Abstract
Newborns with hemophilia are at risk of intracranial hemorrhage, extracranial hemorrhage, and other bleeding complications. The safe delivery of a healthy newborn with hemophilia is a complex process that can begin even before conception, and continues throughout pregnancy, birth, and the newborn period. This process involves the expectant parents and a wide variety of health-care professionals: genetic counselors, obstetricians, neonatologists, pediatricians, radiologists, adult and pediatric hematologists, and nurses with expertise in hemophilia. Because of this multidisciplinary complexity, the relative rarity of births of newborns with hemophilia, and the lack of high-quality evidence to inform decisions, there is considerable variation in practice in this area. We present a comprehensive multidisciplinary approach, from preconception counseling to discharge planning after birth, and describe available options for management decisions. We highlight a number of areas of important uncertainty and controversy, including the preferred mode of delivery, the appropriate use and timing of neuroimaging tests, and the appropriate use of clotting factor concentrates in the newborn period. While the approach presented here will aid clinicians in planning and providing care, further research is required to optimize the care of newborns with hemophilia.
Keywords: hemophilia, gynecology and obstetrics, bleeding
Introduction
Hemophilia A and B are congenital deficiencies of coagulation factor VIII (FVIII) and factor IX (FIX), respectively, and are among the most common inherited bleeding disorders. Newborns with hemophilia are at increased risk of intracranial hemorrhage (ICH) and its sequelae,1-3 extracranial hemorrhage (ECH),1,4 and other bleeding complications.4 The prevention, detection, and treatment of these complications are of foremost importance during and after delivery.
The safe delivery of a newborn with hemophilia involves many decisions and considerable planning. However, even experienced clinicians may not manage newborns with hemophilia frequently,5 and high-quality evidence does not exist to inform many decisions. Guidance documents from the United States (Medical and Scientific Advisory Council of the National Hemophilia Federation),6 the United Kingdom (United Kingdom Hemophilia Centre Doctors’ Organization),7 Australia (Australian Hemophilia Centre Directors’ Organization),8 and the World Federation of Hemophilia9 are available. The breadth and depth of information in some of these documents is limited, particularly with regard to the care of newborns. In addition, some of these documents do not address the most current literature in the area. (They remain useful documents, however, particularly for describing practice in particular settings.) For these reasons, our multidisciplinary group prepared this comprehensive guide for use by clinicians who manage the pregnancies, deliveries, and postnatal care of newborns with hemophilia.
Hemophilia A and B are disorders with X-linked recessive inheritance, and often occur with a family history or in children born to mothers who are known carriers. However hemophilia can occur, as the result of new mutations, in patients without family histories of the disorder. It is not possible to plan for this eventuality, but many of the principles of care described below may be applied to newborns diagnosed with hemophilia after birth.
Some of the principles of care described here may also apply to newborns affected by or at risk of other moderate and severe inherited bleeding disorders. Some disorders, such as type 2A, 2B, or 2 M von Willebrand disease, are inherited in an autosomal dominant fashion: a newborn born to a parent affected by one of these disorders has a 50% chance of being affected themselves. Other disorders, for example type 3 von Willebrand disease or factor XIII deficiency, are generally rare because of autosomal recessive inheritance, and therefore difficult to anticipate before birth. However, if there is already an affected child in a family, the risk for any subsequent newborn is 25%.
Newborns with mild hemophilia (baseline FVIII or FIX levels greater than 5%) can have ICH. This is likely infrequent, but the incidence is difficult to estimate accurately, as patients with mild hemophilia are underrepresented in existing studies.4,10 Other mild bleeding disorders, such as type 1 von Willebrand disease, are not associated with severe bleeding complications, such as ICH, in newborns.11 It may be possible, depending on the clinical circumstance and parent preference, to manage pregnancies involving these mild bleeding disorders without all the special considerations described here.
The obstetric care of women with inherited bleeding disorders, including hemophilia carriers, is an extensive and complex topic. Considerations include the management of bleeding risks associated with invasive prenatal procedures and regional anesthesia, and the increased risk of postpartum hemorrhage. This is all considered in proper detail elsewhere.12,13
This guide incorporates available evidence, which is largely of low quality (ie, observational studies and case reports), as well as expert opinion and common current practice. The primary aims of this guide are therefore to explore management options, describe frameworks for decision-making, and provide guidance, rather than to make firm recommendations. In practice settings where some elements of care are not readily available (eg, factor concentrates, neuroimaging modalities, and specialized obstetric services), the approach described here may require adaptation to the resources at hand.
Longitudinal Multidisciplinary Care Coordinated by a Hemophilia Treatment Center
The care of a newborn with hemophilia begins at the diagnosis of pregnancy or, in some cases, before conception, and continues through delivery and the neonatal period. Many practitioners will be involved: pediatric hematologists, adult hematologists, hemophilia nurses, obstetricians and maternal-fetal specialists, obstetrics nurses, genetic counsellors, neonatologists, pediatricians, radiologists, and experts in blood banking and laboratory medicine. Parents may wish for care to be provided by midwives or doulas; there is no reason to exclude these practitioners, but they should not be considered substitutes for medical professionals with expertise in high-risk obstetrics, neonatology, and hemophilia care. The parents of the newborn are essential members of this team, and their preferences should be solicited and considered throughout.
Ensuring this multidisciplinary care should be the guiding principle, and the Hemophilia Treatment Center (HTC) has a central role in coordinating care during pregnancy, delivery, and the newborn period. Care at an HTC reduces mortality and improves outcomes in patients with hemophilia,14,15 although this has not been specifically demonstrated for newborns or for hemophilia carriers.
Some families may not live near their HTC. Practitioners at the HTC should still assume the role of coordinating care in this circumstance. This will involve thorough communication with local practitioners throughout all stages of the preconception counselling, pregnancy, delivery, and newborn period. Communication with families may be provided via telemedicine when appropriate. However, depending on what resources are available at local facilities, it may be necessary to provide some aspects of care at the HTC. In particular, it may be necessary to have the delivery take place at the HTC, as is discussed below.
There may be obstacles to providing coordinated comprehensive care in a timely manner. For example, pregnant carriers, particularly if they do not have bleeding problems themselves, may not be registered with or aware of the nearest HTC. In this situation, HTC staff might not become aware of a pregnancy involving a hemophilia carrier until very late, and perhaps not until after delivery. If a delivery is occurring at an institution other than the HTC, privacy concerns may make it difficult to inform the HTC of an impending delivery. Finally, if a well-organized registry system or established HTCs do not exist in a particular jurisdiction, the identification of pregnant carriers and the coordination of care may be challenging. When there are such barriers to the provision of comprehensive care, is it important to ensure that communication is as clear and thorough as circumstances will allow. Establishing a written institutional policy for the management of pregnant hemophilia carriers and their newborns is strongly recommended.
Prenatal Care
Genetic Counseling and Prenatal Testing
Care may begin prior to a woman becoming pregnant, if she is known to be a carrier or if there is a family history of hemophilia. Genetic counseling regarding hemophilia would ideally occur before pregnancy,16,17 allowing for all relevant testing options to be made available to parents, with the least time constraints for making decisions.
