected >90 days previously (Fig. 6A).
FIG 6.
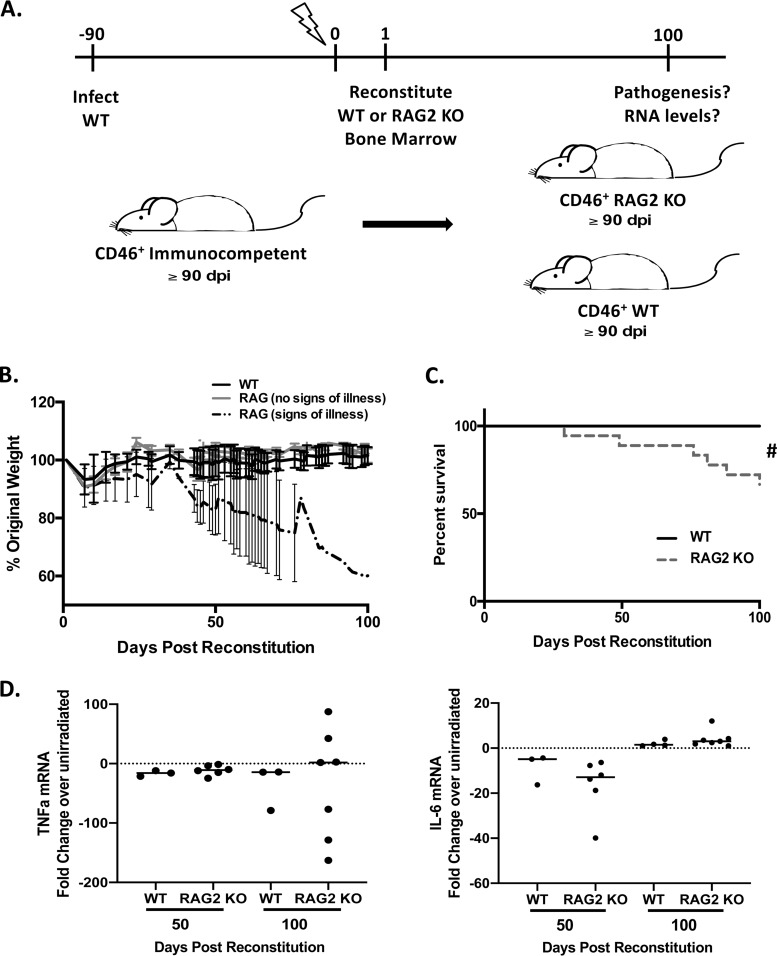
Loss of adaptive immunity after clearance of acute infection leads to pathogenesis. Bone marrow chimeras were generated using NSE-CD46+ mice that had been infected with 1 × 104 PFU of MV-Ed at least 90 days previously. Infected mice were reconstituted with the indicated bone marrow (WT or RAG2 KO) and monitored daily. (A) Schematic of bone marrow chimera generation. (B) Average weight gain or loss of indicated bone marrow recipients. (C) Survival of reconstituted mice (n = 14 to 18/group). (D) RNA was purified from perfused brains of bone marrow recipients (both wild type and RAG2 KO) at 50 and 100 days postreconstitution, and levels of tumor necrosis factor alpha (TNF-α) and IL-6 RNAs were determined. Data are shown as percent change (increase or decrease) compared to mock-infected controls (dashed line). Log rank (Mantel-Cox) test for significance was used. #, P < 0.05.
