Abstract
Our aim is to determine the most appropriate laboratory tests, besides anti-factor Xa (anti-FXa) chromogenic assays, to estimate the degree of anticoagulation with apixaban and compare it with that of rivaroxaban in real-world patients. Twenty patients with nonvalvular atrial fibrillation treated with apixaban 5 mg twice daily and 20 patients on rivaroxaban 20 mg once daily were studied. Conventional coagulation tests, thrombin generation assay (TGA), and thromboelastometry (nonactivated TEM [NATEM] assay) were performed in the 40 patients and 20 controls. The anti-FXa chromogenic assays were used to measure apixaban and rivaroxaban plasma levels. The NATEM measurements showed no significant difference between the 2 groups of patients. Concerning TGA, endogenous thrombin potential (ETP) was significantly decreased in patients on rivaroxaban as compared to those treated with apixaban (P < .003). A statistically significant, strong inverse correlation between apixaban plasma concentrations and ETP (P < .001) was observed. Apixaban significantly reduces ETP compared to controls, but to a lesser extent than rivaroxaban. Thrombin generation assay might provide additional information on apixaban exposure, which is required in order to individualize treatment especially for patients with a high bleeding risk. Our findings have to be further investigated in studies with larger sample sizes, in the entire range of apixaban exposure, with other direct oral anticoagulants, and in relation to clinical outcomes.
Keywords: atrial fibrillation, anticoagulant activity, apixaban, rivaroxaban, thrombin generation assay, thromboelastometry
Introduction
Atrial fibrillation (AF) is the most common chronic cardiac rhythm abnormality with worldwide prevalence ranged between 1% and 2%.1 Direct oral anticoagulants (DOACs), which are inhibitors of both thrombin and factor Xa (FXa), have been developed and demonstrated a favorable benefit–risk profile for primary and secondary prevention of stroke and systemic embolism in patients with nonvalvular atrial fibrillation (NV-AF). Unlike vitamin K antagonists, DOACs (ie, apixaban, dabigatran, edoxaban, and rivaroxaban) are administered in fixed doses and do not usually require routine laboratory monitoring for dose adjustment, due to their more predictable pharmacokinetics and pharmacodynamics.2
However, measuring their anticoagulant activity and/or plasma levels may be helpful in certain clinical circumstances, such as bleeding or thrombosis during treatment, preoperative state, suspected overdose, and in certain populations including those with extremes in body weight, the elderly individuals, patients with renal insufficiency, and patients with AF presenting with acute ischemic stroke prior to administration of thrombolytic therapy.3
The lack of a readily available method to determine the degree of anticoagulation creates challenges to both clinicians and laboratory staff in terms of assessing the risk for bleeding of patients receiving these drugs in emergency. Anti-FXa chromogenic assays seem to be the most appropriate assays for the quantitative measurement of FXa inhibitor plasma levels.4 Nevertheless, this type of assay is time-consuming and not commonly available. More recently, global coagulation assays such as viscoelastic tests (ROTEM and TEG) and thrombin generation assays (TGAs) have also been recommended as potential tests for assessing the anticoagulant effect of DOACs. These assays have several advantages including providing global information on the coagulation process in short turnaround time.5–7 The relatively limited published data on the laboratory testing of apixaban8 suggest that rotational thromboelastometry (ROTEM) and TGA may be useful for screening, with ROTEM providing quick results in emergency situations.9 In most cases, these data were collected from in vitro studies using blood samples spiked with apixaban.6,9–11
Our research group has recently studied the anticoagulant activity of dabigatran and rivaroxaban, as measured by these 2 assays in real-life patients with NV-AF.12,13 The aim of this study is to identify the most appropriate laboratory tests, besides anti-FXa chromogenic assays, to measure the degree of anticoagulation with apixaban 5 mg twice daily and to compare it with that of rivaroxaban 20 mg once daily in real-life patients with NV-AF.
Methods
The study population included patients with NV-AF who were on anticoagulation and were recruited from the Second University Department of Cardiology and the Department of Haemostasis in the “Attiko” University Hospital in Athens, Greece (Supplemental material). The group A consisted of patients on rivaroxaban 20 mg once daily, who were also included in a recently published study by our research team.13 Patients matched on age and sex with those in group A and taking the standard dose of apixaban (5 mg orally twice daily) recommended for patients with high CHA2DS2-VASc score (congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, stroke/transient ischemic attack, vascular disease, age 65-75, and female sex) comprised group B. The same hemostatic parameters were also measured in age- and sex-matched, healthy participants. Individuals with platelet count <100 × 109/L and active thrombosis or bleeding, patients on simultaneous anticoagulant and antiplatelet therapy, those with renal and/or hepatic and/or thyroid dysfunction, those with malignancy or with chronic infectious or autoimmune diseases, and patients with active infection at the start of the study were excluded.