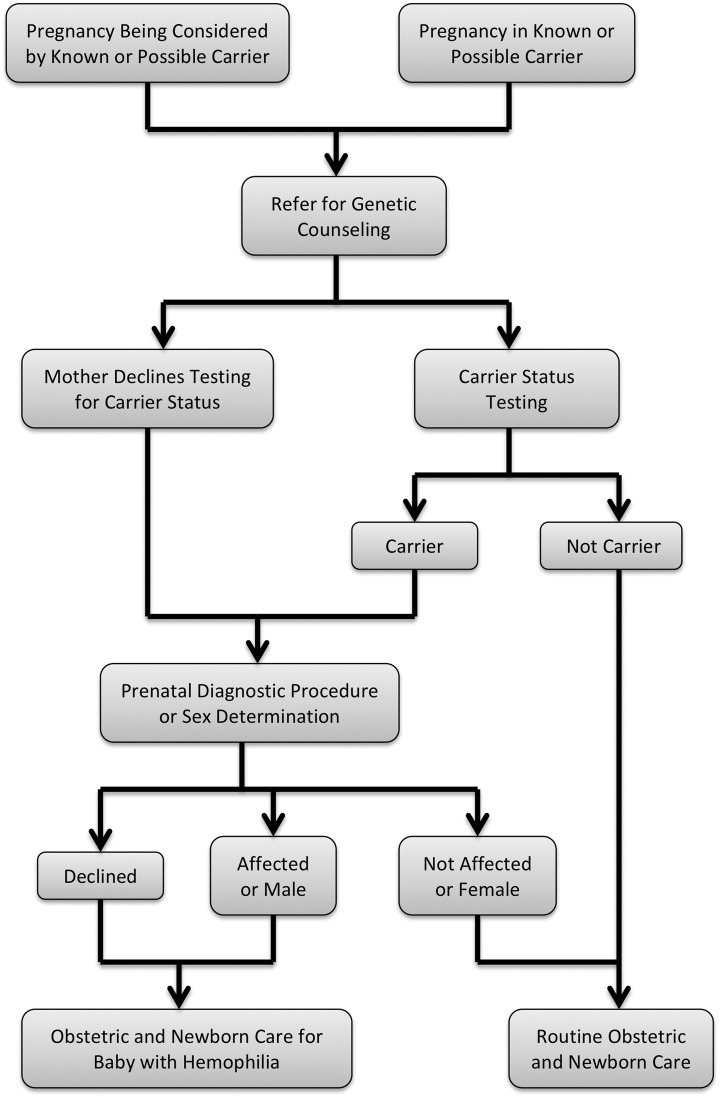
Genetic counseling should be offered to every carrier and possible carrier. This should include a description of hemophilia, a review of the inheritance pattern, and a discussion regarding available testing options9,16 (Figure 1). Applicable prenatal genetic testing of both the mother and the fetus should always be offered, when available.
Figure 1.
Algorithm for prenatal counseling and diagnostic testing for carriers or possible carriers of hemophilia. *See Table 1 for testing options.
Establishing carrier status for women, who may be asymptomatic, can be challenging in X-linked conditions. In the setting of hemophilia, carrier status cannot be excluded by a normal activated partial thromboplastin time (APTT) or normal FVIII or FIX levels,25 and genetic testing is recommended for definitive diagnosis. Unfortunately in some settings. it may be difficult to obtain reimbursement from insurance providers for this testing.
Carriers and possible carriers can opt to conceive naturally, to use an egg donor, to employ preimplantation genetic diagnosis, or to adopt. All of these options are feasible for women at risk to have a child with hemophilia; a successful case of preimplantation genetic diagnosis for hemophilia has been described.18 Once a woman is pregnant, there are a number of testing options that can be used, including noninvasive prenatal testing, ultrasound, chorionic villus sampling, and amniocentesis. First trimester ultrasound for determination of fetal sex may be particularly useful in practice settings with limited resources.22 The information provided by these tests and associated risks are summarized in Table 1. Recently, third trimester amniocentesis has been used for definitive prenatal diagnosis. This may help avoid unnecessary anxiety and intervention when a fetus is proven not to be affected.26,27 When genetic testing is chosen, fetal sex is determined first and then, for male fetuses only, mutation status is determined.12
Table 1.
Reproductive Investigations Available to Women at Risk of Having a Child With Hemophilia.a
| Option | Pregnancy Risks | Timing | Information Provided/Other Details |
|---|---|---|---|
| In-vitro fertilization with preimplantation genetic diagnosis18 | May not result in pregnancy | Before implantation | Sex and genetic status Consider confirmatory chorionic villus sampling or amnio in pregnancy May not be available in all centers |
| No investigation | None | N/A | None Manage pregnancy, delivery, and newborn as if affected until diagnosis is confirmed |
| Noninvasive prenatal testing12,19 | None to pregnancy; same as any blood draw | After 10 weeks gestation | Currently provides sex only Can provide information about common chromosomal aneuploidies |
| Chorionic villus sampling19,20 | Bleedingb
0.5%-1%c risk of miscarriage |
After 10 weeks | Sex and genetic status |
| Amniocentesis19,20 | Bleedingb
0.5%-1%c risk of miscarriaged or preterm deliverye |
After 15 weeks | Sex and genetic status |
| Routine second trimester obstetrical ultrasound21 | None | 18-22 weeks | Sex only Results are not definitive |
| First trimester ultrasound22 | None | After 13 weeks | Sex only Biparietal diameter must be >22 mm to ensure accuracy (99.5%) |
Abbreviation: HTC, Hemophilia Treatment Center.
aGenetic testing is only available if familial F8 or F9 mutation is known. Genetic testing occurs once male sex is established (see Figure 1).
bThe risk of bleeding complications for mothers who are carriers with low factor levels should be assessed and managed in cooperation with the woman’s HTC.12,17
cCurrent literature suggests the risks associated with chorionic villus sampling and amniocentesis may be lower than is stated here.23,24
dFor early amniocentesis, in the first or second trimester.
eFor late amniocentesis, in the third trimester.
Definitive prenatal testing of a fetus cannot occur without a known mutation in the family. It is therefore important to ensure that a mutation has been correctly detected prior to any genetic investigations on a fetus. Genetic testing of mothers can occur during pregnancy.
Noninvasive testing that is currently available for clinical use can determine fetal sex. As technologies develop, more definitive testing by noninvasive methods will likely be possible: accurate noninvasive testing for F8 mutations for families with hemophilia has been successful on a research basis.19
Prenatal Planning
Any carrier or possible carrier who becomes pregnant should be referred to a high-risk obstetrics unit or maternal-fetal medicine unit, for coordination of obstetric care. A referral to an HTC should also be made, so that education can be provided to the parents, and to plan the hematologic care of the mother12 and the fetus/newborn.5
Advance planning for the delivery is an important part of prenatal care. Delivery must occur at a center that can guarantee the availability of obstetrics, neonatology or pediatrics, hematology, and blood bank and laboratory services. The ongoing involvement of the HTC before, during, and after delivery is crucial, and must be ensured even if the HTC is at a different site than the delivery. A written delivery plan (Table 2) should be prepared and given to the parents and to the members of the multidisciplinary team that will care for the mother and the newborn. This plan should include: obstetric care for the mother, planned mode of delivery, and immediate postnatal care for the newborn. Specific topics are discussed in detail below.
Table 2.
