Abstract
Idarucizumab, a humanized monoclonal antibody fragment (Fab), provides rapid and sustained reversal of dabigatran-mediated anticoagulation. Idarucizumab and dabigatran are mainly eliminated via the kidneys. This analysis aimed to characterize the renal elimination of idarucizumab and investigate the influence of idarucizumab on the pharmacokinetics (PK) of dabigatran and vice versa. Studies were conducted in 5/6 nephrectomized rats, in human volunteers with and without renal impairment, and in a porcine liver trauma model. In both rats and humans, renal impairment increased idarucizumab exposure and initial half-life but did not affect its terminal half-life. Urinary excretion of unchanged idarucizumab increased with increasing idarucizumab dose, suggesting saturation of renal tubular reuptake processes at higher doses. The PK of idarucizumab was unaffected by dabigatran. In contrast, idarucizumab administration resulted in redistribution of dabigatran to the plasma, where it was bound and inactivated by idarucizumab. Urinary excretion of dabigatran after administration of idarucizumab was delayed, but total dabigatran excreted in urine was unaffected. Idarucizumab and dabigatran were eliminated together via renal pathways.
Keywords: clinical pharmacokinetics, elimination, renal clearance
Introduction
The oral anticoagulant dabigatran etexilate, prodrug of the direct thrombin inhibitor dabigatran, is widely used for the prevention of stroke and systemic embolism in patients with nonvalvular atrial fibrillation and for the treatment and secondary prevention of venous thromboembolism.1–5 Dabigatran is predominantly eliminated unchanged by renal excretion; hence, dabigatran exposure is increased in patients with renal impairment, and dose adjustment may be required.6,7
Patients being treated with anticoagulant therapies to prevent thromboembolic events are at increased risk of life-threatening bleeding.8 Reversal of anticoagulation may be required in such cases or when urgent surgery is needed. Idarucizumab, a humanized monoclonal antibody fragment (Fab) directed against dabigatran, was developed to provide rapid and sustained reversal of dabigatran-mediated anticoagulation in emergency situations.9–11 The efficacy and safety of idarucizumab have been demonstrated in studies in healthy volunteers12,13 and in patients who have life-threatening bleeds or those in need of emergency interventions.14 Additionally, in a preclinical, blunt-trauma model, idarucizumab significantly reduced blood loss and mortality.15,16
The pharmacokinetics (PK) of idarucizumab in the absence and presence of dabigatran has been studied in human volunteers,12,17 in rat models,11 and in large animal models of severe blood loss15,16,18; these studies showed that idarucizumab is eliminated primarily via the kidney. In the clinical setting, idarucizumab is administered to patients treated with dabigatran, and thus, it is important to understand how the PK and, in particular, elimination of both idarucizumab and dabigatran are affected by the presence of the other drug, especially in patients with impaired renal function. The studies described in this article were therefore conducted to investigate the elimination of idarucizumab both in healthy volunteers and in animal models, focusing on idarucizumab and dabigatran elimination either alone or together, and the influence of renal impairment and the effect of severe hemorrhagic shock. The aim of this analysis was to characterize the renal elimination of idarucizumab and to investigate the influence of idarucizumab on the PK of dabigatran and vice versa.
Materials and Methods
For all of the following studies, idarucizumab (Praxbind) solution for injection/infusion, matching placebo, dabigatran for intravenous infusion, and dabigatran etexilate (Pradaxa) capsules for oral administration were provided by Boehringer Ingelheim Pharma GmbH & Co KG, Biberach an der Riß, Germany. Plasma and urine samples obtained during the studies were analyzed for total dabigatran concentrations using validated liquid chromatography tandem mass spectrometry methods (Nuvisan GmbH; Neu-Ulm, Germany). Total idarucizumab concentrations were determined with validated enzyme-linked immunosorbent assay methods (ELISA; performed at Covance Laboratories, Inc, Chantilly, Virginia, for human samples and at Boehringer Ingelheim Pharmaceuticals Inc, Ridgefield, Connecticut, for animal samples). The concentration of dabigatran that was not bound by idarucizumab (ie, unbound dabigatran), referred to as “active dabigatran,” was determined using a diluted thrombin time clotting assay. These methods are described elsewhere.6,17
Normal and 5/6 Nephrectomized Rat Model
Experiments were conducted in accordance with US legislation governing animal studies or, after approval, by the local institutional animal care and use committee, and animals were allowed food and water ad libitum. The PK and urinary excretion of dabigatran and idarucizumab were examined in Wistar Han rats following the methods described previously,11 and in a 5/6 nephrectomized model of renal impairment, also in Wistar Han rats.19
Six groups of male rats with normal renal function (total N = 21) received an intravenous bolus dose of dabigatran 0.2 mg kg−1, idarucizumab 20 mg kg−1 (ie, equimolar to 0.2 mg kg−1dabigatran), or dabigatran 0.2 mg kg−1 followed 15 minutes later by idarucizumab 20 mg kg−1. In 3 of the groups, urine samples were collected up to 48 hours after dosing to determine urinary excretion of dabigatran (n = 3), idarucizumab (n = 3), and idarucizumab plus dabigatran (n = 3). In the other 3 groups, plasma samples were collected up to 24 hours after dosing to determine the PK of dabigatran (n = 3) and idarucizumab (n = 3) and any PK interaction between idarucizumab and dabigatran (N = 6).
The 5/6 nephrectomized rat study included a total of 10 groups (total N = 40); 5 groups of sham-operated (control) male rats (n = 4 each) and 5 groups of 5/6 nephrectomized male rats (n = 4 each). Two groups each of control and nephrectomized rats received an intravenous bolus of dabigatran 0.2 mg kg−1, 2 groups each received idarucizumab 20 mg kg−1, and 1 group each received indoxyl sulfate 10 mg kg−1 as a positive marker of renal excretion. Serial plasma samples were collected for PK analysis for the different dabigatran and idarucizumab regimens, and urine samples were collected up to 48 hours after dosing from 4 groups of rats for analysis of urinary excretion of all the administered compounds.
