Abstract
Background:
Antithrombin and recombinant human thrombomodulin (rhTM) are individually reported to improve survival in sepsis-induced disseminated intravascular coagulation (DIC). However, continuing controversy exists as to which agent is superior and whether concomitant therapy is superior to individual administration.
Methods:
This post hoc analysis included adult patients with sepsis-induced DIC from a nationwide multicenter registry database in Japan. We categorized patients into 4 groups: patients who received (1) individual administration of antithrombin, (2) individual administration of rhTM, (3) both, and (4) neither. In-hospital mortality was compared between every 2 groups among the 4 groups by Cox proportional hazards model adjusted with propensity scores.
Results:
In total, 1432 patients with sepsis-induced DIC were included. Although both antithrombin and rhTM were associated better outcome compared with no anticoagulants, mortality benefits were similar between each individual anticoagulant. Similarly, no significant difference in mortality was detected between individual administrations and concomitant therapy.
Conclusion:
Antithrombin and rhTM might have comparable efficacy in reducing mortality in patients with sepsis; however, concomitant therapy appeared to offer no additional survival benefit.
Keywords: anticoagulants, sepsis, retrospective studies, disseminated intravascular coagulation, combination
Background
Sepsis almost invariably complicates deranged blood coagulation disorders ranging from subclinical activation to distinct systemic dysfunction of blood coagulation, that is, disseminated intravascular coagulation (DIC). Sepsis-induced DIC plays a crucial role in inducing multiple organ dysfunction syndrome and is associated with an increased risk of death.1,2 Although the management of DIC should be directed primarily at the treatment of the underlying disorders, it is often difficult to control coagulation disorders in patients with sepsis-induced DIC. Therefore, anticoagulant therapy is considered to be effective as supportive therapy against DIC to improve clinical outcomes.3 Actually, much evidence based on previous observational studies and post hoc subgroup analyses of randomized controlled trials suggests that anticoagulant therapies may improve mortality in patients with sepsis-induced DIC.4–11
However, we have little knowledge about which anticoagulant is the most effective for sepsis-induced DIC. Antithrombin and recombinant human thrombomodulin (rhTM) are commonly used anticoagulant agents for sepsis-induced DIC and are often coadministered. According to the recent studies based on the Diagnosis Procedure Combination (DPC) database in Japan, 37.7% of patients treated with antithrombin were reported to receive coadministration of rhTM, and 45.9% of patients treated with rhTM were reported to receive coadministration of antithrombin.12,13 Both agents were reported to be associated with lower risk of death in sepsis-induced DIC by recent large-scale observational studies10,11; however, due to a lack of definitive clinical evidence, continuing controversy exists about which agent is superior and whether concomitant therapy is superior to individual administration.
The present study was designed to compare safety and efficacy between the individual administration of antithrombin or rhTM and to evaluate whether the concomitant administration of both agents over individual administration could reduce the rate of death in sepsis-induced DIC.
Materials and Methods
Study Population
This investigation was a post hoc subgroup analysis of a nationwide multicenter retrospective cohort study conducted in 42 intensive care units (ICUs) in Japan between January 2011 and December 2013.10 Patients were consecutively included in the registry if they were equal to or older than 18 years and diagnosed with having severe sepsis or septic shock according to the Sepsis-1 criteria proposed by the American College of Chest Physicians/Society of Critical Care Medicine (ACCP/SCCM) consensus conference in 1991.14 Among them, we included patients with sepsis-induced DIC in this study. The patients were considered to have DIC if they fulfilled the Japanese Association for Acute Medicine (JAAM) DIC criteria on the day of ICU admission.15
The exclusion criteria were as follows: use of warfarin/acetylsalicylic acid/thrombolytic therapy before study entry; history of fulminant hepatitis, decompensated liver cirrhosis, or other serious liver disorder; history of hematologic malignant disease; other conditions associated with coagulation disorder; treatment with any chemotherapy at study entry; and patients with missing data for main analysis.
This study followed the principles of the Declaration of Helsinki and was approved by the institutional review board of each participating hospital. Because of the anonymous and retrospective nature of this study, the board of each hospital waived the need for informed consent. This study was registered with the University Hospital Medical Information Network Clinical Trial Registry (UMIN-CTR ID: UMIN000012543).