Suggested Contents of the Written Delivery Plan.a
| Content Item | Comments |
|---|---|
| Statement that plan was developed in consultation with parent(s) | |
| Planned site for delivery | |
| Planned mode of delivery | Include indications for deviating from this plan |
| Hemostatic therapy for delivery for mother, if indicated | Develop in consultation with adult hematologist |
| Plans or contraindications to regional anesthesia for mother | Develop in consultation with adult hematologist and obstetric anesthesiologist |
| Services to be notified at onset of labor: NICU, HTC, coagulation laboratory, blood bank (if factor stored there) | Include specific contact information: names, pager, or phone
numbers. Coagulation laboratory should prepare to measure FVIII or FIX level “stat” |
| Avoid operative vaginal delivery (vacuum, forceps) | |
| Avoid invasive monitoring of fetus (scalp electrode) | |
| Obtain cord blood sample for confirmation of diagnosis | Send to coagulation laboratory “stat” |
| Factor concentrate to be used | Identify specific person(s) or other resource (printed or online material) that can give information about reconstitution |
| Location of factor concentrate | |
| Ensure availability of factor concentrate at onset of labor | |
| Plan for prophylactic factor replacement for newborn | Include specific product, dose, vial size, and instruction about whether to use entire vial |
| Plan for empiric factor replacement for newborn | Include specific product, dose, vial size, and instruction about whether to use entire vial |
| Plan for screening neuroimaging for newborn | Include modality and timing |
| Planned route and dose of vitamin K | If PO, include plan for ensuring all doses are given. If IM/SC, apply pressure to the site for 5 minutes |
| Statement about other heel pokes, venipuncture, and other needle pokes | Use smallest possible needle/lancet. Apply pressure to the site for 5 minutes |
Abbreviations: HTC, Hemophilia Treatment Center; IM, intramuscularly; NICU, neonatal intensive care unit; PO, orally; SC, subcutaneously.
aA copy of the written delivery plan should be given to the parents to bring to the delivery. The written delivery plan should be available in the caseroom where delivery is planned to occur, and it should also be on file at the Hemophilia Treatment Centre (HTC).
Management of Delivery
Notification of Team Members
When delivery is imminent, the HTC team, the neonatology service, the hemostasis laboratory, and the blood bank should be notified. The delivery plan should be reviewed. The availability of replacement factor concentrate should be ensured (see below).
Delivery and Monitoring
It is essential to identify a planned mode of delivery, which must be included in the written delivery plan. Minimizing the risk of ICH is the priority during delivery; other severe bleeding complications, such as cephalohematomas, are also possible. The risk of ICH is substantial: there were 14 delivery-associated ICH among 547 newborns (2.6%) in a large registry in the United States.10 It is likely that ICH is more common in newborns with severe hemophilia, as compared to those with moderate or mild disease.10 However, patients with mild disease may be underrepresented in studies, preventing accurate estimation of relative risk among disease severity strata. Intracranial hemorrhage in newborns with hemophilia is associated with permanent morbidity.1–3 Lower risk is observed when the mother is a known carrier,10,28 underscoring the importance of identifying carriers antenatally.
Vaginal delivery is not contraindicated for hemophilia carriers or for affected fetuses. There are conflicting data about whether delivery by caesarian section reduces the risk of ICH.4,10,29 Given that caesarian delivery carries additional risks for both mothers and babies,30 it need not be considered the default mode of delivery.
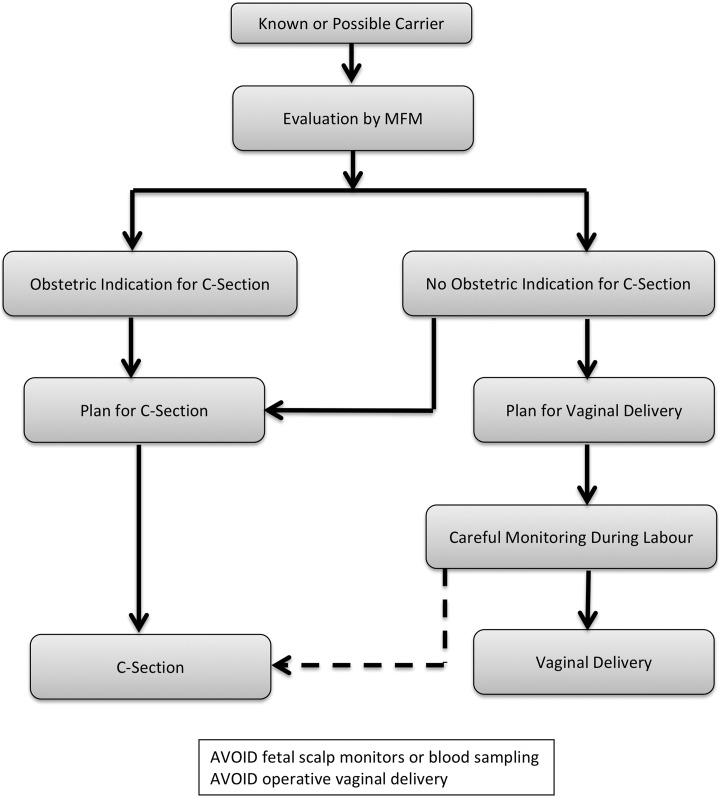
On the other hand, operative vaginal delivery (ie, vaginal delivery with the assistance of vacuum or forceps) is the most important risk factor for ICH and ECH,4 and should be avoided. However, the outcome of labor is unpredictable and if a vaginal delivery is attempted, avoidance of an operative delivery cannot be guaranteed. In some circumstances, such as when the head is impacted after a prolonged second stage of labor, there may be a considerable risk of trauma with caesarean delivery.31 In the absence of conclusive evidence, the safest mode of delivery must be chosen by considering, on an individual basis, the risks and benefits of each mode of delivery to both the mother and the fetus. As a basic approach, if there is no obstetric contraindication to vaginal delivery, an attempt at vaginal delivery can be planned. Careful monitoring during labor is essential. If the need for instrumentation is a possibility, the plan for vaginal delivery should be abandoned early in favor of a caesarean section (Figure 2).
Figure 2.
Algorithm for choosing a mode of delivery. If there is no obstetric contraindication to a vaginal delivery, either a vaginal delivery or a caesarean section may be planned. The dashed pathway indicates that, during labor, a decision may be made to perform a caesarean section in order to avoid a difficult vaginal delivery or an operative vaginal delivery.
Invasive fetal monitoring, such as placement of intrapartum fetal scalp electrodes and fetal scalp blood testing, should be avoided.
The obstetric and analgesic management of those mothers who are carriers with low factor levels is a complex topic that is beyond the scope of this document, and should be provided in close consultation with the HTC.12
Postnatal Care
After delivery, the goals of care are to rapidly confirm a diagnosis of hemophilia if not already done prenatally, to assess the risk of serious bleeding and manage any bleeding that does occur, and to safely provide routine newborn care.
In newborns, bleeding can occur in different sites than in older children with hemophilia. Hemarthroses are uncommon, but bleeding after circumcision can occur frequently. While ICH is the most serious concern, ECH is equally common and can, when severe, result in life-threatening anemia. Bleeding at the sites of heel pokes, intramuscular injections, and venipunctures is possible. Bleeding in the oral mucosa becomes more common after 30 days of age.4 Umbilical stump bleeding can occur but is not common.10 Clinicians caring for newborns with hemophilia must be aware of these patterns of bleeding to provide appropriate clinical monitoring.