Pharmacokinetic data from the rat studies were analyzed using noncompartmental methods. The initial half-life of idarucizumab was calculated by a 2-compartment model using Phoenix WinNonlin (Certara USA Inc, Princeton, New Jersey) software. Urinary excretion of both idarucizumab and dabigatran is presented as a cumulative fraction of the unchanged drug excreted into urine over specified time intervals.
Porcine Model With Blunt Liver Injury
The relationship between urinary output and hemorrhagic shock following the administration of dabigatran and idarucizumab was examined using data from previously described experiments conducted in a lethal porcine blunt liver trauma model.15,16,18 Here, we present previously unpublished data on urinary output and elimination of dabigatran in this model from these studies. Experiments were conducted in accordance with German legislation governing animal studies, and animals were allowed food and water ad libitum. As described in detail elsewhere,18 dabigatran etexilate (30 mg kg−1 twice daily, n = 24) or placebo (n = 6) was administered orally to male pigs for 3 days. On day 4, the pigs were anesthetized and given a 90-minute intravenous infusion of dabigatran (0.38 mg kg−1 over 30 minutes and 0.52 mg kg−1 over 60 minutes), resulting in a peak dabigatran concentration of 1161 ± 372 ng mL−1 prior to induction of a standardized blunt liver injury.15,16 Animals were randomized and then received infusions of 30, 60, or 120 mg kg−1 idarucizumab or placebo (saline) 15 minutes postinjury. Blood loss was recorded at 12, 30, 60, 120, and 240 minutes posttrauma or until death, if sooner. Urine was collected continuously via bilateral catheterization of the ureters. Volume of urinary output was measured for 0 to 60, 60 to 120, and 120 to 240 minutes posttrauma or until death, if sooner. Plasma samples for analysis of total dabigatran and idarucizumab concentrations were also taken at the time of trauma and then at intervals until death or for up to 240 minutes.
Human Volunteers
In humans, the safety, tolerability, and efficacy of idarucizumab for the reversal of dabigatran anticoagulant activity have been studied in a randomized, placebo-controlled, double-blind, phase 1 trial in healthy male volunteers.12 In addition, the effects of age and renal function on idarucizumab PK and idarucizumab-mediated reversal of dabigatran anticoagulant activity have been evaluated in a randomized, double-blind, phase 1b crossover study in middle-aged, elderly, and renally impaired volunteers.20,21 Here, we evaluate the elimination of dabigatran and idarucizumab in these studies, presenting previously unpublished data. The methods for each study are described in detail elsewhere.12,20,21 The protocols for these studies were approved by independent ethics committees, and the studies were carried out in accordance with the principles of the Declaration of Helsinki, the International Conference on Harmonization Good Clinical Practice guidelines, and other applicable regulatory requirements. All volunteers provided written informed consent.
In the healthy, male volunteer study, the cumulative urinary excretion of idarucizumab was determined in 3 groups of volunteers who had received 5-minute idarucizumab infusions of 1 g (n = 6), 2 g (n = 6), or 4 g (n = 6), and in 3 groups of volunteers who had received oral dabigatran etexilate 220 mg twice daily for 3 days, with 5-minute idarucizumab infusions of 1 g (n = 9), 2 g (n = 9), or 4 g (n = 8) administered 1 hour and 55 minutes after a final oral dose of dabigatran etexilate 220 mg on the fourth day. Urine sampling for PK analysis was undertaken over prespecified intervals up to 72 hours after idarucizumab infusion.
The middle-aged, elderly, and renally impaired volunteer study enrolled 46 patients: 12 middle-aged (45-64 years), 16 elderly (65-80 years), 12 with mild renal impairment (creatinine clearance 60-90 mL min−1), and 6 with moderate renal impairment (creatinine clearance 30-60 mL min−1). Volunteers received dabigatran etexilate 220 or 150 mg, according to their renal function, twice daily for 3.5 days. Idarucizumab doses of 1, 2.5, 5, and 2 × 2.5 g 1 hour apart, or placebo, were administered as a rapid (5-minute) infusion 1 hour and 55 minutes after the last dabigatran etexilate dose. Urine sampling for PK analyses in this study was performed over discrete time intervals until 26, 74, and 122 hours after the last intake of dabigatran etexilate. The PK parameter reported for idarucizumab and dabigatran is the fraction of the dose recovered in urine (fe), expressed as the geometric mean.
Statistical Methods
Descriptive statistics were utilized, and data were presented as geometric mean values unless otherwise stated.
Results
Impact of Renal Impairment on the Plasma PK of Dabigatran
In 5/6 nephrectomized rats, dabigatran plasma exposure, which was measured as area under the concentration–time curve (AUC0–∞), increased by 1.89-fold, its clearance decreased by 48%, and terminal half-life increased by 1.65-fold when compared to sham-operated rats with normal renal function (Table 1).
Table 1.