Sepsis Definitions
In this study, “sepsis” meant severe sepsis and septic shock in the conventional criteria (sepsis-1). Severe sepsis was defined according to the definitions set and revised by the ACCP/SCCM consensus conference in 1991: combination with a suspected or proven infection, 3 or more signs of systemic inflammation, and more than 1 organ dysfunction. Septic shock was also defined according to the ACCP/SCCM consensus in 1991 as sepsis-induced hypotension persisting despite adequate fluid resuscitation and requiring catecholamine infusions to improve hemodynamic status.14
Data Collection
Patients were followed up until hospital discharge or death. A case report form was developed for the study, and the following information was obtained: age, sex, scores for illness severity on the day of ICU admission, source of ICU admission, preexisting comorbidities, primary source of infection, and therapeutic interventions such as immunoglobulin, low-dose steroid, renal replacement therapy, and low-dose heparin for prophylaxis against deep vein thrombosis (DVT). We evaluated the severity of illnesses according to the Acute Physiology and Chronic Health Evaluation II score and the Sequential Organ Failure Assessment (SOFA) score at study entry. The primary outcome measure was all-cause in-hospital mortality. We also recorded bleeding complications as secondary outcomes. Bleeding complications included the occurrence of intracranial hemorrhage, transfusion requirements related to bleeding, and bleeding requiring surgical intervention.
Patient Categorization
To compare the efficacy and safety between antithrombin, rhTM, and concomitant therapy with both agents, we categorized the study patients into 4 treatment groups: (1) patients who received individual administration of antithrombin (antithrombin group), (2) patients who received individual administration of rhTM (rhTM group), (3) patients who received both antithrombin and rhTM (concomitant group), and (4) patients who received neither agent (nonanticoagulant group). For study purposes, we compared the primary and secondary outcomes between every 2 groups among these 4 study groups.
Statistical Analysis
Due to the retrospective nature of the study, there were baseline imbalances between the groups; therefore, an adjusted analysis was performed using propensity scores.16–18 The propensity score for the likelihood of undergoing therapies that patients actually received was calculated using multivariable logistic regression analysis including age, sex, disease severity, source of ICU admission, medical history of severe conditions, new organ dysfunctions, types and volume of ICUs, primary source of infection, causal microorganisms, and other therapeutic interventions as covariates. The detailed combinations of the variables are described in Table S1. We compared the in-hospital mortality between every 2 groups among the 4 groups by Cox proportional hazards model adjusted with inverse probability of treatment weighting (IPTW) using the propensity score. For secondary outcomes of bleeding complications, we compared one group to every other group by logistic regression analysis adjusted by IPTW estimation.
Descriptive statistics are summarized as group medians with the first and third quartiles for continuous variables and frequencies with percentages for categorical variables. Univariate differences between groups were assessed using the Kruskal-Wallis test or χ2 test. All hypotheses were 2-sided, and a P value of <.05 indicated statistical significance. All statistical analyses were conducted using STATA Data Analysis and Statistical Software version 14.0 (StataCorp, College Station, Texas).
Results
Study Population and Categorizations
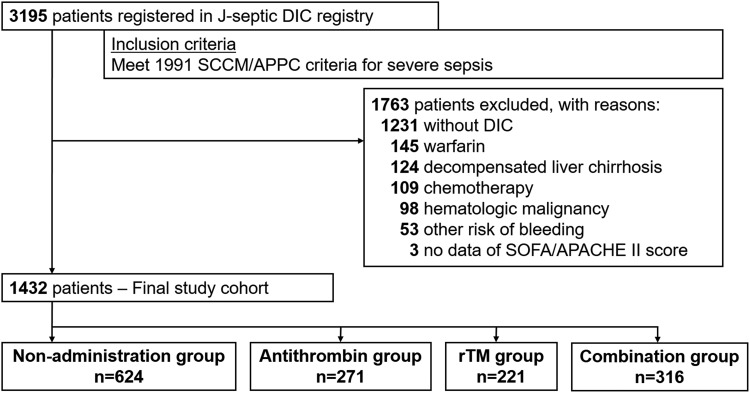
The patient flow diagram is shown in Figure 1. During the study period, 3195 consecutive patients fulfilled the inclusion criteria and were registered in the J-SEPTIC DIC registry database. After excluding 1763 patients without DIC and 352 patients who met at least 1 exclusion criterion, we included 1432 patients as the final study cohort. Among them, 271 (20.6%) patients received individual administration of antithrombin, 221 (14.4%) patients received individual administration of rhTM, 316 (20.6%) patients received both antithrombin and rhTM, and 624 (40.7%) received neither agent.
