Abstract
The prevalence of neurocognitive deficits remains high in patients with stage 5 chronic kidney disease (CKD5D). Major contributors to such deficits include stroke, cervical carotid artery disease (CCAD), and intracranial atherosclerotic disease (ICAD). The risk of developing these dysfunctional vascular processes is facilitated by the chronic inflammation associated with renal failure. Plasma levels of 10 circulating biomarkers in patients with CKD5D (n = 78-90) were quantified using the sandwich enzyme linked immune sorbent assay method. Biomarkers for this study included kidney injury molecule-1, N-terminal prohormone of brain natriuretic peptide (NT-proBNP), neutrophil gelatinase-associated lipocalin, interleukin-18, endothelin 1, calcifediol, parathyroid hormone, platelet-derived growth factor, microparticles-expressing tissue factor, and lipoprotein(a) (Lp(a)). Of the 90 patients with CKD5D, 30 had CCAD, 24 had ICAD, and 22 had stroke. Lp(a) level was significantly elevated in patients with CKD5D with comorbid ICAD compared to those without (125.70 ± 10.03 ng/mL vs 97.16 ± 5.97 ng/mL; P = .0065). NT-proBNP level was also significantly elevated in patients with CKD5D with comorbid stroke diagnosis compared to those without stroke history, once patients with a diagnosis of heart failure (HF) were excluded (14.84 ± 2.80 ng/mL vs 9.06 ± 1.27 ng/mL; P = .0283). Profiling levels of Lp(a) and NT-ProBNP could thus be useful in the risk stratification of ICAD and stroke, respectively, in the CKD5D population.
Keywords: biomarkers, chronic kidney disease, stroke, cervical carotid artery disease, intracranial atherosclerotic disease
Introduction
The prevalence of neurocognitive deficits remains high in patients with stage 5 chronic kidney disease (CKD5D).1-3 Major contributors to such deficits include stroke, cervical carotid artery disease (CCAD), and intracranial atherosclerotic disease (ICAD). Presence of these neurovascular conditions may cause adverse changes in cognitive function, including that of executive and motor skills, attention, and perception.4-7 Dementia, and other cognitive impairments in patients with CKD5D, have remained a serious concern for clinicians due to increased mortality rates associated with these deficits.8,9 Additionally, the National Institute of Diabetes and Digestive and Kidney Diseases noted that 5.4% of the deaths in patients with CKD5D from 2012 to 2014 were caused by stroke alone. Prior studies have reported that the stroke risk in the CKD5D population ranges from 5 to 11 times that of the general population.10,11 The authors of this study suggest that CKD5D ultimately predisposes patients to cerebrovascular events via a multifactorial pathophysiology involving upregulated inflammation, oxidative stress, and reduced toxin filtration within the cerebrorenal axis.
Biomarkers have played a critical role in aiding clinicians with the diagnosis and prognosis of multiple disease processes, yet the role of biomarkers on neurological outcomes in patients with CKD5D is not yet known.12,13 We sought to evaluate the role of 10 individual biomarkers in the CKD5D patient population. Kidney injury molecule-1 (KIM-1) is a protective transmembrane protein of the renal tubular epithelium that is only found to be upregulated in disease states.14-16 N-terminal prohormone of brain natriuretic peptide (NT-proBNP) is the 76 amino-acid long biologically inactive by-product of the brain natriuretic peptide maturation process.17 Utilization of NT-ProBNP as a predictive biomarker for stroke has been postulated in other studies, although translation to the clinical realm requires further investigation.18,19 Neutrophil gelatinase-associated lipocalin (NGAL), also known as siderocalin or lipocalin-2, is a mitogenic growth factor for epithelial development that has been known to be upregulated in the presence of declining renal function.14 Interleukin-18 (IL-18) is a cytokine that is elevated downstream of the inflammasome pathway and has been implicated in the exacerbation of hypertension.16,20 Endothelin 1 (ET-1) is as a marker of endothelial dysfunction and oxidative stress, and is suggested to be a participant in the process of arterial remodeling in CKD5D.21 Calcifediol (25(OH)D) and parathyroid hormone (PTH) are molecules with well-characterized functions related to calcium and phosphate homeostasis. It has been reported that abnormalities within calcium and phosphate metabolism contribute to the progression of CKD, while also providing a favorable environment for vascular calcification and cardiovascular disease.22 Derangements of bone mineral density have also been associated with the risk for stroke via developments of CCAD and ICAD.23-25 Platelet-derived growth factor (PDGF), a molecule known to exert effects on the vascular system (eg smooth muscle cells), has been implicated in the progression of chronic kidney disease.26 Microparticles-expressing tissue factor (MPs-TF) are vascular particles of varying sizes, a feature which depends on the cell of origin. Microparticles-expressing tissue factor have been associated with thrombosis and thromboembolic events.27 Lipoprotein(a) (Lp(a)) is a substance of low-density lipoproteins which has been well-characterized in terms of its participation in atherosclerosis and cardiovascular disease progression.28 Measuring and analyzing these biomarkers may thus contribute to our understanding of the pathogenesis of the cerebrorenal axis in patients with CKD5D.
Methods and Materials
Pre-dialysis blood samples from 90 patients with CKD5D (45 males, 45 females; aged 40-87, mean 62) were collected at Loyola Outpatient Dialysis Center during December 2014. Samples were collected in 3.2% (0.109 mol/L) sodium citrate tubes and centrifuged at 1100×g for 15 minutes within 2 hours of venous sampling. Each resulting plasma was then divided into 10 aliquots of 100 μL plasma, which were then frozen at −70°C for later analysis. Study samples were compared to those collected from healthy volunteers. Control samples originated from 25 male and 25 female, nonsmoking, drug-free healthy volunteers (aged 19-54 years, mean 33), which were purchased from George King Bio-medical, Inc (Overland Park, Kansas). The use of healthy controls would serve to elucidate normal levels of circulating blood biomarkers, as well as confirm the relative elevation of these levels in patients with CKD5D.
Stage 5 chronic kidney disease and control plasma samples were used to profile levels of biomarkers of inflammation, hemostatic dysregulation, or vascular dysfunction. Biomarkers measured in this study included KIM-1, N-terminal prohormone of brain natriuretic peptide (NT-proBNP), NGAL, IL-18, ET-1, 25(OH)D, PTH, PDGF, MP-TF, and Lp(a). Biomarker levels were measured using commercially available sandwich ELISA kits (Hyphen Biomed, Neuville-sur-Oise, France; R&D Systems, Inc., Minneapolis, MN; RayBiotech, Inc., Norcross, Georgia). Reagents, standards, and sample solutions were thawed, diluted, and prepared according to respective ELISA manufacturer’s instructions.