The study was performed in accordance with the Declaration of Helsinki and was approved by the hospital’s institutional review board (approval number: 135, July 10, 2017). Informed consents were obtained from all patients.
For the study participants, the CHA2DS2-VASc score was calculated, detailed personal medical history (related to thrombotic and/or bleeding complications) was obtained, and a thorough clinical examination was performed. At least 7 days after study enrollment, the following laboratory examinations were undertaken: complete blood count, activated partial thromboplastin time (aPTT) and international normalized ratio (INR), fibrinogen, D-dimers, thrombin generation (TG), and ROTEM assay. Chromogenic assays for the in vitro quantitative measurement of FXa direct inhibitor (DiXaI, Biophen; Hyphen BioMed, Neuville-sur-Oise, France, and liquid anti-Xa assay; HemosIL Instrumentation Laboratory Company, Milan, Italy) were used to measure the plasma concentration of rivaroxaban and apixaban, respectively, in the tested samples.
The last dose of antithrombotic medication was administered approximately 3 hours before blood sampling. The ROTEM analysis and conventional coagulation tests were done on the same day, within 2 hours from sampling. Complete blood counts were performed on a Sysmex XE-2100 analyzer (Elgin, Roche, Illinois). The aPTT, PT, and INR, fibrinogen, and d-dimers were all measured on BCS XP System hemostasis analyzer (Siemens Healthcare Diagnostics, Marburg, Germany). Pathromtin* SL (Siemens Healthcare Diagnostics) was used to determine aPTT and the Thromborel S reagent (Siemens Healthcare Diagnostics) was used for PT determination. Plasma concentrations of fibrinogen were measured using a modification of the Clauss method with the Fibrinogen Multifibren* U reagent (Siemens Healthcare Diagnostics). D-dimers were assessed by the Innovance D-dimer assay (Siemens Healthcare Diagnostics), a particle-enhanced immunoturbidimetric method. Blood samples for analysis were anticoagulated with 0.109 mol/L trisodium citrate (9:1, vol/vol blood anticoagulant) and immediately centrifuged at 2500g for 20 minutes.
Liquid Anti-Xa Assay
This automated chromogenic assay is used for measuring direct FXa inhibitor concentrations in human citrated plasma. Factor Xa is neutralized directly by apixaban. Residual FXa is quantified with a synthetic chromogenic substrate. The paranitroaniline (pNA) released is monitored kinetically at 405 nm and is inversely proportional to the apixaban levels in the sample. Apixaban levels in patient plasma were measured automatically on IL (Instrumentation Laboratory) coagulation systems (ACL TOP) when this assay was calibrated with the HemosIL apixaban calibrators.
Biophen, FXa Direct Inhibitor (DiXaI)
This assay is a 2-stage method based on inhibition of a constant and in excess amount of exogenous FXa, by the tested DiXaI, and on hydrolysis of an FXa-specific chromogenic substrate, by the residual FXa. Paranitroaniline is then released from the substrate. The amount of pNA released is in direct relationship with the residual FXa activity. There is an inverse relationship between the concentration of DiXaI in the tested sample and color development measured at 405 nm. The test was performed on the BCS1 XP system hemostasis analyzer.
For both anti-FXa chromogenic assays, blood was collected on 0.109 M trisodium citrate anticoagulant and immediately centrifuged at 2500g for 20 minutes. Then samples were snap frozen in small portions and stored at −20°C until the assay was performed.
Thrombin Generation Assay
INNOVANCE ETP (Siemens Healthcare Diagnostics) is a global hemostasis function test to assess the endogenous thrombin potential (ETP) of plasma samples. The incubation of plasma with phospholipids and activator and calcium ions leads to initiation and propagation of the coagulation processes, eventually resulting in the generation of thrombin. Thrombin generation and the subsequent inactivation were recorded by monitoring the conversion of a specific slow reacting chromogenic substrate at a wavelength of 405 nm over time. The assay was performed using BCS XP system hemostasis. The estimated parameters of the TG curve included area under the curve (AUC), also referred to as ETP; lag time (tlag) that describes the time from initiation of the reaction until TG is observed; time to peak (tmax), which is the time from initiation of the reaction until the maximum TG, is observed; and finally maximum TG depicted by peak height (Cmax). Blood samples were anticoagulated with 0.109 mol/L trisodium citrate (9:1, vol/vol blood anticoagulant) and immediately centrifuged at 2500g for 20 minutes. The supernatant was removed and was then centrifuged again. Plasma was snap frozen in small portions and stored at −20°C until the assay was performed on the BCS XP system hemostasis analyzer.