Figure 1.
Patient flow diagram. APACHE indicates Acute Physiology and Chronic Health Evaluation; DIC, disseminated intravascular coagulation; rTM, recombinant human thrombomodulin; SCCM/ACCP, Society of Critical Care Medicine/American College of Chest Physicians; SOFA, Sequential Organ Failure Assessment.
Baseline Characteristics
Table 1 shows the baseline characteristics of the study population categorized by the presence and types of anticoagulant therapy. There were no significant differences in age and sex distribution between the groups. Baseline disease severity indicated by SOFA and JAAM DIC scores were significantly different between the 4 groups and tended to be lower in the nonadministration group. We also found the percentage of concomitant use of therapeutic interventions other than anticoagulants, such as immunoglobulin, low-dose steroid, renal replacement therapy, and low-dose heparin for prophylaxis against DVT to be significantly different between the groups.
Table 1.
Baseline Characteristics of the 4 Groups.a
| Variable | Nonanticoagulant | Antithrombin | rhTM | Concomitant | P Value |
|---|---|---|---|---|---|
| n = 624 | n = 271 | n = 221 | n = 316 | ||
| Patient characteristics | |||||
| Age, years | 73 (63-81) | 72 (63-80) | 72 (62-78) | 72 (62-80) | .424 |
| Sex, male | 353 (56.6%) | 152 (56.1%) | 122 (55.2%) | 171 (54.1%) | .907 |
| Illness severity | |||||
| APACHE II score | 23 (17-30) | 24 (17-29) | 23 (17-29) | 24 (18-30) | .887 |
| SOFA score | 9 (7-13) | 11 (8-13) | 10 (8-13) | 12 (9-14) | <.001 |
| JAAM DIC score | 5 (4-6) | 6 (4-7) | 6 (5-7) | 6 (5-8) | <.001 |
| ISTH overt-DIC score | 4 (3-5) | 4 (4-5) | 4 (4-5) | 5 (4-6) | <.001 |
| Source of ICU admission | <.001 | ||||
| Emergency department | 307 (49.2%) | 133 (49.1%) | 82 (37.1%) | 134 (42.4%) | |
| Ward | 156 (25.0%) | 74 (27.3%) | 45 (20.4%) | 80 (25.3%) | |
| Other hospital | 161 (25.8%) | 64 (23.6%) | 94 (42.5%) | 102 (32.3%) | |
| Preexisting comorbidities | |||||
| Immunocompromised | 63 (10.1%) | 19 (7.0%) | 31 (14.0%) | 39 (12.3%) | .055 |
| Chronic kidney disease | 71 (11.4%) | 21 (7.7%) | 13 (5.9%) | 16 (5.1%) | .003 |
| Chronic heart failure | 28 (4.5%) | 17 (6.3%) | 16 (7.2%) | 9 (2.8%) | .079 |
| Chronic respiratory disorder | 28 (4.5%) | 10 (3.7%) | 5 (2.3%) | 7 (2.2%) | .223 |
| Liver insufficiency | 7 (1.1%) | 2 (0.7%) | 3 (1.4%) | 4 (1.3%) | .909 |
| Site of infection | .067 | ||||
| Abdomen | 212 (34.0%) | 113 (41.7%) | 67 (30.3%) | 107 (33.9%) | |
| Lung | 131 (21.0%) | 46 (17.0%) | 45 (20.4%) | 51 (16.1%) | |
| Urinary tract | 123 (19.7%) | 44 (16.2%) | 54 (24.4%) | 77 (24.4%) | |
| Bone/soft tissue | 56 (9.0%) | 35 (12.9%) | 25 (11.3%) | 41 (13.0%) | |
| Central nervous system | 19 (3.0%) | 6 (2.2%) | 8 (3.6%) | 7 (2.2%) | |
| Other/unknown | 83 (13.3%) | 27 (10.0%) | 22 (10.0%) | 33 (10.4%) | |
| Therapeutic interventions | |||||
| Immunoglobulin | 96 (15.4%) | 127 (46.9%) | 78 (35.3%) | 196 (62.0%) | <.001 |
| Low-dose steroid | 121 (19.4%) | 81 (29.9%) | 75 (33.9%) | 120 (38.0%) | <.001 |
| Renal replacement therapy | 154 (24.7%) | 115 (42.4%) | 90 (40.7%) | 177 (56.0%) | <.001 |
| Low-dose heparin | 28 (4.5%) | 38 (14.0%) | 8 (3.6%) | 17 (5.4%) | <.001 |
| Interventions for source control | 235 (37.7%) | 141 (52.0%) | 99 (44.8%) | 153 (48.4%) | <.001 |
Abbreviations: APACHE, Acute Physiology and Chronic Health Evaluation; DIC, disseminated intravascular coagulation; ICU, intensive care unit; ISTH, International Society of Thrombosis and Hemostasis; JAAM, Japanese Association for Acute Medicine; rhTM, recombinant human thrombomodulin; SOFA, Sequential Organ Failure Assessment.
