Abstract
Pulmonary embolism represents the third most common cause of cardiovascular death in the United States. Reperfusion therapeutic strategies such as systemic thrombolysis, catheter directed therapies, surgical pulmonary embolectomy, and cardiopulmonary support devices are currently available for patients with high- and intermediate-high–risk pulmonary embolism. However, deciding on optimal therapy may be challenging. Pulmonary embolism response teams have been designed to facilitate multidisciplinary decision-making with the goal to improve quality of care for complex cases with pulmonary embolism. Herein, we discuss the current role and strategies on how to leverage the strengths from pulmonary embolism response teams, its possible worldwide adoption, and implementation to improve survival and change the paradigm in the care of a potentially deadly disease.
Keywords: high-risk pulmonary embolism, intermediate-high–risk pulmonary embolism, reperfusion strategies, pulmonary embolism response team
Introduction
Venous thromboembolism remains a common catastrophic event in the United States, leading to an estimated 100 000 to 200 000 deaths per year.1,2 Pulmonary embolism (PE) is the leading cause of preventable death among hospitalized patients.3-8
Appropriate PE classification remains a critical step to direct clinical decision-making.9 High risk, also known as massive PE, is defined as hemodynamic instability manifested by persistent hypotension with a systolic blood pressure of <90 mm Hg or cardiogenic shock and carries the highest mortality rate exceeding 50%.10,11 Those patients with normotension and presence of right ventricular (RV) strain, by echocardiography, computed tomography angiography (CTA) and/or elevated cardiac biomarkers, are classified as intermediate-risk or submassive PE. Furthermore, recent European Society of Cardiology (ESC) guidelines propose dividing intermediate-risk into intermediate-high and intermediate-low risk.7 The intermediate-high–risk PE have RV strain both by imaging and by positive biomarkers, while the intermediate-low risk may have none or only one of these present.
Most recent PE guidelines, including American College of Chest Physicians (ACCP),12 ESC,7 and American Heart Association (AHA),3 provide recommendations for patients with hemodynamically significant or high-risk PE and highlight the role of systemic thrombolysis.12–15 However, the guidelines are less specific in intermediate-risk PE and differ on the recommendations of advanced reperfusion therapies, including systemic thrombolysis, catheter-directed thrombolysis (CDT), catheter thrombectomy, and/or surgical thrombectomy.
Once the diagnosis of PE is made, appropriate, rapid, and accurate risk stratification of PE subtypes remains crucial to assess the different therapeutic options for each patient.16–19 The pulmonary embolism severity index is a validated clinical prediction rule that uses PE risk factors, including age, chronic heart and lung comorbidities, malignancy, tachycardia, tachypnea, and hypotension, among others, to calculate mortality risk;7 and a simplified version has been validated to estimate 30-day mortality rate.18 Other important prognostic tools are cardiac biomarkers (troponin, brain natriuretic peptide [BNP], and pro-BNP) and presence of RV strain on imaging studies (computed CTA and/or echocardiogram). Patients with both elevated BNP and troponin levels are at risk of worse outcomes.7,20,21
In 2012, the Massachusetts General Hospital (MGH) introduced the Pulmonary Embolism Response Team (PERT).22 Shortly after, other hospitals organized and formed their own institutional PERT. In May 2015, the National PERT Consortium was established when different institutions across the United States met in Boston, Massachusetts, with the intent to guide and advance care for patients with PE. The PERT builds further upon the rapid response team concept by incorporating a process, whereby immediate multidisciplinary consultation is utilized to achieve consensus regarding the optimal, individualized care for each complex clinical scenario. In this review, we will study the structure and impact of the PERT model in the management of acute PE and its expansion worldwide.
Structure and Function of PERT
The PERT team is an innovative systems-based program of multidisciplinary collaboration dedicated to improving care for patients with PE. The aim of PERT is to facilitate rapid consultation and expert consensus with experienced specialists in complex patients.23 Its core mission is to advance patient care with PE through research and quality improvement programs. To achieve this mission, a registry including clinical data may be created. One of the goals of the PERT consortium is the multicenter collaboration nationally and internationally in education, research, and clinical guidelines.24
Pulmonary embolism response team members vary by institution and may involve pulmonary, critical care, cardiology, vascular medicine, vascular surgery, hematology, diagnostic radiology, interventional radiology, emergency medicine, cardiac surgery, and/or pharmacy. In some hospitals, there might be 2 to 3 PERT members, whereas in other centers it might be 5 to 10.
