Abstract
Select patients with acute deep vein thrombosis (DVT) can be managed as outpatients. We sought to conduct a systematic review of real-world studies describing either (1) the clinical characteristics associated with outpatient DVT treatment in all-comers or (2) emergency department (ED) programs designed to facilitate outpatient DVT treatment. MEDLINE and SCOPUS were searched (January 1, 2012, to May 1, 2018) to identify citations meeting the aforementioned criteria. Twenty-one real-world studies were included. The proportion of all-comer patients with DVT managed as outpatients was ≤50% in 11 of 15 studies. With the exception of younger age, no characteristics were consistently associated with outpatient treatment across the 13 studies reporting these characteristics. We identified 8 studies describing ED programs aimed at facilitating DVT outpatient treatment, all of which provided education and included measures to encourage early outpatient follow-up after ED discharge. In conclusion, the proportion of patients with DVT managed as outpatients across real-world studies was low. Several ED programs aimed at facilitating this treatment have been described. It is possible that programs similar to these will increase the proportion of patients with DVT that can be safely managed as outpatients.
Keywords: deep vein thrombosis, venous thrombosis, outpatient, home, patient discharge
Background
Venous thromboembolism (VTE), which includes both deep vein thrombosis (DVT) and pulmonary embolism (PE), results in ∼300 000 admissions to US hospitals each year, with costs of these admissions alone exceeding US$3 billion.1 As early as 1996, randomized controlled trials (RCTs) demonstrated that outpatient treatment is safe and effective in select patients with acute DVT.2-3 However, the adoption of this treatment strategy in clinical practice has been low.4 For example, 1 real-world study showed that only ∼30% of patients presenting with acute DVT are treated in an outpatient setting.4
Since 2012, there have been 2 direct oral anticoagulants (DOACs) approved by the US Food and Drug Administration for acute VTE which do not require initial parenteral anticoagulation.5,6 Removing the need for initial injectable therapy may facilitate outpatient DVT treatment. A 2018 American College of Emergency Physicians (ACEP) clinical policy on acute DVT states DOACs may be a “safe and effective treatment alternative” to low-molecular-weight heparin (LMWH) with a vitamin K antagonist (VKA; level B recommendation) and select patients receiving DOAC therapy can be “directly discharged from the emergency department (ED)” (level C recommendation).7 Directly discharging patients from the ED may result in lower treatment costs, as demonstrated by a case–control study (n = 97) where medical costs in the week following presentation for VTE were ∼2.5 times lower for patients treated with DOACs as outpatients versus those treated with LMWH and a VKA (P < .001).8 In an attempt to increase efficiency and reduce treatment costs, several studies have reported strategies to select and manage those presenting with DVT as outpatients.9,10 However, these studies have yet to be systematically summarized. Therefore, we sought to conduct a systematic review of real-world studies describing either (1) the clinical characteristics associated with or (2) the programs designed to facilitate outpatient DVT treatment.
Methods
MEDLINE and SCOPUS were searched from January 1, 2012, to May 1, 2018, using key words and Medical Subject Heading terms associated with outpatient treatment and DVT (Supplemental Appendix A). We also performed hand searches of the reference sections of eligible studies and relevant review articles (ie, backward citation tracking) to augment our bibliographic database searches.
Identified citations were screened for inclusion by 2 independent investigators. Two investigators determined whether a study met inclusion criteria via a process involving two steps. First, titles/abstracts were assessed and subsequently categorized as “included,” “excluded,” or “unsure.” We obtained the full-text version of each article categorized as “included” or “unsure.” This process was then repeated until each article was marked as either “included” or “excluded.” Disagreements at any stage of the screening process were resolved through discussion. For this systematic review, we only included real-world studies (eg, those including clinical or claims-based data collected prospectively or retrospectively) if they evaluated adult patients (ie, ≥18 years of age) with acute DVT. Studies were also required to either report clinical characteristics associated with outpatient versus inpatient treatment in an all-comer cohort (ie, the population was not selected for the study based on demographics or the presence/absence of comorbidities) or describe a program aimed at facilitating outpatient treatment for those discharged from an ED. Only studies published in the English language were included.
Data were collected from included studies using a standardized data abstraction tool. For each study, the following characteristics were collected: the last name of the first author, year of publication, sample size, country of study conduct, design of the study, data source, timing of the sample, the number of patients treated as outpatients, characteristics associated with or criteria for outpatient treatment, and treatment process (eg, anticoagulant utilized). Each included study was classified into one of 2 categories. Category A studies included those reporting clinical characteristics associated with outpatient DVT treatment, whereas category B included studies of programs aimed at facilitating this outpatient treatment. The validity of included studies was assessed using an adapted version of the AXIS tool.11 Only questions deemed to assess risk of bias based on study design and conduct rather than reporting quality were evaluated.12 The AXIS tool was chosen because it is specifically designed for cross-sectional studies and only includes items relevant to this design.11 In contrast, other tools may also include items relevant to additional observational study designs (eg, cohort studies).13 A total of 8 items were evaluated and are described in Supplemental Appendix B. Each study was awarded a star (*) if it satisfactorily met a criterion, while a minus sign (−) was noted if a study did not meet a criterion.
The proportion of all-comer patients with acute DVT managed as outpatients served as an outcome for this systematic review. For each study in category A, we identified the clinical characteristics associated with outpatient treatment (ie, characteristics present in a significantly higher proportion of outpatients vs inpatients, with a P value <.05 considered significant). For studies in category B, we ascertained the criteria that programs reported to identify patients with DVT who may be ineligible for outpatient treatment (eg, comorbidities that would likely necessitate inpatient treatment). The ED treatment process (eg, anticoagulant utilized and procedures for ensuring follow-up after discharge from the ED) is also summarized for studies in category B. We report a descriptive synthesis of the included studies using tables, with categorical data provided as proportions and continuous data provided as means ± standard deviations (SD).
