Abstract
We aimed to investigate the changes in p-selectin (p-sel), thrombus precursor protein, and D-dimer (D-D) in patients with cirrhosis after portal hypertensive splenectomy and explore its values on the prediction of postoperative portal vein thrombosis (PVT) formation. A total of 144 patients with cirrhosis with portal hypertension who underwent portal hypertensive splenectomy from January 2009 to December 2016 were enrolled in this study and divided into the thrombus and nonthrombus groups. The levels of p-sel, thrombus precursor protein (TpP), and D-D were measured by flow cytometry, enzyme-linked immunosorbent assay, and immunoturbidimetry, respectively. Sensitivity, specificity, and other values for p-sel, TpP, and D-D were calculated. The linear discriminant, logistic regression, and decision tree methods were used to analyze the p-sel value on the prediction of PVT formation. Seventy-nine patients were confirmed having postoperative PVT, with the incidence rate of 54.86%. No significant differences were observed in the p-sel, TpP, and D-D between the thrombus and nonthrombus groups before surgery, but these 3 indexes were obviously elevated in the thrombus group after operation (P < .01). P-selectin level on first day showed the highest positive predictive value (91.0%) and diagnostic coincidence rate (83.3%), while negative expected value (76.6%) was lower than those of TpP and D-D. Multiple analyses showed the prediction accuracy of PVT was 61.1% (P = .023), 97.2% (P < .001), and 97.2% (P < .001), respectively. P-selectin has a significant value in predicting PVT. P-selectin level on first and third day is valuable and feasible for the early prediction of PVT.
Keywords: P-selectin, thrombus precursor protein, D-D, portal vein thrombosis, decision tree
Introduction
Portal hypertension (PHT) is a group of clinical syndromes, which is caused by the portal vein blood flow obstruction and blood stasis, resulting in increased portal vein system pressure.1 Collateral circulation,2 the enlargement and hyperfunction of spleen,3 and ascites4 are the main manifestations of PHT. Almost all patients with PHT show a splenomegaly and varying degrees of hypersplenism. Splenectomy (or combined with devascularization operation) is still the main measure for the treatment of patients with PHT having hypersplenism. Previous study has shown that splenectomy can not only correct hypersplenism but also improve esophageal varices and ascites.5 However, portal vein thrombosis (PVT) is one of the most common complications after portal hypertensive splenectomy, the mechanism of which has not been not fully elucidated.6 It has been reported that dozens of factors such as the hemodynamic changes, blood hypercoagulable state, and irrational use of coagulation drugs are associated with PVT.7–9 In general, the initial symptoms of PVT are not obvious. It cannot be confirmed by imaging examination at early stage and is easy to be misdiagnosed or missed. Literatures reported the natural incidence of PVT in patients with PHT was 0.6% to 2.1%,10,11 which was much higher than in patients with cirrhosis after splenectomy. Recent study has found that cirrhotic PHT is a high-risk factor for PVT.12 Patients with cirrhosis generally have a poor blood clotting function. The local blood hypercoagulable state after splenectomy resulted in the PVT formation, which reduced the liver blood flow, increased liver damage, and even led to liver failure, intestinal necrosis, and other serious consequences.13
P-selectin (p-sel) is a class of glycoproteins that exists on the membrane of rod-like body in vascular endothelial cells and α granules in platelets. It promotes platelets adhesion to endothelial cells and vascular wall inflammation, which plays an important role in atherosclerosis caused by thrombosis.14 Thus, p-sel reflects the platelet activation and functional status, which has a great significance in determining the prethrombotic state and coagulation tendency. In the coagulation process, fibrinogens form soluble fibrin monomers under the catalysis of thrombin. Then, the soluble fibrin monomers such as thrombus precursor protein (TpP) can be converted into the insoluble fibrin. Mega et al reported that plasma TpP levels reflect the endothelial injury, thrombin activation, fibrin peptide release, and so on.15 Elevated plasma TpP level is another molecular marker for the prethrombotic status.16 Fibrinolytic system and coagulation system maintain a dynamic balance under the physiological circumstances. Thrombosis prevents blood out of the damaged blood vessels, while fibrinolysis is responsible for keeping vascular wall permeability and blood flow.17 The fibrinolytic system is also activated to degrade fibrin formation fragments such as D-dimer (D-D) when coagulation occurs. Increased plasma D-D level prompts that there is both acute thrombosis and secondary fibrinolysis in the body. LaCapra et al18 performed TpP test in 14 patients with deep vein thrombosis (DVT), and the results showed a 100% positive rate. However, D-D is not a specific index. Diseases such as trauma, infection, pregnancy, all can lead to secondary D-D elevation.
In the present study, 144 patients with cirrhosis after portal hypertensive splenectomy during the period from 2009 to 2016 in the Huzhou Central Hospital of Zhejiang Province were selected as the objective. The levels of p-sel, TpP, and D-D were measured at different time points before and after operation. We aimed to investigate the changes in p-sel, TpP, and D-D in patients with cirrhosis after portal hypertensive splenectomy and explore its values in PVT prediction.