aData are presented as the median (first and third quartiles) for continuous variables and number (%) for categorical variables. Differences between groups were assessed using the Kruskal-Wallis or χ2 test.
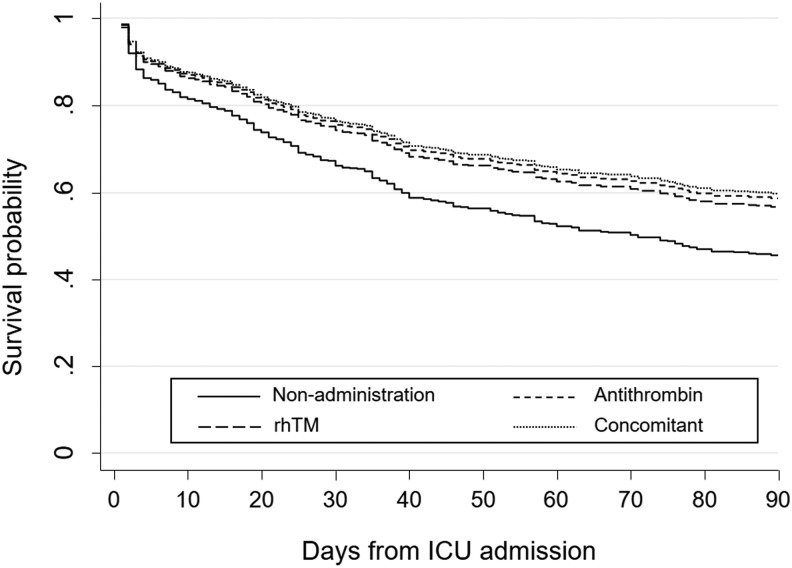
Mortality
The main results in this study using Cox proportional hazards analyses adjusted by propensity score to evaluate the difference in in-hospital mortality between every 2 groups among the 4 groups are shown in Table 2. We also show the propensity score-adjusted survival curves for the 4 groups in Figure 2. The adjusted hazard ratios (HRs) of in-hospital mortality for the antithrombin group and rhTM group compared to the nonanticoagulant group were 0.68 (95% confidence interval [CI]: 0.51-0.91; P = .008) and 0.72 (95% CI: 0.52-0.99; P = .044), respectively, indicating significant survival benefits associated with all 3 types of anticoagulant therapies. In contrast, we detected no significant difference in in-hospital mortality between the antithrombin and rhTM groups (adjusted HR: 0.95, 95% CI: 0.60-1.49; P = .748) or between the individual administration groups and the concomitant group (HR: 1.03, 95% CI: 0.65-1.66; P = .826 for antithrombin vs concomitant, and HR: 1.09, 95% CI: 0.66-1.82; P = .625 for rhTM vs concomitant).
Table 2.
