Abstract
We aimed to examine hypercoagulable and hypocoagulable conditions in patients with prostate cancer using thromboelastography (TEG) and correlate TEG parameters with conventional coagulation test. The t test was used for comparing TEG parameters and routine coagulation results. Spearman rank-order correlation was used to describe the relationship of TEG and conventional tests. Sensitivity, specificity, positive predictive values, and negative predictive values were determined for bleeding and thrombosis. Totally, 20 patients had active bleeding postoperatively, 16 of whom showed hypocoagulation on TEG test and 9 of whom showed hypocoagulation by routine coagulation test (P = .024). Overall, 60 patients did not have active bleeding postoperatively, 51 of whom showed hypercoagulation detected by TEG test and 42 of whom showed hypercoagulation found by routine coagulation test (P = .040). Remarkably, patients had a little higher fibrinogen (FIB) compared to controls. There was no statistical difference in any of the conventional coagulation indexes between the groups. Correlation analysis showed that reaction time (R) and coagulation time (K) were positively correlated with the prothrombin time–international normalized ratio (PT-INR) and negatively correlated with FIB (P < .001). Contrarily, α-angle and maximum amplitude (MA) were negatively correlated with PT-INR but positively correlated with FIB. Significantly, MA showed the strongest correlation with FIB and R exhibited the strongest correlation with PT-INR. Sensitivity and specificity for bleeding and thrombosis in TEG were higher than those in conventional coagulation test. Accordingly, TEG might be superior in evaluating hypercoagulation and detecting the risk of bleeding in patients with prostate cancer.
Keywords: coagulation function, correlation, prostate cancer, routine coagulation test, thrombelastography
Introduction
Postoperative thrombotic complications, for example, vein thrombosis, may be the clinical evidence of hypercoagulability.1 Significantly, thrombosis remains the second most common cause of death in patients with advanced cancer.2,3 Growing evidence from population-based studies has demonstrated that thrombosis is common in prostate cancer.4 Thus, it is vital to identify postoperative prothrombotic condition which is required for adequate anticoagulant prophylaxis.
Previously, a study has demonstrated that standard laboratory tests are nearly incapable to identify the activation phase of the coagulation system and postoperative prothrombotic phase.5 Hence, conventional diagnostic methods remain poor assays for dynamic assessment of clot strength in whole blood. It is pressing to develop a convenient, rapid, and cost-effective approach to predict the risk of thrombosis in patients with cancer.
Thromboelastography (TEG) first described in 1948 is a sensitive assay that analyzes the kinetics of clot formation, from the initial fibrin threads to fibrinolysis.6 Clinically, TEG is used to diagnose coagulopathies, monitor bleeding, evaluate hemostasis, and guide transfusion in patients with trauma and those undergoing surgery.7 More significantly, it has been expanded into oncology area and has been indicated to predict postoperative thromboembolic complications.8,9 Unfortunately, the number of prospective randomized trials evaluating the use of this technology in assessing hypercoagulability in patients with cancer is few and the outcomes are conflicting.10,11 Moreover, only a few researches have studied the correlation between TEG and conventional clotting tests.12,13
Thus, in this study, we planned to observe the preoperative coagulation changes measured by TEG and routine coagulation tests in patients with localized prostate cancer. Moreover, we compared the data obtained by routine laboratory coagulation analysis with TEG tracing values in order to find a correlation between the 2 methods. This may contribute to a more targeted method to transfusion therapy and anticoagulant prophylaxis in patients with prostate cancer in the future.
Materials and Methods
Participants
The protocol of our study was approved by the hospital’s board of ethics. Between January 2010 and December 2014, a suitable group of patients with localized prostate cancer (stages T1B-T2C, life expectancy longer than 10 years) and age-matched controls were included after obtaining informed consent. All patients with localized prostate cancer enrolled in our study were confirmed by magnetic resonance imaging (MRI), ultrasound-guided biopsy, and radionuclide bone scan preoperatively. Patients with blood disease, hepatobiliary disease, liver and kidney dysfunction, and other diseases affecting blood coagulation function were excluded. Furthermore, participants using anticoagulants or drugs affecting fibrinolytic system function or blood products within 2 weeks were excluded. Likewise, healthy age-matched volunteers at the same time were recruited from physical examination center, who constituted the control group. Moreover, the baseline characteristics (age, prostate-specific antigen [PSA], Gleason scores, and preoperative endocrine therapy) were recorded in all participants in our study.
Intervention and Blood Sampling
All eligible patients with localized prostate cancer were treated by extraperitoneal laparoscopic radical prostatectomy. For patients with enlarged lymph nodes detected by MRI or PSA not less than 20 μg/L or Gleason score ≥7, bilateral pelvic lymph nodes dissection was performed. For patients with preoperative sexual function, T1 or T2 stage, PSA <10 μg/L, and Gleason score <7, bilateral neurovascular bundle was kept. After operation, urethral catheter and drainage tube were indwelled.