Thromboelastometry (ROTEM)
Viscoelastic measurements were done using ROTEM (Tem Innovations GmbH, Munich, Germany). For this test, whole blood was collected in 3.8% trisodium citrate, then recalcified and analyzed on the ROTEM analyzer (Tem Innovations GmbH) using the nonactivated TEM (NATEM) assay within 2 hours from blood sampling. The NATEM test is a semiquantitative in vitro diagnostic assay used on the ROTEM delta thromboelastometry system to monitor the coagulation process, contact-activated by the surface of the measurement cell, in citrated whole blood specimens. The following NATEM variables were measured: clotting time (CT, seconds) that was determined by the time elapsed from the start of measurement until the formation of a clot 2 mm in amplitude; clot formation time (CFT, seconds) was the time elapsed from the end of CT (amplitude of 2 mm) until a clot firmness of 20 mm was achieved; a angle (a, o) was the angle between the central line (x axis) and the tangent of the TEM tracing at the amplitude point of 2 mm describing the kinetics of clot formation; maximum clot firmness (MCF, mm) reflects the final strength of the clot; finally, the lysis index at 60 minutes is defined as the percentage of the remaining clot stability in relation to the MCF following the 60-minute observation period after CT and indicates the speed of fibrinolysis.
Statistical Methods
Descriptive statistics are presented as means with standard deviations and as medians with interquartile ranges (IQRs) or proportions with percentages when appropriate. Nonparametric tests were used because most of the variables were not normally distributed. The analyses included the Fisher exact test, the Wilcoxon rank sum (Mann-Whitney) test for differences in continuous variables from 2 sample populations, and the Kruskal-Wallis test for differences in continuous variables from more than 2 sample populations. Correlations were assessed by the Spearman rank correlation coefficient. Spearman ρ <.20 indicates very weak correlation, .21 to .40 weak correlation, .41 to .60 moderate correlation, .61 to .80 strong correlation, and higher than .81 very strong correlation. Test results with P value <.05 were considered as statistically significant. All statistical tests were 2 sided. The analyses were done using STATA 14 (StataCorp LP, College Station, Texas).
Results
The group A included 20 patients on rivaroxaban with a median age of 73 years (IQR: 66.5-78) and half of them being females. The group B consists of 20 patients (70% females) on apixaban with a median age of 77 years (IQR: 63.5-79.5). Descriptive characteristics of patients, associated comorbidities, and their hematological and biochemical parameters are shown in Table 1. The 2 patient groups were similar without statistically significant differences. The anti-FXa levels between the 2 groups were comparable.
Table 1.
Characteristics of Patients on Rivaroxaban (Group A, n = 20) and on Apixaban (Group B, n = 20).a
| Characteristics | Group A | Group B | P Value |
|---|---|---|---|
| Age | 69.7 (13.1); 73.0 (66.5-78.0) | 72.9 (11.7); 77.0 (63.5-79.5) | .40 |
| Gender (females) % | 10 (50) | 14 (70) | .33 |
| CHA2DS2-VASc score | 3.4 (1.8); 3.0 (2.0-5.0) | 4.1 (1.5); 4.0 (3.0-5.0) | .16 |
| Comorbidities | |||
| Diabetes mellitus, n (%) | 3 (15) | 6 (30) | .45 |
| Dyslipidemia, n (%) | 7 (35) | 9 (47) | .52 |
| Smoking status, n (%) | 7 (35) | 3 (15) | .27 |
| Hypertension, n (%) | 14 (70) | 17 (85) | .45 |
| Vascular disease, n (%) | 5 (25) | 6 (30) | 1.00 |
| Biochemical parameters | |||
| Creatinine, mg/dL | 0.9 (0.4); 0.8 (0.8-1.1) | 1.0 (0.3); 0.9 (0.7-1.1) | .72 |
| ALT, U/L | 18.4 (7.6); 18.0 (11.5-24.0) | 26.1 (26.7); 14.0 (12.0-30.5) | .97 |
| AST, U/L | 20.4 (7.4); 20.5 (14.5-23.5) | 20.5 (12.9); 18.0 (13.5-22.0) | .87 |
| TSH, mU/L | 2.0 (1.1); 2.0 (1.1-2.6) | 2.0 (1.2); 1.9 (1.0-3.1) | .46 |
| Hematological parameters | |||
| Hemoglobin, g/dL | 12.6 (2.0); 12.8 (11.5-14.1) | 13.4 (2.4); 13.9 (11.6-15.0) | .29 |
| WBC, ×109 cells/L | 7.33 (1.63); 7.36 (6.00-8.63) | 7.79 (1.68); 7.61 (6.51-9.16) | .41 |
| PLT, ×109 cells/L | 216 (70); 222 (152-275) | 267 (92); 249 (209-301) | .10 |
Abbreviations: ALT, alanine transaminase; AST, aspartate transaminase; CHA2DS2-VASc, congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, stroke/transient ischemic attack, vascular disease, age 65-75, and female sex; PLT, platelets; TSH, thyroid-stimulating hormone; WBC, white blood count.
aData are presented as means with standard deviations; medians and interquartile ranges in parentheses, or percentages when appropriate. Nonparametric statistical tests (Fisher exact test, Wilcoxon rank sum [Mann Whitney] test) were used.