If a diagnosis of hemophilia has not been made prenatally, newborns should be treated with the presumption that they are affected until the diagnosis is confirmed or excluded (Figure 1).
Confirmatory Testing
If a definitive diagnosis of hemophilia has not been made, testing should be done as soon as possible after birth. Both the APTT and FVIII or FIX level should be measured, because an APTT that is normal for age may not exclude the diagnosis of mild hemophilia. Factor IX levels in normal newborns may be low enough to be mistaken for mild hemophilia B,32,33 so that newborns with slightly low FIX levels should be retested at a later time. To avoid the risk of bleeding following venipuncture, umbilical cord blood34 is the preferred source of samples for coagulation tests and for genetic testing, provided that precautions are taken to avoid contamination of the sample with maternal blood.
Disposition
A decision must be made regarding the disposition of a newborn with hemophilia after birth. Those who have had traumatic delivery, are premature, have significant facial bruising, or are thought to be at high risk of bleeding for other reasons should be considered for admission to the neonatal intensive care unit (NICU) for monitoring. This should include the use of a protocol for monitoring of newborns who are at risk of ICH. A term newborn who is well and has none of these risk factors for ICH or other bleeding complications may remain with his mother and be discharged with her, as long as appropriate education is complete and follow-up plans are in place (see below).
Routine Newborn Care
Newborns with hemophilia should receive routine newborn care, although some modifications to this care are required.
Newborns with hemophilia should receive vitamin K. This should not be delayed to wait for results of APTT or factor assays. The route of vitamin K administration should be planned prenatally, and included in the written delivery plan. Vitamin K can be given orally (PO), intramuscularly (IM), or subcutaneously (SC). Clinicians concerned about the small risk4 of an intramuscular hematoma caused by IM injection can consider SC administration of an appropriate formulation of vitamin K.35,36 Vitamin K, IM or SC, should be given with a 27 gauge (or smaller) needle, and direct pressure should be applied for at least 5 minutes afterward. A single dose of IM or SC vitamin K (1 mg) is sufficient. Vitamin K PO must be given in 3 doses: 2 mg at birth, at 1 week of age, and at 4 weeks of age. Therefore, if follow-up care cannot be ensured, IM or SC vitamin K is preferred. Vitamin K PO may be associated with an increased risk of hemorrhagic disease of the newborn, even if all scheduled doses are given.37
The local vaccination schedule should be adhered to. Vaccination is not associated with increased risk of inhibitors in patients with hemophilia A,38 and this theoretical concern should not motivate the delay or omission of required vaccines. Bacille Calmette-Guerin, if required, is always given intradermally. The World Federation of Hemophilia recommends that all other vaccines be given SC to persons with hemophilia,9 but local practice may vary. Hepatitis B vaccine is similarly effective whether given SC or IM.39 Pressure should be applied to the site for at least 5 minutes after either IM or SC vaccination.
Newborns with hemophilia should have blood collected for the routine newborn screening tests at the usual time, as per local practice. A neonatal lancet should be used, and direct pressure applied to the site for at least 5 minutes afterward. If a venipuncture is required, the smallest possible needle should be used, and direct pressure applied to the site for at least 5 minutes afterward.
Availability of Factor Concentrate
The specific factor concentrate to be used, if planned or required, should be determined prenatally, in discussion with the parents, and this information should be included in the written delivery plan. (The selection of a particular concentrate is, in the setting of genuine controversy about the possibility of different risks of inhibitors between different classes of product40,41 and the clinical availability of a number of new modified factor replacement products, well beyond the scope of this guide.) An appropriately sized vial of the chosen concentrate should be made available at the time of delivery. Options for this include: keeping the vial in the caseroom (delivery suite), providing the vial to the parents to bring to the caseroom, or requesting the vial from the blood bank when delivery is imminent. Staff present at the delivery should be familiar with the reconstitution and administration of that concentrate. This may require advance education of caseroom or NICU staff.
Desmopressin should not be used in newborns with hemophilia, because of the risk of symptomatic hyponatremia in younger children.42,43 Cryoprecipitate and fresh frozen plasma should not be used as sources of FVIII and FIX, respectively, unless a concentrated form of the appropriate factor is not available.
A variety of novel factor concentrates and nonfactor therapies are available, and more are in development. These are difficult to apply knowledgeably to newborns, who are underrepresented in the study of new therapies. Every effort should be made to enter newborns into studies, when appropriate.
Factor VIII and FIX Dosing, Pharmacokinetics, and Monitoring
The usual rules-of-thumb regarding incremental recovery can be used to guide dosing of FVIII and FIX concentrates in newborns: expected increases of approximately 2 IU/mL in FVIII activity for every 1 IU/kg of FVIII concentrate, and of approximately 1 IU/mL in FIX activity for every 1 IU/kg of FIX concentrate. However, FVIII may have reduced recovery, increased clearance, and reduced half-life in young children as compared to older persons,44 and these differences may not be predictable. Factor IX pharmacokinetics have not been extensively evaluated in very young children.45 It is therefore not possible to make firm recommendations regarding dosing, particularly if repeated dosing is required. Any newborn who is to receive repeated doses or a continuous infusion of a factor concentrate will require therapeutic monitoring of factor levels. Trough levels, drawn immediately before the next dose of factor concentrate, are used to ensure that factor levels stay above whatever threshold is considered adequate for hemostasis in a given situation (see below). Peak levels, drawn 15 to 30 minutes after a dose has been given, are used to determine recovery. Determining more complex pharmacokinetic parameters (eg, terminal half-life or clearance) requires sampling at multiple time points and may be impractical in newborns. Techniques for the calculation of FVIII and FIX pharmacokinetic parameters from population pharmacokinetic data and Bayesian analysis were not developed with the inclusion of data from newborns,46,47 and this approach should not be considered reliable in these patients.
It is common practice to round the administered dose of factor concentrate up to the nearest vial size, to avoid wastage. In some circumstances—for example a 250 IU vial of FVIII concentrate given to a 3 kg newborn—this could result in factor levels substantially above 100% of normal. Elevated FVIII has been associated with thrombotic events in some populations of pediatric patients,48,49 while elevated FIX has not.48 However, neither association has been examined specifically in newborns, so it is worth considering the potential risks of elevated factor levels as the result of replacement therapy in these patients. There may be less concern for newborns with hemophilia A: FVIII levels above 100% in normal newborns may still be considered physiologic.32 On the other hand, levels of FIX are reduced in the newborn period,32 so that levels greater than 100% might well be functionally abnormal, and caution might be taken when giving FIX doses that might raise the FIX level higher than 100%; discarding part of a vial to avoid this might be appropriate in some circumstances. With either FVIII or FIX replacement therapy, the presence of other risk factors for thrombosis, such as the presence of central arterial or venous catheters, should be considered when determining an acceptable peak factor level.