Thromboelastography and conventional coagulation routine tests were implemented within 24 hours before surgery. Citrated venous blood samples (1:9 trisodium citrate 3.2%:blood) were withdrawn from patients.
Conventional Assay
Routine coagulation analyses were carried out approximately 30 minutes after sampling. Two experienced hematology and coagulation specialists evaluated the presence of coagulopathy based on the results of conventional tests including prothrombin time–international normalized ratio (PT-INR) and activated partial thromboplastin time (APTT) using Sysmex CA-7000 automatic blood coagulation analyzer (Kobe, Japan). Plasma fibrinogen (FIB) was measured using enzyme-linked immunosorbent assay #41-FIBHU-E01 from ALPO Diagnostics (Salem, New Hampshire, USA). When PT-INR or APTT was lower than normal, or FIB was higher than normal, the patients were regarded as hypercoagulable. Similarly, when PT-INR or APTT was higher than normal, or FIB was lower than normal, the patients were regarded as hypocoagulable. One or several of the parameters could be abnormal in this test to indicate coagulopathy. Moreover, if the value was outside the normal range, the test was considered abnormal. Both evaluators were blinded from the results of TEG.
Thromboelastography Test
Thromboelastography analyses were performed at a constant temperature of 37°C at the transfusion medicine laboratory. Venous blood was collected in the morning, after 1:9 3.2% sodium citrate anticoagulation, and completed within 2 hours. Thromboelastography was immediately implemented using Thrombelastograph Analyzer 5000 (Haemoscope Corporation, Braintree, Massachusetts) after sample collection, using disposable standard cuvettes and pins from Medtel (Haemoscope Corporation), according to the manufacturer’s instructions. Specifically, 1 mL of blood was taken from the citrated blood samples and then mixed in a kaolin vial by gentle shaking. Afterward, 0.34 mL of blood was selected with an automatic pipette and placed into a TEG cuvette. Then, 20 µL of 0.2 mol/L calcium was added to the tube to remove the effect of citrate. Then, the test began without delay. The TEG-related parameters were recorded as a representation of hemostasis, which included (1) reaction time (R, normally 3-8 minutes), the time from the start of the analysis till clot formation; (2) coagulation time (K, normally 1-3 minutes), the time from the start of clot formation till the curve reached an amplitude of 20 mm; (3) α-angle, the angle between the baseline and tangent to the TEG curve, meaning the rate of clot formation, reference range of 55° to 78°; (4) maximum amplitude (MA, normally 51-69 mm), reflecting the strength of the clot; (5) coagulation index (CI, reference −3 to 3); and (6) lysis at 30 minutes (LY30; reference range 0%-7.5%), reflecting the degree of fibrinolysis at 30 minutes.
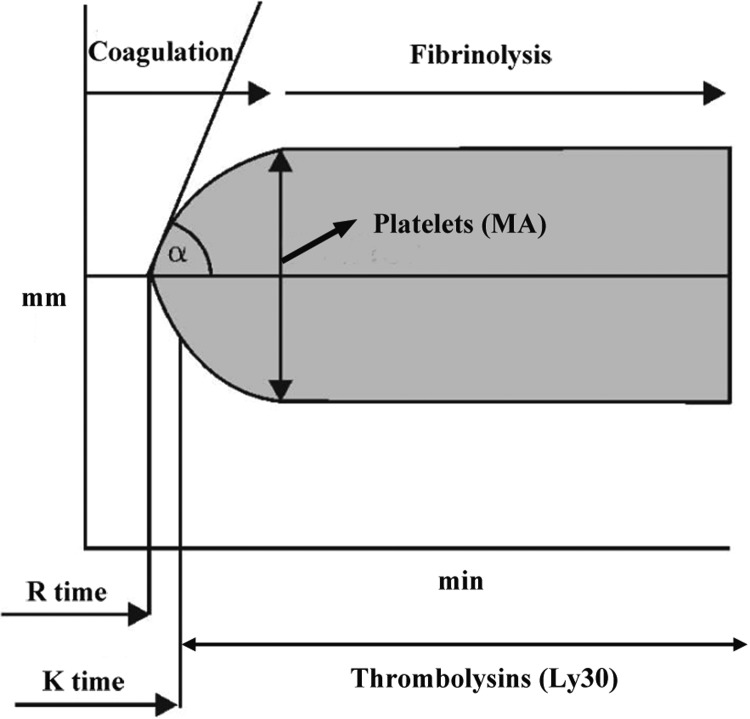
In TEG analysis, hypercoagulability was demonstrated by shorter R and K, increased α-angle, MA, and CI, and LY30 < 0 in our study (herein, any 1 index was abnormal, and this condition was defined as hypercoagulability). Analogously, hypocoagulability was demonstrated by longer R and K, decreased α-angle, MA, and CI, and LY30 > 7.5% in our study (herein, any 1 index was abnormal, and this condition was defined as hypocoagulability). All the TEGs were carried out by the same operator, and the TEG traces were generated over an hour. The dynamic changes in TEG curve and the normal ranges are exhibited in Figure 1.
Figure 1.