Mean (±SD) Plasma PK Parameters of Dabigatran and Idarucizumab Following Intravenous Bolus Dosing in 5/6 Nephrectomized Rats and in Sham-Operated Rats With Normal Renal Function.
| Dabigatran | Idarucizumab | |||||
|---|---|---|---|---|---|---|
| Sham-Operated, n = 3 | 5/6 Nephrectomized, n = 4 | Change in 5/6 Nephrectomized vs Sham | Sham- Operated, n = 3 | 5/6 Nephrectomized, n = 3 | Change in 5/6 Nephrectomized vs Sham | |
| AUC0–∞, nMh | 539 ± 120 | 1020 ± 145 | 1.89-fold increasea | 3700 ± 209 | 8580 ± 1890 | 2.32-fold increasea |
| Clearance, mL min− 1 kg− 1 | 13.6 ± 3.27 | 7.04 ± 1.09 | 48% decreasea | 1.89 ± 0.109 | 0.838 ± 0.168 | 56% decreasea |
| Terminal t1/2, hours | 0.851 ± 0.225 | 1.40 ± 0.256 | 1.65-fold increasea | 6.58 ± 0.514 | 6.29 ± 0.351 | No change |
| Initial t1/2, hours | Not determined | Not determined | Not determined | 0.243 ± 0.017 | 0.605 ± 0.142 | 2.5-fold increasea |
Abbreviations: AUC0–∞, area under the plasma concentration–time curve from time zero to infinity; PK, pharmacokinetics; SD, standard deviation; t1/2, half-life.
aP < .05 for 1-tailed t test.
Impact of Renal Impairment on the Plasma PK of Idarucizumab
Idarucizumab plasma exposure, as measured by AUC0–∞, increased by 2.32-fold and clearance decreased by 56% in 5/6 nephrectomized rats, when compared to sham-operated rats with normal renal function (see Table 1). There was also a 2.5-fold increase in the initial half-life of idarucizumab in 5/6 nephrectomized versus sham-operated rats; however, there was no difference in terminal half-life between nephrectomized and control animals.
Plasma PK of Coadministered Idarucizumab and Dabigatran
When idarucizumab and dabigatran were administered together in rats with normal renal function and would therefore be present in the plasma as a complex, the dabigatran–idarucizumab complex was cleared similar to idarucizumab in the absence of dabigatran. The idarucizumab plasma concentration–time profiles were similar in complex with dabigatran and without dabigatran (see Figure 1a). Immediately after idarucizumab injection, the plasma concentration of total dabigatran increased rapidly (see Figure 1b). Total dabigatran represents both dabigatran bound to idarucizumab and any active, unbound dabigatran, a finding that is consistent with the redistribution of dabigatran from the periphery to plasma due to the formation of idarucizumab–dabigatran complexes in plasma and the corresponding reduction in unbound (ie, active) dabigatran concentrations.
Figure 1.
Mean (±SD) plasma concentration–time profiles of idarucizumab (A) and dabigatran (B) in rats with normal renal function after intravenous bolus administration (separately or together; n = 3 each group). SD indicates standard deviation.
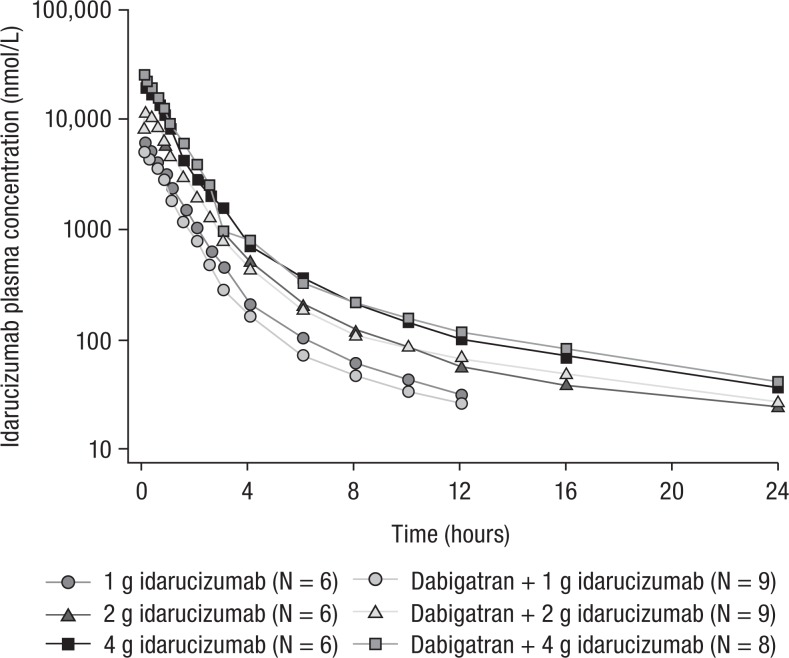
In healthy human volunteers, similar idarucizumab plasma concentration–time profiles with or without dabigatran confirmed that binding to dabigatran does not change the PK of idarucizumab in humans either (see Figure 2).
Figure 2.
Geometric mean idarucizumab plasma concentration–time profiles after a single 5-minute infusion of 1 to 4 g idarucizumab with or without 220 mg dabigatran etexilate twice daily in healthy human volunteers.
Urinary Excretion of Idarucizumab and Dabigatran
In rats with normal renal function, the fraction of both idarucizumab and dabigatran doses excreted into urine was greatest in the first 8 hours after the respective drug administration and then decreased during 48 hours postdose (see Figure 3). The cumulative urinary excretion of idarucizumab was similar between rats given idarucizumab alone and rats given idarucizumab after dabigatran treatment (0-48 hours mean ± standard deviation [SD]: 14.5% ± 6.9% versus 20.8% ± 6.7%, respectively; see Figure 3). The cumulative urinary excretion of dabigatran was also similar in the presence and absence of idarucizumab (0-48 hours mean ± SD: 59.3% ± 17.1% versus 57.2% ± 30.6%, respectively; see Figure 3). However, there appeared to be a trend for a delay in dabigatran excretion in the presence of idarucizumab, with about 10% less of the dose excreted during the first 8 hours and a corresponding increase of approximately 10% in the subsequent 8- to 24-hour interval (see Figure 3).
Figure 3.
Mean (±SD) percentage of idarucizumab and dabigatran excreted into the urine after intravenous dosing of dabigatran (0.2 mg/kg) or idarucizumab (20 mg/kg) alone or together (dabigatran dosing followed by idarucizumab 15 minutes later) in rats with normal renal function. SD indicates standard deviation.