Administration of Factor Concentrate
Factor replacement therapy might be given with a number of different indications: to prevent bleeding (ie, prophylactically), to treat possible bleeding (ie, empirically), or to treat bleeding that is observed or considered very likely to be occurring. There are no evidence-based criteria for identifying newborns who will benefit from prophylactic or empiric therapy, but this has been the subject of several surveys of clinicians: Canadian hematologists uniformly opted not to give prophylactic replacement therapy, and approximately a quarter preferred empiric treatment.5 In the United Kingdom, 19% of surveyed hematologists indicated that they would provide prophylactic treatment, and 38% would provide it empirically.50 In the United States, 60% of surveyed hematologists disagreed that factor concentrates need to be given prophylactically immediately after delivery, but 89% favored “early prophylaxis.”51 These surveys suggest that clinicians are making decisions about providing empiric factor replacement on an individual basis, considering factors such as ease of delivery, the presence of bruising, and prematurity. It is not known if there is reluctance to use prophylactic or empiric factor replacement because of concern over the risk of subsequent development of inhibitors. This may not be an issue: FVIII administration in the first 24 hours of life is not associated with increased inhibitor risk,10 and no association of FIX administration in the perinatal period and FIX inhibitors has ever been observed.
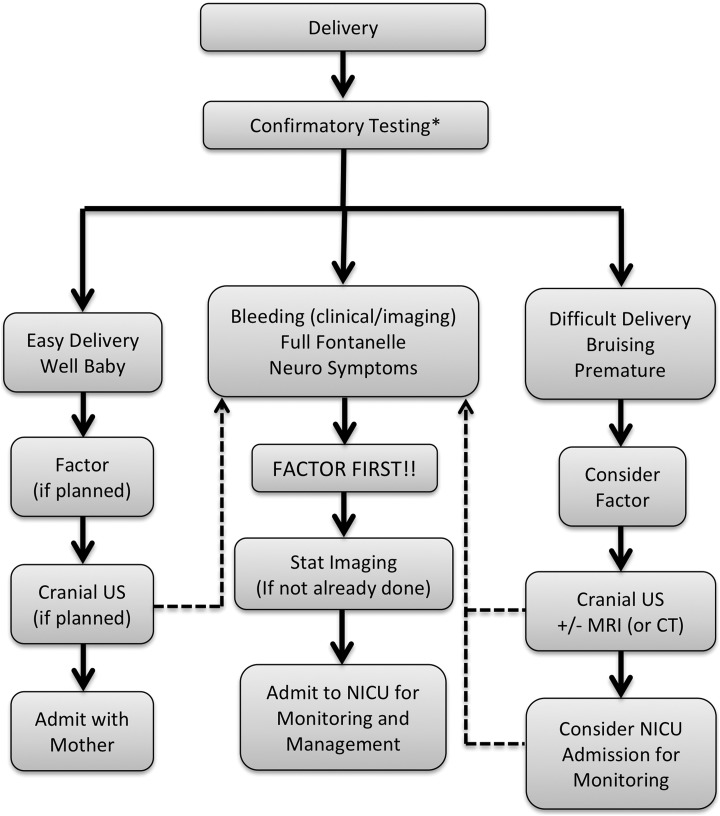
However, since there is no proven benefit of prophylactic factor administration, this may be omitted for a term baby after an uncomplicated delivery. In other situations, however, the “factor first” principle applies, and prophylactic therapy should be strongly considered after a difficult delivery, or if significant facial or cranial bruising is present (Figure 3). Many of the premature newborns with hemophilia reported in the literature have been given prophylactic or empiric factor replacement,52–57 and so prematurity should also prompt consideration of prophylactic factor administration (Figure 3). Plans for prophylactic and empiric therapy should be discussed before birth, and included in the written delivery plan.
Figure 3.
Algorithm for the use of factor concentrates and neuroimaging for newborns with hemophilia. *If a newborn is born to a hemophilia carrier or possible carrier, but a diagnosis of hemophilia was not obtained antenatally, the newborn should be presumed to be affected and appropriate care provided until postnatal testing confirms that the newborn is not affected. The dashed pathways indicate that if, at any time and regardless of the events of birth, a newborn develops concerning symptoms or has bleeding that is observed either clinically or with imaging, the prompt administration of replacement factor is the priority, followed by appropriate investigation. CT indicates computed tomography; neuro, neurological; NICU, neonatal intensive care unit; MRI, magnetic resonance imaging; US, ultrasound.
Newborns with hemophilia may require replacement therapy prior to procedures. Procedures considered to have a high risk of serious bleeding complications (eg, lumbar puncture, peripherally inserted central catheter line insertion with or without cutdown, and chest tube insertion) should be performed under coverage with a factor concentrate. Clinicians may consider performing procedures with low bleeding risk (eg, endotracheal intubation, insertion of umbilical arterial and venous catheters, insertion of nasogastric tubes, and insertion of peripheral intravenous catheters) without coverage with factor replacement therapy.
Surgical procedures must be done under appropriate coverage with factor concentrate. Elective procedures should be avoided. However if a procedure such as circumcision is required for religious, cultural, or medical reasons, this may be performed safely with appropriate factor coverage. Management of circumcision varies in terms of the intensity and duration of factor replacement or other hemostatic therapy58 (which may include fibrin glue or antifibrinolytics).
For newborns with hemophilia who are bleeding or who are suspected to have bleeding (especially ICH), prompt factor replacement therapy is crucial. The “factor first” principle dictates that factor replacement therapy should be given emergently and should not be delayed for procedures or diagnostic tests (Figure 3).
Intracranial hemorrhage and ECH in a newborn with hemophilia require emergent, aggressive treatment. Factor replacement can be given by intermittent dosing or by continuous infusion.59 The immediate goal is to maintain the factor level at 0.5 to 1 IU/mL (50%-100%). Prolonged treatment may be required, followed by a period of prophylactic treatment after the resolution of the hemorrhage.
Bleeding other than ICH or ECH also requires prompt assessment and treatment. In some circumstances, treatment may be less aggressive than is required to treat ICH, and a trough factor level of 0.5 IU/mL (50%) may be adequate.
Intense and prolonged factor replacement therapy is a risk factor for the development of inhibitory antibodies (inhibitors), especially to FVIII.60,61 However this risk should not prevent emergent treatment with FVIII or FIX concentrates when required. Newborns who have had intense factor exposure should be frequently tested for the presence of inhibitors using a Bethesda assay. The prevention and management of inhibitors in newborns and older children is beyond the scope of this guide, and should be done in close collaboration with an HTC.
Neuroimaging
There is no high-quality evidence to inform the use of universal or targeted screening neuroimaging in newborns with hemophilia. Practice in this area is reported to be varied.5,50,51 Screening can be performed within an institutional policy, as a decision made with a family before delivery (and included in the written delivery plan) or on an individual basis. Neuroimaging is required after an operative vaginal delivery (which is supported by a recent cost-utility analysis62), for premature newborns, when the delivery is difficult, or when there is facial bruising or more severe ECH evident clinically. In the absence of any of these circumstances, the yield of screening neuroimaging may be low. If ICH is suspected, diagnostic neuroimaging should be obtained emergently, but should not delay factor replacement therapy (Figure 3).