After a referring physician activates the PERT, a team member gathers relevant patient clinical information and presents it to other team members providing rapid consultation and decision-making. The cornerstone of the model rests on team activation via a single contact number (or pager) notification system and, if available, the utilization of a virtual web-based conference program that enables clinicians to review data and discuss cases in real time. PERT can also coordinate and provide outpatient follow-up as well as serve as a research platform for patient registries or clinical trials.
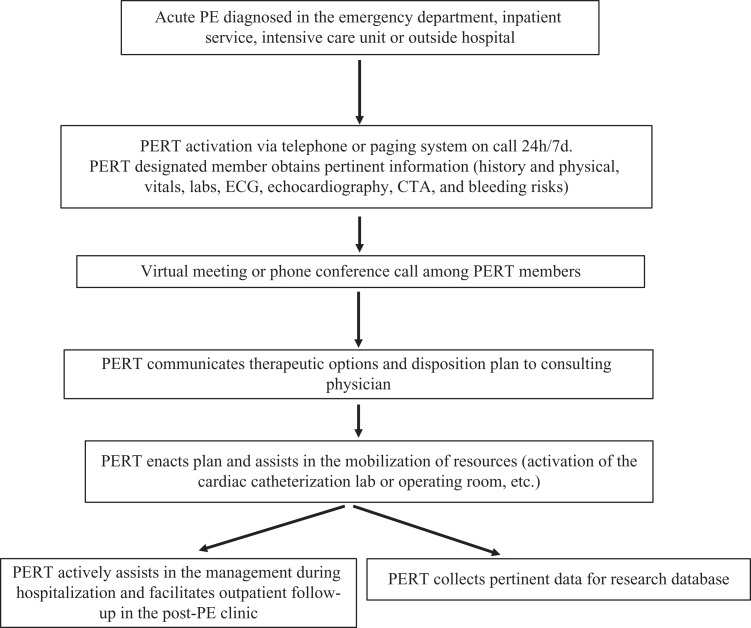
Treatment of patients with intermediate-high–risk and high-risk PE remains controversial and highly variable. A broad range of therapeutic options are available: anticoagulation (AC), systemic thrombolysis, CDT, catheter/mechanical thrombectomy, or surgical thrombectomy; and there is still insufficient data of comparative outcomes for each treatment option.25 The AHA, ESC, and ACCP guidelines do not provide specific recommendations for all these advanced treatment strategies and the clinical scenarios in which they may be appropriate. To address these concerns and with the aim to close the gap in practice variability, PERT was introduced.26 Multidisciplinary, comprehensive, and rapid consultation analyzes individual risks and benefits of each treatment option, sharing decision-making and facilitating consensus. This is particularly beneficial when therapeutic decision must be made on an urgent basis with limited time. Figure 1 shows a proposed flow diagram, showing the dynamic, organized, and function of a PERT.
Figure 1.
Pulmonary embolism response team schematic flow diagram showing the dynamic, organized, and functional role of multidisciplinary expert team members during the care of patients with complex PE. CTA, computed tomography angiogram; ECG, electrocardiogram; PE, pulmonary embolism; PERT, pulmonary embolism response team.
Rationale for PERT
As the number of treatment options, available technology, and scientific knowledge increases, the need for interdisciplinary communication and teamwork grows proportionally. A severely ill patient with PE, whether from emergency department, hospital unit, or referring hospital, may need consult from one or more of the following teams: critical care, vascular medicine, interventional cardiology, interventional radiology, cardiac, and/or vascular surgery.23–26 Coordinating these consults and their recommendations can be time consuming for the primary team and may lead to redundant communication, confusing, or conflicting treatment plans and delays in care. The PERT model streamlines care and interdisciplinary communication. The aim is to construct a system that would allow a rapid multidisciplinary assessment and mobilization of resources to minimize morbidity and decrease mortality. Table 1 outlines the advantages and challenges of the PERT programs.
Table 1.
Advantages and Challenges During the Initiation, Organization, and Performance of Pulmonary Embolism Response Team (PERT).
| Advantages: |
|
|
|
|
|
|
|
|
|
| Challenges: |
|
|
|
|
|
|
|
Advanced Reperfusion Strategies Available for PERT
Systemic Thrombolysis
Guidelines recommend systemic thrombolysis for patients who have high-risk PE and low bleeding risk, as it has been associated with improvement in hemodynamics and right ventricular function and decrease in short-term mortality.3,7,10,12 Guidelines also suggest considering systemic thrombolysis in those patients with intermediate-risk PE with severe RV dysfunction and/or evidence of clinical deterioration (worsening blood pressure, oxygenation, and rising cardiac biomarkers) and low bleeding risk.7,10,12 However, systemic thrombolysis is associated with an increase in major hemorrhagic complications, including intracranial hemorrhage7,10,12; patient characteristics such as younger age (<65 year old), nonobese, absence of active malignancy, and consideration of lower doses of thrombolytic agent represent lower risk for major bleeding events, while selecting appropriate patients for systemic thrombolysis may improve the benefit–risk ratio.
