Abstract
The objective is to determine whether a low serum 25-hydroxyvitamin D (25(OH)D) level is associated with an increased incidence of deep venous thromboembolic events in patients with ischemic stroke. One hundred eighty persons admitted consecutively for inpatient rehabilitation at the Department of Rehabilitation of the First Affiliated Hospital of Wenzhou Medical University with a diagnosis of ischemic stroke from January 2015 to December 2015 were enrolled. The following demographic data were collected: age, sex, body mass index, and history of risk factors. The levels of 25(OH)D and the presence of deep vein thrombosis (DVT) by routine duplex imaging were also recorded. The value of 25(OH)D needed to predict DVT was analyzed using logistic regression analysis, after adjusting for the possible confounders. We found that 80% of patients admitted to the acute inpatient rehabilitation unit had low levels of vitamin D. Forty-seven patients had DVT, and the incidence of DVT was 26.1% within 3 weeks after the stroke event. With all patients taken together, DVT occurrence as a dependent variable with the sufficient group as the reference used for vitamin D levels in the logistic analysis, deficiency of vitamin D was independently associated with the development of DVT (odds ratio = 4.683, 95% confidence interval: 1.396-15.703, P = .012). In conclusion, low serum 25(OH)D levels were independent predictors of DVT in patients with ischemic stroke during inpatient rehabilitation stay in China. This finding reveals the critical role played by 25(OH)D in the pathogenesis of DVT.
Keywords: ischemic stroke, deep venous thrombosis, active vitamin D
Introduction
There is considerable interest in the role vitamin D plays in human health. Vitamin D deficiency, defined as a plasma 25-hydroxyvitamin D (25(OH)D) level ≤20 ng/mL, is highly prevalent, with an incidence of about 30% to 50% worldwide.1–3 A low level of vitamin D is linked to an increased risk of cardiovascular diseases and mortality.2,4–6 The potential effect of vitamin D on the cardiovascular system has been elucidated. Moreover, it has been shown that 1,25-dihydroxyvitamin D (1,25(OH)2D) exerts anticoagulant effects in patients with leukemia through the upregulation of thrombomodulin and downregulation of tissue factors (TFs) in monocytes and myelogenous cells.7 It has also been demonstrated that vitamin D receptors may play an important role in thrombosis.8,9 In addition, it has been demonstrated that an increase in 25(OH)D levels reduces the risk of venous thromboembolism (VTE).10 The use of 1,25(OH)2D has led to a decrease in deep venous thrombosis (DVT) incidence among patients with prostate cancer.11
Deep venous thrombosis is a common complication in patients admitted to the hospital after a stroke. Studies from Western societies have indicated that DVT occurred in up to 80% of patients with ischemic stroke who did not receive prophylactic therapy.12 In general, studies have estimated the overall prevalence of clinically evident DVT after acute stroke to be around 2% to 20%.13,14 Data from the Clots in Legs Or sTockings after Stroke trials, the largest observational report to date, evaluated 5632 immobile patients with acute stroke using duplex ultrasound. The results showed a detection of DVTs within 10 days of enrollment in 11% of the study participants and within 30 days in 15% of the study participants.15 A recent study in an Asian cohort found a high frequency of DVT following acute stroke, which was similar to findings in studies of Caucasian patients.16 Deep venous thromboses can lead to postphlebitic syndrome in the legs and varicose ulcers, in addition to delaying rehabilitation. However, limited information is available regarding the nature of the relationship between vitamin D and DVT in patients with ischemic stroke. Thus, the purpose of this study was to investigate whether vitamin D serum levels, which were measured upon admission, could be a risk factor for DVT in Chinese patients with ischemic stroke during inpatient rehabilitation.