Table 2 presents the results of comparisons of coagulation parameters among the 3 groups (the 2 patient groups and the healthy controls). In terms of the conventional coagulation tests, patients on rivaroxaban had a statistically significant prolongation of aPTT and higher INR values than patients treated with apixaban (P < .001 and P = .036, respectively). Viscoelastic measurements on the ROTEM analyzer showed a statistically significant prolongation of CT and CFT and a decrease in a angle in both groups of patients as compared to controls (P < .05). However, the 2 groups of patients on rivaroxaban and apixaban did not significantly differ, despite the greater impact of rivaroxaban on CT in comparison to apixaban.
Table 2.
Comparison of Coagulation Parameters Among Patients on Rivaroxaban (Group A, n = 20), on Apixaban (Group B, n = 20), and Controls (Control Group, n = 20).a
| Group A | Group B | Control | K-W Test (P Value) | P Value (A vs B; A vs Co; B vs Co) | |
|---|---|---|---|---|---|
| Coagulation parameters | |||||
| INR | 1.58 (0.60); 1.39 (1.15-1.83) | 1.26 (0.29); 1.14 (1.08-1.34) | 0.99 (0.08); 0.99 (0.92-1.04) | <.001 | .036; <.001; <.001 |
| aPTT, seconds | 45.4 (15.3); 42.6 (38.9-48.4) | 31.8 (3.3); 31.5 (29.6-34.0) | 30.5 (3.7); 29.2 (28.0-31.9) | <.001 | <.001; <.001; .070 |
| Fibrinogen, mg/dL | 372.4 (71.4); 369.0 (326.0-407.0) | 446.8 (114.6); 429.5 (358.4-514.2) | 290.2 (94.5); 262.0 (213.0-343.0) | <.001 | .036; .003; <.001 |
| D-dimers, ng/mL | 615.6 (787.0); 296.5 (199.5-754.0) | 1128.3 (2880.2); 447.0 (329.0-779.4) | 317.6 (173.0); 274.5 (170.0-368.5) | .037 | .256; .255; .007 |
| ROTEM (NATEM) | |||||
| CT, seconds | 916 (414); 767 (676-1074) | 750 (150); 763 (660-841) | 499 (116); 491 (412-584) | <.001 | .245; <.001; <.001 |
| CFT, seconds | 310 (265); 188 (151-379) | 232 (128); 205 (157-296) | 133 (31); 125 (113-163) | .001 | .695; .001; .002 |
| a, ° | 50.5 (17.1); 56.0 (39.0-62.0) | 54.5 (12.0); 55.0 (47.0-60.0) | 64.5 (5.0); 65.5 (59.5-68.0) | .002 | .787; .001; .003 |
| MCF, mm | 59.9 (6.2); 59.0 (56.0-64.0) | 60.2 (7.6); 60.0 (56.0-64.5) | 58.8 (4.0); 58.0 (56.0-61.0) | .680 | .839; .524;.401 |
| Li60, % | 95.4 (3.5); 95.5 (92.5-98.5) | 94.6 (3.3); 94.5 (92.0-96.0) | 95.4 (2.8); 95.5 (94.0-97.0) | .652 | .499; .957; .347 |
| Endogenous thrombin potential | |||||
| Lag time, seconds | 48.2 (16.2); 47.5 (35.7-56.2) | 39.7 (7.6); 39.1 (34.4-42.7) | 30.1 (5.0); 29.3 (27.0-31.7) | <.001 | .079; <.001; <.001 |
| Tmax, seconds | 109.3 (47.1); 109.1 (70.6-124.2) | 89.7 (24.5); 89.6 (66.2-107.2) | 83.6 (16.6); 80.3 (70.2-90.3) | .182 | .204; .079; .543 |
| Cmax, %/min | 70.2 (18.8); 71.4 (58.7-84.2) | 86.1 (12.3); 86.5 (75.5-91) | 113.4 (13.2); 111.2 (105.5-119.0) | <.001 | .009; <.001; <.001 |
| AUC, % | 71.4 (18.7); 75.0 (60.5-85.5) | 87.9 (11.6); 86.5 (83.5-95.5) | 101.5 (13.0); 97.5 (92.5-112.5) | <.001 | .003; <.001; .003 |
| Plasma drug concentrations | |||||
| Anti-Xa assays, ng/mL | 217.0 (152.8); 200.0 (85.0-345.0) | 244.9 (120.6); 223.5 (147.0-329.0) | – | – | .387 |
Abbreviations: a, angle; aPTT, activated partial thromboplastin time; AUC, area under the curve; CFT, clot formation time; Cmax, peak height; CT, clotting time; INR, international normalized ratio; LI 60, lysis index at 60 minutes; MCF, maximum clot firmness; Tmax, time to peak.
a Data are presented as means and standard deviations; medians and interquartile ranges in parentheses, or percentages when appropriate. Nonparametric statistical tests (Wilcoxon rank sum [Mann Whitney] test and Kruskal-Wallis rank test) were used.