Materials and Methods
Patients
A total of 144 patients diagnosed with cirrhosis PHT who underwent single splenectomy or splenectomy combined with devascularization during the period from January 2009 to December 2016 at the Huzhou Central Hospital of Zhejiang Province were enrolled in this study. Patients were confirmed by clinical laboratory tests including multislice spiral computed tomography and hepatic venous pressure gradient (HVPG) measurement. Patients were excluded if they fulfilled the following criteria: (1) age of >70 years old or <20 years old; (2) without typical manifestations of PHT or HVPG ≤5 mm Hg; (3) with the incomplete clinicopathologic data; (4) treated with nonselective β-blocker drugs recently; (5) with the deteriorated liver function or aspartate transaminase >5 times of the upper limit, serum bilirubin ≥5 mg/dL (85.65 μmol/L); (6) with cardiovascular, brain, lung, kidney, and other severe organic disease; (7) with hematopoietic system diseases, severe coagulation disorders, congenital thrombotic disease, preoperative PVT, or congenital portal vein malformations.
The retrospective study was approved by the research ethics committee of Huzhou Central Hospital. All participants gave written consent for their clinical information to be used for scientific research.
Portal Vein Thrombosis Diagnosis
Patients were examined by portal vein color Doppler ultrasound (CDU) examination using GE LOGIQE portable color machine (GE Co, Ltd, Chicago, IL, USA) to detect portal diameter, blood flow, and PVT formation at 9 am from the 1st day before surgery to 14th days after surgery. Diagnostic criteria were as follows: (1) portal vein, splenic vein, or superior mesenteric vein became widening, (2) the substantial echo was detected, (3) blood flow became thinning, (4) blood flow signal reduced or disappeared, and (5) distal venous dilatation occurred. Here, the patients were divided into the thrombus (79 cases) and nonthrombus groups (65 cases) according to the formation of postoperative PVT.
Surgical Methods
“Secondary pedicle division strategy” was used for splenectomy, while pericardial devascularization was used for devascularization.19 Here, patients with hemorrhage during the process of splenectomy would undergo the devascularization. All the procedure was performed by the same surgical team of specialists.
P-selectin, TpP, and D-D Examinations
A total of 5 mL venous blood sample was extracted from patients who had fasted for at least 12 hours without pressure band at 1st day before operation and 1st, 3rd, 5th, 7th, and 14th day after operation, then blood sample was divided into 3 vacuum tubes. P-selectin level was measured through evaluating p-sel-positive platelet percentage via flow cytometry method (Beckman Coulter Co, Ltd, Fullerton, CA, USA), and the data were analyzed with CellQuestPlot software to calculate the positive rate of p-sel in platelet. Thrombus precursor protein level was detected by enzyme-linked immunosorbent assay kit (Biotang Co, Ltd, Waltham, MA, USA), and D-D was assayed utilizing immunoturbidimetry kit (Shanghai Xisen Meikang Medical Electronics Co, Ltd, Shanghai, China). All experiments were performed according to the instructions of manufactures.
Statistical Analysis
All data were analyzed utilizing SPSS 19.0 statistical software (IBM, Armonk, New York). The measurement data were presented as means (standard deviation [SD]; normal distribution) or median and interquartile range (nonnormal distribution). Statistically significant differences were evaluated with the Student t test (normal distribution) or nonparameter test (Mann-Whitney U test). P < .05 was considered significant between the 2 groups. Paired t test was used in comparison of different time points within group. Qualitative data were presented as n (%), and statistically significant differences were evaluated using χ2 test. The target parameters were taken as independent variables to perform the receiver operating characteristic (ROC) curve analysis, and area under the ROC curve (AUC) was used to evaluate the diagnostic value. The linear discriminant, logistic regression, and decision tree methods were used to analyze the value of p-sel levels on the first and third day after operation in early thrombus prediction. P value <.05 was considered statistically significant.
Results
Baseline Characteristics
The baseline characteristics of patients are listed in Table 1. There were no significant differences between the thrombus group and nonthrombus group of the preoperative data including sex, Child-Pugh grade,20 and age (P > .05; Table 1). However, the incidence rate of PVT was higher in patients who underwent splenectomy combined with devascularization than those with splenectomy alone (P < .001; Table 1).
Table 1.
Baseline Characteristics of Patients.
| Group | Thrombus Group (n = 79) | Nonthrombus Group (n = 65) | χ2/t | P |
|---|---|---|---|---|
| Sex (n, %) | ||||
| Male | 36 (45.6) | 30 (46.2) | 0.005 | .944 |
| Female | 43 (54.4) | 35 (53.8) | ||
| Child-Pugh grade (n, %) | ||||
| A grade | 48 (60.8) | 41 (63.1) | 0.081 | .776 |
| B grade | 31 (39.2) | 24 (36.9) | ||
| Surgical approach | ||||
| Splenectomy | 36 (45.6) | 53 (81.5) | 19.544 | <.001 |
| Splenectomy + devascularization | 43 (54.4) | 12 (18.5) | ||
| Age | 53.0 ± 12.8 | 50.3 ± 11.7 | 0.760 | .451 |
P-selectin, TpP, and D-D Levels
As shown in Table 2, the levels of p-sel, TpP, and D-D at the 1st, 3rd, 5th, 7th, and 14th day after operation were significantly higher than those before operation in the thrombus and nonthrombus groups (P < .01). In addition, compared with the nonthrombus group, the levels of p-sel, TpP, and D-D were obviously increased in the thrombus group (P < .01). Notably, all the indicators showed a sharp increase in first day after surgery and a melt decrease trend with the change in time (Figure 1). Therefore, the first day after surgery was selected as the key time point for PVT diagnosis. No significant differences were observed in the p-sel, TpP, and D-D values for patients with simple splenectomy and splenectomy combined with devascularization on the first day after surgery (P > .05; Table 3), which indicated the indicators were not affected by the surgical approach.