In healthy volunteers treated with idarucizumab alone, urinary excretion of idarucizumab increased with increasing doses,12,17 from 10.7% of the 1-g dose to 38.9% of the 4-g dose (see Table 2). The presence of dabigatran, and thus excretion of idarucizumab as dabigatran–idarucizumab complexes, did not affect its urinary excretion. Likewise, idarucizumab administration did not change the total fraction of the dabigatran dose excreted in urine during the 72 hours after dosing (see Figure 4).
Table 2.
The Geometric Mean (gCV [Percentage of Dose]) Cumulative Urinary Excretion of Idarucizumab by Treatment (fe) Following Administration of Idarucizumab With or Without Dabigatran Etexilate in Healthy Volunteers 12,17 and in Middle-Aged, Elderly, and Renally Impaired Patients.13,21,a
| Idarucizumab Dose Group | |||||||
|---|---|---|---|---|---|---|---|
| Healthy Male Volunteers | |||||||
| 1 g, 5 minutes, n = 6 | 2 g, 5 minutes, n = 6 | 4 g, 5 minutes, n = 6 | 1 g, 5 minutes + DE, n = 9 | 2 g, 5 minutes + DE, n = 9 | 4 g, 5 minutes + DE, n = 8 | ||
| fe0–4, % | 10.7 (76.3) | 19.1 (73.1) | 38.9 (23.6) | 8.18 (90.6) | 26.2 (23.4) | 40.2 (30.5) | |
| Middle-aged | Elderly | Mild RI | Moderate RI | ||||
| 2.5 g, n = 6 | 5 g, n = 6 | 1 g, n = 8 | 5 g, n = 8 | 1 g, n = 6 | 5 g, n = 6 | 2 × 2.5 g, n = 6 | |
| fe0–6, % | 25.8 (32.6) | 32.1 (60.0) | 9.4 (69.0) | 39.8 (13.7) | 12.4 (46.7) | 32.0 (48.9) | 30.0 (89.0) |
Abbreviations: DE, dabigatran etexilate 220 mg twice daily; feX−Y, fraction of dose excreted in urine from X to Y hours after administration; gCV, geometric mean coefficient of variation; RI, renal impairment.
aThese data have been published elsewhere (for healthy volunteers12,17, middle-aged and elderly renally impaired patients13,21). Healthy male volunteers, middle-aged patients, and elderly patients aged 18 to 45 years, 45 to 64 years and 65 to 80 years, respectively; creatinine clearance 60 to 90 mL/min for mild RI and 30 to 60 mL/min for moderate RI.
Figure 4.
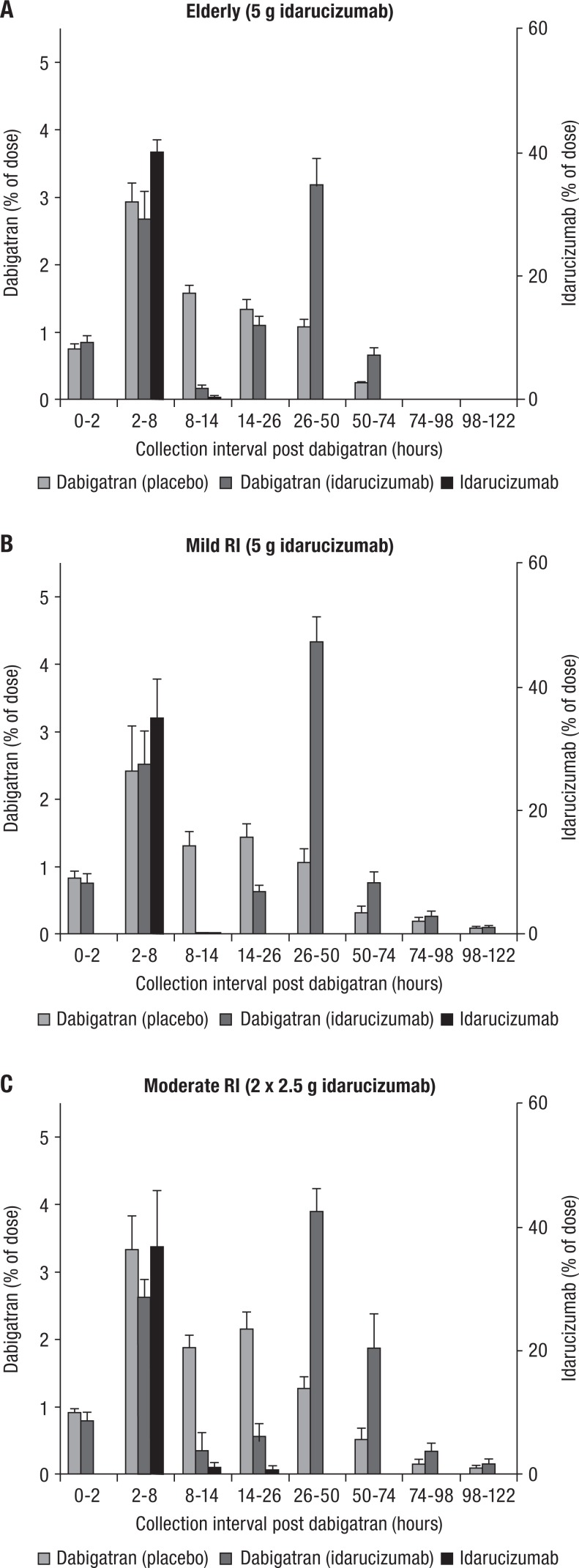
Mean fraction (percentage of dose ± SEM) of dabigatran dose and idarucizumab dose excreted in urine in elderly (A), volunteers with mild RI (B), and moderate RI (C). Volunteers received idarucizumab or placebo 2 hours after the last dabigatran etexilate dose; urine collection time intervals are shown as time from the last dose of dabigatran etexilate. Elderly patients aged 65 to 80 years; creatinine clearance 60 to 90 mL/min for mild RI and 30 to 60 mL/min for moderate RI.