Normal thromboelastography (TEG) tracing and reference ranges for parameters in TEG test. α angle indicates the rate of clot formation; CI, coagulation index; K, coagulation time; LY30, clot lysis at 30 minutes; MA, maximum amplitude; R, reaction time.
Statistical Analyses
SPSS 21.0 software was used to analyze the data. All the data are shown as mean (standard deviation [SD]). The t test was used for the comparison of TEG parameters and routine coagulation results between these 2 groups. Spearman rank-order correlation was employed to study the relationships of coagulation measures (both TEG and conventional tests). Sensitivity, specificity, positive predictive values (PPVs), and negative predictive values (NPVs) were determined for bleeding and thrombosis between TEG and conventional coagulation tests. P < .05 was regarded as statistically significant.
Results
Demographic Characteristics of Patients With Prostate Cancer and Controls
During the study period, a total of 102 patients with prostate cancer were enrolled in our study. However, 22 patients were excluded. In total, 80 patients with prostate cancer (mean age: 64 [5.12]) were included for subsequent analysis. Correspondingly, in the control group, 110 healthy age-matched participants were recruited, but 10 controls were not included. Table 1 indicated clinical data for patients with prostate cancer and controls. From Table 1, we found that 17 patients at T2 stage received the neoadjuvant hormone therapy for 3 to 9 months before surgery and 21 patients were treated by this hormone therapy for less than 3 months.
Table 1.
Baseline Characteristics of the Population of the Study.
| Cases | Age, years | PSA, ng/mL | Clinical Stage | Gleason Score | Preoperative Endocrine Therapy (Cases) | |
|---|---|---|---|---|---|---|
| Prostate group | 80 | 64 (5.12) | 32.1 (10.64) | T1b-T2c | 6.2 (2.5) | 38 (47.5%) |
| Control group | 100 | 61 (8.35) | 1.21 (0.62) | |||
| P values | .85 | .001 | NA | NA | NA |
Abbreviations: NA, not applicable; PSA, prostate-specific antigen.
General Postoperative Data
The operation time was 90 to 190 minutes and the median time was 100 minutes. The bleeding volume was 30 to 800 mL, and the median volume was 80 mL. Postoperative urethral catheterization was used for 6 to 14 days, and the median time was 7 days. Of note, the duration of urethral catheterization was extended to 14 days for 7 cases with postoperative urinary leakage. Postoperative drainage tube was maintained for 3 to 10 days, and the median time was 4 days. Postoperative hospital stay was 7 to 16 days, and the median time was 7 days.
Importantly, active bleeding was observed in 20 cases, of which 2 received blood transfusion because of severe postoperative bleeding and 1 case was treated by antiulcer drug to control gastrointestinal bleeding. No active bleeding after surgery was found in the other 60 cases. Among these 60 patients, 3 cases of deep vein thrombosis were discovered, and these 3 patients were treated with anticoagulant medications without surgical treatment. No rectal injury, lymphocele, infection of incision, incision hernia, postoperative persistent urine incontinence, or other complications occurred.
Comparison of Coagulation Detection Between TEG and Routine Coagulation Test
Descriptive data about coagulation test in 20 cases with active bleeding are presented in Table 2. Among 20 cases with active bleeding, after TEG test, 16 cases were found to be hypocoagulable, 3 cases were hypercoagulable, and 1 case was normocoagulable. The hypocoagulability detection rate was 80.0%. In the routine coagulation tests, 9 cases of hypocoagulability, 5 cases of hypercoagulability, and 6 cases of normocoagulability were found. The hypocoagulability detection rate was 45.0%. Detection for hypocoagulability between the 2 methods was statistically significant (P = .024).
Table 2.
Coagulopathy (Hypocoagulation) in 20 Cases With Active Bleeding and Coagulopathy (Hypercoagulation) in 60 Cases Without Active Bleeding According to Different Coagulation Assays (TEG and Routine Methods).
| Hypocoagulation | Nonhypocoagulation | Hypercoagulation | Nonhypercoagulation | |
|---|---|---|---|---|
| TEG | 16 | 4 | 51 | 9 |
| Routine coagulation tests | 9 | 11 | 42 | 18 |
| P value | .024 | .040 | ||
Abbreviations: TEG, thromboelastography.
Similarly, we also observed the coagulation test in 60 cases without active bleeding (Table 2). Among them, 51 cases were found to have hypercoagulability with TEG test, 7 cases had hypocoagulability, and 2 cases were normal. The hypercoagulability detection rate was 85.0%. In the routine coagulation tests, 42 cases of hypercoagulability, 8 cases of hypocoagulability, and 10 normal cases were found. The hypercoagulability detection rate was 70.0%. Detection for hypercoagulability between the 2 methods was statistically significant (P = .040).
Comparison of Indexes Between TEG and Routine Coagulation Test in Prostate Cancer Group and the Control Group
As shown in Table 3, we found that R and K values of 80 patients with prostate cancer were significantly shortened (P < . 05), relative to the control group. α-Angle, MA, and CI were increased obviously (P < 0.05). Remarkably, patients with prostate cancer had higher level of FIB compared to controls. However, there was no statistical difference in any of the conventional CIs, PT-INR, APTT, and FIB, between the 2 groups (P > .05).