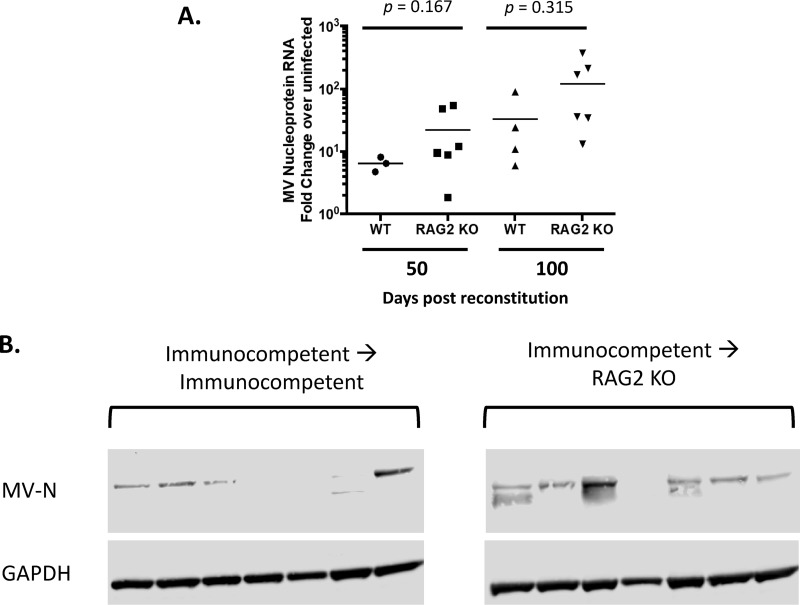
A proportion of long-term-infected mice reconstituted with RAG2 KO bone marrow began to display weight loss and kyphosis by ∼40 to 50 days postreconstitution, not seen in recipients given wild-type bone marrow (Fig. 6B; see also Videos S1 to S4 in the supplemental material). Coincident with these signs of illness, ∼30% of mice displayed spastic paraparesis with dyscoordination of the hindlimbs (Videos S1 to S4), requiring euthanasia (Fig. 6C). As with sublethal irradiation, lethal irradiation prior to bone marrow grafting did not have an appreciable impact on cytokine production within the brain (Fig. 6D). Analysis of whole-brain tissue from long-term-infected mice reconstituted with RAG2 KO bone marrow indicated a trend toward increased detection of viral RNA at both 50 and 100 days postreconstitution (Fig. 7A). Increased viral RNA also was detected in recipients of wild-type bone marrow, perhaps because there was insufficient time to fully repopulate adaptive immunity within the CNS following immune ablation. Moreover, while the presence of MV-N protein was either not detected or barely detected in brains of recipients of wild-type bone marrow, 6/7 RAG2 KO bone marrow chimera recipients had high levels of MV-N protein within the brain (Fig. 7B).
FIG 7.
Loss of adaptive immunity after clearance of acute infection leads to viral reactivation. Bone marrow chimeras were generated using NSE-CD46+ mice that had been infected with 1 × 104 PFU of MV-Ed at least 90 days previously. Infected mice were reconstituted with the indicated bone marrow (WT or RAG2 KO) and monitored daily. (A) RT-qPCR analysis of whole-brain tissue collected from reconstituted mice at the indicated times postreconstitution (n = 3 to 6/group). Data are representative of those from 2 or 3 independent experiments. (B) Western blot analysis of protein purified from whole-brain tissue of individual, reconstituted mice probed for MV nucleoprotein and GAPDH.
Interestingly, the pathogenic motor phenotype observed in BMC RAG2 KO recipients was distinct from the disease seen in acutely infected moribund NSE-CD46+ RAG2 KO animals, in which death occurred by 3 to 4 weeks postinfection (Fig. 1A and B and Video S4). In acutely infected, moribund RAG2 KO mice, illness was characterized by severe weight loss and kyphosis, with no signs of motor dysfunction (Video S4). In contrast, RAG2 KO bone marrow chimera recipients, but not wild-type bone marrow chimera recipients, displayed progressive hindlimb weakness (Videos S1 to S3). Together, these data indicate an essential role for adaptive immunity in controlling a long-term/dormant neurotropic RNA virus infection throughout the life of once-infected mice. Following even transient losses in adaptive immunity, reactivation of viral transcription and translation can occur. Complete ablation of adaptive immunity leads to enhanced viral reactivation and pathogenesis, distinct from that observed during an acute, unrestricted infection.
DISCUSSION
There is substantial ambiguity in how long-lasting viral infections are described in the literature. Often, terms such as “persistent” or “chronic” are used to define a virus that continues to replicate and that remains able to spread to uninfected cells, such as hepatitis B and C virus in humans and lymphocytic choriomeningitis virus in mice. In contrast, “latent” refers to those viruses that do not actively replicate but remain able to reactivate at some point later in life, such as many of the herpesviruses. During latency, RNA and protein syntheses are massively reduced and only selected RNAs are transcribed. It is almost certain that these descriptions define just two of the larger number of long-term viral infection patterns. This may be especially true for RNA viruses. While the field has generally considered these viruses to be cleared from the host soon after an acute infection, it is becoming appreciated that these viruses may be able to establish long-lasting infections. In this study, using a permissive mouse model, we showed that both MV RNA and mRNA remain detectable in apparently healthy mice long after initial immune control of the acute infection; in fact, we were able to detect MV RNA for up to 2 years postinfection in some mice (Fig. 1E). We believe that this is not a traditional persistent infection: all mice are healthy and there is no evidence of viral spread from one host to another after infection. Yet this is also not a latent infection: all viral mRNAs are synthesized (data not shown), albeit to levels far below those seen at the peak of acute infection, implying some low-level translation of the RNA-dependent RNA polymerase. Based on these observations, we believe that MV can enter into a chronic nontransmissible replication state within the CNS, which we refer to as “dormancy” to distinguish it from persistence and latency.