Table 2.
Comparison of p-sel, TpP, and D-D Between the 2 Groups at Different Time Points.
| Group | Thrombus Group (n = 79) | Nonthrombus Group (n = 65) | t | P |
|---|---|---|---|---|
| p-sel (%) | ||||
| 1st day before surgery | 2.55 ± 1.27 | 2.51 ± 1.12 | 0.208 | .835 |
| 1st day after surgery | 14.46 ± 11.00a,b | 3.18 ± 1.67a | 8.992 | <.001 |
| 3rd day after surgery | 15.06 ± 11.72a,b | 3.00 ± 1.40a | 9.068 | <.001 |
| 5th day after surgery | 10.74 ± 7.25a,b | 3.14 ± 1.16a | 9.181 | <.001 |
| 7th day after surgery | 6.48 ± 5.02a,b | 2.75 ± 1.77a | 6.164 | <.001 |
| 14th day after surgery | 5.01 ± 3.52a,b | 3.87 ± 2.84a | 2.100 | .038 |
| TpP (mg/L) | ||||
| 1st day before surgery | 4.42 ± 0.67 | 4.81 ± 1.11 | −2.611 | .010 |
| 1st day after surgery | 12.56 ± 5.08a,b | 6.30 ± 1.29a | 10.556 | <.001 |
| 3rd day after surgery | 12.54 ± 5.03a,b | 6.41 ± 1.17a | 10.494 | <.001 |
| 5th day after surgery | 11.01 ± 3.99a,b | 6.39 ± 1.53a | 9.471 | <.001 |
| 7th day after surgery | 9.47 ± 2.74a,b | 6.36 ± 1.39a | 8.807 | <.001 |
| 14th day after surgery | 7.71 ± 2.23a,b | 6.09 ± 1.14a | 5.613 | <.001 |
| D-D (ng/mL) | ||||
| 1st day before surgery | 261.85 ± 135.00 | 164.15 ± 132.45 | 4.358 | <.001 |
| 1st day after surgery | 859.13 ± 392.52a,b | 348.85 ± 196.63a | 10.115 | <.001 |
| 3rd day after surgery | 1038.32 ± 950.56a,b | 405.08 ± 311.85a | 5.538 | <.001 |
| 5th day after surgery | 1191.68 ± 735.34a,b | 430.40 ± 311.85a | 8.336 | <.001 |
| 7th day after surgery | 875.85 ± 358.18a,b | 454.80 ± 264.89a | 8.098 | <.001 |
| 14th day after surgery | 761.44 ± 256.10a,b | 405.26 ± 237.20a | 8.585 | <.001 |
Abbreviations: D-D, D-dimer; p-sel, p-selectin; TpP, thrombus precursor protein.
a P < .01 compared with the first day before surgery.
b P < .01 compared with nonthrombus group.
Figure 1.
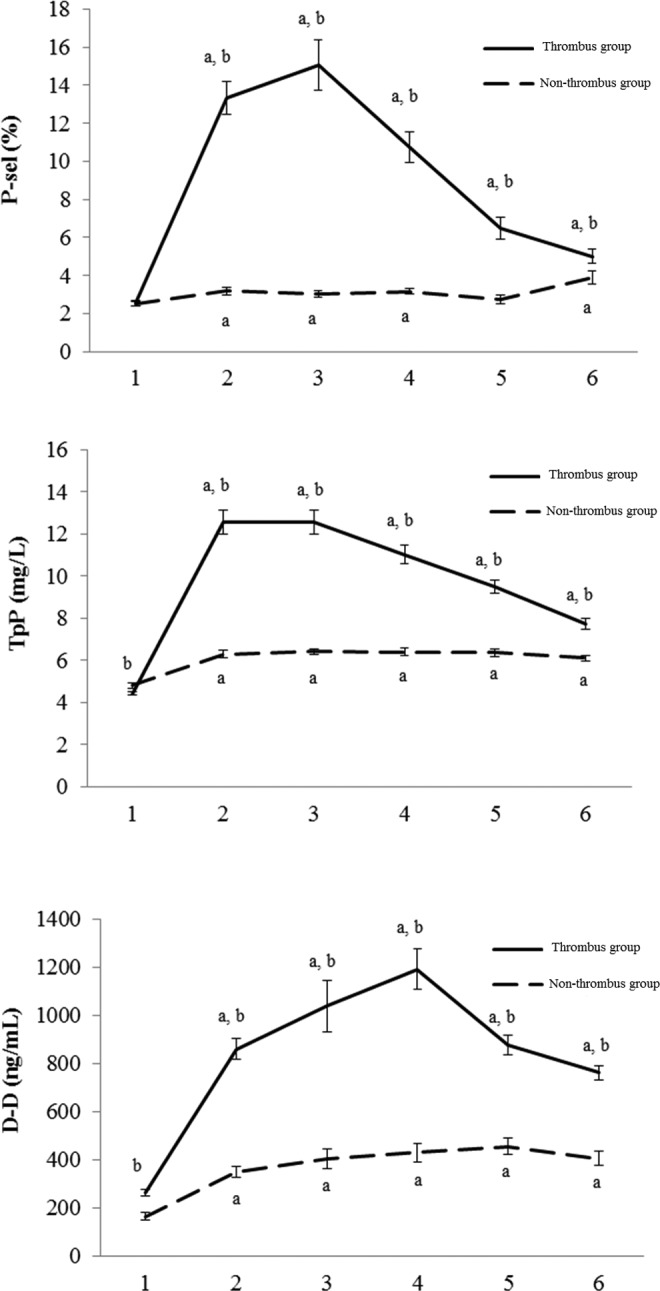
Comparison of p-sel, TpP, and D-D level between thrombus group and nonthrombus group at different time points before and after surgery. A, P < .01 compared with the first day before surgery, B, P < .01 compared with the nonthrombus group; 1. 1st day before surgery; 2. 1st day after surgery; 3. 3rd day after surgery; 4. 5th day after surgery; 5. 7th day after surgery; 6. 14th day after surgery. D-D indicates D-dimer; p-sel, p-selectin; TpP, thrombus precursor protein.