Table 3.
Comparison of Indexes Between Prostate Cancer Group and the Control Group.
| Groups | Cases | R, min | K, min | α Angle, ° | MA, mm | CI | LY30,% | PT-INR | APTT, s | FIB, g/L |
|---|---|---|---|---|---|---|---|---|---|---|
| Prostate group | 80 | 6.12 (1.99) | 1.16 (0.28) | 65.29 (7.12) | 64.28 (4.82) | 1.02 (1.49) | 0.92 (0.29) | 0.65 (0.21) | 27.79 (2.91) | 3.1 (0.7) |
| Control group | 100 | 6.81 (2.11) | 2.21 (0.51) | 60.25 (6.08) | 62.11 (5.02) | −0.96 (1.22) | 0. 87 (0.26) | 0.69 (0.12) | 28.29 (3.12) | 2.9 (0.8) |
| P value | .0128 | .000 | .0015 | .018 | .000 | .1154 | .1319 | .2689 | .0802 |
Abbreviations: APTT, activated partial thromboplastin time; α-angle, the rate of clot formation; CI, coagulation index; FIB, fibrinogen; K, coagulation time; LY30, clot lysis at 30 minutes; MA, maximum amplitude; PT-INR, prothrombin time-international normalized ratio; R, reaction time.
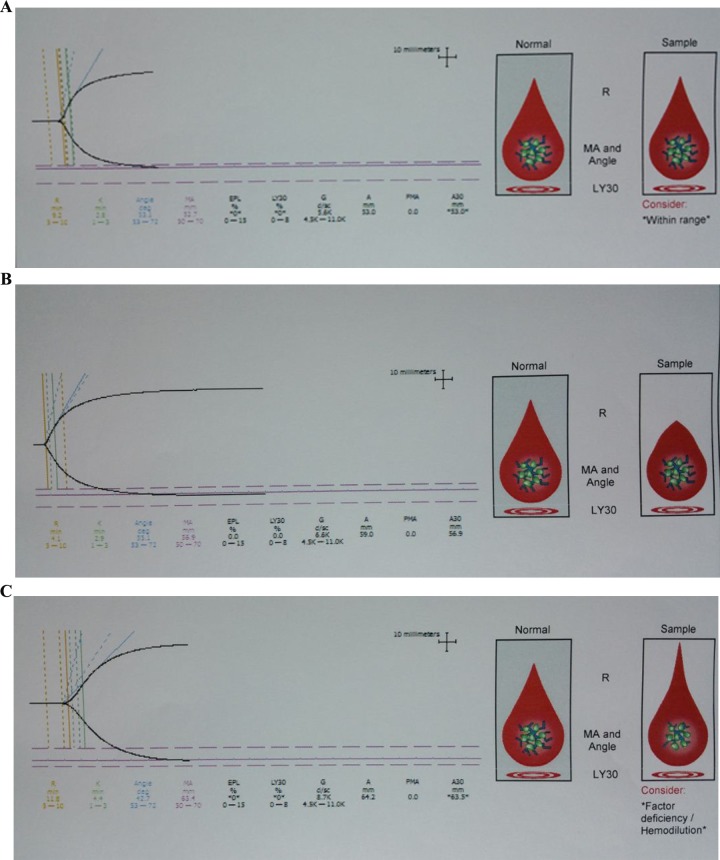
The TEG trace changed toward hypercoagulability as evidenced by shortening of the R and K time, increasing in each of the angle, MA, and CI. Representative normal, hypercoagulable, and hypocoagulable TEG traces are shown in Figure 2.
Figure 2.
Representative normal (A), hypocoagulable (B), and hypercoagulable (C) thromboelastography (TEG) traces.