Available diagnostic imaging modalities are conventional radiographs, ultrasound (US), computed tomography (CT) scans, and magnetic resonance imaging (MRI). Conventional radiographs are not of any use in the diagnosis of ICH. Ultrasound is the first screening modality that should be performed. Ultrasound has the advantage of being available at the bedside, and does not use ionizing radiation. The limitations of US are that it fails to reliably detect small to moderate extra-axial hemorrhages along the lateral surfaces of the brain or in the posterior fossa.63 Intra-axial hemorrhages, large extra-axial hemorrhages which cause midline shift, and intraventricular hemorrhages are reliably detected with US.64 Computed tomography scan is highly sensitive and specific for diagnosis of acute hemorrhage in newborns.65 Computed tomography scan is very fast and state of the art scanners can image the brain in under a minute, but CT is not routinely available at the bedside. There is no need for intravenous contrast to diagnose ICH. The major limitation of CT scan is ionizing radiation. Magnetic resonance imaging is the most sensitive tool for detecting hemorrhage. However, MRI is expensive and not readily available in some settings. Furthermore, performance of brain MRI in newborns requires specific imaging protocols, specialized monitoring, and experienced radiologists to interpret findings. Magnetic resonance imaging should be performed with T1, T2, diffusion weighted imaging, and T2* gradient echo or susceptibility weighted images (SWI). T2* gradient echo images or SWI images, in particular, are sensitive for the detection of hemorrhages. The advantages of MRI are lack of ionizing radiation, multiplanar capability, and the ability to diagnose and detect abnormalities of the brain other than hemorrhage.
Evaluation of the neonatal brain can be complicated by the presence of asymptomatic thin posterior cerebral and posterior fossa subdural hemorrhages after uneventful vaginal deliveries. These hemorrhages can be present in up to 20% to 40% of newborns without hemophilia after vaginal delivery,66,67 and can be presumed to occur at least as often in newborns with hemophilia after vaginal delivery. It is not known how to determine whether these hemorrhages will progress to acutely symptomatic ICH. The incidence of these hemorrhages is much higher than that of symptomatic ICH in newborns with hemophilia, so that most will not progress to symptomatic ICH. The presence of such hemorrhages, particularly in an asymptomatic newborn, is therefore not necessarily an indication for factor replacement therapy. Babies with such hemorrhages should be followed up with close clinical monitoring, and possibly with repeated imaging (US or MRI are preferred, to minimize radiation exposure). Factor replacement therapy should be given promptly if symptoms develop or if there is significant progression on imaging.
Discharge Planning
Bleeding problems that occur during the newborn period do not always manifest immediately following delivery and may become apparent after discharge from hospital. It is therefore crucial that the families of affected newborns receive adequate counseling prior to discharge68 with regard to bleeding risk during early life. Easy bruising can occur, and common sites of significant hemorrhage in children with hemophilia between 1 and 6 months of age include the head, oral mucosa, joints, and soft tissues.4 Recommendations about vaccinations (see above) and medications to avoid (ie, those with antiplatelet effects or other effects on hemostasis) should be given. Prior to discharge, the newborn’s primary care practitioner should be contacted and given information about the child’s diagnosis, the potential risk of bleeding, and plans for follow-up care. Newborns with hemophilia living in remote areas should have arrangements made for a supply of factor concentrate to be available at their local hospital for treatment of bleeding episodes. Registration at an HTC is recommended before discharge, to ensure follow-up and to provide ongoing education for the family and community health-care providers.
Other Issues
Experience with management of premature newborns with hemophilia is limited to case reports.52–57 Management of premature newborns should be determined on an individual basis. Delivery and postnatal care should occur at an HTC.
Girls born to hemophilia carriers do not, in general, require special perinatal management. Females heterozygous for F8 or F9 mutations can have factor levels low enough to cause pathologic bleeding, but this is rarely clinically evident in the neonatal period. However, the possibility of bleeding should be kept in mind. Measurement of FVIII or FIX levels or genetic testing of girls should not be done in the newborn period unless the results would be important for immediate management. Ideally, confirmation of a girl’s carrier status would be deferred, if possible, until she has the capacity to request the testing herself after appropriate discussion.69
Research Priorities
There are a number of areas in which improved understanding is especially needed. Systematic studies should determine the epidemiology and natural history of ICH and subclinical ICH, and should establish the optimal use and timing of different imaging modalities—US and MRI—for newborns with hemophilia. It is also important to determine evidence-based strategies for identifying newborns with hemophilia who will benefit from the prophylactic or empiric administration of factor concentrates. For those who do require replacement therapy, informed dosing decisions will only be possible with improved understanding of the pharmacokinetics of factor concentrates in newborns.
Conclusions
In the effort to deliver a healthy newborn with hemophilia, preparation that incorporates the expertise of a multidisciplinary team associated with an HTC is crucial. This guide provides clinicians with a comprehensive approach to planning for pregnancy, delivery, and the newborn period. However, many important gaps in knowledge exist, and it is hoped that future research will allow for continued improvement in the care of newborns with hemophilia.
Acknowledgments
The authors would like to thank Danielle and Dan McCully for reviewing this manuscript from the perspective of parents of a boy with hemophilia.
Authors’ Note: All authors wrote sections of the manuscript and approved the final draft of the entire manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Yoffe G, Buchanan GR. Intracranial hemorrhage in newborn and young infants with hemophilia. J Pediatr. 1988;113(2):333–336. [DOI] [PubMed] [Google Scholar]
- 2. Stieltjes N, Calvez T, Demiguel V, et al. Intracranial haemorrhages in French haemophilia patients (1991-2001): clinical presentation, management and prognosis factors for death. Haemophilia. 2005;11(5):452–458. [DOI] [PubMed] [Google Scholar]
- 3. Miles BS, Anderson P, Agostino A, et al. Effect of intracranial bleeds on the neurocognitive, academic, behavioural and adaptive functioning of boys with haemophilia. Haemophilia. 2012;18(2):229–234. [DOI] [PubMed] [Google Scholar]
- 4. Kulkarni R, Soucie JM, Lusher J, et al. Sites of initial bleeding episodes, mode of delivery and age of diagnosis in babies with haemophilia diagnosed before the age of 2 years: a report from The Centers for Disease Control and Prevention’s (CDC) Universal Data Collection (UDC) project. Haemophilia. 2009;15(6):1281–1290. [DOI] [PubMed] [Google Scholar]
- 5. Moorehead PC, Ray J, Barrowman NJ, Lemyre B, Klaassen R. A survey of the management of newborns with severe hemophilia in Canada. Paediatr Child Health (Oxford). 2013;18(4):189–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. MASAC. MASAC Guidelines for perinatal management of women with bleeding disorders and carriers of hemophilia A and B. 2017. https://www.hemophilia.org/node/3660. Accessed September 1, 2018. Updated September 17, 2017.