Our findings are likely not unique to this model system as many neurotropic viruses of both mice and humans cannot be recovered in an infectious form following the acute phase of infection in the brain, despite the presence of detectable RNA (8, 13, 33). Therefore, it has generally been assumed that residual viral RNA is a molecular fossil, incapable of reactivation and thus having little impact on host biology. However, we also know that RNA viral infections of the CNS can evoke devastating host diseases later in life, as evidenced by fatal CNS diseases such as subacute sclerosing panencephalitis (SSPE) following acute human MV infection. In this case, SSPE can occur months to years after acute infection (34), and the incidence of SSPE, once thought to occur at a rate of 1:1,700 to 10,000 acute MV infections, has recently been revised to be as many as 1:600 for those infected before 1 year of age. This reevaluation highlights that neuropathogenic consequences temporally distant from an acute infection may be more common than once appreciated (35–37).
In the present study, we showed that following immunosuppression, achieved either transiently by sublethal irradiation or permanently following lethal irradiation and bone marrow reconstitution, levels of viral mRNAs and protein rebound significantly. When we evaluated brains from infected mice at the times most relevant to these studies, we found no global changes in cytokine production, implying that irradiation—at least at the time points tested—did not markedly alter brain homeostasis or produce a proinflammatory state. Nevertheless, we are considering less aggressive strategies to deplete selected immune populations from the CNS, though the blood-brain barrier poses an obstacle for the use of depleting antibodies. Reconstitution of mice with RAG2 KO bone marrow is associated with a unique motor disease that is phenotypically distinct from the disease that results from acute infection in immunocompromised mice. We evaluated brain RNA from these sick mice to determine if particular CNS regions were disproportionally infected compared to the case with acute infection, but no overt differences were noted (data not shown). Efforts to isolate infectious virus from these mice to date have failed, though we cannot rule out that virus is transmitted transsynaptically in the absence of extracellular infectious virus (as occurs during the acute infection), and/or that viral production occurs intermittently throughout life. Alternatively, recrudescence of viral transcription and translation may not be associated with new virus particle production, and thus production of infectious viral progeny may not necessarily be a prerequisite for disease. For example, the expression of viral (non-self) antigens in the CNS may be sufficient to evoke a chronic inflammatory response which may contribute to disease long after initial viral exposure. For example, both mouse hepatitis virus and Theiler’s murine encephalomyelitis virus induce inflammatory disease leading to demyelination and paralysis, in which viral RNA is present, but little or no infectious virus is detected.
The fact that MV RNA can remain in the CNS may not be all that surprising, as clearance and control of MV from CNS neurons are mediated in a noncytolytic fashion (12). Such noncytolytic clearance of viral pathogens preserves the infected neuron while sparing the host the devastating consequences of neuronal loss, creating a potential “harbor” for viral dormancy. Immune-mediated mechanisms may be in place to prevent translation of viral proteins, as well as the resultant host damage that may occur as a result of such an event. This is especially clear in the case of HSV infection of peripheral neurons. Latently infected trigeminal ganglion neurons are the predominant site of HSV latency and persistence, but Trm lymphocytes, adjacent to infected neurons, keep the virus in check. These Trm lymphocytes produce interferons and lytic granules that directly prevent HSV reactivation from latency, while maintaining the integrity of the host neuron (15, 17, 19, 20, 24). While we have not proven a direct effect of Trm lymphocytes in this model, we show that these cells are highly enriched in the MV-infected brain long after viral inoculation and maintain an effector phenotype in the CNS throughout the 3- to 4-month window of observation. Testing the contributions of a brain resident immune population poses some challenges: the blood-brain barrier limits entry of neutralizing antibodies, and adoptive-transfer experiments are limited by the small number of Trm lymphocytes within the brains of dormantly infected mice, coupled with a lack of certainty whether intravenous delivery of these cells would allow them to home to sites of dormant infection. Nevertheless, depletion of adaptive immune cells, which includes CNS-resident Trm lymphocytes, is associated with increased viral transcription and translation within the CNS, causing disease similar to cerebellar ataxia in humans. Ongoing efforts will take advantage of an MV-permissive mouse model in which we can more easily identify infected cells via a color change assay.