Correlation Analysis
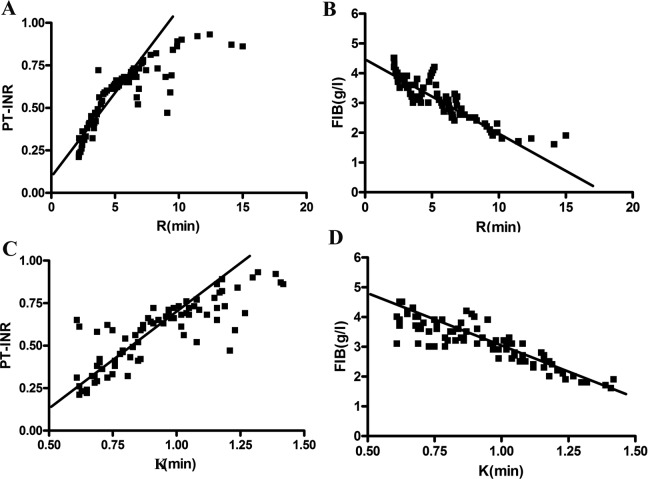
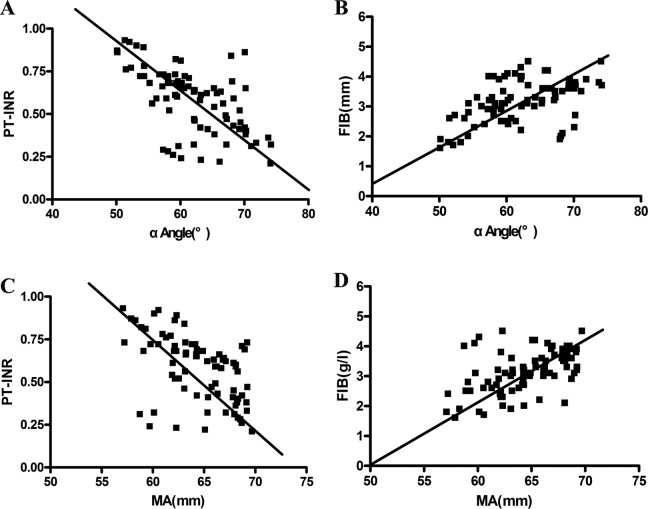
The correlations between the individual parameters of TEG analysis and PT-INR and FIB are shown in Figures 3 and 4. From this image, we observed that R and K were positively correlated with the PT-INR and negatively correlated with FIB (P < .001; Figure 3). On the contrary, α-angle and MA were negatively correlated with the PT-INR and positively correlated with FIB (P < .001; Figure 4). Significantly, MA showed the strongest correlation with FIB with r = .520, and R exhibited the strongest correlation with PT-INR with r = .844. The CI value and PT-INR were weakly correlated with FIB. No obvious correlation was found between LY30 and routine CIs. Similarly, there was no significant correlation between APTT and TEG indexed. Specific correlation coefficients between TEG parameters and routine laboratory indexes are shown in Table 4.
Figure 3.
The correlation between R and K value with PT-INR and FIB. A, R value was significantly positively related to PT-INR. B, R value was significantly negatively correlated with FIB. C, K value was significantly positively related to PT-INR. D, K value was significantly negatively correlated with FIB. FIB indicates fibrinogen; K, coagulation time; MA, maximum amplitude; PT-INR, prothrombin time–international normalized ratio; R, reaction time.
Figure 4.
The correlation between α-angle and MA with PT-INR and FIB. A, α-Angle was significantly positively related to PT-INR. B, α-Angle was significantly negatively correlated with FIB. C, The MA was significantly positively related to PT-INR. D, The MA was significantly negatively correlated with FIB. FIB indicates fibrinogen; K, coagulation time; MA, maximum amplitude; PT-INR, prothrombin time–international normalized ratio; R, reaction time.
Table 4.
Correlation Coefficients Between TEG Parameters and Routine Coagulation Indexes.
| R Value | K Value | α-Angle | MA Value | CI Value | LY30 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| r | P | r | P | r | P | r | P | r | P | r | P | |
| FIB | −.883 | <.001 | −.878 | <.001 | .490 | <.001 | .520 | <.001 | .318 | <.05 | .186 | >.05 |
| PT-INR | .844 | <.001 | .8302 | <.001 | −0.577 | <.001 | −.508 | <.001 | −.329 | <.05 | .024 | >.05 |
| APTT | .149 | >.05 | .101 | >.05 | −.121 | >.05 | −.119 | >.05 | −.126 | >.05 | −.081 | >.05 |
APTT, activated partial thromboplastin time; α-angle, the rate of clot formation; CI, coagulation index; FIB, fibrinogen; K, coagulation time; LY30, clot lysis at 30 minutes; MA, maximum amplitude; PT-INR, prothrombin time-international normalized ratio; R, reaction time
Sensitivity, Specificity, PPV, and NPV for Bleeding and Thrombosis
In detecting bleeding, TEG achieved good ability (16 true-positive, 4 false-negative, 53 true-negative, and 7 false-positive result in 80 patients for TEG analysis and 9 true-positive; 11 false-negative, 52 true-negative, and 8 false-positive result in 80 patients for routine analysis), as shown in Table 5. Similar to the bleeding, the patient number for thrombosis is also shown in Table 5. There were 3 true-positive, 51 false-negative, 17 true-negative, and 9 false-positive result in 80 patients for TEG analysis, and there were 0 true-positive, 42 false-negative, 17 true-negative, and 11 false-positive result in 80 patients for conventional analysis.
Table 5.
Patient Number for the Outcomes of Bleeding and Thrombosis.
| Tests | Bleeding | No Bleeding | Thrombosis | No Thrombosis |
|---|---|---|---|---|
| TEG | 16 (true positive) | 7 (false positive) | 3 (true positive) | 9 (false positive) |
| 4 (false negative) | 53 (true negative) | 51 (false negative) | 17 (true negative) | |
| Conventional coagulation test | 9 (true positive) | 8 (false positive) | 0 (true positive) | 11 (false positive) |
| 11 (false negative) | 52 (true negative) | 42 (false negative) | 17 (true negative) |
Abbreviations: TEG, thromboelastography.