Patients’ demographic data (age, sex, and diagnoses) and comorbid conditions were extracted from electronic medical record. Any documentation of CCAD, ICAD, and stroke for each patient with CKD5D was noted, and it should be noted that some patients had more than one of these diagnoses. For example, 11 patients had all 3 documentations of CCAD, ICAD, and stroke. Two patients had only concurrent CCAD and ICAD without any documentations of stroke. Five patients had only concurrent CCAD and stroke documentations. Three patients had only concurrent ICAD and stroke documentations. Given the patient sample size for this study, stroke events encompass both hemorrhagic and ischemic sub-types. Biomarker and demographic data were collected in Microsoft Excel and analyzed using GraphPad Prism 7[Please provide manufacturer name and location (city and state [if USA] or city and country [if other than USA]) for “GraphPad Prism 7.”]. Mean ± standard error of the mean was used as the standard expression for the quantitative results. Comparisons were performed using Mann-Whitney t tests and Kruskal-Wallis non-parametric analysis of variance. Nonparametric Spearman was used for correlations. P values less than .05 were considered statistically significant.
Results
Table 1 compares the levels of circulating biomarkers between the study CKD5D population and healthy controls. Patients with stage 5 chronic kidney disease had significant elevations in the plasma concentration of most of the inflammatory and hemostatic biomarkers compared to healthy controls (P < .0001 KIM-1, NT-ProBNP, NGAL, IL-18, MP-TF; P = .0087 PTH; P = .0021 Lp(a)), except for ET-1 and PDGF (P = .0507 and P = .4045, respectively). The 25(OH)D, by contrast, was significantly decreased in patients with CKD5D compared to healthy controls (P < .0001). There were no shared correlations between levels of the biomarkers in CKD5D patient samples and healthy controls (Supplemental Table S1). Independently, the healthy controls had moderately strong positive correlations between Lp(a) and NGAL (r = .5579, P = .0131), and between Lp(a) and PTH (r = 0.6845, P = .0012). Stage 5 chronic kidney disease plasma, however, had 4 weak correlations, the strongest of which was between PDGF and PTH (r = −0.3301, P = .0015).
Table 1.
Biomarker Levels Reported as Mean ± SEM for Healthy Controls (n = 19-50) and Total CK5D Patients (n = 78-90). The P Value and Percentage Change (%Δ) between the 2 Groups are Also Reported. Percentage Change in Levels From Healthy Controls to CKD5D is Indicated by %Δ.
| Controls (n) | CKD5D (n) | P Value | %Δ | |
|---|---|---|---|---|
| KIM-1 (ng/mL) | 0.06 ± 0.01 (50) | 0.70 ± 0.13 (90) | <.0001 | +1092 ± 212 |
| NT-ProBNP (ng/mL) | 0.56 ± 0.34 (50) | 12.9 ± 1.19 (90) | <.0001 | +2212 ± 213 |
| NGAL (ng/mL) | 54.7 ± 1.78 (50) | 451 ± 9.67 (90) | <.0001 | +725 ± 17.7 |
| IL-18 (pg/mL) | 259 ± 15.6 (50) | 491 ± 33.4 (90) | <.0001 | +89.8 ± 12.9 |
| ET-1 (ng/mL) | 2.70 ± 0.44 (50) | 2.80 ± 0.16 (90) | .0507 | +3.82 ± 5.89 |
| 25(OH)D (ng/mL) | 31.1 ± 2.00 (50) | 21.9 ± 1.39 (90) | <.0001 | −29.7 ± 4.46 |
| PTH (pg/mL) | 55.3 ± 6.27 (50) | 97.9 ± 17.2 (90) | .0087 | +77.0 ± 31.0 |
| PDGF (pg/mL) | 82.7 ± 16.1 (50) | 116 ± 18.2 (90) | .4045 | +40.3 ± 22.0 |
| MP-TF (pg/mL) | 0.37 ± 0.04 (48) | 3.00 ± 0.16 (78) | <.0001 | +711 ± 43.6 |
| Lp(a) (ng/mL) | 66.1 ± 10.6 (19) | 104 ± 5.27 (90) | .0021 | +58.5 ± 7.98 |
Abbreviations: 25(OH)D, calcifediol; ET-1, endothelin 1; IL-18, interleukin-18; KIM-1, kidney injury molecule-1; Lp(a), lipoprotein(a); MP-TF, microparticles-expressing tissue factor; NT-ProBNP, NT-ProBNP; NGAL, neutrophil gelatinase-associated lipocalin; PTH, parathyroid hormone; PDGF, platelet-derived growth factor.
Electronic medical record data for patients with CKD5D revealed CCAD diagnosis in 30 patients, ICAD in 24 patients, and prior history of stroke in 22 patients. Table 2 compares biomarkers levels in patients with CKD5D with comorbid CCAD to those without. No statistically significant difference was found between these 2 groups. Average percent changes of each biomarker were relatively small, with the highest being that of KIM-1 at +53.5% ± 52.1%. The CKD5D (−)CCAD group demonstrated weak correlations between 5 biomarkers, while the (+)CCAD group revealed 3 correlations (Supplemental Table S1). The most significant correlation, however, was a positive correlation between PTH and NGAL in CKD5D (+)CCAD patients (r = 0.5679, P = .0011).
Table 2.
Levels of Biomarkers in Patients With CKD5D With Respect to Comorbid CCAD. Levels for (+) and (−) CCAD are Reported as Mean ± SEM. Percentage Change in Levels from (−)CCAD to (+)CCAD is Indicated by %Δ.
| (−)CCAD | (+)CCAD | P Value | %Δ | |
|---|---|---|---|---|
| KIM-1 (ng/mL) | 0.60 ± 0.10 | 0.92 ± 0.31 | .4809 | +53.5 ± 52.1 |
| NT-ProBNP (ng/mL) | 12.8 ± 1.54 | 13.3 ± 1.86 | .5597 | +3.81 ± 14.5 |
| NGAL (ng/mL) | 450 ± 12.4 | 454 ± 15.4 | .9813 | +0.75 ± 3.42 |
| IL-18 (pg/mL) | 498 ± 43.8 | 477 ± 49.3 | .6305 | −4.19 ± 9.90 |
| ET-1 (ng/mL) | 2.97 ± 0.23 | 2.48 ± 0.15 | .5612 | −16.3 ± 4.94 |
| 25(OH)D (ng/mL) | 22.3 ± 1.83 | 21.1 ± 2.01 | .9762 | −5.64 ± 8.99 |
| PTH (pg/mL) | 107 ± 25.4 | 79.8 ± 8.57 | .4889 | −25.4 ± 8.01 |
| PDGF (pg/mL) | 111 ± 15.9 | 126 ± 44.8 | .4957 | +13.6 ± 40.4 |
| MP-TF (pg/mL) | 2.93 ± 0.18 | 3.12 ± 0.33 | .9148 | +6.69 ± 11.2 |
| Lp(a) (ng/mL) | 102 ± 6.39 | 111 ± 9.36 | .3366 | +9.67 ± 9.22 |
Abbreviations: 25(OH)D, calcifediol; ET-1, endothelin 1; IL-18, interleukin-18; KIM-1, kidney injury molecule-1; Lp(a), lipoprotein(a); MP-TF, microparticles-expressing tissue factor; NT-ProBNP, NT-ProBNP; NGAL, neutrophil gelatinase-associated lipocalin; PTH, parathyroid hormone; PDGF, platelet-derived growth factor.