Catheter-Directed Thrombolysis
In an effort to reduce the bleeding risk associated with systemic thrombolysis, CDT has emerged as a therapeutic alternative in patients who have intermediate- and high-risk PE.11,27-29 The thrombolytic agent can be administered via a multiside-hole infusion catheter or with the EkoSonic ultrasound-assisted catheter (EKOS, Corporation, Bothell, Washington). Ultrasound-assisted catheter-directed thrombolysis (UCDL) has shown to effectively reverse RV strain in 24 to 48 hours in patients with intermediate- and high-risk PE having low rates of major bleeding.11,27,28 The optimal thrombolytic dosage and duration during UCDL still remains unknown. In a recent study of intermediate-risk PE, 4 different dosing regimens were tested with the tissue plasminogen activator dose ranging from 4 to 12 mg and infusion duration from 2 to 6 hours. All 4 treatment arms showed improved RV function and reduced clot burden.29 In a propensity-matched study of 4426 patients with PE using the National Readmission Database, there were lower mortality and intracranial hemorrhage rates in patients who received CDT compared to systemic thrombolysis30; however. more robust prospective, randomized clinical trial derived evidence is needed.
Catheter Thrombectomy (Aspiration and Mechanical Devices)
Catheter thrombectomy may be considered in patients with intermediate- or high-risk PE, with proximal clot burden and high bleeding risk, contraindications to thrombolysis, or thrombus-in-transit.30–33
The AngioVac catheter (Angiodynamics, Inc, Latham, New York) is a large venous–venous bypass system designed to remove clot via suction and venous reinfusion. Experience with this catheter is limited to case reports and case series, since it has been mostly used within the inferior vena cava, right atrium, and RV but technically difficult to reach the pulmonary vasculature.31 The Flowtriever (Inari Medical, Irvine, California) is a device that mechanically engages thrombus through deployment of 3 self-expanding nitinol disks that retract and entrap thrombus while manual aspiration is performed.31,32 A single arm trial (FLARE [Flowtriever Pulmonary Embolectomy Clinical Study], http://clinicaltrials.gov: NCT02692586) evaluating the safety and efficacy of this device has been completed and preliminary results demonstrate a decrease in RV strain (presented at the Society for Cardiovascular Angiography and Interventions, Scientific Sessions 2018), but publication is pending. The Indigo Thrombectomy System (Penumbra, Inc, Alameda, California) is designed to engage the thrombus and extract thromboembolic material with continuous suction and vacuum pump.31,34 This device recently completed a prospective initial single-center series in 18 patients, demonstrating feasibility,31 and is currently ongoing a multicenter clinical trial investigating its safety and efficacy (NCT03218566).
Surgical Thrombectomy
Surgical thrombectomy may be considered in patients with high-risk PE with high bleeding risk, thrombus-in-transit, failed systemic thrombolysis, or severe shock that is likely to cause death before thrombolysis can take effect.10 This surgical approach in the past was thought to have a high mortality but more recently has demonstrated an improvement in the perioperative mortality of 4% to 6%.35
Hemodynamic Support
Hemodynamically unstable patients may benefit from cardiopulmonary support via extracorporeal membrane oxygenation (ECMO) while they await definitive intervention.36 The ECMO offers potential to stabilize a severely compromised circulation in high-risk PE presenting with severe RV failure, refractory hypoxia, and/or cardiac arrest. The venoarterial configuration (VA-ECMO) may be used as a bridge to surgical embolectomy, endovascular approaches or systemic thrombolysis, or as stand-alone adjunct to anticoagulation.32-34,35,35,36-40 The Impella RP is a 9F catheter designed to be placed at the femoral site, drawing blood from an inlet that sits in the inferior vena cava and expels into the pulmonary artery, generating up to 4.0 L of flow. The experience using this device for PE is limited.8,41 Table 2 summarizes the main advantages and disadvantages of advanced reperfusion strategies for high- and intermediate-risk PE.
Table 2.
