Abstract
For success in clinical trials, eliminating inclusion of patients with irreversible recovery is important. The purpose of this study was to identify the patient population who do not survive for more than 3 days. A total of 449 patients with sepsis suspected of having disseminated intravascular coagulation (DIC) and treated with antithrombin were examined. The patient characteristics, baseline sequential organ failure assessment (SOFA) score, DIC score, and hemostatic markers were retrospectively analyzed in relation to early death (died within 3 days). At the end of day 3, a total of 419 patients had survived and 30 patients had died. A logistic regression analysis revealed a significant association between early death and the baseline prothrombin time-international normalized ratio PT-INR (P <.05) and the total SOFA score (P <.01). In contrast, neither the platelet count, fibrinogen/fibrin degradation products, and antithrombin activity nor the DIC score was associated with early death. Although the accuracy for predicting early death defined by either baseline PT-INR of ≥1.57 or total SOFA score of more than 13 was not high enough, that of “high-risk of early death (PT-INR ≥ 1.57 and SOFA score ≥ 13)” was 83.5%. Furthermore, the negative predictive of this category was 96.0%. The baseline SOFA score and PT-INR were associated with early death among patients with sepsis-associated coagulation disorders. Patients who do not meet the “high-risk of early death” criteria were likely to survive for more than 3 days and therefore should be considered for future therapeutic clinical trials.
Keywords: clinical trial, sepsis, disseminated intravascular coagulation, mortality, antithrombin, sequential organ failure assessment score
Introduction
The cornerstone of treatment for patients with sepsis and coagulopathy is management of the underlying condition. However, the restoration of physiological anticoagulants is reported to have an effect in certain populations.1 KyberSept,2 the largest randomized controlled trial of antithrombin to date, examined the effect of high-dose antithrombin on severe sepsis but failed to demonstrate an effect on 28-day, all-cause mortality. However, a subanalysis demonstrated that such treatment might be effective for patients who also present with coagulopathy.3,4 Other studies have also reported that benefit of anticoagulants was only recognized in patients with sepsis and presenting with disseminated intravascular coagulation (DIC) 5,6; however, anticoagulation therapy is not widely accepted or included in all sepsis management guidelines.7 For patients with septic DIC in Japan,8 the Japanese Clinical Practice Guidelines for the Management of Sepsis and Septic Shock 2016 recommend the use of antithrombin for DIC.6 These guidelines were established based on a meta-analysis reporting the efficacy of antithrombin in patients with septic DIC.9,10
The Japanese Association for Acute Medicine (JAAM) released diagnostic criteria for DIC in 2006, and these criteria have been predominantly used for patient selection in sepsis studies.11 Tagami and Hayakawa et al12,13 have reported the positive effect of antithrombin supplementation in clinical practice using nationwide Per-Diem Payment System data. Umemura and Yamakawa14 reported that in addition to the presence of JAAM DIC, a high risk of death should be another consideration. Similarly, Yamakawa et al.6 and Yoshimura et al15 reported a positive relationship between the effect size and disease severity for antithrombin supplementation. As a result, in high-risk patients having sepsis with or without coagulopathy, the effect may be difficult to determine. Since robust evidence is still lacking, the effect of antithrombin should be evaluated in a properly designed randomized clinical trial that redefines inclusion criteria. For designing such a study, critically ill patients without coagulopathy or who have other underlying cause for immediate death who are unlikely to benefit from antithrombin supplementation should be excluded. In the present study, we hypothesized that patients who do not survive for more than 3 days are not appropriate candidates and examined the prognostic factors for this early death population.
Patients and Methods
Data Set
Data from multi-institutional, postmarketing surveys performed between April 2015 and May 2016 by Nihon Pharmaceutical were used for the analysis. A total of 449 patients with sepsis-associated coagulopathic with decreased antithrombin activity who were treated with antithrombin concentrate (Nihon Pharmaceutical Co. Ltd, Tokyo, Japan) were registered in the survey. The baseline modified sequential organ failure assessment (SOFA) score,16 platelet count, prothrombin time (PT)–international normalized ratio (INR), fibrinogen/fibrin degradation products (FDP), antithrombin activity, and JAAM DIC score were evaluated just before the treatment (baseline, day 1), and their correlations with early death occurring prior to the end of day 3 were examined. The total SOFA score was calculated as the sum of the following 6 items: respiratory SOFA score, cardiovascular SOFA score, hepatic SOFA score, renal SOFA score, neurological SOFA score, and coagulation SOFA score. Standard sepsis care was performed, and platelet concentrate and fresh frozen plasma were used as substitution therapies, if necessary.17
Ethics, Patient Consent, Study Permissions, and Consent to Publish
The survey was conducted in accordance with the Declaration of Helsinki and Good Vigilance Practice and Good Post-marketing Study Practice. Although the Japanese Ministry of Health, Labour and Welfare judged that the patients’ agreement was not necessary for this survey, the patients’ agreement and consent were obtained when required by the ethics committee of each hospital. The complete anonymization of personal data was performed upon data collection, and identification of individual patients was impossible; thus, the Institutional Ethics Committee of Juntendo University judged that consent to publish was not required.