- 7. Chalmers E, Williams M, Brennand J, Liesner R, Collins P, Richards M. Guideline on the management of haemophilia in the fetus and neonate*. Br J Haematol. 2011;154(2):208–215. [DOI] [PubMed] [Google Scholar]
- 8. Dunkley SM, Russell SJ, Rowell JA, et al. A consensus statement on the management of pregnancy and delivery in women who are carriers of or have bleeding disorders. Med J Aust. 2009;191(8):460–463. [DOI] [PubMed] [Google Scholar]
- 9. Srivastava A, Brewer AK, Mauser-Bunschoten EP, et al. Guidelines for the management of hemophilia. Haemophilia. 2013;19(1):e1–e47. [DOI] [PubMed] [Google Scholar]
- 10. Kulkarni R, Presley RJ, Lusher JM, et al. Complications of haemophilia in babies (first two years of life): a report from the centers for disease control and prevention universal data collection system. Haemophilia. 2017;23(2):207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chalmers EA, Alamelu J, Collins PW, et al. Intracranial haemorrhage in children with inherited bleeding disorders in the UK 2003-2015: A National Cohort study. Haemophilia. 2018;24(4):9–13. [DOI] [PubMed] [Google Scholar]
- 12. Kadir RA, Davies J, Winikoff R, et al. Pregnancy complications and obstetric care in women with inherited bleeding disorders. Haemophilia. 2013;19(suppl 4):1–10. [DOI] [PubMed] [Google Scholar]
- 13. Demers C, Derzko C, David M, Douglas J. Society of obstetricians and gynaecologists of Canada. Gynaecological and obstetric management of women with inherited bleeding disorders. Int J Gynaecol Obstet. 2006;95(1):75–87. [DOI] [PubMed] [Google Scholar]
- 14. Smith PS, Levine PH. The benefits of comprehensive care of hemophilia: a five-year study of outcomes. Am J Public Health. 1984;74(6):616–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Soucie JM, Nuss R, Evatt B, et al. Mortality among males with hemophilia: relations with source of medical care. The hemophilia surveillance system project investigators. Blood. 2000;96(2):437–442. [PubMed] [Google Scholar]
- 16. Ludlam CA, Pasi KJ, Bolton-Maggs P, et al. A framework for genetic service provision for haemophilia and other inherited bleeding disorders. Haemophilia. 2005;11(2):145–163. [DOI] [PubMed] [Google Scholar]
- 17. Chi C, Kadir RA. Inherited bleeding disorders in pregnancy. Best Pract Res Clin Obstet Gynaecol. 2012;26(1):103–117. [DOI] [PubMed] [Google Scholar]
- 18. Michaelides K, Tuddenham EGD, Turner C, Lavender B, Lavery SA. Live birth following the first mutation specific pre-implantation genetic diagnosis for haemophilia A. Thromb Haemost. 2006;95(2):373–379. [DOI] [PubMed] [Google Scholar]
- 19. Tsui NBY, Kadir RA, Chan KCA, et al. Noninvasive prenatal diagnosis of hemophilia by microfluidics digital PCR analysis of maternal plasma DNA. Blood. 2011;117(13):3684–3691. [DOI] [PubMed] [Google Scholar]
- 20. Wilson RD, Davies G, Gagnon A, et al. Amended Canadian guideline for prenatal diagnosis (2005) change to 2005-techniques for prenatal diagnosis. J Obstet Gynaecol Can. 2005;27(11):1048–1062. [DOI] [PubMed] [Google Scholar]
- 21. Cargill Y, Morin L, Bly S, et al. Content of a complete routine second trimester obstetrical ultrasound examination and report. J Obstet Gynaecol Can. 2009;31(3):272–275. [DOI] [PubMed] [Google Scholar]
- 22. Mohammed N, Nuruddin R. First trimester sonographic determination of foetal gender: a cost effective non-invasive technique for prenatal screening of haemophilia in low income countries. Haemophilia. 2012;18(2): e49–e50. [DOI] [PubMed] [Google Scholar]
- 23. Akolekar R, Beta J, Picciarelli G, Ogilvie C, D’Antonio F. Procedure-related risk of miscarriage following amniocentesis and chorionic villus sampling: a systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2015;45(1):16–26. [DOI] [PubMed] [Google Scholar]
- 24. Wulff CB, Gerds TA, Rode L, Ekelund CK, Petersen OB, Tabor A. Risk of fetal loss associated with invasive testing following combined first-trimester screening for Down syndrome: a national cohort of 147 987 singleton pregnancies. Ultrasound Obstet Gynecol. 2016;47(1):38–44. [DOI] [PubMed] [Google Scholar]
- 25. Plug I, Mauser-Bunschoten EP, Bröcker-Vriends AHJT, et al. Bleeding in carriers of hemophilia. Blood. 2006;108(1):52–56. [DOI] [PubMed] [Google Scholar]
- 26. Ingerslev MD, Langhoff-Roos J, Soegaard K, Funding E, Diness BR. Prenatal genetic testing by late amniocentesis to guide delivery management in haemophilia carriers. Haemophilia. 2017;23(5):1–3. [DOI] [PubMed] [Google Scholar]
- 27. Cutler J, Chappell LC, Kyle P, Madan B. Third trimester amniocentesis for diagnosis of inherited bleeding disorders prior to delivery. Haemophilia. 2013;19(6):904–907. [DOI] [PubMed] [Google Scholar]
- 28. Maclean PE, Fijnvandraat K, Beijlevelt M, Peters M. The impact of unaware carriership on the clinical presentation of haemophilia. Haemophilia. 2004;10(5):560–564. [DOI] [PubMed] [Google Scholar]
- 29. Davies J, Kadir RA. Mode of delivery and cranial bleeding in newborns with haemophilia: a systematic review and meta-analysis of the literature. Haemophilia. 2016;22(1):32–38. [DOI] [PubMed] [Google Scholar]
- 30. Liu S, Liston RM, Joseph KS, Heaman M, Sauve R, Kramer MS. Maternal mortality and severe morbidity associated with low-risk planned cesarean delivery versus planned vaginal delivery at term. Can Med Assoc J. 2007;176(4):455–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sung JF, Daniels KI, Brodzinsky L, El-Sayed YY, Caughey AB, Lyell DJ. Cesarean delivery outcomes after a prolonged second stage of labor. Am J Obstet Gynecol. 2007;197(3):306.e1–306.e5. [DOI] [PubMed] [Google Scholar]
- 32. Andrew M, Paes B, Milner R, et al. Development of the human coagulation system in the full-term infant. Blood. 1987;70(1):165–172. [PubMed] [Google Scholar]
- 33. Andrew M, Paes B, Milner R, et al. Development of the human coagulation system in the healthy premature infant. Blood. 1988;72(5):1651. [PubMed] [Google Scholar]
- 34. Mibashan RS, Rodeck CH, Thumpston JK, et al. Plasma assay of fetal factors VIIIC and IX for prenatal diagnosis of haemophilia. Lancet. 1979;1(8130):1309–1311. [DOI] [PubMed] [Google Scholar]
- 35. von Kries R, Göbel U, Hachmeister A, Kaletsch U, Michaelis J. Vitamin K and childhood cancer: a population based case-control study in Lower Saxony, Germany. BMJ. 1996;313(7051):199–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Handel J, Tripp JH. Vitamin K prophylaxis against haemorrhagic disease of the newborn in the United Kingdom. Br Med J. 1991;303(6810):1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Busfield A, McNinch A, Tripp J. Neonatal vitamin K prophylaxis in Great Britain and Ireland: the impact of perceived risk and product licensing on effectiveness. Arch Dis Child. 2007;92(9):754–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Platokouki H, Fischer K, Gouw SC, et al. Vaccinations are not associated with inhibitor development in boys with severe haemophilia A. Haemophilia. 2018;24(2):283–290. [DOI] [PubMed] [Google Scholar]