Materials and methods
The study population comprised 180 consecutive patients with a diagnosis of ischemic stroke, who had been referred to the First Affiliated Hospital of Wenzhou Medical University for inpatient rehabilitation from January 2015 to December 2015. In the study patients, the diagnosis of stroke, defined as an acute event of cerebrovascular origin causing focal or global dysfunction lasting >24 hours, was confirmed by both clinical and radiographic evaluation. All patients who were discharged from the Department of Neurology were subsequently admitted to an inpatient rehabilitation unit after completing the stroke treatment. The assessment for DVT occurrence was performed 2 to 3 days after admission by compression ultrasound (CUS) before rehabilitation. Patients were enrolled if they met the following criteria: older than 18 years and had an ischemic stroke within the prior 30 days; modified Rankin Scale score ≥2 before enrollment; weakness in the lower limbs and an NIH Stroke Scale score of ≥1 on item VI; and able to obtain consent from the patient’s legal representative. The exclusion criteria were as follows: presence of transient ischemic attack, subarachnoid hemorrhage, brain tumor, or cerebral venous thrombosis; history of VTE; use of unfractionated heparin or low-molecular-weight heparin; or use of any form of oral anticoagulant in the prior month. The patients who used anticoagulant drugs for the prevention or treatment of venous thrombosis or atrial fibrillation after stroke will be excluded in order to avoid the effects of anticoagulant drugs. The control cases (n = 99) were of similar ages and sex distribution as the patients with ischemic stroke. The control cases had no known diseases and were not using any medications.
At baseline, the demographic data including age and sex, the body mass index, and history of risk factors (hypertension, diabetes mellitus, atrial fibrillation, hyperlipidemia, smoking habit, and alcohol abuse) were obtained. For each patient, the time from stroke onset to hospital admission was also recorded. Upon admission, the neurological deficits were quantified using the National Institutes of Health Stroke Scale (NIHSS). All patients received treatment according to the current guidelines.
The included patients were assessed for DVTs using color Doppler ultrasonography (Phillips Advanced Technology Laboratory 5000 device with a 7.5-MHz linear probe, Amsterdam, Netherlands), regardless of whether they were symptomatic or asymptomatic. We performed color Doppler ultrasonography 2 to 3 days after admission, but before systematic rehabilitation (about 14-21 days after stroke). Two areas of the leg were examined: the common femoral vein at the inguinal ligament and the popliteal vein at the knee joint line traced down to the point of the trifurcation of the calf veins. The diagnosis of DVT was based on either the presence of an incompressible segment (CUS test) or flow impairment on color Doppler imaging.
Fasting venous blood samples were collected from all participants in Vacutainer tubes (Becton-Dickinson, Franklin Lakes, New Jersey) and were quickly centrifuged to avoid glycolysis. Plasma levels of 25(OH)D, the commonly used marker of vitamin D status, was measured by a chemiluminescence immunoassay in a blinded fashion. The 25(OH)D levels ≤20 ng/mL were regarded as vitamin D deficiency, concentrations between 21 and 29 ng/mL were deemed as vitamin D insufficiency, and levels ≥30 ng/mL were considered as vitamin D sufficiency.1,5 Participants gave their written informed consent for blood samples to be obtained for measuring laboratory markers, and the study was approved by our local ethics committee at the First Affiliated Hospital of Wenzhou Medical University.
Data were presented as percentages or frequencies for discrete variables and as medians (interquartile range) for continuous variables. The Mann-Whitney U test, 1-way analysis of variance (ANOVA), Student t test, χ2 test, and Fisher exact test were carried out for comparisons between groups, as appropriate. When ANOVA showed significant differences between the groups, the post hoc Tukey test was used to assess differences in 2-group comparisons. Bonferroni corrections were applied to each test to adjust for multiple testing. Binary logistic regression including all factors significantly different in the univariate analysis was performed to determine significant risk factors for the development of DVT. P Values less than .05 were regarded as statistically significant. All analyses were performed by using SPSS version 19.0 software.