As stated in the introduction, many CNS diseases of unknown etiology have been speculated to have a viral trigger, but the inability to consistently detect viral antigens or recover infectious virus from host tissue has cast doubt on such claims. Nevertheless, some assays are in development to look for viral etiologies for chronic CNS diseases. For example, a positive MRZ reaction (intrathecal antibody directed against 2 of 3 viruses: MV, rubella virus, and varicella-zoster virus) is considered by some to be a prognostic indicator of MS, though this test is not in widespread clinical use. Further, persistent MV infection has been speculated as a causative agent of otosclerosis (localized bone displacement resulting in hearing loss) (38–40), suggesting again that an infection acquired earlier in life can contribute to diseases that are not typically attributed a viral etiology. Finally, a link has been proposed between cytomegalovirus (CMV) and glioblastoma, as anti-CMV treatments led to a reduction in glioblastoma tumor burden (41). In searching for evidence of infectious causality, many of these studies look to recover infectious virus, with various degrees of success. We would suggest that our studies provide proof-of-principle evidence that the presence of non-self proteins in the brain may be sufficient to reawaken memory immune cells specific for those proteins and may thus trigger neuronal dysfunction that may manifest in a manner wholly different from that observed in the acute infection. As a result, persistent RNA viral infections may lead to unanticipated CNS consequences in the host later in life. Thus, the temporal interlude between infection and disease, coupled with disease symptoms distinct from what are observed during acute infection, may have precluded a full understanding of how neurotropic viruses contribute to CNS diseases of unknown etiology.
MATERIALS AND METHODS
Ethics statement.
This study was carried out in accordance with the recommendations provided in the Guide for the Care and Use of Laboratory Animals (42). The protocol was reviewed and approved by the Fox Chase Cancer Center Institutional Animal Care and Use Committee (Office of Laboratory Animal Welfare assurance number A3285-01). All animals were housed in the AALAC-accredited Laboratory Animal Facility at the Fox Chase Cancer Center.
Mice and in vivo infections.
Homozygous NSE-CD46+ transgenic mice (line 18; H-2b) (26) were maintained in the closed breeding colony of the Fox Chase Cancer Center. RAG2 KO mice were a gift from F. W. Alt (Howard Hughes Medical Institute, Boston, MA). Genotypes of mice used in these experiments were confirmed by PCR analysis of tail biopsy specimen DNA and/or blood samples collected from the retro-orbital sinus. Both sexes were used; no differences in pathogenesis have been observed between males and females. All mice were between 6 and 8 weeks of age at the beginning of each experiment.
Isoflurane-anesthetized mice were infected with MV Edmonston (MV-Ed) via intracranial inoculation of 1 × 104 PFU in a volume of 30 μl, delivered along the midline using a 27-gauge needle. Mice were monitored daily postinfection for signs of illness, including weight loss, ruffled fur, ataxia, and seizures. Moribund mice were euthanized in accordance with IACUC guidelines. RNA was isolated from individual mice at the desired times postinfection using TRI reagent (Sigma) and subjected to analysis as described below.
Irradiation and bone marrow chimeras. (i) Sublethal irradiation.
Mice infected for at least 90 days were subjected to sublethal (6.5 gy) panoramic gamma irradiation using a Shepherd model 81-14R Cs-137 panoramic irradiator. Animals were monitored daily for signs of illness until the time of collection. Transient immune depletion was verified by flow cytometric analysis of peripheral blood lymphocytes obtained from the retro-orbital sinus of isoflurane-anesthetized mice.
(ii) Bone marrow chimera generation.