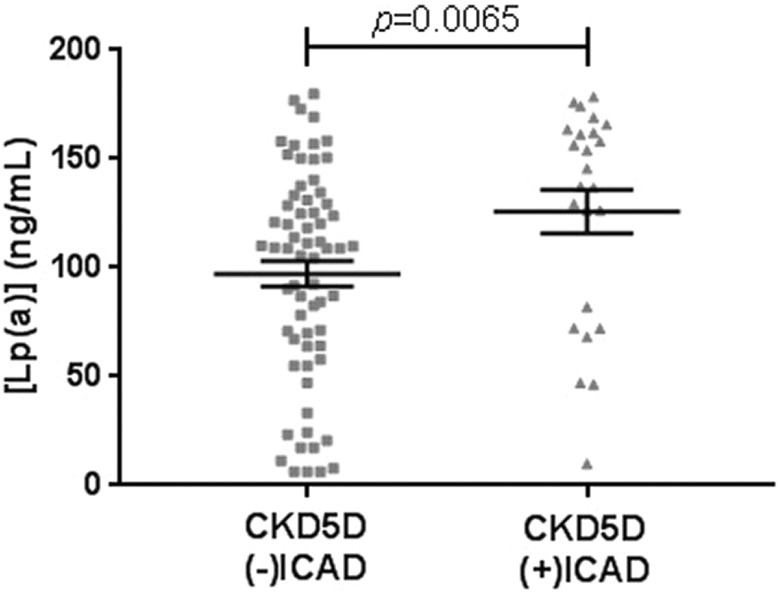
Comparison of biomarker levels in patients with CKD5D with regards to comorbid ICAD is shown in Table 3. Lp(a) levels were significantly elevated in patients with CKD5D with comorbid ICAD compared to those without (125.70 ± 10.03 ng/mL vs 97.16 ± 5.97 ng/mL, P = .0065; Figure 1). The average percent changes of each biomarker were found to be relatively small, and most of them decreased in the (+)ICAD group, with the exception of KIM-1, MP-TF, and Lp(a). The CKD5D (−)ICAD and (+)ICAD groups exhibited 4 weak correlations (Supplemental Table S1). The correlation between PDGF and 25(OH)D is noted, however, to be negative with moderate strength in patients with CKD5D (+)ICAD (r = -−0.5054, P = .0118).
Table 3.
Levels of Biomarkers in Patients With CKD5D with Respect to Comorbid ICAD. Levels for (+) and (−) ICAD are Reported as Mean ± SEM. Percentage Change in Levels from (−)ICAD to (+)ICAD is Indicated by %Δ.
| (−)ICAD | (+)ICAD | P Value | %Δ | |
|---|---|---|---|---|
| KIM-1 (ng/mL) | 0.70 ± 0.16 | 0.71 ± 0.17 | .4420 | +1.00 ± 24.5 |
| NT-ProBNP (ng/mL) | 13.3 ± 1.52 | 12.1 ± 1.60 | .6602 | −9.06 ± 12.1 |
| NGAL (ng/mL) | 455 ± 11.3 | 440 ± 19.0 | .4583 | −3.31 ± 4.17 |
| IL-18 (pg/mL) | 516 ± 43.1 | 423 ± 37.9 | .1766 | −18.0 ± 7.36 |
| ET-1 (ng/mL) | 2.82 ± 0.19 | 2.76 ± 0.29 | .7459 | −2.21 ± 10.3 |
| 25(OH)D (ng/mL) | 23.1 ± 1.73 | 18.5 ± 1.99 | .1949 | −20.1 ± 8.58 |
| PTH (pg/mL) | 104 ± 23.1 | 81.6 ± 11.1 | .6651 | −21.5 ± 10.7 |
| PDGF (pg/mL) | 124 ± 23.0 | 94.6 ± 25.8 | .3434 | −23.5 ± 20.9 |
| MP-TF (pg/mL) | 2.80 ± 0.16 | 3.50 ± 0.39 | .2123 | +25.2 ± 14.0 |
| Lp(a) (ng/mL) | 97.2 ± 5.97 | 126 ± 10.0 | .0065 | +29.4 ± 10.3 |
Abbreviations: 25(OH)D, calcifediol; ET-1, endothelin 1; IL-18, interleukin-18; KIM-1, kidney injury molecule-1; Lp(a), lipoprotein(a); MP-TF, microparticles-expressing tissue factor; NT-ProBNP, NT-ProBNP; NGAL, neutrophil gelatinase-associated lipocalin; PTH, parathyroid hormone; PDGF, platelet-derived growth factor.
Figure 1.
Comparison of Lp(a) levels between CKD5D (−)ICAD (n = 66) and CKD5D (+)ICAD (n = 24). Mean levels were 97.16 ± 5.97 ng/mL for (−)ICAD, and 125.70 ± 10.03 ng/mL for (+)ICAD. CKD5D indicates stage 5 chronic kidney diseas; ICAD, intracranial atherosclerotic disease.
Table 4 shows a comparison of biomarker levels in patients with CKD5D with a documented stroke history compared to those without. There were no statistically significant differences between the 2 groups. Average percent changes in the level of each biomarker were, again, found to be relatively small, with the 2 highest being that of KIM-1 at +63.8% ± 68.8% and NT-ProBNP at +46.9% ± 25.1%. In terms of correlations, the CKD5D (−)stroke group had 4 weak correlations between biomarker levels, while the (+)stroke group had 3 (Supplemental Table S1). The correlation between levels of Lp(a) and NT-ProBNP, however, was noted to be negative and of moderate strength in the CKD5D (+)stroke group (r = −0.5901, P = .0038).
Table 4.