Propensity Score-Adjusted Comparison of In-hospital Mortality.
| Treatment | Reference | HR | 95% CI | P Value | |
|---|---|---|---|---|---|
| Antithrombin | vs | Nonanticoagulant | 0.68 | 0.51-0.91 | .008 |
| rhTM | vs | Nonanticoagulant | 0.72 | 0.52-0.99 | .044 |
| Concomitant | vs | Nonanticoagulant | 0.66 | 0.47-0.91 | .012 |
| Antithrombin | vs | rhTM | 0.95 | 0.60-1.49 | .748 |
| Antithrombin | vs | Concomitant | 1.03 | 0.65-1.66 | .826 |
| rhTM | vs | Concomitant | 1.09 | 0.66-1.82 | .625 |
Abbreviations: CI, confidence interval; HR, hazard ratio; rhTM, recombinant human thrombomodulin.
Figure 2.
Adjusted estimated survival curves according to the types and presence of anticoagulant therapy. rhTM indicates recombinant human thrombomodulin.
Bleeding Complications
We summarized the findings for differences in the risk of bleeding complications between every 2 groups among the 4 groups in Table 3. We observed a significantly higher risk of bleeding complications in the antithrombin group compared to the nonanticoagulant group, whereas the rhTM group was not associated with a higher risk of bleeding complications compared to the nonanticoagulant group. We also found that the risk of bleeding complications in concomitant therapy was not significantly different compared to that for the individual administration of each agent.
Table 3.
Propensity Score-Adjusted Comparison of Bleeding Complications.
| Treatment | Reference | OR | 95% CI | P Value | |
|---|---|---|---|---|---|
| Antithrombin | vs | Nonanticoagulant | 2.10 | 1.30-3.41 | .002 |
| rhTM | vs | Nonanticoagulant | 1.10 | 0.60-2.01 | .749 |
| Concomitant | vs | Nonanticoagulant | 2.12 | 1.17-3.86 | .013 |
| Antithrombin | vs | rhTM | 1.91 | 1.06-3.44 | .031 |
| Antithrombin | vs | Concomitant | 0.99 | 0.55-1.78 | .973 |
| rhTM | vs | Concomitant | 0.52 | 0.26-1.03 | .061 |
Abbreviations: CI, confidence interval; OR, odds ratio; rhTM, recombinant human thrombomodulin.
Discussion
Recently, multiple lines of evidence have clarified that anticoagulant therapy against sepsis-induced DIC can improve mortality.3,8,10,11,19 Antithrombin and rhTM are the most commonly used agents against sepsis-induced DIC, and these 2 agents are often coadministered in Japan. However, a continuing controversy remains about whether 1 agent is superior to the other and whether concomitant therapy with these 2 agents is superior to individual administration. The current propensity score-adjusted study provided the evidence that (1) there was no difference in survival benefit between the individual administration of antithrombin or rhTM, (2) concomitant administration of antithrombin and rhTM showed no additional beneficial effects or interactions over the individual administration of these agents, and (3) concomitant administration of these agents caused no additional adverse effects over those of individual administration.
Differences in Mortality Between Antithrombin and rhTM
Multiple evidence based on previous observational studies and post hoc subgroup analyses of randomized controlled trials suggested the association of survival benefits with anticoagulant therapies for sepsis-induced DIC.4–11 The current study also demonstrated significant reductions in mortality in all 3 groups of anticoagulant therapies compared to the nonanticoagulant group.
However, there has been a great deal of controversy about which agent, antithrombin or rhTM, is more effective in improving patient outcomes because only a few studies have investigated the superiority of 1 agent over the other. Our study showing no significant difference in mortality between antithrombin and rhTM agreed with a recent large-scale observational study based on the Japanese DPC database,20 and these lines of evidence provide a robust indication that these 2 agents offer almost comparable survival benefit for patients with sepsis-induced DIC.
Significance of Concomitant Administration of Anticoagulants
In our study population, 316 patients received the concomitant administration of antithrombin and rhTM, which was equal to 53.8% of the total number of patients who received antithrombin and 58.8% of the total number of patients who received rhTM. Another database in Japan reported by Tagami et al showed that 37.7% of the sepsis-induced DIC patients who received antithrombin were coadministered rhTM, and 45.9% of the sepsis-induced DIC patients who received rhTM were coadministered antithrombin.12,13 These findings might reflect the current Japanese clinical situation in which the concomitant administration of these 2 agents was widely performed as adjutant management for sepsis-induced DIC. Therefore, clinical evidence to evaluate the superiority of concomitant administration over individual administration is strongly required.