Mice infected for at least 90 days were subjected to lethal (2 doses of 5.5 gy separated by 4 h) panoramic gamma irradiation using a Shepherd model 81-14R Cs-137 panoramic irradiator and immediately placed on a medicated diet (Uniprim chow). In parallel, donor animals were euthanized and bone marrow was extracted from the femur and tibia; the marrow was then pooled from individual mice and passed through a 70-μm cell strainer. Red blood cells (RBCs) were depleted using ACK lysis buffer, and the remaining cells were washed twice with sterile PBS and counted using a Countess cell counter. A total of 1 × 107 cells were suspended in 100 to 200 μl of PBS and administered to irradiated recipient mice via retro-orbital sinus 18 h after the final irradiation of the recipients. Reconstituted animals were observed daily for signs of illness. Depletion and reconstitution were confirmed by flow cytometric analysis of splenic and brain lymphocytes. Further confirmation was performed by staining stored splenic tissue from individual mice.
Flow cytometry analysis of purified lymphocytes.
Brains and spleens were removed and pressed through a 70-μm nylon mesh cell strainer in PBS. Dissociated tissue was run over a 70% Percoll gradient for 20 min at 4°C. Mononuclear cells (MNCs) were recovered from the pellet, washed with PBS, treated with 0.84% ammonium chloride to remove contaminating RBCs and washed again. Collected MNCs were counted and plated into a V-bottom 96-well plate for subsequent antibody staining and analysis by multicolor flow cytometry. The following antibodies (purchased from eBioscience and BioLegend) were used: allophycocyanin (APC)-eFluor 780 anti-mouse CD4, phycoerythrin (PE) anti-mouse CD3e, peridinin chlorophyll protein (PerCP)-cyanine 5.5 anti-mouse CD8, APC anti-mouse CD103, Alexa Fluor 700 anti-mouse CD69, PE/Cy5 anti-mouse CD19, Pacific Blue anti-mouse IFN-γ, and PE-cyanine 7 anti-mouse granzyme B. Cells were incubated with antibody for 1 h at 4°C and then washed following the incubation period.
For detection of intracellular proteins, cells were collected and stimulated overnight with 100 ng/ml of mouse recombinant IL-2 (R&D Systems) in a 37°C, 5% CO2 humidified incubator, followed by 4 h of brefeldin A exposure used prior to the initial extracellular staining. After extracellular staining as described above, cells were fixed overnight in 0.5% paraformaldehyde, followed by intracellular staining for 1 h at 4°C. Pelleted, stained cells were resuspended and read in a BD LSR II system. Percentages obtained from flow cytometry were combined with cell counts in order to calculate total cell numbers.
Reverse transcriptase quantitative real-time PCR (RT-qPCR).
RNA was purified from homogenized brain tissue using TRI Reagent as per the manufacturer’s instructions. RNA was quantified using a NanoDrop spectrophotometer. RNA was reverse transcribed using a high-capacity cDNA reverse transcription kit (Applied BioSystems) with random hexamer priming (to detect RNA) or oligo(dT) priming (to detect mRNA). Gene-specific primers were used in combination with probes designed using the universal probe library algorithm (Roche) and universal master mix (Roche); all reactions were run on a Mastercycler Realplex2 system (Eppendorf). Cycling conditions were 50°C for 2 min and 95°C for 10 min, followed by 40 (2-step) cycles of 95°C for 15 s and 60°C for 60 s). Relative quantitation to the control (cyclophilin B) was done using the comparative threshold cycle (ΔΔCT) method. Individual sample PCRs were performed in triplicate. The following gene-specific primers (Integrated DNA Technologies) were used: cyclophilin B forward, 5′-TTC TTC ATA ACC ACA GTC AAG ACC-3′; cyclophilin B reverse, 5′-ACC TTC CGT ACC ACA TCC AT-3′ (UPL 20); MV nucleoprotein forward, 5′-GGA AAC TGC ACC CTA CAT GG-3′; MV nucleoprotein reverse, 5′-GGG TAT GAT CCT GCA CTG AAC T-3′ (UPL 80).
Western blot analyses.
Protein was obtained from homogenized brain tissue using TRI reagent. Samples were suspended in 1× protein solubilization buffer (containing SDS and EDTA) and stored at −80°C until analysis. Protein was electrophoresed into a 10% bis-Tris gel in morpholineethanesulfonic acid (MES) running buffer (Life Technologies) and transferred to an Immobilon membrane (Millipore). Membranes were blocked with Odyssey blocking buffer and immunoblotted for glyceraldehyde-3-phosphate dehydrogenase (GAPDH; 1:10,000, AB2302; Millipore) and MV nucleoprotein (Sigma; 95051114, 1:1,000). Secondary antibodies were obtained from LI-COR (IRDye 680RD donkey anti-chicken IgG [H+L] and IRDye 800CW donkey anti-rabbit IgG [H+L]). Images were captured with a LI-COR Odyssey classic infrared imager.