- 39. Carpenter SL, Soucie JM, Presley RJ, et al. Hepatitis B vaccination is effective by subcutaneous route in children with bleeding disorders: a universal data collection database analysis. Haemophilia. 2015;21(1):e39–e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Peyvandi F, Mannucci PM, Garagiola I, et al. A randomized trial of factor VIII and neutralizing antibodies in hemophilia a. N Engl J Med. 2016;374(21):2054–2064. [DOI] [PubMed] [Google Scholar]
- 41. Gouw SC, van der Bom JG, Ljung R, et al. Factor VIII products and inhibitor development in severe hemophilia A. N Engl J Med. 2013;368(3):231–239. [DOI] [PubMed] [Google Scholar]
- 42. Das P, Carcao M, Hitzler J. DDAVP-induced hyponatremia in young children. J Ped Heme Onc. 2005;27(6):330–332. [DOI] [PubMed] [Google Scholar]
- 43. Sharma R, Stein D. Hyponatremia after desmopressin (DDAVP) use in pediatric patients with bleeding disorders undergoing surgeries. J Ped Heme Onc. 2014;36(6):e371–e375. [DOI] [PubMed] [Google Scholar]
- 44. Björkman S, Blanchette VS, Fische RK, et al. Comparative pharmacokinetics of plasma- and albumin-free recombinant factor VIII in children and adults: the influence of blood sampling schedule on observed age-related differences and implications for dose tailoring. J Thromb Haemost. 2010;8(4):730–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Björkman S. Comparative pharmacokinetics of factor VIII and recombinant factor IX: for which coagulation factors should half-life change with age? Haemophilia. 2013;19(6):882–886. [DOI] [PubMed] [Google Scholar]
- 46. Björkman S, Oh M, Spotts G, et al. Population pharmacokinetics of recombinant factor VIII: the relationships of pharmacokinetics to age and body weight. Blood. 2012;119(2):612–618. [DOI] [PubMed] [Google Scholar]
- 47. Björkman S. Population pharmacokinetics of recombinant factor IX: implications for dose tailoring. Haemophilia. 2013;19(5):753–757. [DOI] [PubMed] [Google Scholar]
- 48. Skouri H, Gandouz R, Abroug S, et al. A prospective study of the prevalence of heparin-induced antibodies and other associated thromboembolic risk factors in pediatric patients undergoing hemodialysis. Am J Hematol. 2006;81(5):328–334. [DOI] [PubMed] [Google Scholar]
- 49. Faustino EVS, Li S, Silva CT, et al. Factor VIII may predict catheter-related thrombosis in critically ill children. Pediatr Crit Care Med. 2015;16(6):497–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chalmers EA, Williams MD, Richards M, et al. Management of neonates with inherited bleeding disorders—a survey of current UK practice. Haemophilia. 2005;11(2):186–187. [DOI] [PubMed] [Google Scholar]
- 51. Kulkarni R, Lusher JM, Lansing E. Current practices regarding newborn intracranial haemorrhage and obstetrical care and mode of delivery of pregnant haemophilia carriers: a survey of obstetricians, neonatologists and haematologists in the United States, on behalf of the National Hemophili. Haemophilia. 1999;5(6):410–415. [DOI] [PubMed] [Google Scholar]
- 52. Fink S, Kunzmann S, Andres O, Eyrich M, Wiegering V. Haemophilia in extreme immature preterm infants: increased risk for intracranial haemorrhage? J Matern Neonatal Med. 2014;27(6):621–624. [DOI] [PubMed] [Google Scholar]
- 53. Bidlingmaier C, Bergmann F, Kurnik K. Haemophilia A in two premature infants. Eur J Pediatr. 2005;164(2):70–72. [DOI] [PubMed] [Google Scholar]
- 54. Gale RF, Hird MF, Colvin BT. Management of a premature infant with moderate haemophilia A using recombinant factor VIII. Haemophilia. 1998;4(6):850–853. [DOI] [PubMed] [Google Scholar]
- 55. Kraft KE, Verlaak R, van Heijst AF, Nováková I, Brons PP. Management of haemophilia in three premature infants. Haemophilia. 2008;14(2):378–380. [DOI] [PubMed] [Google Scholar]
- 56. Gelbart B, Barnes C. Severe haemophilia and extreme prematurity—a case report. Haemophilia. 2009;15(1):352–354. [DOI] [PubMed] [Google Scholar]
- 57. Cartledge P, Deakin K, McKecknie L, Richards M. A case report of a premature infant with haemophilia A and factor VIII inhibitor. Haemophilia. 2011;17(4):711–712. [DOI] [PubMed] [Google Scholar]
- 58. Kearney S, Sharathkumar A, Rodriguez V, et al. Neonatal circumcision in severe haemophilia: a survey of paediatric haematologists at United States Hemophilia Treatment Centers. Haemophilia. 2015;21(1):52–57. [DOI] [PubMed] [Google Scholar]
- 59. Guilcher GMT, Scully MF, Harvey M, Hand JP. Treatment of intracranial and extracranial haemorrhages in a neonate with severe haemophilia B with recombinant factor IX infusion. Haemophilia. 2005;11(4):411–414. [DOI] [PubMed] [Google Scholar]
- 60. Gouw SC, van der Bom JG, Marijke van den Berg H. Treatment-related risk factors of inhibitor development in previously untreated patients with hemophilia A: the CANAL cohort study. Blood. 2007;109(11):4648–4654. [DOI] [PubMed] [Google Scholar]
- 61. Gouw SC, van den Berg HM, Fischer K, et al. Intensity of factor VIII treatment and inhibitor development in children with severe hemophilia A: the RODIN study. Blood. 2013;121(20):4046–4055. [DOI] [PubMed] [Google Scholar]
- 62. Malec LM, Sidonio RF, Smith KJ, Cooper JD. Three cost-utility analyses of screening for intracranial hemorrhage in neonates with hemophilia. J Ped Heme Onc. 2014;36(6):474–479. [DOI] [PubMed] [Google Scholar]
- 63. Blankenberg FG, Norbash AM, Lane B, Stevenson DK, Bracci PM, Enzmann DR. Neonatal intracranial ischemia and hemorrhage: diagnosis with US, CT, and MR imaging. Radiology. 1996;199(1):253–259. [DOI] [PubMed] [Google Scholar]
- 64. Huisman TAGM.Intracranial hemorrhage: ultrasound, CT and MRI findings. Eur Radiol. 2005;15(3):434–440. [DOI] [PubMed] [Google Scholar]
- 65. Blankenberg FG, Loh NN, Bracci P, et al. Sonography, CT, and MR imaging: a prospective comparison of neonates with suspected intracranial ischemia and hemorrhage. Am J Neuroradiol. 2000;21(1):213–218. [PMC free article] [PubMed] [Google Scholar]
- 66. Looney CB, Smith JK, Merck LH, et al. Intracranial hemorrhage in asymptomatic neonates: prevalence on MR images and relationship to obstetric and neonatal risk factors. Radiology. 2007;242(2):535–541. [DOI] [PubMed] [Google Scholar]
- 67. Rooks VJ, Eaton JP, Ruess L, et al. Prevalence and evolution of intracranial hemorrhage in asymptomatic term infants. Am J Neuroradiol. 2008;29(6):1082–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Chalmers EA. Haemophilia and the newborn. Blood Rev. 2004;18(2):85–92. [DOI] [PubMed] [Google Scholar]
- 69. Winikoff R, Lee C. Hemophilia carrier status and counseling the symptomatic and asymptomatic adolescent. J Pediatr Adolesc Gynecol. 2010;23(suppl 6):S43–S47. [DOI] [PubMed] [Google Scholar]