Table 3.
Comparison of p-sel, TpP, and D-D Between Different Surgical Approaches.
| Group | Splenectomy | Splenectomy + Vascular Disconnection | Z/t | P |
|---|---|---|---|---|
| p-sel (%) | 9.71 ± 11.62 | 8.83 ± 6.46 | 0.581 | .562 |
| TpP (mg/L) | 9.35 ± 5.16 | 10.35 ± 4.60 | −1.179 | .240 |
| D-D (ng/mL) | 588.25 ± 465.31 | 694.40 ± 283.55 | −1.701 | .091 |
Abbreviations: D-D, D-dimer; p-sel, p-selectin; TpP, thrombus precursor protein.
Values of p-sel, TpP, and D-D on PVT Prediction
As shown in Table 4, p-sel had the highest specificity (90.8%), highest positive predictive value (91.0%), highest diagnostic coincidence rate (83.3%), and the lowest misdiagnosis rate (9.2%) on PVT prediction. The role of p-sel in predicting PVT was considered more important than TpP and D-D. Therefore, the p-sel was selected for the subsequent analysis.
Table 4.
Values of p-sel, TpP, and D-D in Predicting PVT.a
| Group | Sensitivity (%) | Specificity (%) | Positive Predictive Value (%) | Negative Predictive Value (%) | Misdiagnosis Rate (%) | Missing Diagnosis Rate (%) | Diagnostic Compliance Rate (%) |
|---|---|---|---|---|---|---|---|
| P-sel | 77.2 | 90.8 | 91.0 | 76.6 | 9.2 | 22.8 | 83.3 |
| TpP | 87.3 | 58.5 | 71.9 | 79.2 | 41.5 | 12.7 | 74.3 |
| D-D | 83.5 | 76.9 | 81.5 | 79.4 | 23.1 | 16.5 | 80.6 |
Abbreviations: D-D, D-dimer; p-sel, p-selectin; PVT, portal vein thrombosis; TpP, thrombus precursor protein.
a Positive criteria: p-sel >5%, TpP >6 mg/L, D-D >500 ng/mL.
Value of p-sel Level on PVT Prediction
We noticed that in the thrombus group p-sel level on third day after surgery was even higher than that on first day, and the difference between thrombus group and nonthrombus group was further increased. To avoid missing diagnosis of PVT with single p-sel level on first day after surgery, p-sel on third day after surgery was also combined to predict PVT. Here, the linear discriminant, logistic regression function, and decision classification tree analyses were applied for analyzing the predictive value of p-sel on PVT formation.
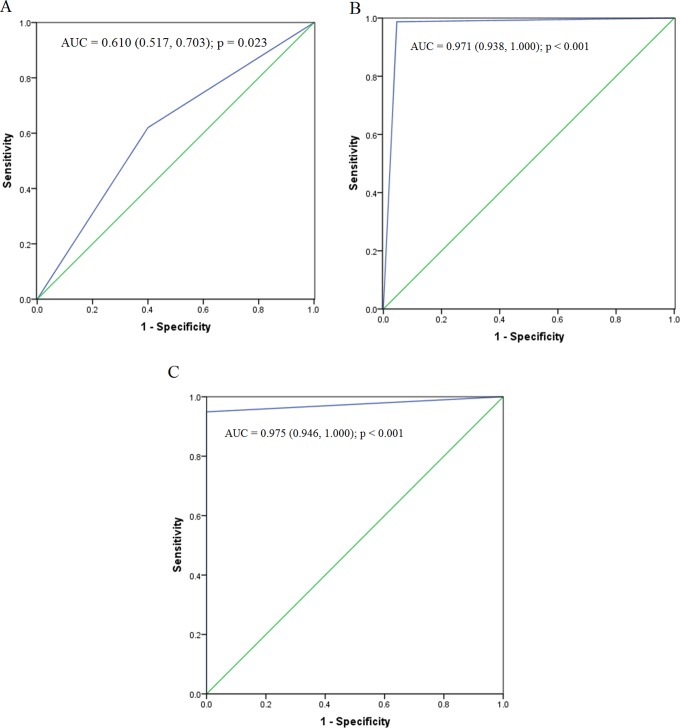
In the linear discriminant analysis, the function formula was p = (−3.062 + 0.024 × p1 + 0.006 × p3). Here, “0” was considered as the cutoff value. Patients were sentenced to thrombus group if the value was greater than 0; otherwise, they were judged as nonthrombus group. The sensitivity, specificity, positive predictive value, negative expected value, and diagnostic accuracy were 62.02% (49/79), 56.52% (39/69), 65.33% (49/75), 60% (39/65), and 61.1%, respectively. The AUC was 0.610 (0.517, 0.703; P = .023; Figure 2A).