Immunofluorescence.
For immunofluorescence staining, 10-μm transverse sections were obtained from flash-frozen brains (dry ice/isopentane) in tissue embedding compound and stained with human polyclonal anti-MV serum (a gift from Michael B. A. Oldstone). Directly conjugated secondary antibodies were used (Hoechst; 1:1,000, goat anti-human AF555, 1:5,000). Sections were mounted to coverslips using Citifluor AF1 (Electron Microscopy Sciences) and sealed. Images were captured at a magnification of ×40 using an inverted TE2000 Nikon C1 confocal microscope.
Statistical analysis and figure preparation.
Data representation and statistical analysis were performed using GraphPad Prism. Statistical analysis was performed using the Mann-Whitney U test unless otherwise specified.
Supplementary Material
ACKNOWLEDGMENTS
We gratefully acknowledge Andreas Solomos for his input on experimental design and manuscript preparation. We also acknowledge the Fox Chase Cancer Center Laboratory Animal Facility, Irradiation Facility, and Flow Cytometry Facility.
This was work supported by F31 NS092307 (K.D.M.), T32 NS007180-32 (K.D.M.), R01 NS060701 (G.F.R.), R21 NS099788 (G.F.R.), a gift from the F. M. Kirby Foundation, and Cancer Center Support Grant 5P30CA006927-53.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JVI.00241-19.
REFERENCES
- 1.Jarius S, Eichhorn P, Franciotta D, Petereit HF, Akman-Demir G, Wick M, Wildemann B. 2017. The MRZ reaction as a highly specific marker of multiple sclerosis: re-evaluation and structured review of the literature. J Neurol 264:453–466. doi: 10.1007/s00415-016-8360-4. [DOI] [PubMed] [Google Scholar]
- 2.Hottenrott T, Dersch R, Berger B, Rauer S, Huzly D, Stich O. 2017. The MRZ reaction in primary progressive multiple sclerosis. Fluids Barriers CNS 14:2. doi: 10.1186/s12987-016-0049-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li W, Lee M-H, Henderson L, Tyagi R, Bachani M, Steiner J, Campanac E, Hoffman DA, Geldern von G, Johnson K, Maric D, Morris HD, Lentz M, Pak K, Mammen A, Ostrow L, Rothstein J, Nath A. 2015. Human endogenous retrovirus-K contributes to motor neuron disease. Sci Transl Med 7:307ra153. doi: 10.1126/scitranslmed.aac8201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jurgens HA, Amancherla K, Johnson RW. 2012. Influenza infection induces neuroinflammation, alters hippocampal neuron morphology, and impairs cognition in adult mice. J Neurosci 32:3958–3968. doi: 10.1523/JNEUROSCI.6389-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brot MD, Rail GF, Oldstone MB, Koob GF, Gold LH. 1997. Deficits in discriminated learning remain despite clearance of long-term persistent viral infection in mice. J Neurovirol 3:265–273. doi: 10.3109/13550289709029467. [DOI] [PubMed] [Google Scholar]
- 6.Gomme EA, Wirblich C, Addya S, Rall GF, Schnell MJ. 2012. Immune clearance of attenuated rabies virus results in neuronal survival with altered gene expression. PLoS Pathog 8:e1002971. doi: 10.1371/journal.ppat.1002971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kinchington PR, Leger AJS, Guedon J-M, Hendricks RL. 2012. Herpes simplex virus and varicella zoster virus, the house guests who never leave. Herpesviridae 3:5. doi: 10.1186/2042-4280-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katayama Y, Kohso K, Nishimura A, Tatsuno Y, Homma M, Hotto H. 1998. Detection of measles virus mRNA from autopsied human tissues. J Clin Microbiol 36:299–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Griffin DE. 2010. Recovery from viral encephalomyelitis: immune-mediated noncytolytic virus clearance from neurons. Immunol Res 47:123–133. doi: 10.1007/s12026-009-8143-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Graham JB, Swarts JL, Wilkins C, Thomas S, Green R, Sekine A, Voss KM, Ireton RC, Mooney M, Choonoo G, Miller DR, Treuting PM, Pardo Manuel de Villena F, Ferris MT, McWeeney S, Gale M, Lund JM. 2016. A mouse model of chronic West Nile virus disease. PLoS Pathog 12:e1005996. doi: 10.1371/journal.ppat.1005996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aronsson F, Lannebo C, Paucar M, Brask J, Kristensson K, Karlsson H. 2002. Persistence of viral RNA in the brain of offspring to mice infected with influenza A/WSN/33 virus during pregnancy. J Neurovirol 8:353–357. doi: 10.1080/13550280290100480. [DOI] [PubMed] [Google Scholar]