Effect of Renal Impairment on Urinary Excretion of Idarucizumab and Dabigatran
The cumulative urinary excretion of dabigatran (% of the dose) was lower in 5/6 nephrectomized rats at 32.1% of the dose, compared to 45.1% in sham-operated rats (see Figure 5a). However, the cumulative percentages of the idarucizumab dose excreted unchanged in the urine were similar between 5/6 nephrectomized and control rats (12.6% and 15.8%, respectively; see Figure 5b), suggesting that urinary excretion of unchanged idarucizumab was not the major mechanism of idarucizumab elimination at the dose administered and that this process was not dependent on renal impairment.
Figure 5.
Mean (±SD) cumulative percent of dose of dabigatran (A) and idarucizumab (B) excreted into the urine in 5/6 nephrectomized rats and in rats with normal renal function. SD indicates standard deviation.
In all of the idarucizumab treatment groups in the human studies, most of the urinary excretion of idarucizumab occurred between 0 and 6 hours postinfusion (corresponding to 2-8 hours after the last dabigatran intake), with negligible amounts detected at subsequent collection intervals (see Figure 4). Urinary excretion of dabigatran in the absence of idarucizumab occurred with a peak between 2 and 8 hours after drug intake and with a slow decrease in the amounts of dabigatran in urine over time over the first 50 hours. Trace amounts of dabigatran were detectable up to 74 to 122 hours, depending on renal function (see Figure 4). In contrast, the time course of dabigatran urinary excretion when complexed with idarucizumab in plasma resulted in 2 peaks, the first peak at 2 to 8 hours after dabigatran intake (0-6 hours post-idarucizumab), and a second large peak at 26 to 50 hours, with relatively lower amounts excreted in between (see Figure 4). This pattern of dabigatran urinary excretion was observed across all ages and levels of renal function tested (see Figure 4), suggesting a partial delay in dabigatran excretion, that is, a second peak. However, the total fraction of dabigatran dose excreted into urine after dosing was similar in the presence or absence of idarucizumab in elderly patients (8.4% and 7.7%, respectively), those with mild renal impairment (8.6% and 6.8%), and those with moderate renal impairment (9.9% and 9.6%).
The urinary excretion of idarucizumab was not greatly affected by older age or renal impairment in humans (see Table 2).20,21 Considering the reduced plasma clearance with renal impairment, this suggests renal catabolism as a major route of elimination. As in healthy volunteers, urinary excretion was also dose dependent in elderly volunteers and volunteers with mild renal impairment, for example, with a fraction of the idarucizumab dose excreted in urine ( fe0–6) of 9.4% and 39.8% following administration of 1 and 5 g idarucizumab to elderly patients, respectively (see Table 2).20,21
Effect of Hemorrhagic Shock on Urinary Excretion of Idarucizumab
As previously reported, in a porcine blunt liver trauma model, blood loss in animals anticoagulated with dabigatran approximately doubled during the first 12 minutes after injury compared to controls.15,16 Hemostasis was restored, and total blood loss was significantly reduced when idarucizumab was administered to reverse the anticoagulant effect of dabigatran.15
In control animals that did not receive dabigatran, urinary output was low during the first 2 hours posttrauma but was restored by 4 hours (see Figure 6). In dabigatran-treated animals, urinary volume remained low over 4 hours (see Figure 6), presumably due to ongoing blood loss and hemorrhagic shock, resulting in low kidney perfusion. Administration of idarucizumab resulted in termination of bleeding and restored kidney perfusion.15,16 Urinary output after administration of idarucizumab was comparable to placebo-treated animals (see Figure 6), presumably due to recovery of hemodynamics and kidney perfusion. The plasma concentration of total dabigatran and idarucizumab decreased in an approximately parallel manner during the terminal 2 to 3.5 hours of elimination, suggesting their elimination as a complex (see Figure 7).
Figure 6.
Mean changes in urinary volume (±SD) as a consequence of trauma-induced blood loss and its reversal with idarucizumab in a porcine trauma model (n = 3-7 per group). SD indicates standard deviation.
Figure 7.
Comparison of plasma concentration–time profiles of total dabigatran, active dabigatran, and idarucizumab after intravenous dosing with idarucizumab 15 minutes after dabigatran administration in a porcine trauma model. Idarucizumab was administered at doses of 30 mg/kg (A), 60 mg/kg (B), and 120 mg/kg (C); data points are mean values (±SD; n = 6 animals for each dose). SD indicates standard deviation.
Discussion
Elimination of both idarucizumab and dabigatran occurs primarily via renal pathways. In clinical use in emergency situations, both compounds are present simultaneously in plasma, and frequently treated patients have compromised renal function. These studies therefore examined the consequences of the binding of idarucizumab to dabigatran with respect to PK in plasma and also to renal elimination. In addition, the impact of renal impairment was investigated.