Summary of Advanced Reperfusion Strategies for High and Intermediate-Risk Pulmonary Embolism.
| Reperfusion Strategy | Indications | Contraindications | Outcomes | Adverse Events/Disadvantages |
|---|---|---|---|---|
| Systemic thrombolysis | High-risk PE with low bleeding risk. Intermediate-high risk PE with signs of clinical deterioration and low bleeding risk.7,10,12 | Active bleeding; prior intracranial hemorrhage; ischemic stroke within 3 months; recent brain or spinal surgery; recent head or facial trauma; aortic dissection. Cautious use in age >75 years old, coagulopathy, thrombocytopenia, recent major surgery.7,10,12 | Decrease in overall and acute PE-related mortality in high-risk PE.7,10,12 Decrease in escalation of cardiorespiratory support therapies (eg, vasopressors and mechanical ventilation) in intermediate-risk PE.7,10,12 | High risk of life-threatening hemorrhage, including major and intracranial hemorrhage; underutilization by clinicians. 7,10,12 |
| Catheter-directed thrombolysis | High-risk PE with high bleeding risk or after failed thrombolysis. Intermediate-risk PE with severe RV dysfunction or signs of clinical deterioration.7,10,12,29 | Significantly high-risk profile for hemorrhagic complications. | Improvement in RV dysfunction by a decrease in RV/LV ratio at 24 to 48 hours; improvement in pulmonary arterial pressures; decreased rates of major or intracranial hemorrhagic events.7,10,12,29 | 5% to 10% nonmajor hemorrhagic events and procedure-related complications.7,10,12,29 Societal guidelines do not fully endorse and precise role; lack of large randomized controlled trials. |
| Catheter Thrombectomy (aspiration and mechanical devices) | High-risk and intermediate-risk PE with high bleeding risk or absolute contraindications for thrombolysis.31–34 | Distal clot burden. | Case series and small studies demonstrate technical success with
clinical benefit in selected patients.31–34
|
Absence of prospective randomized controlled trials.31–34 Catheter manipulation and technical skills limited to expert centers. High-caliber catheters with risk of vascular perforation, dissection, and bleeding. |
| Surgical thrombectomy | High-risk PE or intermediate-risk PE with absolute contraindications for thrombolysis; failed systemic or catheter-directed thrombolysis and/or therapies; active bleeding; thrombus-in-transit; large patent foramen ovale with significant risk for paradoxical embolization.35 | Significant medical comorbidities (ie. advanced age, chronic end-stage disorders); prolonged out of hospital cardiac arrest. | Overall operative mortality of 6.9%; improvement in 3-year survival rates of 64% for high-risk PE and up to 80% for intermediate-risk PE.35 | Lack of randomized clinical trials; mostly observational, case series and retrospective analysis; Limited to expert centers. |
| Hemodynamic support | May be used as a bridge for other reperfusion strategies or as a stand-alone circulatory support36,37 | Not well defined; cautious use after systemic thrombolysis, due to risk of hemorrhagic events | Effective bridging tool while considering definitive reperfusion strategy36,37 | Lack of clinical trials, mostly case reports, case series and retrospective analysis |
Abbreviations: LV, left ventricular; RV, right ventricular; PE, pulmonary embolism.
Initial Outcomes of PERT
The most common treatment delivered by the initial 30-month experience of the MGH PERT in patients with confirmed PE was anticoagulation therapy alone in 69% (219) of patients, followed by reperfusion strategies such as catheter directed thrombolysis in 28 (9%) patients, systemic thrombolysis in 14 (5%), surgical thrombectomy in 8 (3%), and suction thrombectomy in 1 (0.3%) patient.42 The adoption of the PERT program was immediate and sustained. Most of the PERT activations were for intermediate- and high-risk PE, and the number of team activation increased by an average of 16% every 6 months. Fifty-three percent of PERT activations occur on nights and weekends, suggesting the value of this team’s expertise when staffing may be limited. Forty-one percent of MGH activations received a multidisciplinary online meeting. In these cases, the median time to completing the consult was 128 minutes, the average length of virtual meeting was 25 minutes, and the number of physicians on the call ranged from 5 to 15.42
The Beth Israel Deaconess Hospital also described the initial outcomes of their Massive and Submassive Clot On-call Team, where 16% of consults were on high risk and 83% were for patients with intermediate-risk PE. Anticoagulation alone was used in 65% (n = 46), systemic fibrinolysis in 11% (n = 8), catheter-directed therapy in 18% (n = 13), and extracorporeal membrane oxygenation in 3% (n = 2). Survival to discharge was 89% (64% with high-risk PE and 93% with intermediate-risk PE).43
The New York University program reported in 2017 their experience with 124 program activations.44 Over a 20-month period, 87 patients were classified as intermediate-risk (90.8%) and high-risk (9.2%) PE. CDT was administered to 25 (20%) patients, systemic thrombolysis to 6 (5%), and AC alone to 54 (44%). The median intensive care unit length of stay (LOS) and overall LOS were 6 (3-10 IQR [interquartile range]) and 7 (4-14 IQR) days, respectively. There was a 13.7% inpatient mortality. There were 5 major hemorrhagic events: 1 in the CDT group, 1 in the systemic thrombolysis group, and 3 in the AC group. Similar to the MGH PERT experience, their PERT activations increased at 10 to 12 months after their initiation.