Results
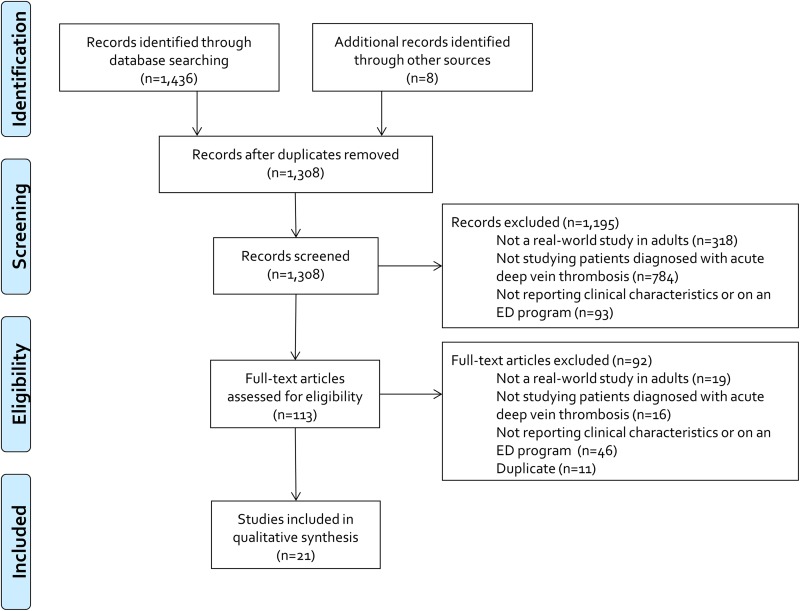
Our search yielded 1308 unique citations, of which 1287 were excluded following title/abstract and full-text review (Figure 1). This left 21 real-world studies evaluating clinical characteristics associated with or programs designed to facilitate outpatient DVT treatment to be included in our systematic review (Table 1).4,8-10,14-37
Figure 1.
Flow diagram of study selection process. ED indicates emergency department.
Table 1.
Characteristics of Real-World Studies of Outpatient Treatment for Deep Vein Thrombosis.
| Author, Year (N) | Country | Study Type | Data Source | Timing of Sample | Male, n (%) | Age, Mean ± SD | Primary Anticoagulant Upon Discharge |
|---|---|---|---|---|---|---|---|
| Chu, 2017 (N = 69) | US | R, clinical | Single-center EHR | 2015-2016 | NR | 53 ± 17 | DOAC |
| Douce, 2017 (N = 141) | US | P, clinical | REGARDS | 2003-2011 | 75 (53) | 67 (median) | NR |
| Kabrhel, 2017 (N = 1112) | US | P, clinical | Multicenter EHR | 2015 | NR | NR | DOAC |
| Mansour, 2017 (N = 23 015) | Canada | R, claims | Alberta administrative databases | 2002-2012 | 10 313 (45) | 56.3 ± NR | NR |
| Mausbach, 2017 (N = 236) | Israel | R, clinical | Single-center EHR | 2013-2015 | 105 (44) | 68 (median) | LMWH and/or VKA |
| Tichter, 2017 (N = 690 000) | US | R, survey | NHAMCS | 2009-2013 | 275 172 (40) | NR | NR |
| Barrett, 2016 (N = 6) | US | P, clinical | Single-center EHR | 2016 | NR | NR | DOAC |
| Lamb, 2016 (N = 1 146 469) | US | R, claims | NEDS | 2006-2012 | NR | NR | NR |
| Singer, 2016 (N = 652 000) | US | R, survey | NHAMCS | 2006-2010 | 325 001 (50) | 58 ± NR | NR |
| Stein, 2016 (N = 2 671 452) | US | R, claims | NEDS/NIS | 2007-2012 | 1 246 129 (47) | NR | NR |
| Beam, 2015 (N = 71) | US | P, clinical | Multicenter EHR | 2013-2014 | NR | 47 ± 16 | DOAC |
| Dentali, 2015 (N = 1452) | Italy | P, clinical | RIETE | 2006-2013 | 753 (52) | 60 ± 18 | LMWH and/or VKA |
| Padron, 2015 (N = 9)a | US | P, clinical | Single-center EHR | 2012-2013 | NR | NR | LMWH and/or VKA |
| Rosa-Salazar, 2015b (N = 1135) | Internationalc | P, clinical | RIETE | 2001-2014 | 573 (51) | 52 ± 18 | LMWH and/or VKA |
| Stein, 2015 (N = 96) | US | R, clinical | Multicenter EHR | 2013-2014 | 43 (50) | 59 ± 16 | NR |
| Trujillo-Santos, 2015 (N = 15 280) | Internationalc | P, clinical | RIETE | 2001-2013 | 7892 (52) | 61 ± 17 | LMWH and/or VKA |
| Falconieri, 2014 (N = 7) | US | R, clinical | Single-center EHR | 2013-2014 | NR | NR | DOAC |
| Lozano, 2014 (N = 13 493) | Internationalc | P, clinical | RIETE | 2001-2012 | 7023 (52) | 62 ± 17 | LMWH and/or VKA |
| Misky, 2014 (N = 107) | US | P, clinical | Single-center EHR | 2011-2012 | NR | 52.4 ± NR | LMWH and/or VKA |
| Davis, 2013 (N = 14) | US | P, clinical | Single-center EHR | NR | NR | NR | LMWH and/or VKA |
| Gibson-Chambers, 2013 (N = 845 000) | US | R, claims | NEDS | 2006-2010 | 397 150 (47) | NR | NR |
Abbreviations: DOAC, direct oral anticoagulant; EHR, electronic health record; LMWH, low-molecular weight heparin; MASTER, Multicenter Advanced Study for a ThromboEmbolism Registry; NEDS, Nationwide Emergency Department Sample; NHAMCS, National Hospital Ambulatory Medical Care Survey; NIS, National Inpatient Sample; NR, not reported; P, prospective; R, retrospective; RIETE, Registro Informatizado de Enfermedad TromboEmbólica; REGARDS, Reasons for Geographic and Racial Differences in Stroke; SD, standard deviation; US, United States; VKA, vitamin K antagonist.
a Reported sample size included patients with both deep vein thrombosis and pulmonary embolism.
b All included patients had upper extremity deep vein thrombosis.
c Countries include Spain, France, Italy, Israel, Germany, Switzerland, Republic of Macedonia, and Brazil.