The sensitivity, specificity, PPV, and NPV for bleeding in the 2 groups are exhibited in Table 6. The sensitivity, specificity, PPV, and NPV of bleeding in the TEG group were 80.0%, 88.3%, 69.5%, and 92.9%, respectively. In conventional test, the sensitivity, specificity, PPV, and NPV were 45.0%, 86.7%, 52.9%, and 82.5%, respectively.
Table 6.
Sensitivity, Specificity, PPV, and NPV for the Outcomes of Bleeding and Thrombosis.
| Tests | Bleeding | Thrombosis | ||||||
|---|---|---|---|---|---|---|---|---|
| Sensitivity | Specificity | PPV | NPV | Sensitivity | Specificity | PPV | NPV | |
| TEG | 80.0% | 88.3% | 69.5% | 92.9% | 5.55% | 65.3% | 25.0% | 25.2% |
| Conventional coagulation test | 45.0% | 86.7% | 52.9% | 82.5% | 0% | 60.7% | 0% | 24.6% |
Abbreviations: TEG, thromboelastography; PPV, positive predictive value; NPV, negative predictive value.
The sensitivity, specificity, PPV, and NPV for thrombosis in the 2 groups are displayed in Table 5. The sensitivity, specificity, PPV, and NPV in the TEG group were 5.55%, 65.3%, 25.0%, and 25.2%, respectively. In conventional coagulation test, the sensitivity, specificity, PPV, and NPV were 0%, 60.7%, 0%, and 24.6%, respectively.
Discussion
As documented, hypercoagulability in patients with malignant neoplasm is related to the tumor’s capacity to overexpress procoagulants, thus resulting in a prothrombotic phenotype.14,15 Although prostate cancer is the second commonest cancer in men in the world, there are small number of studies that analyze the differences between prostate cancer and control with TEG and correlations between TEG parameters and CIs. To achieve this goal, our study was planned to compare results from TEG with routine coagulation tests in patients with prostate cancer. We indicated that, after TEG analysis, the group of patients with prostate cancer had shorter R and reduced K values, increased MA value, and a broad α-angle relative to age-matched controls. By contrast, there were no difference in LY30 between patients with cancer and controls, indicating that increased thrombogenicity observed in patients with prostate cancer resulted from an enhanced tendency to clot formation. Moreover, no differences were found in any standard laboratory index between the 2 groups. Also, our results suggested that R, K, α-angle, and MA were significantly correlated with the most commonly used parameters, PT-INR and FIB, in patients with prostate cancer.
Generally, routine coagulation tests are carried out using centrifuged plasma fractions and only measure isolated portions of coagulation cascade, thus giving no data on important interactions essential to clinical evaluation of clotting syndromes. Fortunately, TEG, by using whole blood, not only measures quantity of clotting but also examines the quality of clotting that is not recorded through routine coagulation assay.16 This hypercoagulable condition was defined as evidenced by shortening R value, a short K value, an increased MA value, and a broad α-angle.17 The utilization of TEG to detect postoperative hypercoagulability has been reported in many fields, for example, ischemic heart disease18 and neurosurgery.19 Significantly, TEG has also been performed on patients with malignant tumor. Previously, Francis and colleagues utilized TEG to assess hypercoagulability in patients with breast and colorectal cancer.20 Although platelets and FIB remained normal, hypercoagulability was identified in a high proportion of patients with breast and colorectal cancer using TEG.20 Moreover, another study has indicated that patients with prostate cancer are hypercoagulable, and this condition is detected by TEG.21 In line with these studies, after analyzing all collected data from our study, we found that significant hypercoagulability was observed in patients with prostate cancer as detected by TEG rather than routine coagulation tests, which indicated that TEG has superior value in evaluating hypercoagulable state in patients with prostate cancer. However, only 3 patients with deep vein thrombosis were found, thereby TEG and conventional testing appear to be much less useful for predicting thrombotic complications.
Thrombocytopenia has been demonstrated to be associated with increased bleeding risk.22 We observed significant hypocoagulant changes in 80.0% of 20 cases with active bleeding using TEG analysis, but 45.0% using routine test. Moreover, the sensitivity, specificity, PPV, and NPV in the TEG group were higher than those in conventional test. Hence, we suppose that TEG analysis may be better in indicating the risk of bleeding in patients with prostate cancer.
When we measured conventional markers of thrombogenesis, we discovered that for patients with prostate cancer, only FIB level was higher than controls, but there was no significant difference in any index between the 2 groups. Our findings were in line with those previously reported in patients with lung cancer.23,24 In the literatures, hyperfibrinogenemia and thrombocytosis have been frequently reported in patients with malignant disorders.25 Thus, we sought to correlate these laboratory parameters with those of TEG. Significantly, we observed that MA had the strongest positive correlation with FIB. Similarly, a former study by Zuckerman et al26 identified the correlations of MA of TEG with the routine common tests in hypercoagulable patients. Moreover, another study correlated the parameters of modified TEG rotational thromboelastometry (ROTEM), with routine coagulation analysis in patients with cancer, which revealed maximum clot firmness (MCF) (meaning MA) showed the strongest correlation with FIB.8 Therefore, MA appears to be the most important TEG parameter of detecting patients with hypercoagulopathic cancer.