The Cleveland Clinic reported recently their initial PERT experience in a retrospective analysis during a 20-month period.45 Eighty (68%) patients were classified as intermediate-risk and 23 (19%) as high-risk PE. Fourteen (12%) patients received CDT, 6 (5%) patients received full doses of alteplase (100 mg), 16 (13%) patients received half doses of alteplase (50 mg), and 6 (5%) patients underwent surgical embolectomy. Fourteen patients had major hemorrhagic events; interestingly, no significant hemorrhagic events were observed in the subgroups treated with either half or full systemic doses of thrombolysis. They believed the reason for less events could be driven by better strategies while assessing hemorrhagic risk profiles in potential candidates for systemic thrombolysis.45
More recently, Rosovsky et al46 published a 10-year retrospective analysis, evaluating the changes in therapeutic patterns and outcomes in the sickest (submassive and massive) subset of patients with PE during pre-PERT (2006-2012) and post-PERT (2012-2016) eras, studying 212 pre-PERT and 228 post-PERT patients. More patients underwent more catheter-directed therapies (1% vs 14%; P = <.0001) or any other advanced therapy (9% vs 19%; P = .002) during the post-PERT era; these changes were more notorious in the submassive PE subgroup. Interestingly, there were no significant differences in the rates of major hemorrhagic events or overall mortality pre- and post-PERT.
In summary, there has been a significant increase in the activation, utilization, and valuable input of PERT in the last 6 years since its inception; there has been an improvement while performing rapid and effective risk stratification for a given patient; moreover, more awareness has been given, and improvement has been made while assessing the particular hemorrhagic risk profile while deciding which patient may benefit the most from systemic thrombolysis or CDT. However, more robust prospective data may be needed to demonstrate a significant net mortality benefit in future clinical trials while minimizing the occurrence of major hemorrhagic events, particularly intracranial hemorrhage.
Pulmonary Embolism Response Team Controversies, Challenges, and Future Perspectives
Over the last years, treatment of acute PE has rapidly expanded, and it has become more apparent that the care for these complex patients requires multidisciplinary management. The implementation of PERT model allows for rapid, multidisciplinary management of this life-threatening condition.23–26,35
Treatment of patients with severe RV dysfunction in the absence of hypotension remains a clinical challenge. The heterogeneous presentation of acute PE and the diversity of available treatments led to the generation of the MGH PERT program, modeled after a rapid response system. Shortly after, multiple other institutions have created their own PERT and have joined the national PERT Consortium with the goal of promoting PE care, facilitating research, and educating health-care professionals.
The impact, mid-, and long-term outcomes of PERT programs remain unknown as well as the cost-effectiveness and quality improvement data. In addition, professional societal guidelines do not yet endorse and recommend the use of PERT for PE care.
Because clinical data and guidelines do not cover all clinical scenarios in treating patients with PE, the PERT concept is designed to apply multispecialty cognitive and procedural expertise to patients. The PERT combines a team approach to generate a prompt and individualized plan without the need to consult multiple individual physicians. The optimal structure of PERT remains unknown. Overutilization of novel technologies, such as catheter-based therapies, for PE treatment is a possibility, and a true assessment of the appropriate use of interventional therapies will have to wait until more robust multicenter studies with strong clinical evidence-based data exist.
Before considering the implementation of PERT programs, institutions should evaluate which departments or groups of physicians are interested in collaborating as a team, including house staff and other trainees. Operational challenges to launch and sustain a PERT include spreading the initiative both intrahospital and with the local network of referring hospitals, routine meetings of involved ongoing quality analyses, and longitudinal and outpatient follow-up. Ensuring 24-hour coverage is a challenge as well as balancing education for house staff with providing rapid consultation and recommendations. Efficacy data and cost analysis will be required to validate the PERT.23–26,35 Future areas for research are recognized and based on the lack of published evidence of critical areas of PE management. Limitations are the small number of patients, the retrospective nature of the literature available, lack of long-term follow-up in most of studies, and the absence of control groups for comparison.