Statistical Analysis
The numerical values in the text and tables represent the median and interquartile range. Univariate associations were evaluated using the Fisher exact test and the unpaired Wilcoxon signed-rank test (Mann-Whitney U test). The correlations between early death and the various predictive factors were analyzed using a logistic regression analysis (enter method). The baseline SOFA score and the PT-INR were analyzed for their possible association with early death. The analysis was conducted using early death (yes, 1; no, 0) as the dependent variable and the baseline SOFA score and PT-INR as additional factors. Results were reported as the odds ratio, Wald result, P values, and 95% confidence interval. A receiver–operating characteristic (ROC) curve analysis was performed to evaluate the areas under the curve (AUCs). The Youden index was calculated for each item as the cutoff offering the best sensitivity and specificity to predict day 3 mortality. A P value <.05 was considered to denote statistical significance. The abovementioned analyses were performed using SPSS version 13.0 for Windows (SPSS Inc, Chicago, Illinois).
Results
Of the 449 patients included in the survey, 419 (93.3%) patients survived for 3 days, while 30 (6.68%) patients died. Table 1 summarizes the baseline characteristics of the survivors and nonsurvivors. The median age of the survivors on day 3 was 74 years while that of the nonsurvivors was 77 years. The gender distribution did not differ between survivors and nonsurvivors. Regarding the coagulation and organ failure profiles, the PT-INR and the total SOFA score were higher among the nonsurvivors (P = .009 and <.001, respectively). No statistical differences in platelet count, FDP, antithrombin activity, or JAAM DIC score were recognized.
Table 1.
Baseline Characteristics of the Patients with Suspected Sepsis-Associated DIC.
| Characteristics | Survivors on Day 3 (n = 419) | Nonsurvivors on Day 3(n = 30) | P Value |
|---|---|---|---|
| Age (years) | 74 (65-82) | 77 (73-82) | .151 |
| Sex (male/female) | 258/161 | 19/11 | 1.000 |
| Baseline values | |||
| Platelet count, × 109/L) | 73.0 (48.0-112.0) | 71. 0 (40.8-120.3) | .989 |
| FDP, μg/mL | 27.6 (14.4-50.5) | 29.1 (21.8-79.8) | .104 |
| PT-INR | 1.39 (1.24-1.69) | 1.62 (1.32-2.35) | .009a |
| Antithrombin activity | 48.0 (39.0-58.7) | 42.0 (32.8-53.8) | .099 |
| JAAM DIC score | 5.0 (4.0-6.0) | 6.0 (5.0-6.3) | .452 |
| Total SOFA score | 11.0 (7.0-13.0) | 13.5 (12.3-16.0) | <.001a |
| Respiratory score | 2.0 (1.0-3.0) | 3.0 (2.4-4.0) | <.001a |
| Cardiovascular score | 3.0 (1.0-4.0) | 4.0 (2.0-4.0) | .024a |
| Hepatic score | 0.0 (0.0-2.0) | 0.5 (0.0-1.0) | .947 |
| Renal score | 1.0 (0.0-2.0) | 2.0 (1.0-3.0) | .007a |
| Neurological score | 2.0 (1.0-3.0) | 4.0 (2.0-4.0) | <.001a |
| Coagulation score | 2.0 (1.0-3.0) | 2.0 (1.0-3.0) | 0.656 |
Abbreviations: DIC, disseminated intravascular coagulation; FDP, fibrinogen and fibrin degradation products; INR, international normalized ratio; JAAM, Japanese association of acute medicine; PT, prothrombin time; SOFA, sequential organ failure assessment.
a P value <.05
A logistic regression analysis showed that both the total SOFA score and the PT-INR were independently associated with early death (P = .031 and <.001, respectively; Table 2).
Table 2.