As documented, androgen deprivation therapy is the mainstay of treatment for patients with advanced prostate cancer.27 However, several previous clinical studies have demonstrated that androgen deprivation therapy may lead to a hypercoagulable state, with a subsequent link to thromboembolism events.21,28,29 Moreover, Klil-Drori et al30 also have shown that patients with prostate cancer on current androgen deprivation therapy use displayed the association with the increased risk of venous thromboembolism, but no association was observed in past use of androgen deprivation therapy. Significantly, Kaur et al31 have implicated that androgen deprivation therapy do not appear to exacerbate hypercoagulability over time as a whole. In our study, a total of 38 patients with prostate cancer received preoperative endocrine therapy, and 3 patients with deep vein thrombosis were discovered. Thus, sensitive monitoring of coagulation of prostate cancer cases on androgen deprivation therapy can help to screen those at risk of developing thromboembolism complications.
Our study had a number of disadvantages. For example, there were small patient size and combining disease stages, yet the results were provocative in their implications. Additional studies with larger numbers will help address whether TEG parameters were related to disease stage, clinical response, and disease surveillance. Later studies might include the measurement of FIB, tissue factor levels, and clotting factors.
In conclusion, our results indicated that certain parameters of TEG were abnormal in patients with prostate cancer, and the significant difference was observed between patients with cancer and controls, particularly MA, demonstrating in vitro that examining the physical properties of clot might be a rewarding area in the future study. Identification of hypercoagulability by TEG in patients with prostate cancer may be helpful to detect those at risk of cancer-caused thrombosis, and detection of hypocoagulability by TEG in patients with prostate cancer may be beneficial to identify those at risk of cancer-caused bleeding. In addition, the TEG test might be more valuable if combined with scoring systems for grading vein leg thrombosis.32
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Clagett GP, Anderson FA, Heit J, et al. Prevention of venous thromboembolism. Chest. 1995;108(4 suppl):312S–334S. [DOI] [PubMed] [Google Scholar]
- 2. Heit JA, Silverstein MD, Mohr DN, et al. Risk factors for deep vein thrombosis and pulmonary embolism: a population-based case-control study. Arch Intern Med. 2000;160(6):809–815. [DOI] [PubMed] [Google Scholar]
- 3. Khorana AA, Francis CW, Culak, ova E, Kuderer NM, Lyman GH. Frequency, risk factors, and trends for venous thromboembolism among hospitalized cancer patients. Cancer. 2007;110(10):2339–2346. [DOI] [PubMed] [Google Scholar]
- 4. Van Hemelrijck M, Adolfsson J, Garmo H, et al. Risk of thromboembolic diseases in men with prostate cancer: results from the population-based PCBaSe Sweden. Lancet Oncol. 2010;11(5):450–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baglin T. Using the laboratory to predict recurrent venous thrombosis. Int J Lab Hematol. 2011;33(4):333–342. [DOI] [PubMed] [Google Scholar]
- 6. Hartert H. Blutgerinnungsstudien mit der Thrombelastographie, einem neuen Untersuchungsverfahren. Klinische Wochenschrift. 1948;26(37):577–583. [DOI] [PubMed] [Google Scholar]
- 7. Sankarankutty A, Nascimento B, Teodoro da Lu, z L, Rizoli S. TEG® and ROTEM® in trauma: similar test but different results. World J Emerg Surg. 2012;7(suppl 1):S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Akay OM, Ustuner Z, Can, turk Z, Mutlu FS, Gulbas Z. Laboratory investigation of hypercoagulability in cancer patients using rotation thrombelastography. Med Oncol. 2009;26(3):358–364. [DOI] [PubMed] [Google Scholar]
- 9. McCrath DJ, Cerboni E, Frume, nto RJ, Hirsh AL, Bennett-Guerrero E. Thromboelastography maximum amplitude predicts postoperative thrombotic complications including myocardial infarction. Anesth Analg. 2005;100(6):1576–1583. [DOI] [PubMed] [Google Scholar]
- 10. Girdauskas E, Kempfert J, Kuntze T, et al. Thromboelastometrically guided transfusion protocol during aortic surgery with circulatory arrest: a prospective, randomized trial. J Thorac Cardiovasc Surg. 2010;140(5):1117–1124.e1112. [DOI] [PubMed] [Google Scholar]
