Abstract
Due to variable pharmacokinetic properties, therapeutic anticoagulation with continuous unfractionated heparin (UFH) requires ongoing laboratory monitoring, generally with activated partial thromboplastin time (aPTT). In the ambulatory setting, clinicians who manage warfarin therapy often use time in the therapeutic range (TTR) to estimate a percentage of time the international normalized ratio is therapeutic. We applied the TTR concept to aPTT monitoring for therapeutic UFH and used 2 methodologies for estimation: percentage of aPTT values in range (%aIR) and a modification of the Rosendaal method (mod-Rosendaal). This study included adult inpatients admitted between September 30, 2015, and September 30, 2016, at Brigham and Women’s Hospital. For each patient, all available aPTT values were extracted to calculate 2 individual TTRs according to each methodology. Comparison between methods was performed using Student t test, and correlation was assessed with simple linear regression. A total of 255 patients were included in this study. The major outcome of TTR estimation was significantly higher using mod-Rosendaal (43.7% [26.5%]) versus %aIR (37.7% [25.7%], P = .012) by a mean difference of 6% points (95% confidence interval: 1.3-10.7). Time in the therapeutic range estimated by mod-Rosendaal significantly correlated with those estimated by %aIR (r = 0.84, P < .001). Further studies should evaluate the correlation between TTR and clinical outcomes and establish a benchmark for quality therapeutic anticoagulation with continuous UFH.
Keywords: anticoagulation, heparin, partial thromboplastin time
Background
Intravenous unfractionated heparin (UFH) infusions are commonly utilized in the hospital setting to achieve therapeutic anticoagulation for a variety of thrombotic and cardiovascular conditions. Its benefits include a rapid onset of action, short half-life, and reversibility; however, UFH exhibits pharmacokinetic properties which result in varied anticoagulant response among individuals.1 Clinical use of UFH, therefore, requires ongoing laboratory monitoring and dose titration to maintain efficacy of the anticoagulant effect and mitigate the risk of bleeding complications. Substantial controversy exists over the preferred assay to monitor UFH therapy. Compared to the activated partial thromboplastin time (aPTT) test, the antifactor Xa activity test is less susceptible to confounding by inflammatory proteins and lupus anticoagulant, and its sensitivity is not affected by choice of reagent.2 However, the aPTT is inexpensive, readily available, and quickly resulted—and ultimately remains the most widely used laboratory assay for UFH monitoring.2
Although UFH is one of the most frequently administered medications among hospitalized patients, there is no standardized method for assessing effectiveness of therapeutic dosing or compliance with nomogram titration. In comparison, measuring time in the therapeutic range (TTR) of the international normalized ratio (INR) is standard practice among ambulatory warfarin-treated patients to assess effectiveness of anticoagulation management. Maintaining tight INR control with appropriate warfarin dose intensity yields a high TTR, which has been associated with both optimal efficacy and safety.3,4
In warfarin-treated patients, 2 common methodologies used to estimate TTR are the percentage of INR values in range and a linear interpolation calculation proposed by Rosendaal et al.4,5 The percentage in range method is a simple fraction that divides the number of INR tests which fall within the defined therapeutic range by the total number of INR tests performed. Although this is an easy calculation, it fails to account for where INR values fall on days between collected lab samples. The Rosendaal calculation is the method most commonly used to report TTR in clinical trials. This method assigns an INR value for each day between collected lab values, assuming linear movement from one INR value to another.5 We speculated that these methodologies could be applied to aPTT monitoring for UFH.