Pulmonary embolism response team may also assist with long-term anticoagulation decision-making based on patient comorbidities and arrange timely follow-up with patients in the outpatient setting. To date, PERT has treated over 700 patients,23 and since its creation has expanded to 140 academic institutions, allowing the generation of the PERT Consortium. An ongoing collaboration among PERT centers provides an infrastructure for large-scale registries, conduct prospective clinical research for better evidence-based medicine, and finally the inclusion of PERT model in multiple societal clinical guidelines for PE.23,24
Conclusion
Pulmonary embolism response team has been developed to provide prompt diagnosis, fast multimodality risk stratification, and individualize the best therapy available for a given patient with significant PE in a multidisciplinary approach, representing a unique, innovative, and continuously evolving concept and protocol, with the main objective to be adopted not only in the United States but worldwide. As PERT model continues to evolve, more robust supporting evidence-based data will generate and in the future may result in a positive outcome by improving quality of life and survival, changing the paradigm of PE care.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Mateo Porres–Aguilar  https://orcid.org/0000-0002-2180-3000
https://orcid.org/0000-0002-2180-3000
References
- 1. US Department of Health and Human Services. The Surgeon General’s Call to Action to Prevent Deep Vein Thrombosis and Pulmonary Embolism. 2008; http://www.surgeonGeneral.gov/library/calls/deepvein/call-to-action-ondvt-2008.pdf. Accessed July 01, 2018. Updated August 01, 2018 [PubMed]
- 2. Wiener RS, Schwartz LM, Woloshin S. Time trends in pulmonary embolism in the United States: evidence of overdiagnosis. Arch Intern Med. 2011;171(9):831–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Go AS, Mozaffarian D, Roger VL, et al. Executive summary: heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014;129(3):399–410. [DOI] [PubMed] [Google Scholar]
- 4. Cohen AT, Agnelli G, Anderson FA, et al. Venous thromboembolism (VTE) in Europe. The number of VTE events and associated morbidity and mortality. Thromb Haemost. 2007;98(4):756–764. [DOI] [PubMed] [Google Scholar]
- 5. Klok FA, van Kralingen KW, van Dijk AP, et al. Quality of life in long-term survivors of acute pulmonary embolism. Chest. 2010;138(6):1432–1440. [DOI] [PubMed] [Google Scholar]
- 6. Kearon C, Akl EA. Duration of anticoagulant therapy for deep vein thrombosis and pulmonary embolism. Blood. 2014;123(12):1794–1801. [DOI] [PubMed] [Google Scholar]
- 7. Konstantinides SV, Torbicki A, Agnelli G, et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014;35:3033–3069. [DOI] [PubMed] [Google Scholar]
- 8. Friedman O, Horowitz JM, Ramzy D. Advanced cardiopulmonary support for pulmonary embolism. Tech Vasc Interv Radiol. 2017;20(3):179–184. [DOI] [PubMed] [Google Scholar]
- 9. Porres-Muñoz M, Porres-Aguilar M. Intermediate-high-risk pulmonary embolism: standardizing definition and optimizing therapeutic strategies. Am J Med. 2017;130(5):e233. [DOI] [PubMed] [Google Scholar]
- 10. Jaff MR, McMurtry MS, Archer SL, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation. 2011;123(16):1788–1830. [DOI] [PubMed] [Google Scholar]
- 11. Teleb M, Porres-Aguilar M, Rivera-Lebron B, et al. Ultrasound-assisted catheter-directed thrombolysis: a novel and promising endovascular therapeutic modality for intermediate-risk pulmonary embolism. Angiology. 2017;68(6):494–501. [DOI] [PubMed] [Google Scholar]
- 12. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guidelines and expert panel report. Chest. 2016;149(2):315–352. [DOI] [PubMed] [Google Scholar]