Relationship between Baseline Characteristics and Early Death (Day 3 Mortality).
| Odds Ratio | Wald | P Value | 95% CI | |
|---|---|---|---|---|
| PT-INR | 0.610 | 4.677 | .031 | 0.390-0.955 |
| Total SOFA score | 0.824 | 12.247 | 0.000 | 0.740-0.918 |
Abbreviations: CI, confidence interval; INR, international normalized ratio; PT, prothrombin time; SOFA, sequential organ failure assessment.
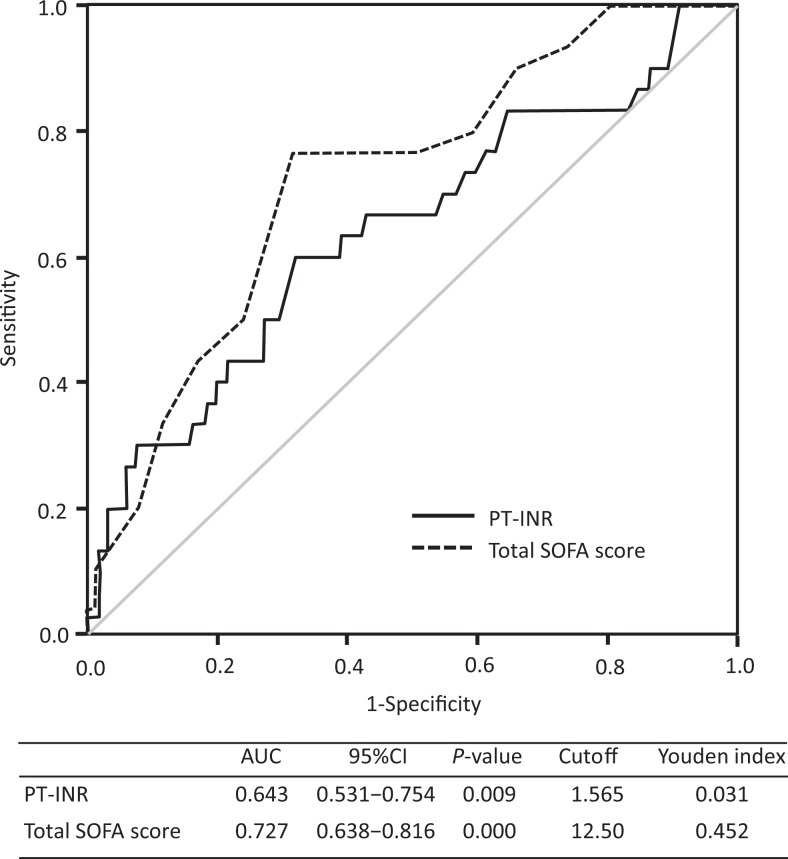
Figure 1 shows the ROC curves of the PT-INR and the total SOFA score for mortality. The AUCs for the 2 indicators were 0.643 and 0.727. The optimal cutoffs were 1.57 for the PT-INR and 12.5 for the total SOFA score.
Figure 1.
Comparison of the receiver–operating characteristic (ROC) curves for the baseline sequential organ failure assessment (SOFA) score and prothrombin time ratio for early death. The ROC curves for day 3 mortality of the prothrombin time (PT)-international normalized ratio (solid line) and the SOFA score (dotted line) are shown. The areas under the ROC curves (AUCs) for the 2 indicators were 0.643 and 0.727, respectively. CI indicates confidence interval; INR, international normalized ratio; PT, prothrombin time.
The positive predictive value (PPV) and the negative predictive value (NPV) of the “high-risk of early death” patients categorized according to a baseline PT-INR of ≥1.57 and a total SOFA score of 13 or more were 20.3% and 96.0% for early death, while the accuracy of the early death category was 83.5%. The sensitivity, specificity, and accuracy of the PT-INR, total SOFA score, and “high-risk of early death” are summarized in Table 3.
Table 3.
Diagnostic Performance of Prothrombin Time, Total SOFA Score and Combination of Both Markers to Early Death (Mortality on Day 3).
| PT-INR > 1.57 (n = 152) | Total SOFA score > 13 (n = 155) | PT-INR > 1.57 and total SOFA score > 13 (n = 74) | |
|---|---|---|---|
| PPV | 11.8% | 14.8% | 20.3% |
| NPV | 96.0% | 97.6% | 96.0% |
| Sensitivity | 60.0% | 76.7% | 50.0% |
| Specificity | 68.0% | 68.5% | 85.9% |
| Accuracy | 67.5% | 69.0% | 83.5% |
Abbreviations: INR, international normalized ratio; NPV, negative predictive value; PT, prothrombin time; PPV, positive predictive value; SOFA, sequential organ failure assessment.