- 12.Patterson CE, Lawrence DMP, Echols LA, Rall GF. 2002. Immune-mediated protection from measles virus-induced central nervous system disease is noncytolytic and gamma interferon dependent. J Virol 76:4497–4506. doi: 10.1128/JVI.76.9.4497-4506.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Griffin DE, Levine B. 1992. Persistence of viral RNA in mouse brains after recovery from acute alphavirus encephalitis. J Virol 66:6429–6435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Divito S, Cherpes TL, Hendricks RL. 2006. A triple entente: virus, neurons, and CD8+ T cells maintain HSV-1 latency. Immunol Res 36:119–126. doi: 10.1385/IR:36:1:119. [DOI] [PubMed] [Google Scholar]
- 15.St Leger AJ, Hendricks RL. 2011. CD8+ T cells patrol HSV-1-infected trigeminal ganglia and prevent viral reactivation. J Neurovirol 17:528–534. doi: 10.1007/s13365-011-0062-1. [DOI] [PubMed] [Google Scholar]
- 16.Jeon S, St Leger AJ, Cherpes TL, Sheridan BS, Hendricks RL. 2013. PD-L1/B7-H1 regulates the survival but not the function of CD8+ T cells in herpes simplex virus type 1 latently infected trigeminal ganglia. J Immunol 190:6277–6286. doi: 10.4049/jimmunol.1300582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knickelbein JE, Khanna KM, Yee MB, Baty CJ, Kinchington PR, Hendricks RL. 2008. Noncytototoxic lytic granule-mediated CD8+ T cell inhibition of HSV-1 reactivation from neuronal latency. Science 322:268–271. doi: 10.1126/science.1164164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khanna KM, Bonneau RH, Kinchington PR, Hendricks RL. 2003. Herpes simplex virus-specific memory CD8+ T cells are selectively activated and retained in latently infected sensory ganglia. Immunity 18:593–603. doi: 10.1016/S1074-7613(03)00112-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu T, Khanna KM, Chen X, Fink DJ, Hendricks RL. 2000. CD8+ T cells can block herpes simplex virus type 1 (HSV-1) reactivation from latency in sensory neurons. J Exp Med 191:1459–1466. doi: 10.1084/jem.191.9.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wakim LM, Woodward-Davis A, Liu R, Hu Y, Villadangos J, Smyth G, Bevan MJ. 2012. The molecular signature of tissue resident memory CD8 T cells isolated from the brain. J Immunol 189:3462–3471. doi: 10.4049/jimmunol.1201305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Theil D, Derfuss T, Paripovic I, Herberger S, Meinl E, Schueler O, Strupp M, Arbusow V, Brandt T. 2003. Latent herpesvirus infection in human trigeminal ganglia causes chronic immune response. Am J Pathol 163:2179–2184. doi: 10.1016/S0002-9440(10)63575-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu T, Khanna KM, Carriere BN, Hendricks RL. 2001. Gamma interferon can prevent herpes simplex virus type 1 reactivation from latency in sensory neurons. J Virol 75:11178–11184. doi: 10.1128/JVI.75.22.11178-11184.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu T, Tang Q, Hendricks RL. 1996. Inflammatory infiltration of the trigeminal ganglion after herpes simplex virus type 1 corneal infection. J Virol 70:264–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wakim LM, Woodward-Davis A, Bevan MJ. 2010. Memory T cells persisting within the brain after local infection show functional adaptations to their tissue of residence. Proc Natl Acad Sci U S A 107:17872–17879. doi: 10.1073/pnas.1010201107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wakim LM, Gebhardt T, Heath WR, Carbone FR. 2008. Cutting edge: local recall responses by memory T cells newly recruited to peripheral nonlymphoid tissues. J Immunol 181:5837–5841. doi: 10.4049/jimmunol.181.9.5837. [DOI] [PubMed] [Google Scholar]
- 26.Rall GF, Manchester M, Daniels LR, Callahan EM, Belman AR, Oldstone M. 1997. A transgenic mouse model for measles virus infection of the brain. Proc Natl Acad Sci U S A 94:4659–4663. doi: 10.1073/pnas.94.9.4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lawrence DMP, Vaughn MM, Belman AR, Cole JS, Rall GF. 1999. Immune response-mediated protection of adult but not neonatal mice from neuron-restricted measles virus infection and central nervous system disease. J Virol 73:1795–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reference deleted.