The aim of this study was to compare 2 methods of TTR estimation to evaluate therapeutic anticoagulation quality for patients at our institution who received continuous UFH infusions. The setting of our study follows recent implementation of institution-wide, nurse-driven titration nomograms to standardize practice for UFH administration and improve time to reach therapeutic anticoagulation.6
Methods
This was a single-center retrospective analysis conducted at Brigham and Women’s Hospital, a 793-bed tertiary academic medical center located in Boston, Massachusetts. The study protocol was approved by the Partners HealthCare Institutional Review Board (Protocol #: 2016P001416). Patients were identified from computer-generated reports of active heparin orders. All adult inpatients who received a continuous UFH infusion between September 30, 2015, and September 30, 2016, were considered for inclusion. Depending on the indication, providers at our institution may order 1 of 2 hospital-approved nurse-titration nomograms targeting aPTT values of either 50 to 70 seconds or 60 to 80 seconds, or place a provider-driven custom order.6 Our institution-specific UFH nomograms and aPTT assay have previously been described in detail.6 Our aPTT assay uses a silica activator (PTT Automate; Diagnostica Stago Inc, Parsippany, New Jersey) run on the STA-R Evolution (Diagnostica Stago Inc). Patients were excluded if they received UFH for nontherapeutic anticoagulation or per a custom provider-driven order. Baseline patient characteristics, indication for anticoagulation, and documented lab values were collected from the electronic health record (EHR). The major outcome was percentage of TTR using the following methodologies: percentage of aPTT values in range (%aIR), calculated by (number of aPTT tests within therapeutic range/total number of aPTT tests performed) × 100; and a modified Rosendaal (mod-Rosendaal) linear interpolation method, which revised the original method to calculate time per minute rather than per day.5 For each patient, all available aPTT values were extracted to calculate 2 individual TTRs according to each methodology. After initial evaluation, we restricted the analysis to subgroups with a duration of therapy of at least 24 hours or with at least 4 aPTT tests documented to see how results were affected. Difference in TTR estimation between methodologies was assessed using a paired Student t test. Correlation was assessed with simple linear regression using Pearson r. P values <.05 were considered statistically significant.
Results
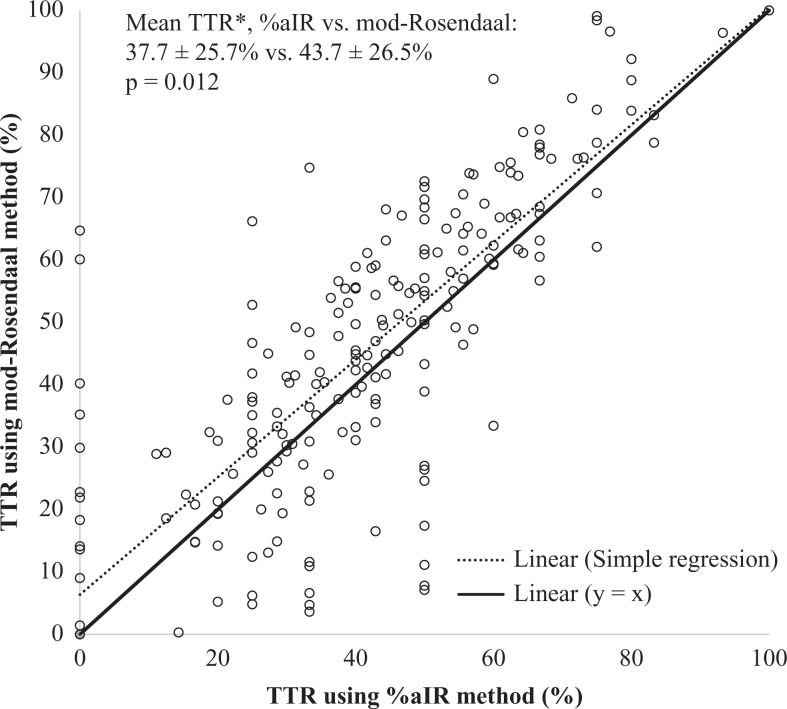
A total of 255 patients were included in this study. Baseline patient characteristics and indications for therapeutic anticoagulation with UFH are described in Table 1. The major outcome of TTR% was significantly higher using mod-Rosendaal (43.7% [26.5%]) versus %aIR (37.7% [25.7%], P = .012) by a mean difference of 6% points (95% confidence interval, 1.3-10.7; Table 2). Time in the therapeutic ranges estimated by mod-Rosendaal significantly correlated with those estimated by %aIR (r = 0.84, P < .001; Figure 1).
Table 1.