Levels of Biomarkers in Patients With CKD5D With Respect to Comorbid Stroke. Levels for (+) and (−)Stroke are Reported as Mean ± SEM. Percentage Change in Levels From (−)Stroke to (+)stroke is Indicated by %Δ.
| (−)Stroke | (+)Stroke | P Value | %Δ | |
|---|---|---|---|---|
| KIM-1 (ng/mL) | 0.61 ± 0.10 | 1.00 ± 0.42 | .6098 | +63.8 ± 68.8 |
| NT-ProBNP (ng/mL) | 11.6 ± 1.24 | 17.1 ± 2.91 | .0849 | +46.9 ± 25.1 |
| NGAL (ng/mL) | 445 ± 11.4 | 472 ± 17.9 | .1990 | +5.99 ± 4.02 |
| IL-18 (pg/mL) | 498 ± 40.6 | 470 ± 54.8 | .7267 | −5.65 ± 11.0 |
| ET-1 (ng/mL) | 2.73 ± 0.18 | 3.04 ± 0.34 | .4317 | +11.5 ± 12.6 |
| 25(OH)D (ng/mL) | 23.0 ± 1.74 | 18.5 ± 1.72 | .3725 | −19.3 ± 7.48 |
| PTH (pg/mL) | 97.7 ± 22.2 | 98.7 ± 16.1 | .2899 | +1.01 ± 16.5 |
| PDGF (pg/mL) | 123 ± 22.6 | 93.4 ± 25.7 | .4055 | −24.2 ± 20.8 |
| MP-TF (pg/mL) | 2.98 ± 0.18 | 3.06 ± 0.35 | .9425 | +2.78 ± 11.6 |
| Lp(a) (ng/mL) | 101 ± 5.97 | 116 ± 11.1 | .2111 | +14.4 ± 11.0 |
Abbreviations: 25(OH)D, calcifediol; ET-1, endothelin 1; IL-18, interleukin-18; KIM-1, kidney injury molecule-1; Lp(a), lipoprotein(a); MP-TF, microparticles-expressing tissue factor; NT-ProBNP, NT-ProBNP; NGAL, neutrophil gelatinase-associated lipocalin; PTH, parathyroid hormone; PDGF, platelet-derived growth factor.
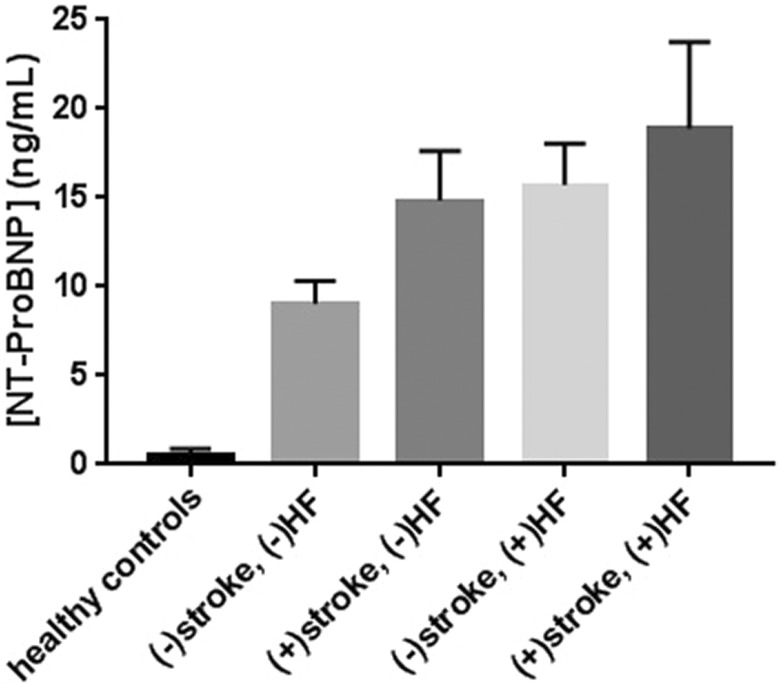
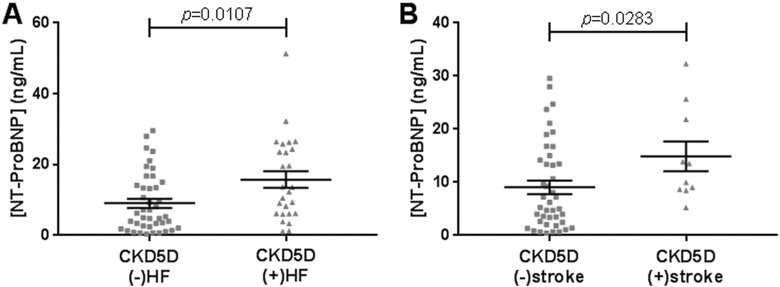
In relation to stroke history and HF diagnosis, 42 patients with CKD5D had neither a history of stroke nor HF, 10 patients with CKD5D had a prior diagnosis of stroke without documented HF, 26 patients with CKD5D had a HF diagnosis without experiencing a prior stroke event, and 12 patients with CKD5D had both a stroke history and HF comorbidity. Figure 2 shows the mean levels of NT-ProBNP in healthy controls and patients with CKD5D with or without prior diagnosis of stroke and/or HF. N-terminal prohormone of brain natriuretic peptide levels were found to be significantly elevated in nonstroke patients with CKD5D with comorbid HF compared to those without (P = .0107; Figure 3A). N-terminal prohormone of brain natriuretic peptide levels were also significantly elevated in patients with CKD5D with a history of stroke diagnosis compared to those without, once patients with HF diagnosis were excluded (P = .0283; Figure 3B).
Figure 2.
Levels of circulating NT-ProBNP in healthy controls and patients with CKD5D with or without prior diagnosis of stroke and/or HF. Mean levels were 0.56 ± 0.01 ng/mL for healthy controls (n = 50), 9.06 ± 1.27 ng/mL for CKD5D without comorbid HF or stroke history (n = 42), 14.8 ± 2.8 ng/mL for CKD5D with stroke history only (n = 10), 15.7 ± 2.3 ng/mL for CKD5D with comorbid HF only (n = 26), and 18.9 ± 4.9 ng/mL for CKD5D with dual stroke history and HF comorbidity (n = 12). CKD5D indicates stage 5 chronic kidney disease.
Figure 3.
A, Comparison of NT-ProBNP levels between (−)HF (n = 42) and (+)HF (n = 26) groups in nonstroke CKD5D patients. Mean levels were 9.06 ± 1.27 ng/mL for (−)HF, and 15.74 ± 2.32 ng/mL for (+)HF. B, Comparison of NT-ProBNP levels between (−)stroke (n = 42) and (+)stroke (n = 10) groups in patients with non-HF CKD5D. Mean levels were 9.06 ± 1.27 ng/mL for (−)stroke, and 14.84 ± 2.80 ng/mL for (+)stroke. CKD5D indicates stage 5 chronic kidney disease; NT-ProBNP, N-terminal prohormone of brain natriuretic peptide.
The prevalence of various vascular abnormalities present within the study CKD5D cohort were enumerated and stratified by the relevant neurovascular disease subcategories in Table 5. HF, carotid artery disease (CAD), and peripheral vascular disease (PVD) had the highest prevalence within this CKD5D population. The prevalence of a vascular pathology within this cohort is also greater in patients with CKD5D with a neurovascular disease, with the exception of deep venous thrombosis and the broad vasculitides. Finally, similar percentages of CAD diagnosis seem to be apparent across the positive neurovascular disease categories, and also this holds true for the diagnoses of ACS, HF, and PVD.