- 29.Reference deleted.
- 30.Himmelein S, Leger AJS, Knickelbein JE, Rowe A, Freeman ML, Hendricks RL. 2011. Circulating herpes simplex type 1 (HSV-1)-specific CD8+ T cells do not access HSV-1 latently infected trigeminal ganglia. Herpesviridae 2:5. doi: 10.1186/2042-4280-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Solomos AC, O’Regan KJ, Rall GF. 2016. CD4+ T cells require either B cells or CD8+ T cells to control spread and pathogenesis of a neurotropic infection. Virology 499:196–202. doi: 10.1016/j.virol.2016.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tishon A, Lewicki H, Andaya A, McGavern D, Martin L, Oldstone M. 2006. CD4 T cell control primary measles virus infection of the CNS: regulation is dependent on combined activity with either CD8 T cells or with B cells: CD4, CD8 or B cells alone are ineffective. Virology 347:234–245. doi: 10.1016/j.virol.2006.01.050. [DOI] [PubMed] [Google Scholar]
- 33.Katayama Y, Hotta H, Nishimura A, Tatsuno Y, Homma M. 1995. Detection of measles virus nucleoprotein mRNA in autopsied brain tissues. J Gen Virol 76:3201–3204. doi: 10.1099/0022-1317-76-12-3201. [DOI] [PubMed] [Google Scholar]
- 34.Anlar B, Pinar A, Yaşar Anlar F, Engin D, Ustaçelebi Ş, Kocagöz T, Us D, Akduman D, Yalaz K. 2002. Viral studies in the cerebrospinal fluid in subacute sclerosing panencephalitis. J Infect 44:176–180. doi: 10.1053/jinf.2002.0974. [DOI] [PubMed] [Google Scholar]
- 35.Ludlow M, McQuaid S, Milner D, de Swart RL, Duprex WP. 2015. Pathological consequences of systemic measles virus infection. J Pathol 235:253–265. doi: 10.1002/path.4457. [DOI] [PubMed] [Google Scholar]
- 36.Campbell B. 2016. Fatal measles complication more common than thought: U.S. study. Clin Infect Dis Advance Access. [Google Scholar]
- 37.Gutierrez J, Issacson RS, Koppel BS. 2010. Subacute sclerosing panencephalitis: an update. Dev Med Child Neurol 52:901–907. doi: 10.1111/j.1469-8749.2010.03717.x. [DOI] [PubMed] [Google Scholar]
- 38.Potocka-Bakłażec M, Sakowicz-Burkiewicz M, Kuczkowski J, Pawełczyk T, Stankiewicz C, Sierszeń W, Jankowski Z, Buczny J. 2014. Expression of TNF-α, OPG, IL-1β and the presence of the measles virus RNA in the stapes of the patients with otosclerosis. Eur Arch Otorhinolaryngol doi: 10.1007/s00405-014-3008-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cohen BE, Durstenfeld A, Roehm PC. 2014. Viral causes of hearing loss: a review for hearing health professionals. Trends Hear 18:233121651454136–233121651454117. doi: 10.1177/2331216514541361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rudic M, Keogh I, Wagner R, Wilkinson E, Kiros N, Ferrary E, Sterkers O, Grayeli AB, Zarkovic K, Zarkovic N. 2015. The pathophysiology of otosclerosis: review of current research. Hear Res 330:51–56. doi: 10.1016/j.heares.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 41.Söderberg-Nauclér C, Johnsen JI. 2015. Cytomegalovirus in human brain tumors: role in pathogenesis and potential treatment options. World J Exp Med 5:1–11. doi: 10.5493/wjem.v5.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed National Academies Press, Washington, DC. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.