A total of 15 studies evaluated patients from the United States, while most patients in the remaining studies were treated in Europe. Data were collected in all studies between the years 2001 and 2016, with 11 studies evaluating ≥5 years of data. The average age of patients ranged from 47 to 68 years across studies, and ∼50% (range: 40%-53%) of patients were male. Eleven studies were prospective and collected clinical data. The remaining retrospective studies consisted of claims (n = 4), clinical (n = 4), and national survey (n = 2) data.
Upon quality assessment, only 2 of the 21 studies were given a minus sign for >1 of the 8 evaluated items (Supplemental Table 1). Studies were most commonly given a minus sign because variables in the study were not measured in a way that would minimize misclassification bias (n = 6).
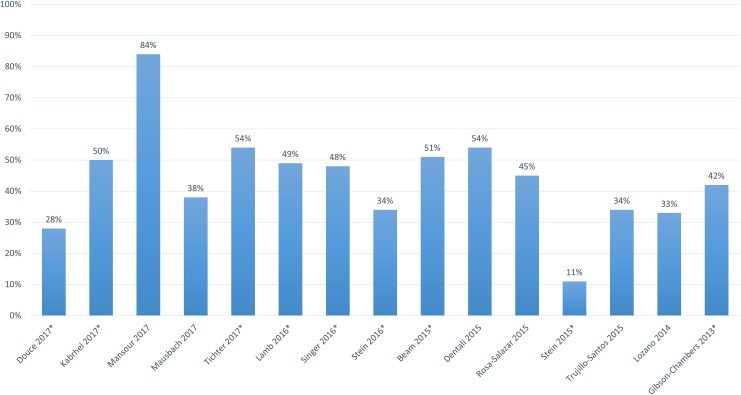
The proportion of all-comer patients with DVT managed as outpatients was reported in 15 studies and ranged from 11% to 84% but was ≤50% in 11 studies (Figure 2). Of the 9 studies evaluating US patients, the proportion of those treated as outpatients ranged from 11% to 54%, with 7 studies reporting that ≤50% of patients were managed as outpatients. The study with the highest occurrence of outpatient treatment (ie, 84%) included patients treated in Canada, while in the studies consisting of mainly European patients, 33% to 54% of patients were managed in an outpatient setting. The proportion of patients treated in an outpatient setting did not appear to correlate with study publication year.
Figure 2.
The proportion of patients with deep vein thrombosis treated as outpatients across studies. *Study included patients treated in the United States.
Thirteen studies reported clinical characteristics associated with outpatient compared to inpatient treatment (ie, category A studies; Table 2 and Supplemental Table 2). The only characteristic that was consistently associated with management in an outpatient setting was younger age, which was associated with outpatient treatment in 9 (69%) studies. Sex was not consistently associated with treatment setting. The comorbidities evaluated and found to be associated with treatment setting varied across the studies.
Table 2.
Factors Associated With Outpatient Versus Inpatient Treatment for Deep Vein Thrombosis.
| Author, Year (N) | Country | Proportion Treated as Outpatients, % (n/N) | Primary Anticoagulant Upon Discharge | Characteristics Associated With Outpatient Treatment | Characteristics not Associated With Treatment Setting |
|---|---|---|---|---|---|
| Douce, 2017 (N = 141) | US | 28% (39/141) | NR |
|
|
| Mansour 2017,a (N = 23 015) | Canada | 84% (19 306/23 015) | NR |
|
|
| Mausbach, 2017a (N = 236) | Israel | 38% (89/236) | LMWH and/or VKA |
|
|
| Tichter, 2017 (N = 690 000) | US | 54% (374 670/690 000) | NR |
|
|
| Lamb, 2016 (N = 1 146 469) | US | 49% (559 477/1 146 469) | NR |
|
|
| Singer, 2016 (N = 652 000) | US | 48% (312 960/652 000) | NR |
|
|
| Stein, 2016 (N = 2 671 452) | US | 34% (905 152/2 671 452) | NR |
|
|
| Dentali, 2015 (N = 1452) | Italy | 54% (780/1452) | LMWH and/or VKA |
|
|
| Rosa-Salazar, 2015a,c (N = 1135) | International | 45% (515/1135) | LMWH and/or VKA |
|
|
| Stein, 2015 (N = 96) | US | 11% (11/96) | NR |
|
|
| Trujillo-Santos, 2015 (N = 15 280) | International | 34% (5164/15 280) | LMWH and/or VKA |
|
|
| Lozano, 2014 (N = 13 493) | International | 33% (4456/13 493) | LMWH and/or VKA |
|
|
| Gibson-Chambers, 2013 (N = 845 000) | US | 42% (358 280/845 000) | NR |
|
|
Abbreviations: COPD, chronic obstructive pulmonary disease; DVT, deep vein thrombosis; LMWH, low-molecular-weight heparin; NR, not reported; US, United States; PVD, peripheral vascular disease; VKA, vitamin K antagonist; VTE, venous thromboembolism.
a Statistical significance was not reported for desired outcomes; thus, we independently analyzed the data to generate a P value, with values <.05 considered statistically significant.
b Defined by the Charlson comorbidity index. The proportion of patients with no comorbidities treated as outpatients was higher than the number of patients with comorbid conditions treated as outpatients.
c All included patients had deep vein thrombosis in the upper extremity.
d The most common comorbid conditions were diabetes and chronic obstructive pulmonary disease. Other comorbidities included dementia, cancer, and cerebral vascular disease.
e Defined by the Charlson comorbidity index and measured as a continuous variable.
We identified 8 studies describing a program aimed at facilitating outpatient treatment for patients with DVT presenting to an ED (ie, category B studies). Of these, 4 reported criteria used to identify patients who might be ineligible for outpatient treatment (Table 3). Elevated bleeding risk (eg, recent gastrointestinal bleeding, coagulopathy, and thrombocytopenia) was consistently reported as a factor that would make patients with DVT ineligible for outpatient treatment across the studies, with all studies reporting ≥4 criteria assessing this. Similarly, all studies reported comorbidities (eg, renal or liver disease) that may make patients ineligible for outpatient treatment. Three (75%) studies listed unreliable follow-up or inability to obtain medication as a reason to exclude patients from outpatient DVT treatment. Other criteria included extensive or recurrent DVT and pregnancy.