Table 5.
Prevalence of Various Vascular Pathologies Present Within the CKD5D Cohort and its Neurovascular Disease Subcategories. Coronary Artery Disease (CAD), Acute Coronary Syndrome (ACS), Peripheral Vascular Disease (PVD), Deep Venous Thrombosis (DVT), Pulmonary Embolism (PE).
| CAD | ACS | HF | PVD | DVT | PE | Vasculitides | |
|---|---|---|---|---|---|---|---|
| CKD5D (n = 90) | 37 (41.1%) | 24 (26.7%) | 38 (42.2%) | 27 (30.0%) | 12 (13.3%) | 1 (1.1%) | 8 (8.9%) |
| (−)CCAD (n = 60) | 17 (28.3%) | 12 (20.0%) | 23 (38.3%) | 15 (25.0%) | 10 (16.7%) | 0 | 5 (8.3%) |
| (+)CCAD (n = 30) | 20 (66.7%) | 12 (40.0%) | 15 (50.0%) | 12 (40.0%) | 2 (6.7%) | 1 (3.3%) | 3 (10.0%) |
| (−)ICAD (n = 66) | 21 (31.8%) | 14 (21.2%) | 24 (36.4%) | 18 (27.3%) | 9 (13.6%) | 0 | 5 (7.6%) |
| (+)ICAD (n = 24) | 16 (66.7%) | 10 (41.7%) | 14 (58.3%) | 9 (37.5%) | 3 (12.5%) | 1 (41.7%) | 3 (12.5%) |
| (−)stroke (n = 68) | 21 (30.9%) | 14 (20.6%) | 26 (38.2%) | 17 (25.0%) | 10 (14.7%) | 0 | 7 (10.3%) |
| (+)stroke (n = 22) | 16 (72.7%) | 10 (45.5%) | 12 (54.5%) | 10 (45.5%) | 2 (9.1%) | 1 (4.5%) | 1 (4.5%) |
Abbreviations: CKD5D stage 5 chronic kidney disease; CCAD, cervical carotid artery disease; ICAD, intracranial atherosclerotic disease.
Discussion
Comparisons between the plasma of patients with CKD5D and that of healthy controls appear to show changes in circulating levels of the biomarkers of this study within the CKD5D population. Of the biomarkers which have further alterations in plasma concentrations in those patients with CKD5D with concurrent neurovascular disease, Lp(a) and NT-ProBNP show potential utility in risk-stratification of ICAD and stroke, respectively. Other biomarkers may be of limited value in this regard. It is noted that some correlations among the biomarkers were relatively stronger with r values >0.5, whereas others were relatively weaker. Regardless of the strengths of the correlations among these biomarkers, it is difficult to establish their pathophysiology in the CKD5D cohort due to the small sample size of this study. However, this study is suggestive of the importance of these biomarkers in predicting their relevance to the development of stroke, CCAD, and ICAD if these studies were performed in a larger CKD5D cohort.
It can be expected that levels of circulating biomarkers of inflammation and hemostasis are elevated in patients with CKD5D when compared to the general population. Stage 5 chronic kidney disease subjects patients to a state of constant systemic inflammation via impaired removal of endogenous and exogenous toxins.29,30 Such continual vascular insults promote the induction of proinflammatory cytokines, activation of the renin-angiotensin-aldosterone-system, and dysregulation of the metabolic milieu.21,31 The resulting maladaptation of the vascular system favors the development of calcific atherosclerosis and exacerbation of hypertension.31 Additionally, patients with CKD5D rely on maintenance hemodialysis therapy for survival, which itself has been implicated with arterial stiffness and inflammation.32 The combination of CKD5D-associated systemic inflammation and long-term hemodialysis treatment has been implicated in the greater prevalence of cognitive dysfunction in the CKD5D population.3,33-35 Potential reasons for this increased prevalence include uremic toxicity, the progressive stiffening of the arterial system, anemia, systemic inflammation, and the inherent nature of conventional hemodialysis therapy thought to directly induce acute cerebral ischemic states.33,36-38 The dynamic connections between renal and cognitive functions, maintained through the vascular system, have given rise to the idea of a cerebrorenal axis similar to that of the established cardiorenal axis.3,39-41 Derangements of the systemic vasculature, therefore, ultimately contribute to the pathogenesis of neurovascular diseases in patients with CKD5D.
Cervical carotid artery disease and ICAD share similar risk factors, pathogeneses, treatment options and both contribute significantly to the development of acute and recurrent cerebrovascular accidents, yet subtle differences support investigation of these 2 conditions as separate entities.42,43 It has been suggested that prevalence of these 2 neurovascular diseases differ amongst racial and ethnic groups, as well as by gender.23,44-46 It has been reported that being of a male gender is an independent risk factor for the development of CCAD, while ICAD is more prevalent in females, possibly due to factors related to bone mineral density.23,47 Reasons for such differences may extend to factors beyond the scope of this study, including genetic susceptibility and differences in lifestyle.42
Although the mean level of circulating ET-1 in patients with CKD5D was higher than that of healthy controls, the differences were not statistically significant. This contrast with reports from other studies which found ET-1 levels to be significantly increased in the plasma of predialysis patients with CKD5D.48,49 It is possible that differences in ET-1 levels could be better appreciated with a larger size of the CKD5D study cohort. Another explanation is that the normally elevated levels of ET-1 in our CKD5D population were artificially lowered due to anticoagulant administration, particularly that of heparin.50
There is also a lack of significant difference in PDGF levels between patients with CKD5D and healthy controls. One explanation is that the PDGF levels in our CKD5D population were decreased by repeat maintenance hemodialysis. It has been reported that long-term patients of hemodialysis experience activation of platelets, as well as aggregations of platelets with erythrocytes, such that thrombocytopenia could be transiently-induced.51-53 However, it is debatable as to the extent a thrombocytopenic state influences PDGF levels in patients with CKD5D. This mitogen is known to be expressed by a variety of cell types, including endothelial cells, which experience notable fluctuations in vascular pressure during hemodialysis.54 The resulting activation of endothelial cells may modulate PDGF expression, thus altering its plasma concentration.55
In consideration of neurovascular diseases in patients with CKD5D, this study reports that Lp(a) levels are significantly elevated in the presence of comorbid ICAD. Lp(a) is known to contribute to the increased prevalence of atherosclerotic and cardiovascular diseases in patients with CKD5D, mainly due to reduced renal clearance of lipoprotein molecules.56,57 It has been reported that high Lp(a) levels are also a prominent independent risk factor for ICAD, especially that of large-artery occlusive disease.58 Given the predilection of patients with CKD5D for the development of concurrent dyslipidemia, monitoring and reducing Lp(a) levels, as well as that of other uremic lipids, may be an integral step toward the prevention of cerebrovascular events in patients with CKD5D. Additionally, a reduction in pro-atherogenic particles may also buffer against the repeat vascular burden incurred by maintenance hemodialysis and lessen the risk for developing vascular dementia.38,59
There was no statistically significant difference in circulating levels of NT-ProBNP when considering stroke history in patients with CKD5D. However, NT-ProBNP has been previously assessed for clinical use as a diagnostic and prognostic marker of HF.60-62 In this regard, the patients with CKD5D of this study were categorized according to presence of a HF diagnosis, and subsequently, levels of NT-ProBNP were found to be significantly elevated in patients with CKD5D with comorbid HF when compared to those without. Studies have shown that NT-ProBNP levels were also elevated following acute ischemic strokes, with suggestions that transient cardiac dysfunction occurs concurrently with cerebrovascular events63,64 It has also been postulated that acute ischemic stroke may induce neurohormonal modulation in order to counter cerebral vasodilation, at the expense of cardiac ventricular function.64 This study has found that NT-ProBNP levels in patients with CKD5D were significantly elevated with comorbid stroke diagnosis compared to those without stroke history, once HF diagnoses were excluded. Interestingly, it has also been reported that NT-ProBNP could be an independent risk factor for congestive HF even after a stroke event or transient ischemic attack.65 Overall, the potential utilization of NT-ProBNP with respect to HF and cerebrovascular accidents within the CKD5D patient population should be further investigated.