Figure 2.
Receiver operating characteristic curve of different analytical methods. A, Linear discriminant analysis. B, Logical regression analysis. C, Decision tree analysis.
The logistic regression function formula was p = exp (−10.611 + 0.687 × P1 + 1.203 × P3)/[1 + exp(−10.611 + 0.687 × P1 + 1.203 × P3)]. Here, 0.5 was regarded as the cutoff value. Patients were judged as thrombus group if the probability value was greater than .5; otherwise, they were judged as nonthrombus group. The sensitivity, specificity, positive predictive value, negative expected value, and diagnostic accuracy were 98.73% (78/79), 95.38% (62/65), 96.30% (78/81), 98.41% (62/63), and 97.2%, respectively. The AUC was 0.971 (0.938, 1.000) (P < .001; Figure 2B).
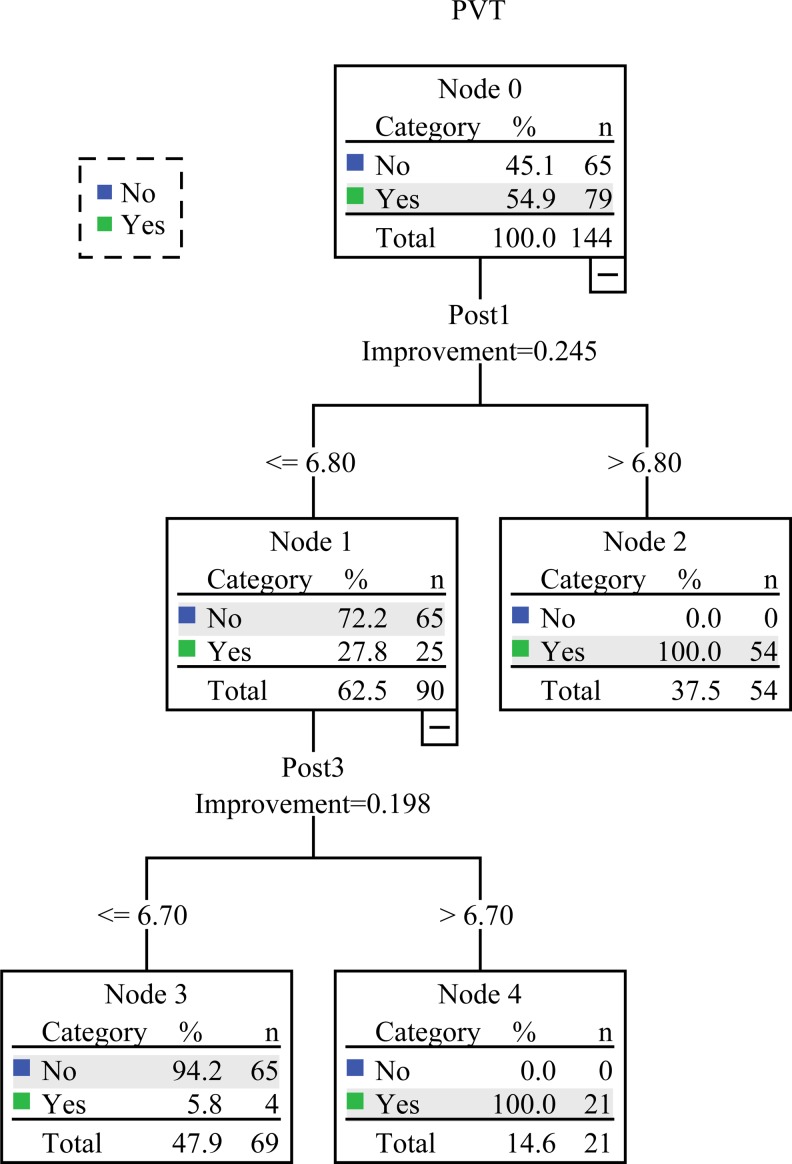
Decision classification tree analysis was performed in a chronological order. Here, the optimal cutoff value was 6.8 at first day after surgery and was 6.7 at third day after surgery. Patients were directly classified into the thrombus group if p-sel level was greater than or equal to 6.80 on first day after surgery; otherwise, we continued to monitor p-sel level at third day. If p-sel level was greater than or equal to 6.70 on third day after surgery, patients were judged as thrombus group; otherwise, they were judged as nonthrombus group (Figure 3). The sensitivity, specificity, positive predictive value, negative expected value, and diagnostic accuracy were 94.94% (75/79), 100% (65/65), 100.00% (75/75), 94.20% (65/69), and 97.2%, respectively. The AUC was 0.975 (0.946, 1.000; P < .001; Figure 2C).