Table 3.
Criteria Used to Deem Patients With Deep Vein Thrombosis Ineligible for Outpatient Treatment in Studies of Emergency Department Programs.
| Criteriaa | Barrett (2016) | Beam (2015)b | Falconieri (2014)c | Davis (2013)c |
|---|---|---|---|---|
| Active or high risk for bleeding | ||||
| Active bleeding | Yes | Yes | Yes | Yes |
| Recent GI bleeding | Yes | NR | Yes | NR |
| Recent surgery | Yes | Yes | Yes | Yes |
| Recent stroke or thrombolytic therapy | Yes | NR | Yes | Yes |
| Recent trauma or hospitalization | NR | NR | Yes | Yes |
| Coagulopathy | Yes | Yes | Yes | Yes |
| Thrombocytopenia | NR | Yes | Yes | Yes |
| High risk for fall or trauma | NR | NR | Yes | Yes |
| Comorbidities | ||||
| Decreased renal function | Yes | Yes | Yes | Yes |
| Liver disease/dysfunction | Yes | Yes/Nod | Yes | Yes |
| Overweight/obese | Yes | NR | Yes | Yes |
| Chronic lung disease | Yes | Yes/No d | NR | NR |
| Heart failure | Yes | Yes/No d | NR | NR |
| HIT | NR | Yes | Yes | NR |
| Receiving chemotherapy for cancer | NR | Yes/Noe | Yes | Yes |
| Immobilization | NR | Yes/No d | Yes | Yes |
| Social factors | ||||
| Unreliable follow-up or unable obtain medication | Yes | Yes | Yes | NR |
| Incarcerated | Yes | Yes | NR | NR |
| Psychosis | NR | Yes | Yes | NR |
| Drug/alcohol dependence | NR | Yes | NR | NR |
| Presentation | ||||
| Iliofemoral DVT | Yes | NR | NR | NR |
| Extensive or bilateral DVT | Yes | NR | Yes | NR |
| Recurrent DVT | NR | NR | Yes | Yes |
| DVT developed while on anticoagulation | Yes | NR | Yes | Yes |
| Intractable pain | NR | Yes | NR | NR |
| SBP <100 or >180 mm Hg | NR | Yes | Yes | NR |
| Other factors | ||||
| Pregnancy | Yes | Yes | Yes | Yes |
| Drug interactions | Yes | NR | NR | Yes |
Abbreviations: DVT, deep vein thrombosis; GI, gastrointestinal; HIT, heparin induced thrombocytopenia; NR, not reported; SBP, systolic blood pressure
a“Yes” indicates criteria used to deem patients ineligible for outpatient treatment. Chu and colleagues as well as Karbhel and colleagues reported selecting patients for outpatient treatment via clinical gestalt.
b This criterion was also applied to patients with pulmonary embolism.
cThis criterion is based on criterion from InterQual software. Padron and colleagues also report that criterion from InterQual software was used to identify patients who may be ineligible for outpatient treatment.
dThis study listed any medical condition requiring hospital treatment (as judged by the clinician) as a criteria that would deem a patient ineligible for outpatient treatment.
e Patients with cancer underwent additional risk stratification via the POMPEC clinical prediction rule.
The DVT treatment strategies across the 8 identified programs are described in Table 4. All programs included the following 4 components: (1) patient education, (2) measures to encourage early outpatient follow-up (eg, scheduling appointments prior to ED discharge), (3) assessment of medication access (eg, checking insurance coverage), and (4) a multidisciplinary team (eg, consisting of the treating ED clinician as well as pharmacists [n = 7] and social workers or case managers [n = 5]). Further, 7 of the 8 programs reported calling patients in the weeks following their presentation for acute DVT. Half (n = 4) of the programs provided standardized educational handouts upon discharge, and 3 programs provided patients with a supply of the anticoagulant before ED discharge.
Table 4.
Process for Outpatient Treatment of Acute Deep Vein Thrombosis Across Emergency Department Programs.
| Author, Year | ED Treatment | Primary Anticoagulant Upon Discharge | Postdischarge Follow-Up |
|---|---|---|---|
| Chu, 2017 | Pharmacist reviews patient chart (eg, baseline laboratory values, comorbidities) for contraindication to anticoagulation and provides advice on dosing, provides education and medication counseling, maintains awareness of underinsured patients, facilitates prior authorization paperwork if needed for anticoagulant and anticoagulant filled through outpatient pharmacy and delivered to patient in ED (30-day supply) | DOAC | Outpatient follow-up established prior to discharge with assistance from social work services. Follow-up visit within 1-2 weeks encouraged. Pharmacist calls patients in the weeks following discharge until follow-up confirmed |
| Kabrhel, 2017 | ED clinicians and case managers educate patients about the importance of follow-up. Use of case managers to check if medications are covered by insurance and assess adherence is encouraged | DOAC | Clinicians and case managers make every effort to ensure follow-up appointment with PCP or designated VTE clinic within 1 week. Patients called at 7 and 30 days |
| Barrett, 2016 | Baseline CBC and BMP obtained, patient given first dose of anticoagulant, ED pharmacist dispenses 7- to 14-day supply of anticoagulant and provides education and medication counseling. ED pharmacist also consulted to determine which anticoagulant can be prescribed based on a patient’s insurance | DOAC | Appointment (within 3-7 days) scheduled prior to discharge with assistance from social work |
| Beam, 2015a | Baseline CBC and BMP obtained, patient given 1 dose of DOAC (1 time dose of LMWH optional), a prescription for a DOAC, and discharge instructions (which included contact information for physician) provided | DOAC | Patients seen in designated VTE clinic at 3 weeks and 3-6 months. Patients called 1-2 days after discharge to confirm that they filled the medication and to answer any questions |
| Padron, 2015 | Pharmacist drops off prescription at outpatient pharmacy and provides patient with a slip to pick up the medication, provides education and medication counseling, and instructs patients to call pharmacist with questions after discharge | LMWH and/or VKA | Appointment at anticoagulation clinic scheduled prior to discharge. Follow-up appointments occur at 1, 3 and 6 months and patients called prior to appointments to remind them of the visit |
| Falconieri, 2014 | Baseline laboratory values obtained, appropriate anticoagulant selected, medication access assessed with assistance from social work, prescription given (1 time dose of LMWH was optional), and education (which included patient handouts) and medication counseling provided. Observation unit was utilized for patients when more extensive discharge planning was required | DOAC | Appointment scheduled with PCP or antithrombotic service. Patients called by pharmacist in first 3-5 days and then again at 30 days |