Limitations of this study include the relatively small size of our CKD5D cohort, as well as differences in the timing of neurovascular disease events experienced by the patients, particularly in terms of stroke. Additionally, there were overlaps of neurovascular diseases in some of the patients, and not all patients received equal opportunities to undergo imaging studies. As such, the robustness of this study can be going so far as to the extent of proper documentations and diagnostic evaluations performed by the clinicians who manage the CKD5D cohort. Another factor is that samples were gathered only from the Loyola Outpatient Dialysis Center, the patient demographics of which might not reflect that of other communities. Due to the relatively small number of stroke patients within this CKD5D cohort, we defined stroke diagnosis to encompass both the hemorrhagic and ischemic subtypes. A larger study population could allow these 2 stroke subtypes to be analyzed as separate entities and potentially illuminate differences in levels of circulating biomarkers between them.
Conclusions
This study suggests that Lp(a) and NT-ProBNP are biomarkers with potential utility in the risk stratification of ICAD and stroke, respectively, in the CKD5D population. As Lp(a) is both a prominent risk factor for ICAD and has been known to have elevated levels during renal disease, monitoring Lp(a) should be explored as part of primary prevention of neurovascular events in patients with CKD5D. For NT-ProBNP, while it is unclear as to what contributions neurohormonal modulation may have on this biomarker’s level during a stroke event, the fact that NT-ProBNP has been reported to be significantly elevated in patients with CKD5D with comorbid stroke diagnosis once HF diagnoses were excluded warrants further investigation. The other biomarkers measured in this study may be of limited value in the stratification of neurovascular deficits in CKD5D. Further investigation should consider the impact that other vascular diseases may have on biomarker levels within patients with CKD5D, particularly those with concurrent diagnoses of atrial fibrillation and peripheral vascular disease.
Supplemental Material
Supplemental Material, Table_S1_(1) for Circulating Biomarker Levels in Patients With Stage 5 Chronic Kidney Disease With Respect to Neurovascular Diseases by Justin Lee, Ryan McMillan, Leonidas Skiadopoulos, Vinod Bansal, José Biller, Debra Hoppensteadt, and Jawed Fareed in Clinical and Applied Thrombosis/Hemostasis
Acknowledgments
The authors gratefully acknowledge the staff of the Department of Pathology at the Loyola University Medical Center and the Loyola Outpatient Dialysis Center for the expert collection of blood samples. We would also like to thank members of the Hemostasis and Thrombosis laboratories for their continued guidance and collaboration.
Authors’ Note: Ethical approval to report this case was obtained from Loyola University Chicago Institutional Review Board. Written informed consent was obtained from the patients for their anonymized information to be published in this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Research reported in this manuscript was supported by the National Heart, Lung and Blood Institute of the National Institutes of Health under award number T35HL120835.
Supplemental Material: Supplemental material for this article is available online.
ORCID iD: Justin Lee  https://orcid.org/0000-0002-2231-4831
https://orcid.org/0000-0002-2231-4831
References
- 1. Miranda AS, Cordeiro TM, Dos Santos Lacerda Soares TM, Ferreira RN, Simões E Silva AC. Kidney-brain axis inflammatory cross-talk: from bench to bedside. Clin Sci. 2017;131(11):1093–1105. [DOI] [PubMed] [Google Scholar]
- 2. Kurella TM, Wadley V, Yaffe K, et al. Kidney function and cognitive impairment in US adults: the reasons for geographic and racial differences in stroke (REGARDS) study. Am J Kidney Dis. 2008;52(2):227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hung PH, Yeh CC, Hsiao CY, et al. End stage renal disease is associated with development of dementia. Oncotarget. 2017;8(64):107348–107355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Blazer D. Neurocognitive disorders in DSM-5. Am J Psychiatry. 2013;170(6):585–587. [DOI] [PubMed] [Google Scholar]
- 5. Johnston SC, Omeara ES, Manolio TA, et al. Cognitive impairment and decline are associated with carotid artery disease in patients without clinically evident cerebrovascular disease. Ann Intern Med. 2004;140(4):237–247. [DOI] [PubMed] [Google Scholar]
- 6. Dolan H, Crain B, Troncoso J, Resnick SM, Zonderman AB, Obrien RJ. Atherosclerosis, dementia, and Alzheimer disease in the Baltimore Longitudinal Study of Aging cohort. Ann Neurol. 2010;68(2):231–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sachdev PS, Lo JW, Crawford JD, et al. STROKOG (stroke and cognition consortium): An international consortium to examine the epidemiology, diagnosis, and treatment of neurocognitive disorders in relation to cerebrovascular disease. Alzheimers Dement (Amst). 2017;7:11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rakowski DA, Caillard S, Agodoa LY, Abbott KC. Dementia as a predictor of mortality in dialysis patients. Clin J Am Soc Nephrol. 2006;1(5):1000–1005. [DOI] [PubMed] [Google Scholar]
- 9. Griva K, Stygall J, Hankins M, Davenport A, Harrison M, Newman SP. Cognitive impairment and 7-year mortality in dialysis patients. Am J Kidney Dis. 2010;56(4):693–703. [DOI] [PubMed] [Google Scholar]
- 10. Krishna PR, Naresh S, Krishna GS, et al. Stroke in chronic kidney disease. Indian J Nephrol. 2009;19(1):5–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Masson P, Kotwal S, Kelly PJ, et al. Risk factors for stroke in people with end-stage kidney disease: a cohort study. Cerebrovasc Dis. 2016;42(5-6):428–438. [DOI] [PubMed] [Google Scholar]