Results
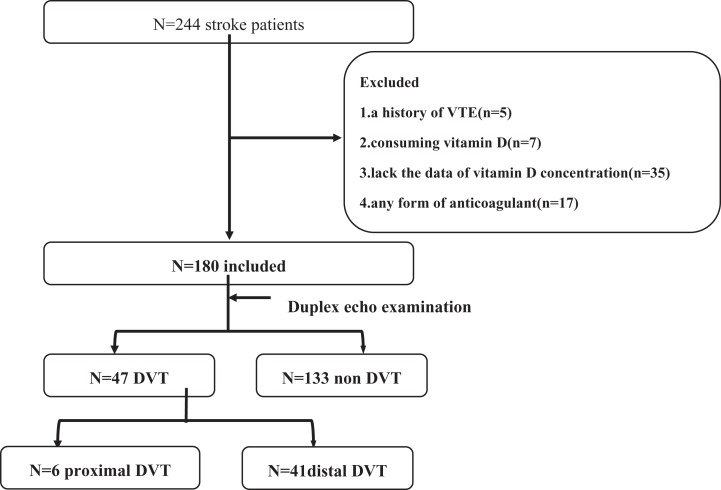
Sixty-four patients were excluded from the analysis based on the exclusion criteria. Data from 180 patients were thus included in the analysis (Figure 1). Table 1 shows the baseline characteristics of the 180 eligible patients (demographics, clinical features, lower limb paresis, varicosities, and presence or absence of DVT). The mean (SD) age of the patients was 64.83 (10.95) years, and 36.1% were women. Forty-seven patients had a DVT, and the incidence of DVT was 26.1% within 3 weeks after the stroke event. Forty-one patients displayed a distal DVT in this study. Among the crural veins, the soleal vein was particularly important as an initial site of the DVTs. The mean (SD) vitamin D level for all patients with stroke was 55.90 (24.84) nmol/L. The mean (SD) vitamin D levels in normal participants, non-DVT patients, and patients with DVT were 64.70 (19.57), 58.35 (25.39), and 48.99 (22.06) nmol/L, respectively. These results are similar to the findings of a previous study in an Asian population.17
Figure 1.
Patient flowchart.
Table 1.
Clinical and Demographic Characteristics of the Samples Under Study.a
| Variables | Patients With Ischemic Stroke (n = 180) | Normal Controls (n = 99) | P Value | |
|---|---|---|---|---|
| DVT Group (n = 47) | Non-DVT Group (n = 133) | |||
| Demographic characteristics | ||||
| Age (years), means (SD) | 68.02 (7.86)b,c | 63.35 (11.51) | 63.02 (12.60) | .007 |
| Sex (female/male) | 23/24b,c | 92/41 | 63/36 | .046 |
| BMI (kg/m2), means (SD) | 23.65 (3.54) | 23.71 (3.36) | .923 | |
| Vascular risk factors | ||||
| Current smoking | 10 (21.3)b | 50 (37.6) | .041 | |
| History of hypertension | 40 (85.1)b | 91 (68.4) | .027 | |
| History of diabetes | 21 (44.7)b | 34 (25.6) | .014 | |
| History of hyperlipidemia | 23 (48.9) | 70 (52.6) | .663 | |
| Atrial fibrillation | 1 (2.1) | 8 (6.4) | .267 | |
| Clinical features | ||||
| Course (days), median (IQR) | 19 (16-25) | 18 (16-22) | .142 | |
| NIHSS scores, median (IQR) | 9 (7-12) | 8 (6-11) | .222 | |
| Lower limb NIHSS score ≥2 | 29 (61.7)b | 50 (37.6) | .004 | |
| MBI scores, median (IQR) | 35 (20-45) | 35 (20-50) | .820 | |
| Varix of lower limb | 22 (46.8)b | 25 (18.8) | .013 | |
| Laboratory results | ||||
| PLT (×109/L), means (SD) | 252 (83) | 249 (85) | .813 | |
| HCT (L/L), means (SD) | 0.38 (0.03) | 0.39 (0.04) | .257 | |
| FIB (g/L), means (SD) | 4.08 (0.37) | 3.88 (1.28) | .383 | |
| Vitamin D (nmol/L), means (SD) | 48.99 (22.06)b,c | 58.35 (25.39) | 64.70 (19.57) | .001 |
Abbreviations: BMI, body mass index; DVT, deep vein thrombosis; FIB, fibrinogen; HCT, hematocrit; IQR, interquartile range; MBI, modified Barthel Index; NIHSS, National Institutes of Health Stroke Scale; PLT, platelet; SD, standard deviation.
a Values are shown as number (percentage) except where indicated.
b P < .05 compared with non-DVT.
c P < .05 compared with normal controls.
A significant intergroup difference in serum vitamin D levels within 24 hours of admission was found (P = .001). Indeed, serum vitamin D was significantly lower in patients who had a stroke, with or without DVT, than it was in the normal controls. Serum vitamin D levels were significantly lower in the DVT group than in the non-DVT group. The DVT group had more conventional vascular risk factors such as current smoking status, hypertension, diabetes mellitus, poor lower limb function, and varicosity. Patients in the DVT group were also more likely to be female and of older age.