Figure 3.
Flowchart of decision tree analysis. Here, “no” represents that the patients were classified into the nonthrombus group, and “yes” represents that the patients were classified into the thrombus group.
Discussion
Previous studies reported that the natural incidence of PVT in patients with cirrhosis is 0.6% to 2.1%; however, the incidence rate is often more than 20% after patients underwent PHT, even up to 91.6%.21,22 In the current study, the incidence rate of PVT was 54.86%, which was consistent with the previous reports. Due to the low diagnostic specificity, PVT may rapidly progress, thereby resulting in a mass of intestinal veins thrombosis, even fatal consequences.23 Therefore, it is important for the prevention of PVT. Currently, several methods have been applied for preventing the PVT formation, such as heparin therapy24 and CDU. Nevertheless, these methods cannot commendably prevent or predict the formation of PVT. Thus, it is essential to explore some indicators which can be used for early diagnosis of PVT. Here, we evaluated the changes in p-sel, TpP, and D-D in patients with cirrhotic PHT and its values on the prediction of PVT formation.
P-selectin is a glycoprotein that is expressed at the surface of platelet membrane and endothelial cells,25 reflecting platelet activation and functional status. Several studies revealed that p-sel may be associated with the pathogenesis of PVT.26–28 Blann et al29 found that soluble p-sel is significantly elevated in patients with DVT, which is likely to be correlated with excessive platelet activation. Myers and his colleagues30 presented that p-sel enlarges the effect of vein wall inflammation caused by thrombotic stimuli, promoting venous thrombosis. Moreover, Panicker et al31 found that soluble p-sel could trigger neutrophil intracellular signal transduction and promote cell adhesion aggregation in mice. There is a positive correlation between the infiltration degree of inflammation cells in venous wall and the plasma soluble p-sel level. Simultaneously, soluble p-sel caused by platelet activation usually promotes venous thrombosis, and the blood clot size positively correlated with plasma soluble p-sel levels. These findings suggested that p-sel play a potential role in both inflammation and thrombosis. Our study showed that the levels of p-sel were significantly higher in the thrombus group than those in nonthrombus group at all time points postoperatively, especially at first day after operation. The specificity of p-sel on the prediction of PVT formation was as high as 90.8%, indicating that p-sel is likely to be served as an early predicative indicator for PVT formation. In addition, we noticed that the differences in p-sel on third day after surgery continued to increase between the 2 groups, suggesting that it might be more valuable and significant in predicting and discriminating PVT. Hence, the linear discriminant, logistic regression, and decision classification tree analyses were performed to explore the optimal time point and method for the prediction of PVT formation. The linear discriminant and logistic regression analyses showed that the accuracy rate of postoperative PVT was 61.1% and 97.2%, respectively. Decision classification tree analysis had the same accuracy rate with logistic regression method. In addition, the AUC of linear discriminant analysis was only 0.610, which was 0.971 in logistic regression and 0.975 in decision classification tree analysis. Our results revealed that the linear discriminant analysis is not an ideal method in predicting PVT, while both logical regression and classification of trees have a good discriminant performance. In logistic regression analysis, p-sel level on both first day and third day after surgery is needed for making a judgment for all patients. The decision tree analysis is usually based on a time order. P-selectin level would not be monitored in the next days if PVT can be confirmed by p-sel level on first day after surgery. Otherwise, p-sel level would be checked on third day after surgery to make the final judgment. This is more conducive for early detection of PVT. Considering the discriminant effect and feasibility, the decision classification tree is the optimal and preferred method in PVT early prediction. It has been shown that insoluble cross-linked fibrinogen multimer converted from soluble fibrinogen have crucial roles in PVT formation.32,33 Thrombus precursor protein is a kind of soluble fibrinogen; its plasma level reflects the activity of thrombin in circulation system. The increase in TpP level can be detected in thrombosis or prethrombotic state caused by many diseases such as myocardial infarction, disseminated intravascular coagulation, diabetes, and malignant tumors. It was reported that TpP had slightly increased in the early stage of tissue injury or organ dysfunction and had significantly increased in the early stage of DVT, which provides the possibility of early prevention and treatment of PVT. Our results presented that TpP levels were significantly increased in the thrombus group compared with the nonthrombus group at all time points after surgery, especially at first day after surgery. We proposed that surgery-induced venous intimal injury activated the coagulation system. The TpP level >6 mg/L was regarded as the positive standard; the sensitivity of TpP for PVT diagnosis was 87.3% under the standard, but the specificity was only 58.5%.
The fibrinolysis activation system is activated when the PVT is formed, and the fibrinolytic enzyme, plasmin is generated to degrade the cross-linked fibrin. D-dimer, small peptide fragments of early thrombosis, is one of the degradation products of fibrin. Prandoni et al34 reported that D-D detection contributes to monitor the recurrence of venous thromboembolism after systemic anticoagulation therapy for patients. Zhang and his colleagues35 revealed that D-D level has potential roles in the formation of PVT. Additionally, D-D can act as a sensitive indicator for coagulation and fibrinolytic systems, thereby predict the PVT formation.36 Our results showed that the specificity and sensitivity of D-D level on PVT prediction were 76.9% and 83.5%, respectively, suggesting D-D may be used as a monitoring indicator for the PVT formation. However, D-D value on the PVT formation was still inferior to the p-sel value.