- 13. Teleb M, Porres-Aguilar M, Anaya-Ayala JE, Rodriguez-Castro C, Porres-Muñoz M, Mukherjee D. Potential role of systemic thrombolysis in acute submassive intermediate risk pulmonary embolism: review and future perspectives. Ther Adv Cardiovasc Dis. 2016;10(2):103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Virk HUH, Chatterjee S, Sardar P, Bavishi C, Giri J, Chatterjee S. Systemic thrombolysis for pulmonary embolism: evidence, patient selection, and protocols for management. Interv Cardiol Clin. 2018;7(1):71–80. [DOI] [PubMed] [Google Scholar]
- 15. Tapson VF, Friedman O. Systemic thrombolysis for pulmonary embolism: who and how. Tech Vasc Interv Radiol. 2017;20(3):162–174. [DOI] [PubMed] [Google Scholar]
- 16. Pollack JS. Catheter-Based Therapies for Pulmonary Emboli. Clin Chest Med. 2018;39(3):651–658. [DOI] [PubMed] [Google Scholar]
- 17. Neely RC, Byrne JG, Gosev I, et al. Surgical embolectomy for acute massive and submassive pulmonary embolism in a series of 115 patients. Ann Thorac Surg. 2015;100(4):1245–1251. [DOI] [PubMed] [Google Scholar]
- 18. Kosova EC, Desai KR, Schimmel DR. Endovascular management of massive and submassive acute pulmonary embolism: current trends in risk stratification and catheter-directed therapies. Curr Cardiol Rep. 2017;19(6):54. [DOI] [PubMed] [Google Scholar]
- 19. Aujesky D, Roy PM, Le Manach CP, et al. Validation of a model to predict adverse outcomes in patients with pulmonary embolism. Eur Heart J. 2006;27(4):476–481. [DOI] [PubMed] [Google Scholar]
- 20. Bondarsky E, Schaikewitz M, Filopei J, Steiger D. Brain natriuretic peptide/troponin i ratio in pulmonary embolism. Chest. 2017;152(4):A1040. [Google Scholar]
- 21. Jiménez D, Aujesky D, Moores L, et al. Simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism. Arch Intern Med. 2010;170(15):1383–1389. [DOI] [PubMed] [Google Scholar]
- 22. Provias T, Dudzinski DM, Jaff MR, et al. The Massachusetts General Hospital Pulmonary Embolism Response Team (MGH PERT): creation of a multidisciplinary program to improve care of patients with massive and submassive pulmonary embolism. Hosp Pract (1995). 2014;42(1):31–37. [DOI] [PubMed] [Google Scholar]
- 23. Zern EK, Young MN, Rosenfield K, Kabrhel C. A pulmonary embolism response team: initial experiences and future directions. Expert Rev Cardiovasc Ther. 2017;15(6):481–489. [DOI] [PubMed] [Google Scholar]
- 24. Barnes G, Giri J, Courtney DM, et al. Nuts and bolts of running a pulmonary embolism response team: results from an organizational survey of the National PERT™ Consortium members. Hosp Pract (1995). 2017;45(3):76–80. [DOI] [PubMed] [Google Scholar]
- 25. Monteleone PP, Rosenfield K, Rosovsky RP. Multidisciplinary pulmonary embolism response teams and systems. Cardiovasc Diagn Ther. 2016;6(6):662–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Barnes GD, Kabrhel C, Courtney DM, et al. Diversity in the pulmonary embolism response team model: an organizational survey of the national PERT consortium members. Chest. 2016;150(6):1414–1417. [DOI] [PubMed] [Google Scholar]
- 27. Kucher N, Boekstegers P, Müller OJ, et al. Randomized, controlled trial of ultrasound-assisted catheter-directed thrombolysis for acute intermediate-risk pulmonary embolism. Circulation. 2014;129(4):479–486. [DOI] [PubMed] [Google Scholar]
- 28. Piazza G, Hohlfelder B, Jaff MR, et al. A prospective, single-arm, multicenter trial of ultrasound-facilitated, catheter-directed, low-dose fibrinolysis for acute massive and submassive pulmonary embolism: the SEATTLE II study. JACC Cardiovasc Interv. 2015;8(10):1382–1392. [DOI] [PubMed] [Google Scholar]
- 29. Tapson VF, Sterling K, Jones N, et al. A randomized trial of the optimum duration of acoustic pulse thrombolysis procedure in acute intermediate-risk pulmonary embolism: the OPTALYSE PE trial. JACC Cardiovasc Interv. 2018;11(14):1401–1410. [DOI] [PubMed] [Google Scholar]