The 25(OH)D levels were categorized into 3 groups: (1) sufficient group: 25(OH)D ≥ 30 ng/mL (≥75 nmol/L), (2) insufficient group: 25(OH)D = 21 to 29 ng/mL (50-75 nmol/L), and (3) deficient group: 25(OH)D ≤ 20 ng/mL (≤50 nmol/L). Deficiency of 25(OH)D was the most frequently occurring 25(OH)D status in both the DVT and the non-DVT groups among patients with ischemic stroke (59.57% vs 35.33%, respectively). Whereas, a sufficient 25(OH)D level was the least frequently occurring status in the DVT group compared to that in the non-DVT group (8.51% vs 24.06%, respectively). Accordingly, the number of participants in the 25(OH)D subgroups was found to be significantly different between the DVT group and non-DVT group (P = .008; Table 2).
Table 2.
Vitamin D Status of Patients.a
| Variables | DVT Group (n = 47) | Non-DVT Group (n = 133) | χ2 | P Value |
|---|---|---|---|---|
| Vitamin D status | 9.778 | .008 | ||
| Deficient | 28 (59.6) | 47 (35.3) | ||
| Insufficient | 15 (31.9) | 54 (40.6) | ||
| Sufficient | 4 (8.5) | 32 (24.1) | ||
aValues are shown as numbers (percentage). The 25(OH)D levels were categorized into 3 groups: (1) sufficient group, 25(OH)D ≥ 30 ng/mL (≥75 nmol/L); (2) insufficient group, 25(OH)D = 21 to 29 ng/mL (50-75 nmol/L); and (3) deficient group, 25(OH)D ≤ 20 ng/mL (≤50 nmol/L).
With all patients taken together, DVT occurrence as a dependent variable with the sufficient group as the reference used for vitamin D levels in the logistic regression model, deficiency of vitamin D was independently associated with the development of DVT (odds ratio [OR] = 4.683, 95% confidence interval [CI]: 1.396-15.703, P = .012). However, older age, lower limb NIHSS score ≥2, and varix of lower limb were each associated with a greater risk of developing DVT after ischemic stroke (Table 3). In another logistic analysis, no interactions were found between age, lower limb NIHSS score ≥2, and varix of lower limb and the levels of vitamin D.
Table 3.
Multivariate Logistic Model of the Clinical Determinants of DVT in Ischemic Stroke Patients.
| Variables | OR (95% CI) | P Value |
|---|---|---|
| Vitamin D | .029 | |
| Sufficient (reference) | ||
| Insufficient | 1.875 (0.512-6.870) | .165 |
| Deficient | 4.683 (1.396-15.703) | .012 |
| Age | 1.817 (1.212-2.722) | .004 |
| Sex | 1.662 (0.646-4.071) | .55 |
| Current smoking | 0.606 (0.214-1.718) | .067 |
| History of hypertension | 2.330 (0.824-6.585) | .052 |
| History of diabetes | 1.674 (0.746-3.757) | .128 |
| Varix of lower limb | 2.778 (1.269-6.084) | .011 |
| Lower limb NIHSS score ≥ 2 | 2.322 (1.096-4.921) | .028 |
Abbreviations: CI, confidence interval; DVT, deep vein thrombosis; NIHSS, National Institutes of Health Stroke Scale; OR, odds ratio.
Discussion
To the best of our knowledge, this is the first report exploring the possible association between serum vitamin D levels and the development of venous thromboembolic events in patients with ischemic stroke during an inpatient rehabilitation stay. This finding is compatible with the various large-scale studies that have reported findings of vitamin D deficiency as a risk factor for thrombosis.10,18
In the present study, 26.1% of patients with ischemic stroke presented with a DVT within 3 weeks poststroke, which is higher than that in previous studies.15,19 Possible reasons for this may be based on the fact that most of the research comes from the neurology department, where the DVTs were diagnosed by ultrasonography within 2 weeks of the stroke onset. All patients in this study were admitted to receive inpatient rehabilitation after completing treatment for the stroke and were followed from admission to discharge. We performed color Doppler ultrasonography about 14 to 21 days after stroke, but prior to systematic rehabilitation. A recent study detected lower extremity DVT in 34% of patients, and 23% of patients displayed isolated calf vein thrombosis among patients admitted to inpatient rehabilitation programs.20 All patients were admitted to the hospital for systematic rehabilitation training because of poor functioning. The severity of leg weakness also increased the risk of poststroke DVT, as expected.14,15,19 Therefore, this study excludes the patients who used anticoagulant drugs for the prevention or treatment of venous thrombosis after stroke. Estimates of frequency in cohorts and trials vary widely and depend on the characteristics of patients and the timing and method of screening and treatment.