Antithrombin exerts its anticoagulant properties by controlling the activity of thrombin and a large number of coagulation factors including factors VIIa, IXa, Xa, XIa, and XIIa.21 In contrast, rhTM forms a high-affinity complex with circulating thrombin and activates protein C, which inactivates coagulation factors Va and VIIIa, thereby suppressing further thrombin generation.22 Previous studies reported that these 2 independent anticoagulant pathways neither affect nor disturb each other.23 Therefore, the concomitant administration of antithrombin and rhTM could possibly exert additive and synergistic anticoagulant effects. Actually, several animal studies demonstrated that concomitant therapy with antithrombin and rhTM could modulate cell death, decrease the circulating levels of damage-associated molecular patterns, and lead to reduced organ damage and mortality.24,25
Nevertheless, despite these theoretical advantages of the concomitant administration of antithrombin and rhTM, the efficacy of concomitant therapy remains a topic of controversy because of a lack of definitive clinical evidence.26,27 We have provided the best evidence so far that the survival benefit associated with concomitant therapy was almost comparable to that of the individual administration of antithrombin or rhTM in the clinical situation. However, we cannot offer a clear explanation for why concomitant therapy was not associated with a greater effect on mortality despite the line of pathophysiological advantage reported by past basic studies. One possible reason might be that the individual administration of these agents already has sufficient efficacy to improve the patient’s condition, so that any additional benefit from coadministration of the other agent might be concealed.
Adverse Events
Bleeding was the most significant adverse event associated with anticoagulant therapy. In this study, we showed that the individual administration of antithrombin was associated with a higher risk of bleeding complications compared to nonuse of an anticoagulant. These findings agreed with those of previous studies showing an association between antithrombin administration and an increased risk of bleeding complications.28,29 Therefore, anticoagulant therapy including antithrombin should be administered only inpatients who can be expected to receive sufficient survival benefits despite the potentially increased risk of bleeding.
Limitations of This Study
Because of the retrospective study design, baseline characteristics and therapeutic interventions, including the concomitant use of low-dose heparin for prophylaxis of DVT, were different between the 4 groups. Therefore, we developed a propensity score approach to cope with the nonrandomized effect of anticoagulant therapies. However, it is hard to remove the effects of observed confounding completely because multiple unmeasured variables may account for the outcome differences observed in this study. Further multicenter prospective randomized trials are therefore required to gather definitive evidence of the difference in clinical outcomes between individual and concomitant anticoagulant therapy against sepsis-induced DIC.
Conclusion
The present study using the multicenter nationwide J-Septic DIC registry in Japan suggested that individual administration of antithrombin or rhTM had comparable efficacy in reducing mortality, but concomitant administration of these 2 agents might offer no additional survival benefit. Our findings raise some concerns about the routine use of concomitant anticoagulant therapy against sepsis-induced DIC.
Supplemental Material
Supplementary_material for Concomitant Versus Individual Administration of Antithrombin and Thrombomodulin for Sepsis-Induced Disseminated Intravascular Coagulation: A Nationwide Japanese Registry Study by Yutaka Umemura, Kazuma Yamakawa, Mineji Hayakawa, Daisuke Kudo, and Satoshi Fujimi in Clinical and Applied Thrombosis/Hemostasis
Acknowledgments
The authors thank all of the regional J-Septic DIC study coordinators, nurses, physicians, and patients who contributed to the success of this study.
Authors’ Note: Y.U. contributed to the study design; acquisition, analysis, and interpretation of the data; and drafting of the manuscript. K.Y. conceived and designed this study and was responsible for drafting, editing, and submission of the manuscript. M.H., D.K., and S.F. assisted with the design of the study and interpretation of the data. All authors reviewed and revised the manuscript and approved the final version of the manuscript. The data sets supporting the conclusions of this article are available in the University Hospital Medical Information Network Individual Case Data Repository (UMIN000012543, http://www.umin.ac.jp/icdr/index-j.html). Please contact the corresponding author to access the data. Trial registration: UMIN000012543. Retrospectively registered 10 December 2013. All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author).