Concerning TGA, the procoagulant activity was significantly decreased in patients on rivaroxaban than those on apixaban (AUC: P < .003; Cmax: P < .009). Both tlag and tmax were also affected more by rivaroxaban than apixaban, but this difference was not statistically significant.
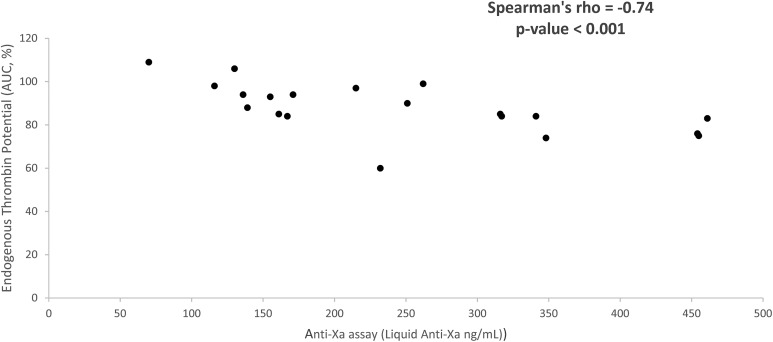
The correlation of apixaban plasma values, as determined by the anti-FXa chromogenic assay, with all hemostatic parameters is presented in Table 3. There is statistically significant, strong inverse correlation between apixaban plasma concentrations and AUC (P < .001; Figure 1) or Cmax (P < .001). No correlation was found between ETP and rivaroxaban levels. The plasma levels of apixaban and rivaroxaban were not significantly associated with NATEM variables.
Table 3.
Group B: Correlation of Plasma Apixaban Levels (ng/mL) With Hemostatic Parameters.
| Parameter | Spearman ρ | P Value | Interpretation |
|---|---|---|---|
| Coagulation | |||
| INR | +0.47 | .037 | Statistically significant, moderate positive correlation |
| aPTT, seconds | +0.51 | .021 | Statistically significant, moderate positive correlation |
| Fibrinogen, mg/dL | +0.27 | .257 | No correlation |
| D-dimers, ng/mL | +0.16 | .492 | No correlation |
| ROTEM (NATEM) | |||
| CT, seconds | +0.29 | .212 | No correlation |
| CFT, seconds | +0.12 | .600 | No correlation |
| a, ° | −0.09 | .716 | No correlation |
| MCF, mm | +0.33 | .151 | No correlation |
| LI 60, % | −0.08 | .743 | No correlation |
| Endogenous thrombin potential | |||
| Lag time, seconds | +0.60 | .005 | Statistically significant, moderate positive correlation |
| Tmax, seconds | +0.40 | .081 | No correlation |
| Cmax, seconds | −0.74 | <.001 | Statistically significant, strong inverse correlation |
| AUC, % | −0.74 | <.001 | Statistically significant, strong inverse correlation |
Abbreviations: a, angle; aPTT, activated partial thromboplastin time; AUC, area under curve; CFT, clot formation time; Cmax, peak height; CT, clotting time; INR, international normalized ratio; LI 60, lysis index at 60 minutes; MCF, maximum clot firmness; Tmax, time to peak.
Figure 1.
Endogenous thrombin potential (AUC, %) and plasma apixaban levels (group B) determined by anti-Xa assay (liquid anti-Xa, ng/mL). AUC indicates area under the curve.
Discussion
This study shows a stronger procoagulant effect of rivaroxaban than of apixaban and highlights the potential role of TGA in assessing the anticoagulant activity of apixaban in vivo in certain clinical contexts. To the best of our knowledge, a direct comparison of the anticoagulant activity of different DOACs using hemostatic variables measured in real-life settings has not been done.