| Misky, 2014 | Baseline laboratory values obtained, prescription and educational handouts given, education and medication counseling provided by nurse and/or pharmacist. Case management helps with discharge planning. Low-income patients received medication assistance for anticoagulants | LMWH and/or VKA | Providers submit a standardized electronic form that ensures a follow-up appointment with a pharmacist-run anticoagulation clinic is scheduled. Patients called within 3 days to confirm they have obtained the medication and are taking it correctly and to screen for adverse events and disease progression. Patients are re-educated about disease state, importance of follow-up, and medication during call |
| Davis, 2013 | Patients managed in observation care unit while arrangements for discharge made. Pharmacist recommends anticoagulant dose, provides education and medication counseling (which includes informational kit with educational material), and instructs patients to call pharmacist with questions after discharge. Anticoagulation either delivered to patient in ED or pharmacist instructs where it can be filled. For patients unable to afford anticoagulant regimen, hospital clinicians and administrators determine if medication costs can be waived | LMWH and/or VKA | Appointment at anticoagulation clinic scheduled prior to discharge and patients are called prior to this appointment to remind them of the visit |
Abbreviations: CBC, complete blood count; BMP, basic metabolic panel; DOAC, direct oral anticoagulant; ED, emergency department; LMWH, low-molecular-weight heparin; PCP, primary care physician; VKA, vitamin K antagonist; VTE, venous thromboembolism
aOutcomes of this program are also reported by DiRenzo and colleagues, Kahler and colleagues, Kline and colleagues, and Hall and colleagues. Direnzo and colleagues report on the outcomes of a pharmacist-managed outpatient follow-up clinic after ED discharge for patients selected using the protocol by Beam and colleagues.
Discussion
In this systematic review, we evaluated the use of outpatient treatment for acute DVT across 21 real-world studies. The proportion of all-comer patients treated in an outpatient setting was low (ie, <55% in all but 1 study). The only characteristic that was consistently associated with outpatient rather than inpatient treatment in these all-comer patients was younger age, which was associated with outpatient treatment in ∼70% of studies. We identified 8 studies describing a program aimed at facilitating outpatient treatment for patients with DVT presenting to an ED. Four of these studies reported criteria used to identify patients who might be ineligible for outpatient treatment (eg, elevated bleeding risk and comorbidities). Strengths of our systematic review include that it summarizes real-world data, which increases applicability. Moreover, the review could serve as a resource for clinicians developing ED programs aimed at facilitating outpatient DVT treatment. For instance, all ED programs in our systematic review included the following components: (1) patient education, (2) measures to encourage early outpatient follow-up, (3) assessment of medication access, and (4) a multidisciplinary team. Clinicians developing DVT outpatient treatment programs should ensure that these aforementioned components are incorporated.
The use of the outpatient setting to treat acute DVT was studied as early as the 1990s and was first mentioned in the American College of Chest Physicians (ACCP) clinical practice guidelines in 2001.2,3,38 Between 1996 and 2005, there were 7 RCTs comparing outpatient versus inpatient DVT treatment.39 In a meta-analysis including 6 of these trials (n = 1708), outpatient treatment resulted in fewer recurrent VTE events compared to inpatient treatment (relative risk [RR] = 0.58; 95% confidence interval [CI] = 0.39 to 0.86). Mortality (RR = 0.69; 95% CI = 0.44 to 1.09) and major bleeding (RR = 0.67; 95% CI = 0.33 to 1.36) did not differ between groups. Based on several of these trials, the 2004 ACCP guidelines recommended outpatient treatment for acute DVT “if possible” and inpatient treatment “if necessary.”40 Although these guideline recommendations have existed for nearly 15 years, the implementation of outpatient treatment for DVT appears to be low, as demonstrated by the low proportion of all-comer patients with DVT treated in an outpatient setting across the real-world studies included in our systematic review (ie, <55% in all but 1 study).
The proportion of patients with DVT treated in an outpatient setting ranged from 11% to 84% across the studies included in our systematic review. This wide range can be partially explained by the fact that studies were conducted in different countries. For instance, the study with the highest proportion of outpatient treatment (ie, 84%) was conducted in Canada, whereas 11% to 54% of patients in US studies were managed in an outpatient setting. Similar trends have been observed with PE treatment.20,41-44 Studies suggest approximately half of all patients presenting with PE in Canada are treated as outpatients,42-43 whereas ≤10% of patients with PE in several US studies received outpatient management.20,44 It has been hypothesized that factors contributing to these observations include differences in health systems and malpractice litigation.41
Although we restricted our systematic review to studies published after 2012, the majority of the included studies still collected data in the years prior to the widespread use of DOACs for the management of acute VTE.45 As such, LMWHs and VKAs were the anticoagulants prescribed upon ED or hospital discharge in most studies. Low-molecular-weight heparin requires patients to commit to daily injections, and as VKAs require initial overlap with an injectable agent until a therapeutic international normalized ratio (INR) is obtained, outpatient treatment with a VKA also requires a patient to commit to LMWH injections for ∼5 or more days.46-47 Moreover, VKAs require frequent INR monitoring and dose adjustments in the initial treatment period.47 These properties of LMWH and VKAs could have created a barrier to outpatient treatment. There are 2 DOACs (ie, apixaban and rivaroxaban) that do not require initial treatment with an injectable anticoagulant or frequent dose adjustments in the initial treatment period.5,6 When compared to LMWH and VKAs for the acute treatment of VTE, these agents have been associated with reductions in length of stay.48 Moreover, according to a clinical policy on acute DVT published by ACEP in 2018, DOACs may be a “safe and effective treatment alternative to LMWH/VKA” (level B recommendation) and select patients receiving DOAC therapy can be “directly discharged from the ED” (level C recommendation).7 It is possible that the availability of DOACs could lead to a higher amount of outpatient treatment for DVT than was observed in our systematic review.