- 12. Apple FS, Collinson PO, IFCC Task Force on Clinical Applications of Cardiac Biomarkers. Analytical characteristics of high-sensitivity cardiac troponin assays. Clin Chem. 2012;58(1):54–61. [DOI] [PubMed] [Google Scholar]
- 13. Shi Y, Xu YC, Rui X, Zhang HM, Wang Y, Du W. Procalcitonin kinetics and nosocomial pneumonia in older patients. Respir Care. 2014;59(8):1258–1266. [DOI] [PubMed] [Google Scholar]
- 14. Rysz J, Gluba-brzózka A, Franczyk B, Jabłonowski Z, Ciałkowska-Rysz A. Novel biomarkers in the diagnosis of chronic kidney disease and the prediction of its outcome. Int J Mol Sci. 2017;18(8):E1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ichimura T, Asseldonk EJ, Humphreys BD, Gunaratnam L, Duffield JS, Bonventre JV. Kidney injury molecule-1 is a phosphatidylserine receptor that confers a phagocytic phenotype on epithelial cells. J Clin Invest. 2008;118(5):1657–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Teo SH, Endre ZH. Biomarkers in acute kidney injury (AKI). Best Pract Res Clin Anaesthesiol. 2017;31(3):331–344. [DOI] [PubMed] [Google Scholar]
- 17. Weber M, Hamm C. Role of B-type natriuretic peptide (BNP) and NT-proBNP in clinical routine. Heart. 2006;92(6):843–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Llombart V, Antolin-fontes A, Bustamante A, et al. B-type natriuretic peptides help in cardioembolic stroke diagnosis: pooled data meta-analysis. Stroke. 2015;46(5):1187–1195. [DOI] [PubMed] [Google Scholar]
- 19. García-Berrocoso T, Giralt D, Bustamante A, et al. B-type natriuretic peptides and mortality after stroke: a systematic review and meta-analysis. Neurology. 2013;81(23):1976–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Krishnan SM, Sobey CG, Latz E, Mansell A, Drummond GR. IL-1β and IL-18: inflammatory markers or mediators of hypertension?. Br J Pharmacol. 2014;171(24):5589–5602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Briet M, Burns KD. Chronic kidney disease and vascular remodelling: molecular mechanisms and clinical implications. Clin Sci. 2012;123(7):399–416. [DOI] [PubMed] [Google Scholar]
- 22. Felsenfeld AJ, Levine BS, Rodriguez M. Pathophysiology of Calcium, Phosphorus, and Magnesium Dysregulation in Chronic Kidney Disease. Semin Dial. 2015;28(6):564–577. [DOI] [PubMed] [Google Scholar]
- 23. Kang K. Low bone mineral density is associated with intracranial posterior circulation atherosclerosis in women. Bone. 2015;81:669–674. [DOI] [PubMed] [Google Scholar]
- 24. Myint PK, Clark AB, Kwok CS, et al. Bone mineral density and incidence of stroke: European prospective investigation into cancer-norfolk population-based study, systematic review, and meta-analysis. Stroke. 2014;45(2):373–382. [DOI] [PubMed] [Google Scholar]
- 25. Jørgensen L, Joakimsen O, Rosvold Berntsen GK, Heuch I, Jacobsen BK. Low bone mineral density is related to echogenic carotid artery plaques: a population-based study. Am J Epidemiol. 2004;160(6):549–556. [DOI] [PubMed] [Google Scholar]
- 26. Boor P, Ostendorf T, Floege J. PDGF and the progression of renal disease. Nephrol Dial Transplant. 2014;29(suppl 1):i45–i54. [DOI] [PubMed] [Google Scholar]
- 27. Zwicker JI, Trenor CC, Furie BC, Furie B. Tissue factor-bearing microparticles and thrombus formation. Arterioscler Thromb Vasc Biol. 2011;31(4):728–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Verhoye E, Langlois MR; Asklepios Investigators. Circulating oxidized low-density lipoprotein: a biomarker of atherosclerosis and cardiovascular risk?. Clin Chem Lab Med. 2009;47(2):128–137. [DOI] [PubMed] [Google Scholar]
- 29. Stevens PE, Levin A; Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158(11):825–830. [DOI] [PubMed] [Google Scholar]
- 30. Brunet P, Gondouin B, Duval-Sabatier A, et al. Does uremia cause vascular dysfunction?. Kidney Blood Press Res. 2011;34(4):284–290. [DOI] [PubMed] [Google Scholar]
- 31. Hénaut L, Mary A, Chillon JM, Kamel S, Massy ZA. The impact of uremic toxins on vascular smooth muscle cell function. Toxins (Basel). 2018;10(6):E218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Laucyte-Cibulskiene A, Rimsevicius L, Gumbys L, Valanciene D, Miglinas M. Two years of maintenance hemodialysis has a pronounced effect on arterial stiffness progression [Published ahead of print May 19, 2018]. Aging Clin Exp Res. doi:10.1007/s40520-018-0971-4 [DOI] [PubMed] [Google Scholar]
- 33. Kurella M, Chertow GM, Fried LF, et al. Chronic kidney disease and cognitive impairment in the elderly: the health, aging, and body composition study. J Am Soc Nephrol. 2005;16(7):2127–2133. [DOI] [PubMed] [Google Scholar]
- 34. Fukunishi I, Kitaoka T, Shirai T, Kino K, Kanematsu E, Sato Y. Psychiatric disorders among patients undergoing hemodialysis therapy. Nephron. 2002;91(2):344–347. [DOI] [PubMed] [Google Scholar]
- 35. Kurella M, Chertow GM, Luan J, Yaffe K. Cognitive impairment in chronic kidney disease. J Am Geriatr Soc. 2004;52(11):1863–1869. [DOI] [PubMed] [Google Scholar]
- 36. Seifter JL, Samuels MA. Uremic encephalopathy and other brain disorders associated with renal failure. Semin Neurol. 2011;31(2):139–143. [DOI] [PubMed] [Google Scholar]
- 37. Angermann S, Baumann M, Wassertheurer S, et al. Pulse wave velocity is associated with cognitive impairment in hemodialysis patients. Clin Sci. 2017;131(13):1483–1493. [DOI] [PubMed] [Google Scholar]
- 38. Murray AM. Cognitive impairment in the aging dialysis and chronic kidney disease populations: an occult burden. Adv Chronic Kidney Dis. 2008;15(2):123–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ikram MA, Vernooij MW, Hofman A, et al. Kidney function is related to cerebral small vessel disease. Stroke. 2008;39(1):55–61. [DOI] [PubMed] [Google Scholar]