Few studies have investigated the value of elastography or elastometry in the context of apixaban therapy. In most cases, in vitro experiments were done that demonstrated a significant impact of apixaban on CT or reaction time.6,7,9,11,14 In our study, NATEM CT and CFT were also significantly prolonged in patients on apixaban or rivaroxaban compared to controls. However, there was not any correlation between apixaban/rivaroxaban plasma concentrations and NATEM parameters. A strong correlation between apixaban and rivaroxaban plasma concentrations and the LowTF-ROTEM CT in in vitro experiments have been noted by Adelmann et al, but such a relation was not reported in ex vivo samples.7 Several studies have been conducted to evaluate the role of thromboelastometry in the setting of rivaroxaban therapy, yielding however inconsistent results.7,15–17 In a recent study of our team, a significant prolongation of CT and CFT was observed, but no association was found between ROTEM variables and anti-Xa values in real-world patients.13 The available data insofar do not support the use of viscoelastic coagulation assays either for excluding the presence of rivaroxaban or for determining rivaroxaban concentration, due to the lack of adequate sensitivity. Thus, although viscoelastic coagulation tests could play a role in detecting the presence of direct FXa inhibitors, more evidence is needed before these tests are considered appropriate assays for detecting and measuring the anticoagulant effect of these inhibitors.18
Regarding TGA, few in vitro studies have assessed the results of this assay in relation to plasma levels of apixaban.10,11,14,19 Apixaban influenced all parameters in a dose-dependent manner, but the sensitivity varied. Thus, there is insufficient evidence to recommend the use of TGA for assessing apixaban effect in clinical practice.18 In this study, apixaban, compared to controls, reduced TG, with prolongations of lag time and a decrease in peak thrombin level. Moreover, significant correlations between apixaban plasma levels and almost all parameters of the ETP assay were also observed. The impact of rivaroxaban on TG has been more extensively evaluated. Rivaroxaban produced dose-dependent effects on all TG parameters with variable sensitivity, but ETP (AUC) appeared to have lower responsiveness to rivaroxaban plasma levels than did the other TG parameters.13,18,20 This does not seem to hold for apixaban. Unlike rivaroxaban, a strong, statistically significant, inverse correlation between ETP and apixaban plasma concentrations was observed in our study. Moreover, apixaban resulted in a significantly lower reduction in ETP and Cmax, compared to rivaroxaban, indicating a different effect of the 2 FXa inhibitors on TG curve. The strong correlation between ETP and apixaban plasma levels in real-life patients suggests that TGA may be useful in quantifying the anticoagulant activity of apixaban. It is noteworthy that dabigatran plasma levels were also reported to be strongly inversely correlated with ETP in real-world patients.12,21 This is in accordance with the findings of a recent study, which showed that TG can measure the anticoagulation effect of commonly used DOACs, with each drug having a unique TG profile.22
Based on the effect of the 2 direct FXa inhibitors on ROTEM and TGA parameters, the anticoagulant effect of rivaroxaban 20 mg once daily is stronger than that of apixaban 5 mg twice daily. Rivaroxaban use, compared to apixaban, resulted in significant prolongations of lag time, decreases in peak thrombin level and ETP, prolonged aPTT, and higher INR values.
Since the introduction of the DOACs, it has been attempted to compare and rank them in terms of their safety and efficacy. In the absence of head-to-head randomized controlled trials, alternative methods of comparisons using existing trial data have been used, including cross-study comparisons using meta-analyses and network meta-analyses.23–26 Based on current evidence, the treatment with apixaban has the most favorable safety profile in terms of risk for bleeding among the DOACs and is considered to have the optimal benefit to risk ratio. These findings are in line with current American Heart Association/American Stroke Association recommendations that classify apixaban as the DOAC with the highest level of evidence (class I; level of evidence A) for prevention of recurrent stroke in patients with a history of stroke or transient ischemic attack in comparison with dabigatran (class I; level of evidence B) and rivaroxaban (class IIa; level of evidence B).27
To the best of our knowledge, no direct comparisons among DOACs, regarding their anticoagulant activity in real-life patients, are available. This is the first attempt to directly compare anticoagulant effects in patients with NV-AF between the 2 standard, direct FXa inhibitors. Based on TGA, laboratory findings are in accordance with clinical evidence that suggests that apixaban has the best benefit/risk profile among the 3 NOACs. Apixaban significantly reduces ETP compared to controls, but to a lesser extent than rivaroxaban and dabigatran, which both have a comparable effect on the amount of thrombin generated in real-life patients.13 This might be the optimal level of anticoagulant activity in order to achieve the best benefit to risk ratio for ischemic stroke prevention in patients with NV-AF. If apixaban is found to affect ETP in a dose-dependent manner, TGA could be used for assessing apixaban effect in clinical practice, by defining relative reference ranges in order to individualize treatment in certain clinical settings, especially in patients with high bleeding risk.
Limitations of our study are the small sample size and the absence of long-term follow-up to evaluate the clinical relevance of the laboratory parameters that were tested. On the other hand, our analyses are based on real-life patients, and it is also important that the laboratory findings based on TGA support current clinical evidence that classifies apixaban as the DOAC with the optimal benefit/risk profile.