Patient Characteristics.
| Characteristic | Population (N = 255) |
|---|---|
| Median age (year; IQR) | 66 (56-74) |
| Male sex (no.; %) | 144 (56.5) |
| Weight (kg)a | 83.3 (23.1) |
| Body mass indexa | 29.1 (7.8) |
| Median SCr (mg/dL; IQR) | 1.05 (0.81-1.51) |
| ICU admission (no.; %) | 20 (7.8) |
| Ethnicity | |
| White | 210 (82.4) |
| Black | 20 (8.9) |
| Other | 16 (6.3) |
| Medical history (no.; %) | |
| Previous VTE | 54 (21.2) |
| Active cancer | 63 (24.7) |
| Previous ACS | 77 (30.2) |
| Previous stroke | 32 (12.5) |
| Home anticoagulant (no.; %) | 101 (39.6) |
| Warfarin | 59 (23.1) |
| Dabigatran | 3 (1.2) |
| Rivaroxaban | 10 (3.9) |
| Apixaban | 10 (3.9) |
| Enoxaparin | 17 (6.7) |
| Fondaparinux | 2 (0.8) |
| Baseline coagulation labs | |
| aPTT (seconds)a | 33.9 (6.5) |
| Hemoglobin (g/dL)a | 11.4 (2.3) |
| Hematocrit (%)a | 35.1 (6.5) |
| Indication for UFHb (no.; %) | |
| VTE | 91 (35.7) |
| ACS | 80 (31.4) |
| Stroke prevention in AF | 51 (20.0) |
| Stroke | 9 (3.5) |
| Otherc | 46 (18.0) |
| Nomogram goal (no.; %) | |
| 50 to 70 seconds | 77 (30.2) |
| 60 to 80 seconds | 178 (69.8) |
| Initial UFH dose (units/kg)a | 15.6 (2.9) |
| Received initial bolus UFH dose (no; %) | 165 (64.7) |
| 60 units/kg | 56 (22.0) |
| 80 units/kg | 98 (38.4) |
| Other | 11 (4.3) |
| Maintenance UFH dosed (units/kg)a | 14.4 (3.8) |
| Median duration (hour; IQR) | 66 (34-144) |
| Median total aPTTs documented (no.; IQR) | 7 (3-14) |
Abbreviations: ACS, acute coronary syndrome; AF, atrial fibrillation; aPTT, activated partial thromboplastin time; ICU, intensive care unit; IQR, interquartile range; SCr, serum creatinine; UFH, unfractionated heparin; VTE, venous thromboembolism.
aValues signify mean (standard deviation [SD]).
bSome patients had multiple indications for UFH therapy and were counted more than once.
cThe most common indications in this category include history of mechanical valve replacement, prophylactic anticoagulation for hypercoagulable states, left ventricle thrombus, and critical limb ischemia.
dDefined as the dose when the patient achieved ≥2 consecutive aPTT results in therapeutic range.
Table 2.
Estimation of TTR by Method.
| Population | %aIRa | mod-Rosendaala | Mean Difference | CI | P Value |
|---|---|---|---|---|---|
| All patients | 37.7 (25.7) | 43.7 (26.5) | 6.0 | 1.3 to 10.7 | .012 |
| ICU admission | 39.6 (16.6) | 41.2 (18.7) | 1.6 | −5.9 to 9.2 | .66 |
| Non-ICU admission | 38.8 (26.4) | 43.9 (27.1) | 5.1 | 2.3 to 6.2 | <.001 |
| ≥24-hour duration | 42.3 (22.0) | 46.3 (25.0) | 4.0 | 2.2-5.8 | <.001 |
| ≥4 aPTTs documented | 41.9 (20.6) | 46.7 (23.5) | 4.8 | 3.0 to 6.5 | <.001 |
aValues signify mean (standard deviation [SD]).
Abbreviations: %aIR, percentage of activated partial thromboplastin time values in range; aPTT, activated partial thromboplastin time; CI, confidence interval; ICU, intensive care unit; mod-Rosendaal, modified Rosendaal method; SD, standard deviation; TTR, time in the therapeutic range.
Figure 1.
Correlation between TTR methods. Time in the therapeutic range (TTR) using mod-Rosendaal versus %aIR methods, correlation: r = 0.84, P < .001; linear regression: y (mod-Rosendaal) = 0.941 × (%aIR) + 6.634, r2 = 0.71, P < .001. Solid y = x line added as reference to perfect correlation. *Values signify mean (standard deviation [SD]). %aIR indicates percentage of activated partial thromboplastin time values in range; mod-Rosendaal, modified Rosendaal method.