There are several possible explanations for the considerable variability in the clinical characteristics associated with outpatient versus inpatient treatment of DVT observed across the 13 category A studies in our systematic review. First, unlike PE where clinical prediction rules can be used to identify patients who may qualify for outpatient treatment,49-51 there are few extensively validated rules to select those who may qualify for outpatient DVT treatment. This could lead to variability in the comorbidities used by clinicians to select patients for inpatient management. Second, studies in our systematic review utilized both claims and clinical data, which often measure patient characteristics through diagnostic billing codes and manual review of patient charts, respectively. This may have led to differences in the types of characteristics available to authors for analysis. For instance, while several clinical studies reported the proportion of outpatients and inpatients with immobility, it is unlikely that this characteristic was available in the included studies using claims data. Similarly, some included studies used univariate analysis, while others used multivariable analysis, to compare clinical characteristics among outpatients versus inpatients, which could have also contributed to differences in the clinical characteristics associated with treatment setting across studies.
Of the studies of programs aimed at facilitating outpatient treatment of acute DVT for patients presenting to the ED identified in our systematic review, one (Beam and colleagues) reported outcomes among outpatients (n = 71) that were prospectively followed for ∼1 year (mean = 389 days) after their acute DVT.8-9,35-37 None of these patients had recurrent VTE or major bleeding while receiving DOAC therapy. Decreased costs and favorable health-related quality of life scores have also been reported among patients selected for outpatient treatment using the criteria described by Beam and colleagues.8-9,35-37 In a case–control study, treatment costs were compared between patients receiving outpatient treatment with a DOAC (ie, cases) and 47 controls treated with initial LMWH and a VKA following an acute VTE.8 At 6 months, median VTE-related treatment costs were US$1446 (interquartile range [IQR] = US$1143-US$2842) for patients receiving a DOAC versus US$4006 (IQR = US$2692-US$8476) in the control group (P < .001). Further, the Venous Insufficiency Epidemiological and Economic Study Quality of Life questionnaire (VEINES QoL) and the physical component summary (PCS) from the 36-item Short-Form Health Survey were administered to 106 patients managed in an outpatient setting for acute DVT at 2 to 4 weeks (ie, baseline) and 3 to 6 months following ED discharge.35 Mean ± SD VEINES QoL and PCS scores at baseline were 48 ± 6 and 37.2 ± 13.9, respectively, which are similar to scores previously reported among patients with acute DVT.52 At 3 to 6 months, VEINES QoL scores increased to 73 ± 7 (P < .001), while PCS scores increased to 42.2 ± 12.9 (P < .05).35 Although this program designed by Beam and colleagues resulted in favorable outcomes,8-9,35-37 whether similar programs will increase the proportion of patients with acute DVT that can be safely managed as outpatients has yet to be determined.
This systematic review has several limitations. Outpatient treatment requires adequate social support.38-40,45 Patients must be able to attend follow-up visits, have access to anticoagulant treatment, and be able to easily return to the hospital if they were to deteriorate. Unfortunately, these factors were not reported in the majority of studies classified as category A in our systematic review, and thus, there was no way to summarize the impact that they might have had on disposition decisions. Second, the sample size of some included studies in category A was small (ie, in 3 studies, <300 patients were evaluated). These smaller studies may have had lower power to show a difference in clinical characteristics among outpatients versus inpatients. Third, outpatient DVT ED programs may be created as quality improvement projects at single institutions. These initiatives may not be disseminated outside the institution or only presented at local meetings and thus could not be captured in our literature search. Finally, many studies did not report outcomes (ie, mortality, major bleeding, and recurrent VTE) among patients with DVT treated as outpatients, and thus, we could not report these outcomes in our systematic review.
Conclusion
Although RCT evidence demonstrating the safety and efficacy of outpatient treatment for acute DVT has existed for >20 years, the proportion of all-comer patients with DVT managed as outpatients across real-world studies in this systematic review was low (ie, ≤50% all but 4 studies). While the clinical characteristics associated with outpatient treatment in these all-comer patients varied considerably, several programs aimed at facilitating outpatient DVT treatment have been described. It is possible that programs similar to these will increase the proportion of patients with DVT who can be safely managed as outpatients.
Supplemental Material
Supplementary_material_(2)_(1) for Systematic Review of Real-World Studies Evaluating Characteristics Associated With or Programs Designed to Facilitate Outpatient Management of Deep Vein Thrombosis by Erin R. Weeda, and Sofia Butt in Clinical and Applied Thrombosis/Hemostasis
Acknowledgments
The authors would like to thank David Bart Lawrence, Marcia Wright, Patrick Hlavacek, Nicole Abolins, and Anu Gupta for their support.
Authors’ Note: Informed consent was not applicable for this systematic review summarizing already published studies.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: E.R.W. received research support for this systematic literature review from Pfizer and Bristol-Myers Squibb. SB has nothing to disclose.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: E.R.W. received research support for this systematic literature review from Pfizer and Bristol-Myers Squibb
Supplemental Material: Supplemental material is available for this article online.