The PKs of idarucizumab were unaffected by the presence of dabigatran. In contrast, idarucizumab administration resulted in redistribution of dabigatran to the plasma, where it was bound and inactivated by idarucizumab. Idarucizumab and dabigatran appear to be eliminated as a complex, and total dabigatran excreted in urine was unaffected by idarucizumab. At higher doses of idarucizumab, saturation of renal tubular reuptake processes seems to occur, resulting in increased urinary excretion of idarucizumab with increasing dose. Renal impairment increased both dabigatran and idarucizumab exposures. In the blunt liver injury animal model, the profound decrease in renal function associated with extreme blood loss was restored after idarucizumab treatment reestablished normal hemostasis in animals treated with dabigatran. These findings were consistent across animal models and human volunteer studies and build on existing knowledge of the PK of dabigatran and its reversal agent idarucizumab.7,17,22
Idarucizumab is a humanized monoclonal Fab.9 Although whole immunoglobulin G (IgG) molecules may be retained in the body for days or weeks (eg, mean residence time ∼8 days), Fab molecules can be cleared up to 35 times faster.23 In contrast to whole IgGs, smaller proteins such as Fab with molecular weights lower than ∼60 kDa can undergo glomerular filtration and are consequently excreted into the urine or catabolized and recycled back into the blood through the kidney.24 The renal elimination of Fabs appears to be mediated by a number of pathways. In mouse models, radiolabeled Fab was taken up in the renal cortical region and subsequently catabolized into amino acids.25 Findings in other studies in mice and human patients have also shown that radiolabeled Fab was predominantly taken up and subsequently catabolized in the kidneys.26,27 This occurred via receptor-mediated endocytosis (“reuptake”) in the proximal tubule and subsequent catabolism in lysosomes.28 If this receptor-mediated process was saturated due to excess Fab or other protein/amino acids, then increased urinary excretion of unchanged Fab would result.26,27 Reuptake of filtered proteins can be mediated by megalin and cubilin receptors on the apical membranes of renal tubular cells in mouse models,28,29 and there is evidence that reuptake of Fabs may also be facilitated by these receptors.30
Idarucizumab is a 47.8-kDa protein and is therefore a candidate for elimination via renal catabolism, in which renal filtration in the glomeruli is followed by reuptake in the proximal renal tubular cells and subsequent catabolism. When the renal tubular reuptake mechanism becomes saturated, for example, following administration of the 5-g dose, excess idarucizumab will be excreted in the urine. This model therefore explains the dose-dependent increase in the fraction of idarucizumab dose excreted into urine.20 The systemic clearance of idarucizumab was found to be constant across the dose levels investigated, which indicates that although a reduced fraction of the dose is catabolized as a result of saturation, this is compensated for by excretion of an increased fraction of the dose unchanged in the urine.
In animal models and human volunteers with renal impairment and slower glomerular filtration, due to a reduced number of functional nephrons, there was reduced plasma clearance of idarucizumab. This observation suggests that idarucizumab elimination is largely controlled by the rate of glomerular filtration. This is consistent with findings both in volunteers with renal impairment20,21 and in 5/6 nephrectomized rats, where the time taken to clear the bulk of the idarucizumab dose (ie, the initial half-life of the drug) is prolonged. During the subsequent terminal phase of excretion in these studies, however, when the plasma concentration of idarucizumab was extremely low, idarucizumab was not detected in urine. The observation that the terminal half-life of idarucizumab during this phase was not affected by renal impairment suggests that distributional processes rather than renal elimination were rate limiting for idarucizumab elimination.
Renal impairment also increased dabigatran exposure in the rat model. These observations are consistent with findings of the increased dabigatran exposure in human volunteers with renal impairment in the absence of idarucizumab. In these subjects, after treatment with dabigatran etexilate 150 mg twice daily, peak total dabigatran plasma levels were greater with moderate renal impairment than with mild impairment,20,21 with geometric mean concentrations of up to ∼300 and ∼195 ng mL−1, respectively.
Idarucizumab binds dabigatran in plasma to form a stable complex with minimal dissociation.9,11 Urinary excretion of idarucizumab was unaffected by coadministration of dabigatran, which suggests that idarucizumab elimination either alone or as dabigatran–idarucizumab complexes occurs by the same mechanism. This is consistent with the fact that the idarucizumab molecule is ∼100-fold larger than the dabigatran molecule, and the size of the dabigatran–idarucizumab complex is essentially the same as that of unbound idarucizumab.
In humans, in the absence of idarucizumab, ∼80% to 85% of dabigatran in the plasma is excreted into urine, proportionally to plasma concentrations.6 Importantly, the same amount of dabigatran was recovered in urine irrespective of whether idarucizumab was present or not. The partial delay in dabigatran urinary excretion when idarucizumab is present provides indirect evidence that dabigatran and idarucizumab are eliminated together, and dabigatran is not released from the dabigatran–idarucizumab complexes or excreted in the urine until idarucizumab is catabolized. This is supported by the observation that no release of dabigatran back into plasma following renal catabolism of idarucizumab was observed. In addition, there was no evidence of any safety concerns or treatment-related adverse events associated with idarucizumab administration observed in the studies reported.
During major bleeding episodes, a number of compensatory physiological changes take place in an attempt to maintain blood pressure and blood flow to prevent hypoxia in vital organs.31,32 Decreased circulating volume as a result of hemorrhage triggers neural reflexes that result in an increase in heart rate, vasoconstriction, and redistribution of blood flow away from nonvital organs including the skin, gastrointestinal tract, and kidneys.33 At the same time, stimulation of corticotrophin-releasing hormone leads to the release of a number of hormones, including vasopressin that acts on the kidneys, causing water retention at the distal tubules; in addition, renin is released, leading to increased aldosterone secretion and consequently to sodium ion and water resorption.33 As a result, urinary output also decreases in order to maintain the circulating blood volume in a fluid-centralization process32 as was observed in the porcine trauma model. Restoration of hemostasis in the dabigatran-treated porcine trauma model following idarucizumab treatment resulted in the return of urinary volume to levels comparable to those in control animals, although it is important to note that this effect was due to the restoration of fluid balance from stopping blood loss rather than from any direct effect of idarucizumab on renal function. The elimination of idarucizumab and dabigatran showed parallel plasma concentration–time profiles in this model, suggesting again that the 2 agents were cleared from plasma together as dabigatran–idarucizumab complexes.