Serum levels of vitamin D within 24 hours of admission were found to be significantly lower in stroke patient groups than in normal controls, which is consistent with findings of previous studies, which include the Asian population.17,21 An association between low vitamin D levels and the development of DVT was also found. In a large study comprising 18 791 participants with a 30-year follow-up, decreased 25(OH)D levels during the seasonal change from summer to winter led to an increase in the risk of VTE adjusted for age, sex and other factors.22 One cohort study consisted of patients with idiopathic DVT, in which the effects of other factors on DVT incidence were excluded. The results showed that the level of 25(OH)D was significantly lower in patients who had idiopathic DVT compared to the controls (P = .004).23 Vitamin D receptor−/− mice, which display vitamin D deficiency, have increased thrombogenic activity.24 Recently, vitamin D has been found to play an important role in the pathogenesis of DVT of the lower extremity secondary to other diseases. A negative correlation between low vitamin D levels and the presence of DVT has also been found in patients with a spinal cord injury during acute inpatient and rehabilitation stays.25 Similarly, low serum vitamin D levels are common in primary antiphospholipid syndrome (PAPS) with thrombotic disease, which may suggest that the lack of vitamin D could be one of the many factors involved in the thrombotic process.26 Moreover, mounting evidence has shown a high prevalence of vitamin D deficiency during pregnancy.27 Some experts have suggested that hypovitaminosis D should represent a potentially modifiable risk factor to be targeted at the beginning of and during pregnancy to prevent thromboembolic complications.28 In addition, it has been demonstrated that an increase in 25(OH)D levels reduces the risk of VTE.10 A randomized controlled study of <250 patients with prostate cancer, half of whom were given 45 mg of 1,25(OH)2D<weekly, found an unexpected, significantly lower risk of thrombotic events (2 vs 11).11 The same result also was found in kidney disease.29
Whether low vitamin D has a role in VTE, that is, venous thrombosis and pulmonary embolism, is largely unexplored. The Tromsø study, involving 6021 participants during a 10-year follow-up, demonstrated that there was no correlation between VTE and levels of 25(OH) D (28 vs 18 ng/mL). They also concluded that vitamin D deficiency did not have a pathological role in the development of VTE.30 Low levels of 25(OH)D were not a risk factor for VTE in African Americans over Caucasians in the Atherosclerosis Risk in Communities Study.31 However, along with lower mean levels of 25(OH)D, recent evidence suggests that African Americans also tend to have lower levels of vitamin D-binding protein, and hence, the mean levels of bioavailable vitamin D are similar to those in Caucasians.32 The results from the Multiple Environmental and Genetic Assessment (MEGA) case–control study indicate that generalized multivitamin use (which includes vitamin D) yielded a 37% lower risk of venous thrombosis than no vitamin use (OR = 0.63; 95% CI: 0.57-0.70) when comparing patients with random-digit dialing controls. However, when patients were compared with partner controls, ORs attenuated to unity, which means that vitamin supplementation was no longer associated with a decreased risk of venous thrombosis.33 However, owing to the lack of data about vitamin status before or after vitamin consumption, whether some vitamins would be beneficial against venous thrombosis in individuals with vitamin deficiency at baseline could not be evaluated. In addition, a study of PAPS patients discovered that low doses of vitamin D (average 400 UI/d) were insufficient to enable systemic levels to rise above 30 ng/mL.26 A similar observation was made in patients with systemic lupus erythematosus who received an even higher dosage (800 UI/ d). After supplementation, 70% of participants still had insufficient levels of vitamin D.34 All of these factors may have an effect on whether or not vitamin supplementation is associated with a decrease in venous thrombosis risk.