- 11. Capraro L, Kuitunen A, Salmenperä M, Kekomäki R. On-site coagulation monitoring does not affect hemostatic outcome after cardiac surgery. Acta Anaesthesiol Scand. 2001;45(2):200–206. [DOI] [PubMed] [Google Scholar]
- 12. Moganasundram S, Hunt BJ, Sykes K, et al. The relationship among thromboelastography, hemostatic variables, and bleeding after cardiopulmonary bypass surgery in children. Anesth Analg. 2010;110(4):995–1002. [DOI] [PubMed] [Google Scholar]
- 13. Herbstreit F, Winter E, Pete, rs J, Hartmann M. Monitoring of haemostasis in liver transplantation: comparison of laboratory based and point of care tests. Anaesthesia. 2010;65(1):44–49. [DOI] [PubMed] [Google Scholar]
- 14. Cloonan ME, DiNapoli M, Mousa SA. Efficacy of anticoagulants and platelet inhibitors in cancer-induced thrombosis. Blood Coagul Fibrinolysis. 2007;18(4):341–345. [DOI] [PubMed] [Google Scholar]
- 15. Mannucci P. The measurement of multifactorial thrombophilia. Thromb Haemost. 2002;88(1):1–2. [PubMed] [Google Scholar]
- 16. Traverso CI, Caprini JA, Arcelus JI. The normal thromboelastogram and its interpretation. Semin Thromb Hemost. 1995;21(suppl 4):7–13. [DOI] [PubMed] [Google Scholar]
- 17. Mallet S, Cox D. Thromboelastography. Br J Anaesth. 1992;69(3):307–313. [DOI] [PubMed] [Google Scholar]
- 18. Artang R, Jensen E, Pede, rsen F, Frandsen NJ. Thrombelastography in healthy volunteers, patients with stable angina and acute chest pain. Thromb Res. 2000;97(6):499–503. [DOI] [PubMed] [Google Scholar]
- 19. Goh KY, Tsoi WC, Fe, ng CS, Wickham N, Poon WS. Haemostatic changes during surgery for primary brain tumours. J Neurol Neurosurg Psychiatry. 1997;63(3):334–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Francis JL, Francis DA, Gunathilagan GJ. Assessment of hypercoagulability in patients with cancer using the sonoclot analyzer and thromboelastography. Thromb Res. 1994;74(4):335–346. [DOI] [PubMed] [Google Scholar]
- 21. Toukh M, Siemens DR, Black A, et al. Thromboelastography identifies hypercoagulablilty and predicts thromboembolic complications in patients with prostate cancer. Thromb Res. 2014;133(1):88–95. [DOI] [PubMed] [Google Scholar]
- 22. Elting LS, Rubenstein EB, Martin CG, et al. Incidence, cost, and outcomes of bleeding and chemotherapy dose modification among solid tumor patients with chemotherapy-induced thrombocytopenia. J Clin Oncol. 2001;19(4):1137–1146. [DOI] [PubMed] [Google Scholar]
- 23. Davies NA, Harrison NK, Sabra A, et al. Application of ROTEM to assess hypercoagulability in patients with lung cancer. Thromb Res. 2015;135(6):1075–1080. [DOI] [PubMed] [Google Scholar]
- 24. Marinho FC, Takagaki TY. Hypercoagulability and lung cancer. J Bras Pneumol. 2008;34(5):312–322. [DOI] [PubMed] [Google Scholar]
- 25. Sun NC, McAfee WM, H, um GJ, Weiner JM. Hemostatic abnormalities in malignancy, a prospective study of one hundred eight patients. Part I. Coagulation studies. Am J Clin Pathol. 1979;71(1):10–16. [DOI] [PubMed] [Google Scholar]
- 26. Zuckerman L, Cohen E, Vagher J, Woodward E, Caprini JA. Comparison of thrombelastography with common coagulation tests. Thromb Haemost. 1981;46(4):752–756. [PubMed] [Google Scholar]
- 27. Bekelman JE, Mitra N, Handorf EA, et al. Effectiveness of androgen-deprivation therapy and radiotherapy for older men with locally advanced prostate cancer. J Clin Oncol. 2015;33(7):716–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Malan NT, Känel RV, Schutte AE, et al. Testosterone and acute stress are associated with fibrinogen and von Willebrand factor in African men: The SABPA study. Int J Cardiol. 2013;168(5):4638–4642. [DOI] [PubMed] [Google Scholar]
- 29. Brodin E, Vikan T, Hansen JB, Svartberg J. Testosterone, hemostasis, and cardiovascular diseases in men. Semin Thromb Hemost. 2011;37(1):87–94. [DOI] [PubMed] [Google Scholar]
- 30. Klil-Drori AJ, Yin H, Tagalakis V, Aprikian A, Azoulay L. Androgen deprivation therapy for prostate cancer and the risk of venous thromboembolism. Eur Urol. 2015;70(1):56–61. [DOI] [PubMed] [Google Scholar]
- 31. Kaur H, Siemens DR, Black A, et al. Effects of androgen-deprivation therapy on hypercoagulability in prostate cancer patients: a prospective, longitudinal study. Can Urol Assoc J. 2017;11(1-2):33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Björgell O, Robertson F. Scoring systems for grading deep leg vein thrombosis. Acta Radiol. 2002;43(3):299–305. [DOI] [PubMed] [Google Scholar]