- 30. Arora S, Panaich SS, Ainani N, et al. Comparison of in-hospital outcomes and readmission rates in acute pulmonary embolism between systemic and catheter-directed thrombolysis (from the national readmission database). Am J Cardiol. 2017;120(9):1653–1661. [DOI] [PubMed] [Google Scholar]
- 31. Dudzinski DM, Giri J, Rosenfield K. Interventional treatment of pulmonary embolism. Circ Cardiovasc Interv. 2017;10(2):e004345 [E-pub online] [DOI] [PubMed] [Google Scholar]
- 32. Dumantepe M, Teymen B, Akturk U, Seren M. Efficacy of rotational thrombectomy on the mortality of patients with massive and submassive pulmonary embolism. J Card Surg. 2015;30(4):324–332. [DOI] [PubMed] [Google Scholar]
- 33. Ciampi-Dopazo JJ, Romeu-Prieto JM, Sanchez-Casado M, et al. Aspiration thrombectomy for treatment of acute massive and submassive pulmonary embolism: initial single-center prospective experience. J Vasc Interv Radiol. 2018;29(1):101–106. [DOI] [PubMed] [Google Scholar]
- 34. Jaber WA, Fong PP, Weisz G, et al. Acute pulmonary embolism: with an emphasis on an interventional approach. J Am Coll Cardiol. 2016;67(8):991–1002. [DOI] [PubMed] [Google Scholar]
- 35. LeVarge BL, Wright CD, Rodriguez-Lopez JM. Surgical management of acute and chronic pulmonary embolism. Clin Chest Med. 2018;39(3):659–667. [DOI] [PubMed] [Google Scholar]
- 36. Yusuff HO, Zochios V, Vuylsteke A. Extracorporeal membrane oxygenation in acute massive pulmonary embolism: a systematic review. Perfusion. 2015;30(8):611–616. [DOI] [PubMed] [Google Scholar]
- 37. Lodewyks CL, Bednarczyk JM, Mooney OT, Arora RC, Singal RK. Extracorporeal membrane oxygenation, pulmonary embolectomy, and right ventricular assist device for massive pulmonary embolism. Can J Cardiol. 2017;33(7):e950. [DOI] [PubMed] [Google Scholar]
- 38. Dolmatova EV, Moazzami K, Cocke TP, et al. Extracorporeal membrane oxygenation in massive pulmonary embolism. Heart Lung. 2017;46(2):106–109. [DOI] [PubMed] [Google Scholar]
- 39. Maggio P, Hemmila M, Haft J, Bartlett R. Extracorporeal life support for massive pulmonary embolism. J Trauma. 2007;62(3):570–576. [DOI] [PubMed] [Google Scholar]
- 40. Malekan R, Saunders PC, Yu CJ, et al. Peripheral extracorporeal membrane oxygenation: comprehensive therapy for high-risk massive pulmonary embolism. Ann Thorac Surg. 2012;94(1):104–108. [DOI] [PubMed] [Google Scholar]
- 41. Cheung AW, White CW, Davis MK, Freed DH. Short-term mechanical circulatory support for recovery from acute right ventricular failure: clinical outcomes. J Heart Lung Transplant. 2014;33(8):794–799. [DOI] [PubMed] [Google Scholar]
- 42. Kabrhel C, Rosovsky R, Channick R, et al. A multidisciplinary pulmonary embolism response team: initial 30-month experience with a novel approach to delivery of care to patients with submassive and massive pulmonary embolism. Chest. 2016;150(2):384–393. [DOI] [PubMed] [Google Scholar]
- 43. Carroll BJ, Pemberton H, Bauer KA, et al. Initiation of a multidisciplinary, rapid response team to massive and submassive pulmonary embolism. Am J Cardiol. 2017;120(8):1393–1398. [DOI] [PubMed] [Google Scholar]
- 44. Sista AK, Friedman OA, Dou E, et al. A pulmonary embolism response team’s initial 20-month experience treating 87 patients with submassive and massive pulmonary embolism. Vasc Med. 2018;23(1):65–71. [DOI] [PubMed] [Google Scholar]
- 45. Mahar JH, Haddadin I, Saddana D, et al. A pulmonary embolism response team (PERT) approach: initial experience from the cleveland clinic [published online 2018]. J Thromb Thrombolysis. 2018;46(2):186–192. doi:10.1007/s11239-018-1686-2. [DOI] [PubMed] [Google Scholar]
- 46. Rosovsky R, Chang Y, Rosenfield K, et al. Changes in treatment and outcomes after creation of a pulmonary embolism response team (PERT); a 10-year analysis [published online 2018]. J Thromb Thrombolysis. 2018. doi: 10.1007/s11239-018-1737-8. [DOI] [PubMed] [Google Scholar]