In order to verify the potential impact of apixaban or other DOACs on specific coagulation assays, like TGA or the viscoelastic coagulation tests, in a valid and reliable way, several critical issues have to be addressed. The large number of specialized and modified assays that are available, the different reagents used by several laboratories, the limited use of ex vivo samples, and the lack of standardization probably cause delays in the development and establishment of new methods that can appropriately detect and quantify the anticoagulant activity of DOACs.
Conclusively, it seems that TGA can provide additional information on apixaban exposure and the benefit–risk profile, which are needed to individualize treatment under certain clinical circumstances, especially in patients with high bleeding risk. Hypotheses based on our findings have to be further investigated in studies with larger sample sizes, in the entire range of apixaban exposure, with other DOACs, and in relation to clinical outcomes.
Supplemental Material
Supplemental Material, DATA_(1) for Laboratory Assessment of the Anticoagulant Activity of Apixaban in Patients With Nonvalvular Atrial Fibrillation by Elias Kyriakou, Konstantinos Katogiannis, Ignatios Ikonomidis, George Giallouros, Georgios K. Nikolopoulos, Evdoxia Rapti, Maria Taichert, Katerina Pantavou, Argiri Gialeraki, Foteini Kousathana, Aristarchos Poulis, Andreas G. Tsantes, Stefanos Bonovas, Violetta Kapsimali, Georgios Tsivgoulis, and Argirios E. Tsantes in Clinical and Applied Thrombosis/Hemostasis
Footnotes
Authors’ Note: Ethical approval to report this case series was obtained from the institutional review board of “Attiko” University Hospital (approval number: 135, July 10, 2017). Written informed consent was obtained from the patients for their anonymized information to be published in this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Stefanos Bonovas  http://orcid.org/0000-0001-6102-6579
http://orcid.org/0000-0001-6102-6579
Supplemental Material: Supplemental material is available for this article online.
References
- 1. Danelich IM, Reed BN, Hollis IB, Cook AM, Rodgers JE. Clinical update on the management of atrial fibrillation. Pharmacotherapy. 2013;33(4):422–446. [DOI] [PubMed] [Google Scholar]
- 2. Ageno W, Gallus AS, Wittkowsky A, Crowther M, Hylek EM, Palareti G. American College of Chest Physicians. Oral anticoagulant therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl 2):e44S–e88S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tripodi A. The laboratory and the direct oral anticoagulants. Blood. 2013;121(20):4032–4035. [DOI] [PubMed] [Google Scholar]
- 4. Samama MM. Which test to use to measure the anticoagulant effect of rivaroxaban: the anti-factor Xa assay. J Thromb Haemost. 2013;11(4):579–580. [DOI] [PubMed] [Google Scholar]
- 5. Oswald E, Velik-Salchner C, Innerhofer P, et al. Results of rotational thromboelastometry, coagulation activation markers and thrombin generation assays in orthopedic patients during thromboprophylaxis with rivaroxaban and enoxaparin: a prospective cohort study. Blood Coagul Fibrinol. 2015;26(2):136–144. [DOI] [PubMed] [Google Scholar]
- 6. Dias JD, Norem K, Doorneweerd DD, et al. Use of thromboelastography (TEG) for detection of new oral anticoagulants. Arch Pathol Lab Med. 2015;139(5):665–673. [DOI] [PubMed] [Google Scholar]
- 7. Adelmann D, Wiegele M, Wohlgemuth RK, et al. Measuring the activity of apixaban and rivaroxaban with rotational thrombelastometry. Thromb Res. 2014;134(4):918–923. [DOI] [PubMed] [Google Scholar]
- 8. Favaloro EJ, Lippi G. Laboratory testing in the era of direct or nonvitamin K antagonist oral anticoagulants: a practical guide to measuring their activity and avoiding diagnostic errors. Semin Thromb Hemost. 2015;41(2):208–227. [DOI] [PubMed] [Google Scholar]
- 9. Eller T, Busse J, Dittrich M, et al. Dabigatran, rivaroxaban, apixaban, argatroban and fondaparinux and their effects on coagulation POC and platelet function tests. Clin Chem Lab Med. 2014;52(6):835–844. [DOI] [PubMed] [Google Scholar]
- 10. Tripodi A, Padovan L, Veena C, Scalambrino E, Testa S, Peyvandi F. How the direct oral anticoagulant apixaban affects thrombin generation parameters. Thromb Res. 2015;135(6):1186–1190. [DOI] [PubMed] [Google Scholar]
- 11. Martin AC, Gouin-Thibault I, Siguret V, et al. Multimodal assessment of non-specific hemostatic agents for apixaban reversal. J Thromb Haemost. 2015;13(3):426–436. [DOI] [PubMed] [Google Scholar]