References
- 1. HCUPnet, Healthcare Cost and Utilization Project. Agency for Healthcare Research and Quality. 2014. http://hcupnet.ahrq.gov/. Accessed October 25, 2018. [PubMed]
- 2. Koopman MM, Prandoni P, Piovella F, et al. Treatment of venous thrombosis with intravenous unfractionated heparin administered in the hospital as compared with subcutaneous low-molecular-weight heparin administered at home. The Tasman Study Group. N Engl J Med. 1996;334(11):682–687. [DOI] [PubMed] [Google Scholar]
- 3. Levine M, Gent M, Hirsh J, et al. A comparison of low-molecular-weight heparin administered primarily at home with unfractionated heparin administered in the hospital for proximal deep-vein thrombosis. N Engl J Med. 1996;334(11):677–681. [DOI] [PubMed] [Google Scholar]
- 4. Lozano F, Trujillo-santos J, Barrón M, et al. Home versus in-hospital treatment of outpatients with acute deep venous thrombosis of the lower limbs. J Vasc Surg. 2014;59(5):1362–1367.e1. [DOI] [PubMed] [Google Scholar]
- 5. Eliquis [package insert]. Princeton, NJ: Bristol-Myers Squibb; New York, NY: Pfizer; 2017. [Google Scholar]
- 6. Xarelto [package insert]. Titusville, NJ: Janssen Pharmaceuticals; 2017. [Google Scholar]
- 7. Wolf SJ, Hahn SA, Nentwich LM, et al. Clinical policy: critical issues in the evaluation and management of adult patients presenting to the emergency department with suspected acute venous thromboembolic disease. Ann Emerg Med. 2018;71(5):e59–e109. [DOI] [PubMed] [Google Scholar]
- 8. Kahler ZP, Beam DM, Kline JA. Cost of treating venous thromboembolism with heparin and warfarin versus home treatment with rivaroxaban. Acad Emerg Med. 2015;22(7):796–802. [DOI] [PubMed] [Google Scholar]
- 9. Beam DM, Kahler ZP, Kline JA. Immediate discharge and home treatment with rivaroxaban of low-risk venous thromboembolism diagnosed in two U.S. emergency departments: a one-year preplanned analysis. Acad Emerg Med. 2015;22(7):788–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Barrett TW, Wrenn KD, Slovis CM, et al. An outpatient management protocol for emergency department patients with a newly diagnosed lower extremity deep venous thrombosis. Crit Pathw Cardiol. 2016;15(3):75–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Downes MJ, Brennan ML, Williams HC, Dean RS. Development of a critical appraisal tool to assess the quality of cross-sectional studies (AXIS). BMJ Open. 2016;6(12):e011458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Viswanathan M, Patnode CD, Berkman ND, et al. Recommendations for assessing the risk of bias in systematic reviews of health-care interventions. J Clin Epidemiol. 2018;97:26–34. [DOI] [PubMed] [Google Scholar]
- 13. National Institutes of Health. NIH quality assessment tool for observational cohort and cross-sectional studies. https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools. 2014. Accessed October 25, 2018.
- 14. Dentali F, Di micco G, Giorgi pierfranceschi M, et al. Rate and duration of hospitalization for deep vein thrombosis and pulmonary embolism in real-world clinical practice. Ann Med. 2015;47(7):546–554. [DOI] [PubMed] [Google Scholar]
- 15. Douce D, Mcclure LA, Lutsey P, Cushman M, Zakai NA. Outpatient treatment of deep vein thrombosis in the United States: the reasons for geographic and racial differences in stroke study. J Hosp Med. 2017;12(10):826–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rosa-salazar V, Trujillo-santos J, Díaz peromingo JA, et al. A prognostic score to identify low-risk outpatients with acute deep vein thrombosis in the upper extremity. J Thromb Haemost. 2015;13(7):1274–1278. [DOI] [PubMed] [Google Scholar]
- 17. Mansour S, Alotaibi G, Wu C, Mcmurtry MS. Trends in admission rates and in-hospital stay for venous thromboembolism. Thromb Res. 2017;156:149–154. [DOI] [PubMed] [Google Scholar]
- 18. Mausbach LS, Avnery O, Ellis MH. Ambulatory versus in-hospital treatment of proximal lower-limb deep vein thrombosis in adults: a retrospective cohort study. Clin Appl Thromb Hemost. 2017;23(7):859–864. [DOI] [PubMed] [Google Scholar]
- 19. Lamb KM, Neylan C, Jackson BM, et al. Risk factors for inpatient admission for deep venous thrombosis: the impact of structural factors. J Vasc Surg. 2016;63:211S. [Google Scholar]
- 20. Singer AJ, Thode HC, Peacock WF. Admission rates for emergency department patients with venous thromboembolism and estimation of the proportion of low risk pulmonary embolism patients: a US perspective. Clin Exp Emerg Med. 2016;3(3):126–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stein PD, Matta F, Hughes PG, et al. Home treatment of deep venous thrombosis in the era of new oral anticoagulants. Clin Appl Thromb Hemost. 2015;21(8):729–732. [DOI] [PubMed] [Google Scholar]
- 22. Stein PD, Matta F, Hughes MJ. Home treatment of deep venous thrombosis according to comorbid conditions. Am J Med. 2016;129(4):392–397. [DOI] [PubMed] [Google Scholar]
- 23. Trujillo-santos J, Lozano F, Lorente MA, et al. A prognostic score to identify low-risk outpatients with acute deep vein thrombosis in the lower limbs. Am J Med. 2015;128(1):90.e9–90.e15. [DOI] [PubMed] [Google Scholar]
- 24. Gibson Chambers J, Kabrhel C, Venkatesh AK, Schuur JD. Prevalence and patient predictors in outpatient treatment of venous thromboembolic disease. Ann Emerg Med. 2013;62:S36. [Google Scholar]
- 25. Chu A, Limberg J. Rivaroxaban program for acute venous thromboembolism upon ED discharge, with focus on utility of commercially available dose pack. Am J Emerg Med. 2017;35(12):1910–1914. [DOI] [PubMed] [Google Scholar]
- 26. Misky GJ, Carlson T, Thompson E, Trujillo T, Nordenholz K. Implementation of an acute venous thromboembolism clinical pathway reduces healthcare utilization and mitigates health disparities. J Hosp Med. 2014;9(7):430–435. [DOI] [PubMed] [Google Scholar]
- 27. Davis KA, Miyares MA, Price-goodnow VS. Optimizing transition of care through the facilitation of a pharmacist-managed deep vein thrombosis treatment program. J Pharm Pract. 2013;26:438–441. [DOI] [PubMed] [Google Scholar]
- 28. Falconieri L, Thomson L, Oettinger G, et al. Facilitating anticoagulation for safer transitions: preliminary outcomes from an emergency department deep vein thrombosis discharge program. Hosp Pract (1995). 2014;42(4):16–45. [DOI] [PubMed] [Google Scholar]
- 29. Padron M, Miyares MA. Development of an anticoagulation stewardship program at a large tertiary care academic institution. J Pharm Pract. 2015;28(1):93–98. [DOI] [PubMed] [Google Scholar]
- 30. Tichter AM, Nassef Y, Suh E, et al. Demographics, treatment, and disposition of patients diagnosed with deep vein thrombosis in United States emergency departments. Acad Emerg Med. 2017;70:S61. [Google Scholar]
- 31. Kabrhel C, Rosovsky R, Baugh C, et al. A novel protocol increases the proportion of pulmonary embolism patients safely discharged from the emergency department without hospital admission. Ann Emerg Med. 2017;70:S161. [Google Scholar]
- 32. Kabrhel C, Rosovsky R, Baugh C, et al. The creation and implementation of an outpatient pulmonary embolism treatment protocol. Hosp Pract (1995). 2017;45(3):123–129. [DOI] [PubMed] [Google Scholar]
- 33. White B, Rosovsky R, Parry BA, Kabrhel C. The outpatient treatment of venous thromboembolism: operational impact and the role of novel anticoagulants. Semin Thromb Hemost. 2016;42(8):846–856. [DOI] [PubMed] [Google Scholar]
- 34. Massachusetts General Hospital . Outpatient treatment of PE and DVT in the emergency department. In: ClinicalTrials.gov. Bethesda, MD: National Library of Medicine (US); 2000. https://clinicaltrials.gov/ct2/show/NCT02532387NLMIdentifier:NCT02532387. Accessed October 25, 2018.