Data Limitations
Only subjects with mild and moderate renal impairment have been included in the present studies, and the effects in patients with severe renal impairment are not known.1 Animal models may not accurately reflect the human PK of dabigatran or idarucizumab. For example, the proportion of dabigatran cleared via biliary excretion is larger in rats than in humans. However, as the effects of trauma or of severe loss of renal function cannot be evaluated in humans, the cross-species models employed provide insights that cannot be otherwise obtained in humans and enable reproducible evaluation of the effects of such injuries.
Conclusion
Idarucizumab and dabigatran are both eliminated via the renal system. When coadministered, they are eliminated together in the form of dabigatran–idarucizumab complexes. Reuptake followed by catabolism in the proximal renal tubules appears to be a key elimination mechanism for idarucizumab; however, saturation of renal tubular reuptake after the clinical dose of idarucizumab also results in direct excretion into the urine. The partial delay in dabigatran elimination in the presence of idarucizumab is likely to result from reuptake of the complex into the renal tubule cells followed by catabolism of idarucizumab and consequent release of dabigatran from the complex into the urine. Importantly, the total amount of dabigatran excreted in the urine was unaffected by the presence of idarucizumab. In a model of severe trauma-associated bleeding, renal function could also be restored by reversing dabigatran activity and subsequently stopping bleeding.
Acknowledgments
The authors would like to thank the following for their contributions to the data and studies described in this manuscript: Stephen Norris and Kelly Coble (Boehringer Ingelheim Pharmaceuticals, Inc) and Dietmar Gansser (Boehringer Ingelheim GmbH & Co KG) for PK measurements and Benjamin Lang (Boehringer Ingelheim GmbH & Co KG) for statistical analyses. We also thank Wouter Haazen (previously at SGS Life Science Services; currently at Janssen Belgium), who was the principal investigator in the age and renal impairment study. Medical writing assistance, supported financially by Boehringer Ingelheim Pharma GmbH & Co KG, was provided by Emma Fulkes of PAREXEL during the preparation of this article.
Footnotes
Author Contributions: GG, who was the principal investigator for studies in rats, worked as a pharmacokineticist for the studies in the porcine model; SG, VM, and PR contributed to planning, conduct, data analysis, and interpretation of the clinical studies and undertook critical review of the manuscript. OG and MH conducted the porcine trauma model studies. All authors contributed to the interpretation of the mechanism of elimination for idarucizumab and dabigatran and manuscript preparation.
Declaration of Conflicting Interests: The author(s) declared the following potential conflict of interest with respect to the research, authorship, and/or publication of this article: Stephan Glund, Viktoria Moschetti, and Joanne van Ryn are employees of Boehringer Ingelheim GmbH & Co KG. Guanfa Gan and Paul Reilly are employees of Boehringer Ingelheim Pharmaceuticals, Inc. Markus Honickel has received travel support from Boehringer Ingelheim GmbH & Co KG. Oliver Grottke has received research funding from Boehringer Ingelheim GmbH & Co KG, Novo Nordisk, Biotest, CSL Behring, and Nycomed; and honoraria for lectures and consultancy support from Baxalta, Bayer HealthCare, Boehringer Ingelheim GmbH & Co, CSL Behring, Octapharma, Sanofi, Pfizer, and Portola Pharmaceuticals Inc.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was funded by Boehringer Ingelheim Pharma GmbH & Co KG.
References
- 1. Pradaxa. Summary of product characteristics. Boehringer Ingelheim. http://www.medicines.org.uk/emc/medicine/24839. Updated October 19, 2017. Accessed November 23, 2017.
- 2. You JJ, Singer DE, Howard PA, et al. Antithrombotic therapy for atrial fibrillation: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl 2):e531S–e575S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schulman S, Kearon C, Kakkar AK, et al. ; RE-COVER Study Group. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med. 2009;361(24):2342–2352. [DOI] [PubMed] [Google Scholar]
- 4. Schulman S, Kearon C, Kakkar AK, et al. ; RE-MEDY Trial Investigators; RE-SONATE Trial Investigators. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N Engl J Med. 2013;368(8):709–718. [DOI] [PubMed] [Google Scholar]
- 5. Schulman S, Kakkar AK, Goldhaber SZ, et al. ; RE-COVER II Trial Investigators. Treatment of acute venous thromboembolism with dabigatran or warfarin and pooled analysis. Circulation. 2014;129(7):764–772. [DOI] [PubMed] [Google Scholar]
- 6. Stangier J, Rathgen K, Stahle H, Gansser D, Roth W. The pharmacokinetics, pharmacodynamics and tolerability of dabigatran etexilate, a new oral direct thrombin inhibitor, in healthy male subjects. Br J Clin Pharmacol. 2007;64(3):292–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stangier J. Clinical pharmacokinetics and pharmacodynamics of the oral direct thrombin inhibitor dabigatran etexilate. Clin Pharmacokinet. 2008;47(5):285–295. [DOI] [PubMed] [Google Scholar]
- 8. Ageno W, Gallus AS, Wittkowsky A, Crowther M, Hylek EM, Palareti G. Oral anticoagulant therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl 2):e44S–e88S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Eikelboom JW, Quinlan DJ, van Ryn J, Weitz JI. Idarucizumab: the antidote for reversal of dabigatran. Circulation. 2015;132(25):2412–2422. [DOI] [PubMed] [Google Scholar]
- 10. Praxbind. Summary of product characteristics. Boehringer Ingelheim. http://www.medicines.org.uk/emc/medicine/31243. Updated November 9, 2017. Accessed December 1, 2017.