Discussion
Coagulopathy is recognized as a response to systemic infection and is a common cause of DIC,18,19 characterized by the systemic intravascular activation of coagulation.20 Sepsis-induced DIC leads to the consumption of physiologic anticoagulants and widespread deposition of fibrin in the vasculature, resulting in multiple organ dysfunction and death.21 Understanding this important pathogenetic mechanism of DIC22 supports the use of anticoagulant therapy with physiological anticoagulants in managing patients in addition to treating sepsis.1,23 However, despite multiple clinical trials, the only sepsis-specific therapy for which a phase 3 study has been successfully completed is recombinant activated protein C24 but later discontinued after a follow-up clinical trial in septic shock.25 One of the ongoing concerns in sepsis trials is including patients with a high probability of early mortality where underlying disease or factors influence mortality. In patients with sepsis having DIC, the efficacy of anticoagulant therapy appears to be limited to patients with coagulopathy,3–6 and the beneficial effects of antithrombin supplementation are in patients with sepsis-associated DIC.26–28
Another noteworthy issue is the relationship between effect and disease severity, and recent studies suggest the efficacy of antithrombin was greater in selected critically ill patients.6,15 Determining what group of patients may best benefit from a therapy is important not only for clinical trials but also for the clinical practice. Since total SOFA score and PT-INR were shown to be useful to estimate the patients’ outcome in this study, we recommend to measure these markers in the daily practice.
Certain patients may present with irreversible injury or other underlying conditions with multiorgan failure that are beyond any possible benefit, despite therapeutic interventions. Yamakawa et al6 demonstrated that anticoagulant therapy was not effective in such cases. Wiedermann et al29 also reported that the optimal candidates for antithrombin therapy were patients with an estimated 28-day mortality of 30% to 60%, and no difference in mortality was recognized if the estimated mortality was over 60%. Indeed, previous studies have not established a specific upper limit of severity for study inclusion. Even in the latest clinical trial examining the effect of thrombomodulin defines the related exclusion criteria as platelet count <30 000/mm3 and life expectancy <90 days (https://clinicaltrials.gov/ct2/show/NCT01598831?%20cond=ART-123&rank=4).
In this study, we established exclusion criteria for disease severity and hypothesized that patients who did not survive for more than 3 days were not appropriate candidates because the antithrombin supplementation is usually performed for 3 consecutive days in Japan. However, when we assume other therapeutic modalities, we probably need to set the different time point. Our data suggest the total SOFA score and PT-INR were helpful parameters; however, the PPVs of these indices were very low (< 15%), and the value remained low even when the parameters were combined. Thus, we determined that “high risk of early death” was not appropriate as an exclusion criterion. Instead, both the PT-INR and the total SOFA score demonstrated high NPVs (>95%), and the combination of both indicators improved the specificity (85.9%), leading to an improved accuracy (83.5%). Since a “high risk of early death” had an NPV of more than 95% and a specificity of more than 85%, the patient population that does not fit this criterion (375 cases, 83.5% of total) is highly likely to survive for more than 3 days, and such patients may be appropriate candidates for enrollment in clinical studies. Since no such index has been used in former clinical trials, we believe that a “high risk of early death” criterion might contribute to the success of future clinical trials. From the aspect of clinical practice, “high risk of early death” criterion will be useful because we can apply more intensive and more comprehending treatments to the patients from the early stage of sepsis.
This study has several limitations. First, we defined inappropriate candidates as those who did not survive for more than 3 days because antithrombin supplementation which is usually continued for 3 days would not be completed in these patients. Macias and Nelson30 reported that mortality due to refractory shock also occurred during the initial hospitalization as defined as 5 days. Second, since many of our patients (394/449, 87.8%) met the JAAM DIC criteria at baseline, a selection bias might have existed. More patients with less severe coagulopathy should be included in future studies. Third, although only the baseline data were utilized, all the patients were treated with antithrombin, which might have influenced the timing of death. Moreover, since the data were obtained from actual clinical practice, the patients who were estimated to survive <3 days might not have been included. Finally, this was a retrospective analysis, and the results should be confirmed in a prospective study.