- 35. Kline JA, Kahler ZP, Beam DM. Outpatient treatment of low-risk venous thromboembolism with monotherapy oral anticoagulation: patient quality of life outcomes and clinician acceptance. Patient Prefer Adherence. 2016;10:561–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Direnzo BM, Beam DM, Kline JA, et al. Implementation and preliminary clinical outcomes of a pharmacist-managed venous thromboembolism clinic for patients treated with rivaroxaban post emergency department discharge. Acad Emerg Med. 2018;25(6):634–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hall CL, Garrett JS, Wang H, et al. Dissemination of a protocol to expedite home treatment of low-risk patients with venous thromboembolism with monotherapy anticoagulation. Ann Emerg Med. 2017;70:S4. [Google Scholar]
- 38. Hyers TM, Agnelli G, Hull RD, et al. Antithrombotic therapy for venous thromboembolic disease. Chest. 2001;119(suppl 1):176S–193S. [DOI] [PubMed] [Google Scholar]
- 39. Othieno R, Okpo E, Forster R. Home versus in-patient treatment for deep vein thrombosis. Cochrane Database Syst Rev. 2018;1:CD003076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Büller HR, Agnelli G, Hull RD, Hyers TM, Prins MH, Raskob GE. Antithrombotic therapy for venous thromboembolic disease: the seventh ACCP conference on antithrombotic and thrombolytic therapy. Chest. 2004;126(suppl 3):401S–428S. [DOI] [PubMed] [Google Scholar]
- 41. Vinson DR, Zehtabchi S, Yealy DM. Can selected patients with newly diagnosed pulmonary embolism be safely treated without hospitalization? A systematic review. Ann Emerg Med. 2012;60(5):651–662. e4. [DOI] [PubMed] [Google Scholar]
- 42. Kovacs MJ, Hawel JD, Rekman JF, Lazo-langner A. Ambulatory management of pulmonary embolism: a pragmatic evaluation. J Thromb Haemost. 2010;8(11):2406–2411. [DOI] [PubMed] [Google Scholar]
- 43. Erkens PM, Gandara E, Wells P, et al. Safety of outpatient treatment in acute pulmonary embolism. J Thromb Haemost. 2010;8(11):2412–2417. [DOI] [PubMed] [Google Scholar]
- 44. Pollack CV, Schreiber D, Goldhaber SZ, et al. Clinical characteristics, management, and outcomes of patients diagnosed with acute pulmonary embolism in the emergency department: initial report of EMPEROR (Multicenter Emergency Medicine Pulmonary Embolism in the Real World Registry). J Am Coll Cardiol. 2011;57(6):700–706. [DOI] [PubMed] [Google Scholar]
- 45. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149(2):315–352. [DOI] [PubMed] [Google Scholar]
- 46. Lovenox [package insert]. Bridgewater, NJ: Sanofi-Aventis U.S. LLC; 2009. [Google Scholar]
- 47. Coumadin [package insert]. Princeton, NJ: Bristol-Myers Squibb; 2017. [Google Scholar]
- 48. Saint CA, Castelli MR, Crannage AJ, Stacy ZA, Hennessey EK. Comparison of hospital length of stay in patients treated with non-vitamin K oral anticoagulants or parenteral agents plus warfarin for venous thromboembolism. SAGE Open Med. 2017;5:2050312117719628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Weeda ER, Kohn CG, Peacock WF, et al. External validation of the Hestia criteria for identifying acute pulmonary embolism patients at low risk of early mortality. Clin Appl Thromb Hemost. 2016;23(7):769–774. [DOI] [PubMed] [Google Scholar]
- 50. Weeda ER, Kohn CG, Fermann GJ, et al. External validation of prognostic rules for early post-pulmonary embolism mortality: assessment of a claims-based and three clinical-based approaches. Thromb J. 2016;14:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kohn CG, Mearns ES, Parker MW, Hernandez AV, Coleman CI. Prognostic accuracy of clinical prediction rules for early post-pulmonary embolism all-cause mortality: a bivariate meta-analysis. Chest. 2015;147(4):1043–1062. [DOI] [PubMed] [Google Scholar]
- 52. Kahn SR, Lamping DL, Ducruet T, et al. VETO Study investigators. VEINES-QOL/Sym questionnaire was a reliable and valid disease-specific quality of life measure for deep venous thrombosis. J Clin Epidemiol. 2006;59(10):1049–1056. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary_material_(2)_(1) for Systematic Review of Real-World Studies Evaluating Characteristics Associated With or Programs Designed to Facilitate Outpatient Management of Deep Vein Thrombosis by Erin R. Weeda, and Sofia Butt in Clinical and Applied Thrombosis/Hemostasis