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Supplemental Material: Supplementary material for this article is available online.
References
- 1. Zeerleder S, Hack CE, Wuillemin WA. Disseminated intravascular coagulation in sepsis. Chest. 2005;128(4):2864–2875. [DOI] [PubMed] [Google Scholar]
- 2. Levi M, de Jonge E, van der Poll T. Sepsis and disseminated intravascular coagulation. J Thromb Thrombolysis. 2003;16(1-2):43–47. [DOI] [PubMed] [Google Scholar]
- 3. Umemura Y, Yamakawa K, Ogura H, Yuhara H, Fujimi S. Efficacy and safety of anticoagulant therapy in three specific populations with sepsis: a meta-analysis of randomized controlled trials. J Thromb Haemost. 2016;14(3):518–530. [DOI] [PubMed] [Google Scholar]
- 4. Kienast J, Juers M, Wiedermann CJ, et al. KyberSept investigators. Treatment effects of high-dose antithrombin without concomitant heparin in patients with severe sepsis with or without disseminated intravascular coagulation. J Thromb Haemost. 2006;4(1):90–97. [DOI] [PubMed] [Google Scholar]
- 5. Dhainaut JF, Yan SB, Joyce DE, et al. Treatment effects of drotrecogin alfa (activated) in patients with severe sepsis with or without overt disseminated intravascular coagulation. J Thromb Haemost. 2004;2(11):1924–1933. [DOI] [PubMed] [Google Scholar]
- 6. Yamakawa K, Fujimi S, Mohri T, et al. Treatment effects of recombinant human soluble thrombomodulin in patients with severe sepsis: a historical control study. Crit Care. 2011;15(3):R123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ogawa Y, Yamakawa K, Ogura H, et al. Recombinant human soluble thrombomodulin improves mortality and respiratory dysfunction in patients with severe sepsis. J Trauma Acute Care Surg. 2012;72(5):1150–1157. [DOI] [PubMed] [Google Scholar]
- 8. Yamakawa K, Ogura H, Fujimi S, et al. Recombinant human soluble thrombomodulin in sepsis-induced disseminated intravascular coagulation: a multicenter propensity score analysis. Intensive Care Med. 2013;39(4):644–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ferrer R, Artigas A, Suarez D, et al. Edusepsis Study Group. Effectiveness of treatments for severe sepsis: a prospective, multicenter, observational study. Am J Respir Crit Care Med. 2009;180(9):861–866. [DOI] [PubMed] [Google Scholar]
- 10. Hayakawa M, Yamakawa K, Saito S, et al. ; Japan Septic Disseminated Intravascular Coagulation (JSEPTIC DIC) study group. Recombinant human soluble thrombomodulin and mortality in sepsis-induced disseminated intravascular coagulation. A multicentre retrospective study. Thromb Haemost. 2016;115(6):1157–1166. [DOI] [PubMed] [Google Scholar]
- 11. Hayakawa M, Kudo D, Saito S, et al. Antithrombin supplementation and mortality in sepsis-induced disseminated intravascular coagulation: a multicenter retrospective observational study. Shock. 2016;46(6):623–631. [DOI] [PubMed] [Google Scholar]
- 12. Tagami T, Matsui H, Horiguchi H, Fushimi K, Yasunaga H. Antithrombin and mortality in severe pneumonia patients with sepsis-associated disseminated intravascular coagulation: an observational nationwide study. J Thromb Haemost. 2014;12(9):1470–1479. [DOI] [PubMed] [Google Scholar]
- 13. Tagami T, Matsui H, Horiguchi H, Fushimi K, Yasunaga H. Recombinant human soluble thrombomodulin and mortality in severe pneumonia patients with sepsis-associated disseminated intravascular coagulation: an observational nationwide study. J Thromb Haemost. 2015;13(1):31–40. [DOI] [PubMed] [Google Scholar]
- 14. Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101(6):1644–1655. [DOI] [PubMed] [Google Scholar]