In conclusion, our results showed that the levels of p-sel, TpP, and D-D alone on first day after surgery all have a certain value to monitor the formation of PVT. Generally, p-sel has a higher value than TpP and D-D alone on PVT prediction. In particular, p-sel level on first day and third day is valuable and feasible for the early prediction of PVT. However, the sample size was small in the current study, and further studies with the large sample size will be performed in the future.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Social Public Technology Research and Development Program from Science and Technology Department of Zhejiang Province, China (Program No. 2014C33137), and General Science and Research Project Program from Science and Technology Bureau of Huzhou City, China (Program No. 2010YSB08).
References
- 1. Khodayar-Pardo P, Pena Aldea A, Ramirez Gonzalez A, Meseguer Carrascosa A, Calabuig Bayo C. Very early presentation of extrahepatic portal vein obstruction causing portal hypertension in an infant: uncertainties in the management and therapeutic limitations. Case Rep Gastroenterol. 2016;10(2):360–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Moreau R. VEGF-induced angiogenesis drives collateral circulation in portal hypertension. J Hepatol. 2005;43(1):6–8. [DOI] [PubMed] [Google Scholar]
- 3. Jha AK, Ranjan R, Priyadarshi RN. Wandering spleen and portal hypertension: a vicious interplay. ACG Case Rep J. 2017;4:e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Garcia-Tsao G. Current management of the complications of cirrhosis and portal hypertension: variceal hemorrhage, ascites, and spontaneous bacterial peritonitis. Dig Dis. 2016;34(4):382–386. [DOI] [PubMed] [Google Scholar]
- 5. Batista-Neto J, Tognetti LB, Ribeiro LT, Balwani Mdo C, Muritiba T, Alves EE. Evolutional profile of the esophageal varices after splenectomy associated with ligation of the left gastric vein and sclerotherapy in schistosomal portal hypertension. Arq Bras Cir Dig. 2013;26(1):49–53. [DOI] [PubMed] [Google Scholar]
- 6. Yang S, He C, Fan X, Ding W, Wu X, Li J. Early prophylactic anticoagulation via transjugular intrahepatic route for portal vein thrombosis after splenectomy in cirrhotic portal hypertension. J Vasc Interv Radiol. 2015;26(7):1009–1017. [DOI] [PubMed] [Google Scholar]
- 7. Chandra S, Dutta U, Das R, Vaiphei K, Nagi B, Singh K. Mesenteric venous thrombosis causing jejunal stricture: secondary to hypercoagulable states and primary portal hypertension. Dig Dis Sci. 2002;47(9):2017–2019. [DOI] [PubMed] [Google Scholar]
- 8. Garbuzenko DV, Arefyev NO, Belov DV. Restructuring of the vascular bed in response to hemodynamic disturbances in portal hypertension. World J Hepatol. 2016;8(36):1602–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vilaseca M, Garcia-Caldero H, Lafoz E, et al. The anticoagulant rivaroxaban lowers portal hypertension in cirrhotic rats mainly by deactivating hepatic stellate cells. Hepatology. 2017;65(6):2031–2044. [DOI] [PubMed] [Google Scholar]
- 10. Winslow ER, Brunt LM, Drebin JA, Soper NJ, Klingensmith ME. Portal vein thrombosis after splenectomy. Am J Surg. 2002;184(6):631–635; discussion 635-636. [DOI] [PubMed] [Google Scholar]
- 11. Webster GJ, Burroughs AK, Riordan SM. Review article: portal vein thrombosis—new insights into aetiology and management. Aliment Pharmacol Ther. 2005;21(1):1–9. [DOI] [PubMed] [Google Scholar]
- 12. Jiang GQ, Bai DS, Chen P, Qian JJ, Jin SJ, Wang XH. Risk factors for portal vein system thrombosis after laparoscopic splenectomy in cirrhotic patients with hypersplenism. J Laparoendosc Adv Surg Tech A. 2016;26(6):419–423. [DOI] [PubMed] [Google Scholar]
- 13. Filik L. Coagulation parameters and portal vein thrombosis in cirrhosis. Hepatol Res. 2010;40(5):555. [DOI] [PubMed] [Google Scholar]
- 14. Shi D, Xu X, Xu Z, et al. P-selectin: an unpredicted factor for deep vein thrombosis after total hip arthroplasty. Biomed Res Int. 2014;2014:783967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mega JL, Morrow DA, de Lemos JA, Mohanavelu S, Cannon CP, Sabatine MS. Thrombus precursor protein and clinical outcomes in patients with acute coronary syndromes. J Am Coll Cardiol. 2008;51(25):2422–2429. [DOI] [PubMed] [Google Scholar]
- 16. Kozlova TV. [The prognostic value of thrombus precursor protein in the assessment of the probability for venous thrombosis recurrence after the completion of anticoagulatory therapy with warfarin]. Klin Med (Mosk). 2006;84(1):51–53. [PubMed] [Google Scholar]