Vitamin D is produced through diet and ultraviolet irradiation and is converted to 25(OH)D in the liver, which is clinically used for evaluating vitamin D status.1 Currently, the potential effect of vitamin D on the cardiovascular system has been elucidated. Moreover, the association of vitamin D deficiency and the incidence of thromboembolism has been described in various studies.10,11,18,35 The main suggested mechanisms for the antithrombotic properties of vitamin D include the “upregulation of thrombomodulin” and the “downregulation of TF.”35,36 On the other hand, vitamin D can upregulate and increase the level of the anti-inflammatory cytokine interleukin 10.37,38 In addition, atherosclerosis is associated with the development of thrombosis as a result of platelet activation and consequent thrombotic adverse events.39 It has been demonstrated that vitamin D’s anticoagulant properties play a role in atherothrombosis.7–9,36
There are several limitations in our study. First, there is increasing evidence in terms of the seasonal effects on 25(OH)D levels. It has been demonstrated that vitamin D concentration is at its lowest in the winter months compared to summer.40 On the other hand, a recent study has found that an increase in sunbathing and consequent elevated vitamin D levels contributes to a decrease in the risk of VTE.10 However, in our study, we could not investigate the seasonal impact on DVT incidence because the patients in our study generally had to limit their exposure to the sun because of poor limb function. Second, patients included in the study had a high NIHSS score or a poor limb function, which might make us overestimate the actual incidence of DVT after ischemic stroke and may impose some bias to the results. Third, subclinical DVT may have been present prior to the ischemic stroke event. In such cases, reverse causality cannot be excluded, as vitamin D levels were measured after occurrence of the stroke event. Therefore, the small sample size of our study was another restriction—large-scale studies are needed to clarify the relationship between vitamin D and DVT among patients after experiencing a stroke.
In conclusion, our study demonstrates an important association between serum vitamin D levels at the time of admission and the incidence of DVT after ischemic stroke. Moreover, further prospective studies and randomized controlled trials are needed to establish determinants and the probable causative role of 25(OH)D in DVT, irrespective of whether it is primary or secondary to other diseases.
Acknowledgments
The authors thank the staff and participants of the study for their important contributions.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by a grant from Wenzhou Municipal Sci-Tech Bureau Program (Y20150028). These sources had no further role in study design, data collection and analysis, decision to publish, or preparation of the article.
References
- 1. Holick MF. Vitamin D deficiency. Nederlands Tijdschrift Voor Geneeskunde. 2006;150(23):1315–1316. [PubMed] [Google Scholar]
- 2. Anderson JL, May HT, Horne BD, et al. ; Intermountain Heart Collaborative (IHC) Study Group. Relation of vitamin D deficiency to cardiovascular risk factors, disease status, and incident events in a general healthcare population. Am J Cardiol. 2010;106(7):963–968. [DOI] [PubMed] [Google Scholar]
- 3. Vacek JL, Vanga SR, Good M, Lai SM, Lakkireddy D, Howard PA. Vitamin D deficiency and supplementation and relation to cardiovascular health. Am J Cardiol. 2012;109(3):359–363. [DOI] [PubMed] [Google Scholar]
- 4. Wang TJ, Pencina MJ, Booth SL, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;17(4):503–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lee JH, O’Keefe JH, Bell D, Hensrud DD, Holick MF. Vitamin D deficiency an important, common, and easily treatable cardiovascular risk factor? J Am Coll Cardiol. 2008;52(24):1949–1956. [DOI] [PubMed] [Google Scholar]