- 12. Kyriakou E, Ikonomidis I, Stylos D, et al. Laboratory assessment of the anticoagulant activity of dabigatran. Clin Appl Thromb Hemost. 2015;21(5):434–445. [DOI] [PubMed] [Google Scholar]
- 13. Tsantes AE, Kyriakou E, Ikonomidis I, et al. Comparative assessment of the anticoagulant activity of rivaroxaban and dabigatran in patients with nonvalvular atrial fibrillation: a noninterventional study. Medicine (Baltimore). 2016;95(14):e3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Escolar G, Fernandez-Gallego V, Arellano-Rodrigo E, et al. Reversal of apixaban induced alterations in hemostasis by different coagulation factor concentrates: significance of studies in vitro with circulating human blood. PLoS One. 2013;8(11):e78696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Casutt M, Konrad C, Schuepfer G. Effect of rivaroxaban on blood coagulation using the viscoelastic coagulation test ROTEM™. Anaesthesist. 2012;61(11):948–953. [DOI] [PubMed] [Google Scholar]
- 16. Oswald E, Velik-Salchnera C, Innerhofera P, et al. Results of rotational thromboelastometry, C assays in orthopedic patients during thromboprophylaxis with rivaroxaban and enoxaparin: a prospective cohort study. Blood Coagul Fibrinol. 2015;26(2):136–144. [DOI] [PubMed] [Google Scholar]
- 17. Chojnowski K, Gorski T, Robak M, Trelinski J. Effects of rivaroxaban therapy on ROTEM coagulation parameters in patients with venous thromboembolism. Adv Clin Exp Med. 2015;24(6):995–1000. [DOI] [PubMed] [Google Scholar]
- 18. Samuelson BT, Cuker A, Siegal DM, Crowther M, Garcia DA. Laboratory assessment of the anticoagulant activity of direct oral anticoagulants: a systematic review. Chest. 2017;151(1):127–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jourdi G, Siguret V, Martin AC, et al. Association rate constants rationalise the pharmacodynamics of apixaban and rivaroxaban. Thromb Haemost. 2015;114(1):78–86. [DOI] [PubMed] [Google Scholar]
- 20. Rathbun S, Tafur A, Grant R, Esmon N, Mauer K, Marlar RA. Comparison of methods to determine rivaroxaban anti-factor Xa activity. Thromb Res. 2015;135(2):394–397. [DOI] [PubMed] [Google Scholar]
- 21. Tsantes AE, Kyriakou E, Bonovas S, et al. Impact of dabigatran on platelet function and fibrinolysis. J Neurol Sci. 2015;357(1-2):204–208. [DOI] [PubMed] [Google Scholar]
- 22. Rigano J, Ng C, Nandurkar H, Ho P. Thrombin generation estimates the anticoagulation effect of direct oral anticoagulants with significant interindividual variability observed. Blood Coagul Fibrinol. 2018;29(2):148–154. [DOI] [PubMed] [Google Scholar]
- 23. Almutairi AR, Zhou L, Gellad WF, et al. Effectiveness and safety of non-vitamin k antagonist oral anticoagulants for atrial fibrillation and venous thromboembolism: a systematic review and meta-analyses. Clin Ther. 2017;39(7):1456–1478. [DOI] [PubMed] [Google Scholar]
- 24. Sterne JA, Bodalia PN, Bryden PA, et al. Oral anticoagulants for primary prevention, treatment and secondary prevention of venous thromboembolic disease, and for prevention of stroke in atrial fibrillation: systematic review, network meta-analysis and cost-effectiveness analysis. Health Technol Assess. 2017;21(9):1–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Katsanos AH, Mavridis D, Parissis J, et al. Novel oral anticoagulants for the secondary prevention of cerebral ischemia: a network meta-analysis. Ther Adv Neurol Disord. 2016;9(5):359–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cohen AT, Hamilton M, Bird A, et al. Comparison of the non-VKA oral anticoagulants apixaban, dabigatran, and rivaroxaban in the extended treatment and prevention of venous thromboembolism: systematic review and network meta-analysis. PLoS One. 2016;11(9):e0163386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kernan W, Ovbiagele B, Black H, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(7):2160–2236. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, DATA_(1) for Laboratory Assessment of the Anticoagulant Activity of Apixaban in Patients With Nonvalvular Atrial Fibrillation by Elias Kyriakou, Konstantinos Katogiannis, Ignatios Ikonomidis, George Giallouros, Georgios K. Nikolopoulos, Evdoxia Rapti, Maria Taichert, Katerina Pantavou, Argiri Gialeraki, Foteini Kousathana, Aristarchos Poulis, Andreas G. Tsantes, Stefanos Bonovas, Violetta Kapsimali, Georgios Tsivgoulis, and Argirios E. Tsantes in Clinical and Applied Thrombosis/Hemostasis