Conclusion
The effectiveness of antithrombin has not yet been proven. For success in future clinical trials, appropriate patient selection is crucial. To define appropriate candidates, a “high risk of early death” designation consisting of the total SOFA score and the PT-INR might be helpful.
Acknowledgment
The authors thank K. Kinoshita for the operation of the infrastructure that was used to collect the data. The authors also thank all the institutes that cooperated with this study.
Authors’ Note: Our institution does not require ethical approval for reporting individual cases or case series. Informed consent for patient information to be published in this article was not obtained because the complete anonymization of personal data was performed upon data collection and the identification of individual patients was impossible.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: This work was performed using data from a postmarketing surveillance conducted by Nihon Pharmaceutical Co. Ltd. MA is an employee of Nihon Pharmaceutical Co. Ltd. The other authors state that they have no conflicts of interest.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Ministry of Education, Culture, Sports, Science and Technology-Supported Program for the Strategic Research Foundation at Private Universities 2018.
ORCID iD: Toshiaki Iba http://orcid.org/0000-0002-0255-4088
http://orcid.org/0000-0002-0255-4088
References
- 1. Levi M, Scully M. How I treat disseminated intravascular coagulation. Blood. 2017;131(8):845–854. [DOI] [PubMed] [Google Scholar]
- 2. Warren BL, Eid A, Singer P, et al. ; KyberSept Trial Study Group. Caring for the critically ill patient. High-dose antithrombin III in severe sepsis: a randomized controlled trial. JAMA. 2001;286(15):1869–1878. [DOI] [PubMed] [Google Scholar]
- 3. Kienast J, Juers M, Wiedermann CJ, et al. ; KyberSept investigators. Treatment effects of high-dose antithrombin without concomitant heparin in patients with severe sepsis with or without disseminated intravascular coagulation. J Thromb Haemost. 2006;4(1):90–97. [DOI] [PubMed] [Google Scholar]
- 4. Angstwurm M, Hoffmann J, Ostermann H, Frey L, Spannagl M. Severe sepsis and disseminated intravascular coagulation. Supplementation with antithrombin. Anaesthesist. 2009;58(2):171–179. [DOI] [PubMed] [Google Scholar]
- 5. Umemura Y, Yamakawa K, Ogura H, Yuhara H, Fujimi S. Efficacy and safety of anticoagulant therapy in three specific populations with sepsis: a meta-analysis of randomized controlled trials. J Thromb Haemost. 2015;14(3):518–530. [DOI] [PubMed] [Google Scholar]
- 6. Yamakawa K, Umemura Y, Hayakawa M, et al. ; Japan Septic Disseminated Intravascular Coagulation (J-Septic DIC) study group. Benefit profile of anticoagulant therapy in sepsis: a nationwide multicentre registry in Japan. Crit Care. 2016;20(1):229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43(3):304–377. [DOI] [PubMed] [Google Scholar]
- 8. Iba T, Thachil J. Present and future of anticoagulant therapy using antithrombin and thrombomodulin for sepsis-associated disseminated intravascular coagulation: a perspective from Japan. Int J Hematol. 2016;103(3):253–261. [DOI] [PubMed] [Google Scholar]
- 9. Nishida O, Ogura H, Egi M, et al. The japanese clinical practice guidelines for management of sepsis and septic shock 2016 (J-SSCG 2016). Acute Med Surg. 2018;5(1):3–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wiedermann CJ. Antithrombin concentrate use in disseminated intravascular coagulation of sepsis: meta-analyses revisited. J Thromb Haemost. 2018;16(3):455–457. [DOI] [PubMed] [Google Scholar]
- 11. Gando S, Iba T, Eguchi Y, et al. ; Japanese Association for Acute Medicine Disseminated Intravascular Coagulation (JAAM DIC) Study Group. A multicenter, prospective validation of disseminated intravascular coagulation diagnostic criteria for critically ill patients: comparing current criteria. Crit Care Med. 2006;34(3):625–631. [DOI] [PubMed] [Google Scholar]
- 12. Tagami T. Antithrombin concentrate use in sepsis-associated disseminated intravascular coagulation: re-evaluation of a ‘pendulum effect’ drug using a nationwide database. J Thromb Haemost. 2018;16(3):458–461. [DOI] [PubMed] [Google Scholar]