Results were similar across different levels of patient acuity after stratification based on intensive care unit (ICU) admission versus non-ICU admission. Estimated TTRs were slightly higher after restricting the analysis to subgroups with a duration of therapy at least 24 hours or with at least 4 aPTT tests documented (Table 2).
Discussion
To the authors’ knowledge, this is the first study to perform estimations of TTR as a metric of therapeutic UFH quality. In previous studies of patients anticoagulated with warfarin, TTR has been validated as a surrogate measure of efficacy and safety, with the greatest clinical benefit seen when the INR is in therapeutic range at least 70% of the time.3 In this retrospective study of patients receiving continuous UFH, we identified an average TTR at our institution of approximately 40% using either methodology.
Maintenance of therapeutic aPTT is a known challenge of UFH therapy. In one retrospective study comprising patients receiving UFH for acute thrombosis, only 29% of patients who obtained a therapeutic aPTT result were able to maintain it for the next 2 consecutive measurements.7 Besides the pharmacokinetic limitations of UFH, patients who receive therapeutic UFH infusions are acutely ill and less clinically stable than outpatients taking oral anticoagulants. The acute nature of hospitalized management may also expose patients to inconsistencies in therapy such as bolus dose administration and prolonged or frequent interruptions. In the setting of these inconsistencies, the accuracy of aPTT draws is contingent upon appropriate timing since the last titration.
In warfarin-treated patients, studies assessing TTR using more than one methodology have found nonequivalent results.4,8–10 Similarly, both of our TTR strategies for monitoring UFH are prone to biases. For an ideal %aIR calculation, aPTT should be drawn at a regular frequency to accurately predict time. However, in clinical practice, the frequency of lab draws is increased at times when anticoagulation is unstable. Our institution-wide protocol specifies that aPTT draws should be performed every 6 hours until 2 consecutive results in therapeutic range are obtained, after which the frequency may be extended to every 12 hours. Presence of multiple out-of-range values drawn over a relatively short period of time skews the %aIR toward one direction. The mod-Rosendaal method is expected to be more accurate because it factors in time elapsed since the last test, thereby avoiding the effect of varied test frequency. However, its assumption of linear movement between aPTT values may pose a limitation, as UFH has a short half-life, and its anticoagulant effect is known to be nonlinear at therapeutic doses.1 Out-of-range aPTT results may normalize more quickly following dose adjustment than as predicted by linear interpolation. Thus, extreme deviations from the therapeutic range dramatically lower the overall TTR. Validity of the linear movement assumption may be even further diminished in higher acuity patients prone to more variable aPTT values, such as in the case of organ dysfunction and presence of inflammatory markers.
In this study, the mod-Rosendaal estimation captured significantly more time in range than the simpler %aIR estimation by 6% points. This aligns well with results from studies of warfarin-treated patients, which have reported modest underestimation of TTR using the percentage of INR values in range method by 5% to 6% points.4,8 This magnitude of difference is small and the association with clinical outcomes remains unknown. Additionally, we found that the 2 methods strongly correlated, which suggests that %aIR is a good predictor of the higher quality mod-Rosendaal TTR estimation. Due to the tedious calculations involved in the latter method, taking a simple %aIR may be sufficient for an institution to benchmark quality.