- 17. Olson JD. D-dimer: an overview of hemostasis and fibrinolysis, assays, and clinical applications. Adv Clin Chem. 2015;69:1–46. [DOI] [PubMed] [Google Scholar]
- 18. LaCapra S, Arkel YS, Ku DH, Gibson D, Lake C, Lam X. The use of thrombus precursor protein, D-dimer, prothrombin fragment 1.2, and thrombin antithrombin in the exclusion of proximal deep vein thrombosis and pulmonary embolism. Blood Coagul Fibrinolysis. 2000;11(4):371. [DOI] [PubMed] [Google Scholar]
- 19. Zhou J, Liu P, Yin Z, Zhao Y, Wang X. Safety and cost-effectiveness analysis of laparoscopic splenectomy by secondary pedicle division using monopolar electrocautery. Hepatogastroenterology. 2013;60(126):1302–1306. [DOI] [PubMed] [Google Scholar]
- 20. Spray J, Willett K, Chase D, Sindelar R, Connelly S. Dosage adjustment for hepatic dysfunction based on Child-Pugh scores. Am J Health Syst Pharm. 2007;64(7):690, 692–693. [DOI] [PubMed] [Google Scholar]
- 21. Kinjo N, Kawanaka H, Akahoshi T, et al. Risk factors for portal venous thrombosis after splenectomy in patients with cirrhosis and portal hypertension. Br J Surg. 2010;97(6):910–916. [DOI] [PubMed] [Google Scholar]
- 22. Yoshida M, Watanabe Y, Horiuchi A, Yamamoto Y, Sugishita H, Kawachi K. Portal and splenic venous thrombosis after splenectomy in patients with hypersplenism. Hepatogastroenterology. 2009;56(90):538–541. [PubMed] [Google Scholar]
- 23. Ponziani F, Zocco M, Campanale C, et al. Portal vein thrombosis: insight into physiopathology, diagnosis, and treatment. World J Gastroenterol. 2010;16(2):143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lisman T. Low molecular weight heparin in management and prevention of portal vein thrombosis. Thromb Res. 2014;134(4):761–762. [DOI] [PubMed] [Google Scholar]
- 25. Pan J, Xia L, McEver R. Comparison of promoters for the murine and human P-selectin genes suggests species-specific and conserved mechanisms for transcriptional regulation in endothelial cells. J Biol Chem. 1998;273(16):10058–10067. [DOI] [PubMed] [Google Scholar]
- 26. Kyrle P, Hron G, Eichinger S, Wagner O. Circulating P-selectin and the risk of recurrent venous thromboembolism. Thromb Haemost. 2007;97(6):880–883. [PubMed] [Google Scholar]
- 27. Gok F, Ugur Y, Ozen S, Dagdeviren A. Pathogenesis-related adhesion molecules in Henoch-Schonlein vasculitis. Rheumatol Int. 2008;28(4):313–316. [DOI] [PubMed] [Google Scholar]
- 28. Ay C, Jungbauer L, Sailer T, et al. High concentrations of soluble P-selectin are associated with risk of venous thromboembolism and the P-selectin Thr715 variant. Clin Chem. 2007;53(7):1235–1243. [DOI] [PubMed] [Google Scholar]
- 29. Blann A, Noteboom W, Rosendaal F. Increased soluble P-selectin levels following deep venous thrombosis: cause or effect? Br J Haematol. 2000;108(1):191–193. [DOI] [PubMed] [Google Scholar]
- 30. Myers D, Hawley A, Farris D, et al. P-selectin and leukocyte microparticles are associated with venous thrombogenesis. J Vasc Surg. 2003;38(5):1075–1089. [DOI] [PubMed] [Google Scholar]
- 31. Panicker SR, Mehta-D’souza P, Zhang N, Klopocki AG, Shao B, McEver RP. Circulating soluble P-selectin must dimerize to promote inflammation and coagulation in mice. Blood. 2017;130(2):181–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Carville D, Dimitrijevic N, Walsh M, Digirolamo T, Brill E, Drew N, Gargan P. Thrombus precursor protein (TpP): marker of thrombosis early in the pathogenesis of myocardial infarction. Clin Chem. 1996;42(9):1537–1541. [PubMed] [Google Scholar]
- 33. Song K, Kim H, Song J. Measurement of thrombus precursor protein in septic patients with disseminated intravascular coagulation and liver disease. Haematologica. 2002;87(10):1062–1067. [PubMed] [Google Scholar]
- 34. Prandoni P, Vedovetto V, Ciammaichella M, et al. Residual vein thrombosis and serial D-dimer for the long-term management of patients with deep venous thrombosis. Thromb Res. 2017;154:35–41. [DOI] [PubMed] [Google Scholar]
- 35. Zhang D, Hao J, Yang N. Protein C and D-dimer are related to portal vein thrombosis in patients with liver cirrhosis. J Gastroenterol Hepatol. 2010;25(1):116–121. [DOI] [PubMed] [Google Scholar]
- 36. Cosmi B, Legnani C, Tosetto A, et al. Usefulness of repeated D-dimer testing after stopping anticoagulation for a first episode of unprovoked venous thromboembolism: the PROLONG II prospective study. Blood. 2010;115(3):481–488. [DOI] [PubMed] [Google Scholar]