- 13. Hayakawa M, Kudo D, Saito S, et al. Antithrombin supplementation and mortality in sepsis-induced disseminated intravascular coagulation: a multicenter retrospective observational study. Shock. 2016;46(6):623–631. [DOI] [PubMed] [Google Scholar]
- 14. Umemura Y, Yamakawa K. Optimal patient selection for anticoagulant therapy in sepsis: an evidence-based proposal from Japan. J Thromb Haemost. 2018;16(3):462–464. [DOI] [PubMed] [Google Scholar]
- 15. Yoshimura J, Yamakawa K, Ogura H, et al. Benefit profile of recombinant human soluble thrombomodulin in sepsis-induced disseminated intravascular coagulation: a multicenter propensity score analysis. Crit Care. 2015;19:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the working group on sepsis-related problems of the European society of intensive care medicine. Intensive Care Med. 1996;22(7):707–710. [DOI] [PubMed] [Google Scholar]
- 17. Wada H, Asakura H, Okamoto K, et al. ; Japanese Society of Thrombosis Hemostasis/DIC subcommittee. Expert consensus for the treatment of disseminated intravascular coagulation in Japan. Thromb Res. 2010;125(1):6–11. [DOI] [PubMed] [Google Scholar]
- 18. Simmons J, Pittet JF. The coagulopathy of acute sepsis. Curr Opin Anaesthesiol. 2015;28(2):227–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gaertner F, Massberg S. Blood coagulation in immunothrombosis-At the frontline of intravascular immunity. Semin Immunol. 2016;28(6):561–569. [DOI] [PubMed] [Google Scholar]
- 20. Taylor FB, Jr, Toh CH, Hoots WK, Wada H, Levi M; Scientific Subcommittee on Disseminated Intravascular Coagulation (DIC) of the International Society on Thrombosis and Haemostasis (ISTH). Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost. 2001:86(5):1327–1330. [PubMed] [Google Scholar]
- 21. Frantzeskaki F, Armaganidis A, Orfanos SE. Immunothrombosis in acute respiratory distress syndrome: cross talks between inflammation and coagulation. Respiration. 2017;93(3):212–225. [DOI] [PubMed] [Google Scholar]
- 22. Iba T, Levy JH. Inflammation and thrombosis: roles of neutrophils, platelets and endothelial cells and their interactions in thrombus formation during sepsis. J Thromb Haemost. 2017;16(2):231–241. [DOI] [PubMed] [Google Scholar]
- 23. Levi M, van der Poll T. Disseminated intravascular coagulation: a review for the internist. Intern Emerg Med. 2013;8(1):23–32. [DOI] [PubMed] [Google Scholar]
- 24. Bernard GR, Vincent JL, Laterre PF, et al. ; Recombinant human protein C Worldwide Evaluation in Severe Sepsis (PROWESS) study group. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344(10):699–709. [DOI] [PubMed] [Google Scholar]
- 25. Ranieri VM, Thompson BT, Barie PS, et al. ; PROWESS-SHOCK Study Group. Drotrecogin alfa (activated) in adults with septic shock. N Engl J Med. 2012;366(22):2055–2064. [DOI] [PubMed] [Google Scholar]
- 26. Gando S, Saitoh D, Ishikura H, et al. ; Japanese Association for Acute Medicine Disseminated Intravascular Coagulation (JAAM DIC) Study Group for the JAAM DIC Antithrombin Trial (JAAMDICAT). A randomized, controlled, multicenter trial of the effects of antithrombin on disseminated intravascular coagulation in patients with sepsis. Crit Care. 2013;17(6):R297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tagami T, Matsui H, Horiguchi H, Fushimi K, Yasunaga H. Antithrombin and mortality in severe pneumonia patients with sepsis-associated disseminated intravascular coagulation: an observational nationwide study. J Thromb Haemost. 2014;12(9):1470–1479. [DOI] [PubMed] [Google Scholar]
- 28. Tagami T, Matsui H, Fushimi K, Yasunaga H. Supplemental dose of antithrombin use in disseminated intravascular coagulation patients after abdominal sepsis. Thromb Haemost. 2015;114(3):537–545. [DOI] [PubMed] [Google Scholar]
- 29. Wiedermann CJ, Hoffmann JN, Juers M, et al. ; KyberSept Investigators. High-dose antithrombin III in the treatment of severe sepsis in patients with a high risk of death: efficacy and safety. Crit Care Med. 2006;34(2):285–292. [DOI] [PubMed] [Google Scholar]
- 30. Macias WL, Nelson DR. Severe protein C deficiency predicts early death in severe sepsis. Crit Care Med. 2004;32(5 suppl):S223–S228. [DOI] [PubMed] [Google Scholar]