Our study has several limitations. First, due to the single-center and retrospective design, results are reflective of our institution-specific UFH protocols, and aPTT values were contingent upon accurate EHR documentation. Second, there may have been changes in a patient’s anticoagulation therapy throughout an admission, including interruptions. Third, some patients received UFH very briefly while ruling out venous thromboembolism or acute coronary syndrome. Due to this limitation, we restricted the analysis to subgroups with a duration of therapy of at least 24 hours or with at least 4 aPTT tests documented. However, TTRs improved only modestly to the mid-40% range, while maintaining an approximate 5% underestimation with the %aIR method. Finally, because a much larger sample size is required to compare very low incidence rates, as with thromboembolic and hemorrhagic events, we did not evaluate clinical event rates for this study in an attempt to correlate TTRs with efficacy or adverse events. Thus, we are unable to recommend use of these methods to predict clinical outcomes. For now, hospitals may optimize outcomes by practicing good adherence to standardized UFH titration nomograms. Institutions may also use internal quality assurance data to track alternative metrics which have been associated with clinical outcomes, such as time to therapeutic anticoagulation.11
Larger studies should seek to correlate TTR for UFH with clinical event rates and establish a quality benchmark for inpatients receiving continuous UFH infusions across institutions, as is commonly done with warfarin in the ambulatory setting. Due to the marked variability between institutions in UFH monitoring protocols, it may also be valuable to study TTR using antifactor Xa values rather than aPTT values. Data from our study suggest that although the mod-Rosendaal method yielded a higher TTR estimation, its strong correlation with %aIR supports this simpler alternative.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Katelyn W. Sylvester has participated on a scientific advisory board for Bristol Meyers Squibb/Pfizer.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Clara Ting  http://orcid.org/0000-0003-0376-6717
http://orcid.org/0000-0003-0376-6717
James W. Schurr  http://orcid.org/0000-0001-5330-4604
http://orcid.org/0000-0001-5330-4604
References
- 1. Hirsh J, Warkentin TE, Shaughnessy SG, et al. Heparin and low-molecular-weight heparin: mechanisms of action, pharmacokinetics, dosing, monitoring, efficacy, and safety. Chest. 2001;119(Suppl 1):64S–94S. [DOI] [PubMed] [Google Scholar]
- 2. Douxfils J, Tamigniau A, Chatelain B, Goffinet C, Dogné JM, Mullier F. Measurement of non-VKA oral anticoagulants versus classic ones: the appropriate use of hemostasis assays. Throm J. 2014;12:24 doi: 10.1186/1477-9560-12-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gallagher AM, Setakis E, Plumb JM, Clemens A, van Staa TP. Risks of stroke and mortality associated with suboptimal anticoagulation in atrial fibrillation patients. J Thromb Haemost. 2011;106(5):968–977. [DOI] [PubMed] [Google Scholar]
- 4. Wan Y, Heneghan C, Perera R, et al. Anticoagulation control and prediction of adverse events in patients with atrial fibrillation: a systematic review. Circ Cardiovasc Qual Outcomes. 2008;1(2):84–91. [DOI] [PubMed] [Google Scholar]
- 5. Rosendaal FR, Cannegieter SC, van der Meer FJ, Brit E. A method to determine the optimal intensity of oral anticoagulant therapy. J Thromb Haemost. 1993;69(3):236–239. [PubMed] [Google Scholar]
- 6. Schurr JW, Stevens CA, Bane A, et al. Description and evaluation of the implementation of a weight-based, nurse-driven heparin nomogram in a tertiary academic medical center. Clin Appl Thromb Hemost. 2018;24(2):248–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hylek EM, Regan S, Henault LE, et al. Challenges to the effective use of unfractionated heparin in the hospitalized management of acute thrombosis. Arch Intern Med. 2003;163(5):621–627. [DOI] [PubMed] [Google Scholar]
- 8. Caldeira D, Cruz I, Morgado G, et al. Is the time in therapeutic range using the ratio of tests equivalent to the Rosendaal method? Blood Coagul Fibrinolysis. 2015;26(8):972–976. [DOI] [PubMed] [Google Scholar]
- 9. Schmitt L, Speckman J, Ansell J. Quality assessment of anticoagulation dose management: comparative evaluation of measures of time-in-therapeutic range. J Thromb Thrombolysis. 2003;15(3):213–216. [DOI] [PubMed] [Google Scholar]
- 10. Biss TT, Avery PJ, Walsh PM, Kamali F. Comparison of time within therapeutic INR range with percentage INR within therapeutic range for assessing long-term anticoagulation control in children. J Thromb Haemost. 2011;9(5):1090–1092. [DOI] [PubMed] [Google Scholar]
- 11. Hull RD, Raskob GE, Brant RF, Pineo GF, Valentine KA. Relation between the time to achieve the lower limit of the APTT therapeutic range and recurrent venous thromboembolism during heparin treatment for deep vein thrombosis. Arch Intern Med. 1997;157(22):2562–2568. [PubMed] [Google Scholar]