- 40. Watanabe K, Watanabe T, Nakayama M. Cerebro-renal interactions: impact of uremic toxins on cognitive function. Neurotoxicology. 2014;44:184–193. [DOI] [PubMed] [Google Scholar]
- 41. Tanphaichitr VS, Chaiprasert A, Suvatte V, Thasnakorn P. Subcutaneous mucormycosis caused by Saksenaea vasiformis in a thalassaemic child: first case report in Thailand. Mycoses. 1990;33(6):303–309. [DOI] [PubMed] [Google Scholar]
- 42. Holmstedt CA, Turan TN, Chimowitz MI. Atherosclerotic intracranial arterial stenosis: risk factors, diagnosis, and treatment. Lancet Neurol. 2013;12(11):1106–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wabnitz AM, Turan TN. Symptomatic carotid artery stenosis: surgery, stenting, or medical therapy?. Curr Treat Options Cardiovasc Med. 2017;19(8):62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Caplan LR, Gorelick PB, Hier DB. Race, sex and occlusive cerebrovascular disease: a review. Stroke. 1986;17(4):648–655. [DOI] [PubMed] [Google Scholar]
- 45. Fustinoni O, Biller J. Ethnicity and stroke: beware of the fallacies. Stroke. 2000;31(5):1013–1015. [DOI] [PubMed] [Google Scholar]
- 46. Cruz-Flores S, Rabinstein A, Biller J, et al. Racial-ethnic disparities in stroke care: the American experience: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(7):2091–2116. [DOI] [PubMed] [Google Scholar]
- 47. Mathiesen EB, Joakimsen O, Bønaa KH. Prevalence of and risk factors associated with carotid artery stenosis: the Tromsø Study. Cerebrovasc Dis. 2001;12(1):44–51. [DOI] [PubMed] [Google Scholar]
- 48. El-Shafey EM, El-Nagar GF, Selim MF, El-Sorogy HA, Sabry AA. Is there a role for endothelin-1 in the hemodynamic changes during hemodialysis?. Clin Exp Nephrol. 2008;12(5):370–375. [DOI] [PubMed] [Google Scholar]
- 49. Erkan E, Devarajan P, Kaskel F. Role of nitric oxide, endothelin-1, and inflammatory cytokines in blood pressure regulation in hemodialysis patients. Am J Kidney Dis. 2002;40(1):76–81. [DOI] [PubMed] [Google Scholar]
- 50. Kuwahara-Watanabe K, Hidai C, Ikeda H, et al. Heparin regulates transcription of endothelin-1 gene in endothelial cells. J Vasc Res. 2005;42(3):183–189. [DOI] [PubMed] [Google Scholar]
- 51. Sirolli V, Strizzi L, Di Stante S, Robuffo I, Procopio A, Bonomini M. Platelet activation and platelet-erythrocyte aggregates in end-stage renal disease patients on hemodialysis. Thromb Haemost. 2001;86(3):834–839. [PubMed] [Google Scholar]
- 52. Hakim RM, Schafer AI. Hemodialysis-associated platelet activation and thrombocytopenia. Am J Med. 1985;78(4):575–580. [DOI] [PubMed] [Google Scholar]
- 53. Daugirdas JT, Bernardo AA. Hemodialysis effect on platelet count and function and hemodialysis-associated thrombocytopenia. Kidney Int. 2012;82(2):147–157. [DOI] [PubMed] [Google Scholar]
- 54. Dicorleto PE, Bowen-Pope DF. Cultured endothelial cells produce a platelet-derived growth factor-like protein. Proc Natl Acad Sci USA. 1983;80(7):1919–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bao X, Lu C, Frangos JA. Temporal gradient in shear but not steady shear stress induces PDGF-A and MCP-1 expression in endothelial cells: role of NO, NF kappa B, and egr-1. Arterioscler Thromb Vasc Biol. 1999;19(4):996–1003. [DOI] [PubMed] [Google Scholar]
- 56. Kaysen GA. Lipid and lipoprotein metabolism in chronic kidney disease. J Ren Nutr. 2009;19(1):73–77. [DOI] [PubMed] [Google Scholar]
- 57. Kwan BC, Kronenberg F, Beddhu S, Cheung AK. Lipoprotein metabolism and lipid management in chronic kidney disease. J Am Soc Nephrol. 2007;18(4):1246–1261. [DOI] [PubMed] [Google Scholar]
- 58. Arenillas JF, Molina CA, Chacón P, et al. High lipoprotein (a), diabetes, and the extent of symptomatic intracranial atherosclerosis. Neurology. 2004;63(1):27–32. [DOI] [PubMed] [Google Scholar]
- 59. Murray AM, Tupper DE, Knopman DS, et al. Cognitive impairment in hemodialysis patients is common. Neurology. 2006;67(2):216–223. [DOI] [PubMed] [Google Scholar]
- 60. Oremus M, Don-Wauchope A, Mckelvie R, et al. BNP and NT-proBNP as prognostic markers in persons with chronic stable heart failure. Heart Fail Rev. 2014;19(4):471–505. [DOI] [PubMed] [Google Scholar]
- 61. Bombelli M, Maloberti A, Rossi S, et al. Clinical value of NT-proBNP assay in the emergency department for the diagnosis of heart failure (HF) in very elderly people. Arch Gerontol Geriatr. 2015;61(2):296–300. [DOI] [PubMed] [Google Scholar]
- 62. Hill SA, Booth RA, Santaguida PL, et al. Use of BNP and NT-proBNP for the diagnosis of heart failure in the emergency department: a systematic review of the evidence. Heart Fail Rev. 2014;19(4):421–438. [DOI] [PubMed] [Google Scholar]
- 63. Etgen T, Baum H, Sander K, Sander D. Cardiac troponins and N-terminal pro-brain natriuretic peptide in acute ischemic stroke do not relate to clinical prognosis. Stroke. 2005;36(2):270–275. [DOI] [PubMed] [Google Scholar]
- 64. Giannakoulas G, Hatzitolios A, Karvounis H, et al. N-terminal pro-brain natriuretic peptide levels are elevated in patients with acute ischemic stroke. Angiology. 2005;56(6):723–730. [DOI] [PubMed] [Google Scholar]
- 65. Campbell DJ, Woodward M, Chalmers JP, et al. Prediction of heart failure by amino terminal-pro-B-type natriuretic peptide and C-reactive protein in subjects with cerebrovascular disease. Hypertension. 2005;45(1):69–74. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, Table_S1_(1) for Circulating Biomarker Levels in Patients With Stage 5 Chronic Kidney Disease With Respect to Neurovascular Diseases by Justin Lee, Ryan McMillan, Leonidas Skiadopoulos, Vinod Bansal, José Biller, Debra Hoppensteadt, and Jawed Fareed in Clinical and Applied Thrombosis/Hemostasis