- 6. Pilz S, Tomaschitz A, März W, et al. Vitamin D, cardiovascular disease and mortality. Clin Endocrinol (Oxf). 2011;75(5):575–584. [DOI] [PubMed] [Google Scholar]
- 7. Koyama T, Shibakura M, Ohsawa M, Kamiyama R, Hirosawa S. Anticoagulant effects of 1alpha,25-dihydroxyvitamin D3 on human myelogenous leukemia cells and monocytes. Blood. 1998;92(1):160–167. [PubMed] [Google Scholar]
- 8. Wu-wong JR, Nakane M, Ma J. Vitamin D analogs modulate the expression of plasminogen activator inhibitor-1, thrombospondin-1 and thrombomodulin in human aortic smooth muscle cells. J Vas Res. 2007;44(1):11–18. [DOI] [PubMed] [Google Scholar]
- 9. Aihara K, Azuma H, Akaike M, et al. Disruption of nuclear vitamin D receptor gene causes enhanced thrombogenicity in mice. J Biol Chem. 2004;279(34):35798–35802. [DOI] [PubMed] [Google Scholar]
- 10. Lindqvist PG, Epstein E, Olsson H. Does an active sun exposure habit lower the risk of venous thrombotic events? A D-lightful hypothesis. J Thromb Haemost. 2009;7(4):605–610. [DOI] [PubMed] [Google Scholar]
- 11. Beer TM, Venner PM, Ryan CW, et al. High dose calcitriol may reduce thrombosis in cancer patients. Br J Haematol. 2006;135(3):392–394. [DOI] [PubMed] [Google Scholar]
- 12. Johnston KC, Li JY, Lyden PD, et al. Medical and neurological complications of ischemic stroke: experience from the RANTTAS trial. RANTTAS Investigators. Stroke. 1998;29(2):447–453. [DOI] [PubMed] [Google Scholar]
- 13. Kamran SI, Downey D, Ruff RL. Pneumatic sequential compression reduces the risk of deep vein thrombosis in stroke patients. Neurology. 1998;50(6):1683–1688. [DOI] [PubMed] [Google Scholar]
- 14. Kelly J, Rudd A, Lewis RR, Coshall C, Moody A, Hunt BJ. Venous thromboembolism after acute ischemic stroke: a prospective study using magnetic resonance direct thrombus imaging. Stroke. 2004;35(10):2320–2325. [DOI] [PubMed] [Google Scholar]
- 15. Dennis M, Mordi N, Graham C, Sandercock P; CLOTS Trials Collaboration. The timing, extent, progression and regression of deep vein thrombosis in immobile stroke patients: observational data from the CLOTS multicenter randomized trials. J Thromb Haemost. 2011;9(11):2193–2200. [DOI] [PubMed] [Google Scholar]
- 16. De Silva DA, Pey HB, Wong MC, Chang HM, Chen CP. Deep vein thrombosis following ischemic stroke among Asians. Cerebrovasc Dis. 2006;22(4):245–250. [DOI] [PubMed] [Google Scholar]
- 17. De Silva DA, Talabucon LP, Ng EY, Ang ES, Tan EK, Lee WL. Vitamin D deficiency and its relation to underlying stroke etiology in ethnic Asian ischemic stroke patients. Int J Stroke. 2013;8(5):E18. [DOI] [PubMed] [Google Scholar]
- 18. Agmon-Levin N, Blank M, Zandman-Goddard G, et al. Vitamin D: an instrumental factor in the anti-phospholipid syndrome by inhibition of tissue factor expression. Ann Rheum Dis. 2011;70(1):145–150. [DOI] [PubMed] [Google Scholar]
- 19. Liu LP, Zheng HG, Wang DZ, et al. Risk assessment of deep-vein thrombosis after acute stroke: a prospective study using clinical factors. CNS Neurosci Ther. 2014;20(5):403–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sachdev U, Teodorescu VJ, Shao M, et al. Incidence and distribution of lower extremity deep vein thrombosis in rehabilitation patients: implications for screening. Vasc Endovascular Surg. 2006;40(3):205–211. [DOI] [PubMed] [Google Scholar]
- 21. Poole KE, Loveridge N, Barker PJ, et al. Reduced vitamin D in acute stroke. Stroke. 2006;37(1):243–245. [DOI] [PubMed] [Google Scholar]
- 22. Brøndum-Jacobsen P, Benn M, Tybjaerg-Hansen A, Nordestgaard BG. 25-Hydroxyvitamin D concentrations and risk of venous thromboembolism in the general population with 18,791 participants. J Thromb Haemost. 2013;11(3):423–431. [DOI] [PubMed] [Google Scholar]
- 23. Khademvatani K, Seyyed-Mohammadzad MH, Akbari M, Rezaei Y, Eskandari R, Rostamzadeh A. The relationship between vitamin D status and idiopathic lower-extremity deep vein thrombosis. Int J Gen Med. 2014;7:303–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wu-Wong JR. Are vitamin D receptor activators useful for the treatment of thrombosis? Curr Opin Investig Drugs. 2009;10(9):919–927. [PubMed] [Google Scholar]