- 15. Gando S, Iba T, Eguchi Y, et al. Japanese Association for Acute Medicine Disseminated Intravascular Coagulation (JAAM DIC) Study Group. A multicenter, prospective validation of disseminated intravascular coagulation diagnostic criteria for critically ill patients: comparing current criteria. Crit Care Med. 2006;34(3):625–631. [DOI] [PubMed] [Google Scholar]
- 16. Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70(1):41–55. [Google Scholar]
- 17. Gayat E, Pirracchio R, Resche-Rigon M, Mebazaa A, Mary JY, Porcher R. Propensity scores in intensive care and anaesthesiology literature: a systematic review. Intensive Care Med. 2010;36(12):1993–2003. [DOI] [PubMed] [Google Scholar]
- 18. D’Agostino RB, Jr, D’Agostino RB., Sr Estimating treatment effects using observational data. JAMA. 2007;297(3):314–316. [DOI] [PubMed] [Google Scholar]
- 19. Yamakawa K, Umemura Y, Hayakawa M, et al. ; Japan Septic Disseminated Intravascular Coagulation (J-Septic DIC) study group. Benefit profile of anticoagulant therapy in sepsis: a nationwide multicenter registry in Japan. Crit Care. 2016;20(1):229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Murata A, Okamoto K, Mayumi T, Muramatsu K, Matsuda S. Observational study to compare antithrombin and thrombomodulin for disseminated intravascular coagulation. Int J Clin Pharm. 2015;37(1):139–147. [DOI] [PubMed] [Google Scholar]
- 21. Roemisch J, Gray E, Hoffmann JN, Wiedermann CJ. Antithrombin: a new look at the actions of a serine protease inhibitor. Blood Coagul Fibrinolysis. 2002;13(8):657–670. [DOI] [PubMed] [Google Scholar]
- 22. Ito T, Maruyama I. Thrombomodulin: protectorate God of the vasculature in thrombosis and inflammation. J Thromb Haemost. 2011:9(suppl 1):168–173. [DOI] [PubMed] [Google Scholar]
- 23. Aritomi M, Watanabe N, Ohishi R, et al. Recombinant human soluble thrombomodulin delivers bounded thrombin to antithrombin III: thrombomodulin associates with free thrombin and is recycled to activate protein c. Thromb Haemost. 1993;70(3):418–422. [PubMed] [Google Scholar]
- 24. Iba T, Nakarai E, Takayama T, Nakajima K, Sasaoka T, Ohno Y. Combination effect of antithrombin and recombinant human soluble thrombomodulin in a lipopolysaccharide induced rat sepsis model. Crit Care. 2009;13(6):R203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Iba T, Miki T, Hashiguchi N, Tabe Y, Nagaoka I. Combination of antithrombin and recombinant thrombomodulin modulates neutrophil cell-death and decreases circulating DAMPs levels in endotoxemic rats. Thromb Res. 2014;134(1):169–173. [DOI] [PubMed] [Google Scholar]
- 26. Sawano H, Shigemitsu K, Yoshinaga Y, et al. Combined therapy with antithrombin and recombinant human soluble thrombomodulin in patients with severe sepsis and disseminated intravascular coagulation. Nihon Kyukyu Igakukai Zasshi. 2013;24(3):119–131. [Google Scholar]
- 27. Sakurai T, Yamada S, Kitada M, et al. A comparative study of the efficacy of recombinant thrombomodulin monotherapy intravascular coagulation. Nihon Kyukyu Igakukai Zasshi. 2013;24(3):132–140. [Google Scholar]
- 28. Warren BL, Eid A, Singer P, et al. KyberSept trial study group. Caring for the critically ill patient. High-dose antithrombin III in severe sepsis: a randomized controlled trial. JAMA. 2001;286(15):1869–1878. [DOI] [PubMed] [Google Scholar]
- 29. Allingstrup M, Wetterslev J, Ravn FB, Møller AM, Afshari A. Antithrombin III for critically ill patients: a systematic review with meta-analysis and trial sequential analysis. Intensive Care Med. 2016;42(4):505–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary_material for Concomitant Versus Individual Administration of Antithrombin and Thrombomodulin for Sepsis-Induced Disseminated Intravascular Coagulation: A Nationwide Japanese Registry Study by Yutaka Umemura, Kazuma Yamakawa, Mineji Hayakawa, Daisuke Kudo, and Satoshi Fujimi in Clinical and Applied Thrombosis/Hemostasis