- 11. Schiele F, van Ryn J, Canada K, et al. A specific antidote for dabigatran: functional and structural characterization. Blood. 2013;121(18):3554–3562. [DOI] [PubMed] [Google Scholar]
- 12. Glund S, Stangier J, Schmohl M, et al. Safety, tolerability, and efficacy of idarucizumab for the reversal of the anticoagulant effect of dabigatran in healthy male volunteers: a randomised, placebo-controlled, double-blind phase 1 trial. Lancet. 2015;386(9994):680–690. [DOI] [PubMed] [Google Scholar]
- 13. Glund S, Stangier J, van Ryn J, et al. Restarting dabigatran etexilate 24 h after reversal with idarucizumab and redosing idarucizumab in healthy volunteers. J Am Coll Cardiol. 2016;67(13):1654–1656. [DOI] [PubMed] [Google Scholar]
- 14. Pollack CV, Jr, Reilly PA, Eikelboom J, et al. Idarucizumab for dabigatran reversal. N Engl J Med. 2015;373(6):511–520. [DOI] [PubMed] [Google Scholar]
- 15. Grottke O, Honickel M, van RJ, ten Cate H, Rossaint R, Spronk HM. Idarucizumab, a specific dabigatran reversal agent, reduces blood loss in a porcine model of trauma with dabigatran anticoagulation. J Am Coll Cardiol. 2015;66(13):1518–1519. [DOI] [PubMed] [Google Scholar]
- 16. Honickel M, Treutler S, van RJ, Tillmann S, Rossaint R, Grottke O. Reversal of dabigatran anticoagulation ex vivo: porcine study comparing prothrombin complex concentrates and idarucizumab. Thromb Haemost. 2015;113(4):728–740. [DOI] [PubMed] [Google Scholar]
- 17. Glund S, Moschetti VF, Norris S, et al. A randomized study in healthy volunteers to investigate the safety, tolerability and pharmacokinetics of idarucizumab, a specific antidote to dabigatran. Thromb Haemost. 2015;133(5):943–951. [DOI] [PubMed] [Google Scholar]
- 18. Grottke O, van Ryn J, Spronk HM, Rossaint R. Prothrombin complex concentrates and a specific antidote to dabigatran are effective ex-vivo in reversing the effects of dabigatran in an anticoagulation/liver trauma experimental model. Crit Care. 2014;18(1):R27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Devada S, Patel M, Mishra V, et al. Novel model for renal failure and anaemia induced by 5/6 nephrectomy in Wistar rat. Int J Vet Sci. 2012;1:83–88. [Google Scholar]
- 20. Glund S, Stangier J, van Ryn J, et al. Effect of age and renal function on idarucizumab pharmacokinetics and idarucizumab-mediated reversal of dabigatran anticoagulant activity in a randomized, double-blind, crossover phase Ib study. Clin Pharmacokinet. 2017;56(1):41–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Glund S, Stangier J, Schmohl M, et al. Idarucizumab, a specific antidote for dabigatran: immediate, complete and sustained reversal of dabigatran induced anticoagulation in elderly and renally impaired subjects. Presented at: 56th ASH Annual Meeting and Exposition; December 6-9, 2014; San Francisco, CA, USA Abstract 344. [Google Scholar]
- 22. Blech S, Ebner T, Ludwig-Schwellinger E, Stangier J, Roth W. The metabolism and disposition of the oral direct thrombin inhibitor, dabigatran, in humans. Drug Metab Dispos. 2008;36(2):386–399. [DOI] [PubMed] [Google Scholar]
- 23. Covell DG, Barbet J, Holton OD, Black CD, Parker RJ, Weinstein JN. Pharmacokinetics of monoclonal immunoglobulin G1, F(ab’)2, and Fab’ in mice. Cancer Res. 1986;46(8):3969–3978. [PubMed] [Google Scholar]
- 24. Meibohm B, Zhou H. Characterizing the impact of renal impairment on the clinical pharmacology of biologics. J Clin Pharmacol. 2012;52(Suppl 1):54S–62S. [DOI] [PubMed] [Google Scholar]
- 25. Tsai SW, Li L, Williams LE, Anderson AL, Raubitschek AA, Shively JE. Metabolism and renal clearance of 111In-labeled DOTA-conjugated antibody fragments. Bioconjug Chem. 2001;12(2):264–270. [DOI] [PubMed] [Google Scholar]
- 26. Behr TM, Sharkey RM, Juweid ME, et al. Reduction of the renal uptake of radiolabeled monoclonal antibody fragments by cationic amino acids and their derivatives. Cancer Res. 1995;55(17):3825–3834. [PubMed] [Google Scholar]
- 27. Behr TM, Becker WS, Sharkey RM, et al. Reduction of renal uptake of monoclonal antibody fragments by amino acid infusion. J Nucl Med. 1996;37(5):829–833. [PubMed] [Google Scholar]
- 28. Christensen EI, Birn H, Storm T, Weyer K, Nielsen R. Endocytic receptors in the renal proximal tubule. Physiology (Bethesda). 2012;27(4):223–236. [DOI] [PubMed] [Google Scholar]
- 29. Amsellem S, Gburek J, Hamard G, et al. Cubilin is essential for albumin reabsorption in the renal proximal tubule. J Am Soc Nephrol. 2010;21(11):1859–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nagai J, Sato K, Yumoto R, Takano M. Megalin/cubilin-mediated uptake of FITC-labeled IgG by OK kidney epithelial cells. Drug Metab Pharmacokinet. 2011;26(5):474–485. [DOI] [PubMed] [Google Scholar]
- 31. Chatrath V, Khetarpal R, Ahuja J. Fluid management in patients with trauma: restrictive versus liberal approach. J Anaesthesiol Clin Pharmacol. 2015;31(3):308–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gutierrez G, Reines HD, Wulf-Gutierrez ME. Clinical review: hemorrhagic shock. Crit Care. 2004;8(5):373–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Udeani J. Hemorrhagic shock. Medscape. http://emedicine.medscape.com/article/432650-overview#a5. Updated March 27, 2015. Accessed September 9, 2016.