- 25. Timmerman MA, Crew J, Shem K, et al. Low Vitamin D levels in persons with spinal cord injury and increased incidence of venous thromboembolic events during acute inpatient and rehabilitation stay. PM R. 2013;5(9):S140. [Google Scholar]
- 26. Piantoni S, Andreoli L, Allegri F, Meroni PL, Tincani A. Low levels of vitamin D are common in primary antiphospholipid syndrome with thrombotic disease. Reumatismo. 2012;64(5):307–313. [DOI] [PubMed] [Google Scholar]
- 27. van der Meer IM, Karamali NS, Boeke AJ, et al. High prevalence of vitamin D deficiency in pregnant non-Western women in The Hague, Netherlands. Am J Clin Nutr. 2006;84(2):350–353. [DOI] [PubMed] [Google Scholar]
- 28. Mascitelli L, Grant WB, Goldstein MR. The role of hypovitaminosis D in pregnancy-related venous thromboembolism. Int J Clin Pract. 2013;67(1):97. [DOI] [PubMed] [Google Scholar]
- 29. Moscarelli L, Zanazzi M, Bertoni E, et al. Renin angiotensin system blockade and activated vitamin D as a means of preventing deep vein thrombosis in renal transplant recipients. Clin Nephrol. 2011;75(5):440–450. [DOI] [PubMed] [Google Scholar]
- 30. Brodin E, Lerstad G. Serum levels of vitamin D are not associated with future risk of venous thromboembolism. The Tromsø Study. Thromb Haemost. 2013;109(5):885–890. [DOI] [PubMed] [Google Scholar]
- 31. Folsom AR, Roetker NS, Rosamond WD, et al. Serum 25-hydroxyvitamin D and risk of venous thromboembolism: the Atherosclerosis Risk in Communities (ARIC) study. J Thromb Haemost. 2014;12(9):1455–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Powe CE, Evans MK, Wenger J, et al. Vitamin D-binding protein and vitamin D status of black Americans and white Americans. N Engl J Med. 2013;369(21):1991–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vučković BA, Van RN, Cannegieter SC, Rosendaal FR, Lijfering WM. Vitamin supplementation on the risk of venous thrombosis: results from the MEGA case–control study. Am J Clin Nutr. 2015;101(3):606–612. [DOI] [PubMed] [Google Scholar]
- 34. Ruiz-Irastorza G, Gordo S, Olivares N, Egurbide MV, Aguirre C. Changes in Vitamin D levels in patients with systemic lupus erythematosus: effects on fatigue, disease activity, and damage. Arthritis Care Res (Hoboken). 2010;62(8):1160–1165. [DOI] [PubMed] [Google Scholar]
- 35. Koyama T, Hirosawa S. Anticoagulant effects of synthetic retinoids and activated vitamin D3 . Semin Thromb Hemost. 1998;24(3):217–226. [DOI] [PubMed] [Google Scholar]
- 36. Ohsawa M, Koyama T, Yamamoto K, Hirosawa S, Kamei S, Kamiyama R. 1alpha,25-dihydroxyvitamin D3 and its potent synthetic analogs downregulate tissue factor and upregulate thrombomodulin expression in monocytic cells, counteracting the effects of tumor necrosis factor and oxidized LDL. Circulation. 2000;102(23):2867–2872. [DOI] [PubMed] [Google Scholar]
- 37. Michel G, Gailis A, Jarzebska-Deussen B, Müschen A, Mirmohammadsadegh A, Ruzicka T. 1,25-(OH)2-vitamin D3 and calcipotriol induce IL-10 receptor gene expression in human epidermal cells. Inflamm Res. 1996;46(1):32–34. [DOI] [PubMed] [Google Scholar]
- 38. Schleithoff SS, Zittermann A, Tenderich G, Berthold HK, Stehle P, Koerfer R. Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: a double-blind, randomized, placebo-controlled trial. Am J Clin Nutr. 2006;83(4):754–759. [DOI] [PubMed] [Google Scholar]
- 39. Duggirala MK, Cook DA, Mauck KF. An association between atherosclerosis and venous thrombosis. N Engl J Med. 2003;349(4):401–402. [DOI] [PubMed] [Google Scholar]
- 40. Hovsepian S, Amini M, Aminorroaya A, Amini P, Iraj B. Prevalence of vitamin D deficiency among adult population of Isfahan City, Iran. J Health Popul Nutr. 2011;29(2):149–155. [DOI] [PMC free article] [PubMed] [Google Scholar]