Abstract
Global hemostatic assays including thromboelastography (TEG), Innovance ETP (endogenous thrombin potential), and Thrombinoscope could measure thrombin generation potential and be useful to guide management of patients with factor VIII (FVIII) inhibitors. However, the performance characteristics of these global assays in the presence of FVIII inhibitors are incompletely characterized. In this study, the normal range of thrombin generation potential was measured in 20 healthy individuals by all 3 assays. In 5 commercial and 7 clinical samples with FVIII inhibitors, it was shown that PPP-reagent thrombinoscope shows a dose-dependent response to different levels of FVIII inhibitors from the same patients, while Innovance ETP shows virtually no response to FVIII inhibitors. The TEG is more sensitive to FVIII inhibitors than thrombinoscope. Importantly, we show the same levels of FVIII inhibitor from different patients results in different levels of inhibition for thrombin generation potential by thrombinoscope, which potentially explains the phenotypic heterogeneity of patients with FVIII inhibitors. Global assays such as thrombinoscope, but not Innovance ETP, show appropriate sensitivity to FVIII inhibitors that could offer an objective and clinically relevant marker to guide patient management.
Keywords: hemophilia, factor VIII inhibitor, global assays, thromboelastography, Innovance ETP, Thrombinoscope
Introduction
Patients with hemophilia A display phenotypic heterogeneity: Patients with the same levels of factor VIII (FVIII) activity or inhibitor titers may present with different bleeding tendencies.1 Blood coagulation is a complex physiological process, and the generation of thrombin is the outcome of coagulation cascade activation. The most widely used coagulation tests including the prothrombin time (PT) and the activated partial thromboplastin time (APTT) mainly assess the initiation phase of coagulation during which only 3% to 5% of thrombin is formed.2 Thus, these in vitro tests as well as clot-based FVIII activity and FVIII inhibitor assays do not always reflect the in vivo hemostatic conditions. In contrast, global hemostatic assays measure total thrombin generation, and thus recent guidelines have proposed using global coagulation assays to assess the risk of bleeding or thrombosis.3
Currently, there are 3 global assays that can measure or estimate thrombin generation potential, either from direct measurement or derivation from coagulation wave form analysis: thromboelastography (TEG), Innovance ETP (endogenous thrombin potential), and Thrombinoscope. The TEG can assess the efficiency of whole blood coagulation including the process of clot formation and fibrinolysis, reflecting the function of clotting factors, platelets, fibrinogen, and the fibrinolytic proteins. The kinetics of the process are recorded graphically.4 The parameter total thrombus generation (TTG), the total area under the velocity curve, is a derivative of the TEG waveform and a reasonable parameter for the assessment of thrombin generation. The Thrombinoscope assay is based on Calibrated Automated Thrombography (CAT). The Thrombinoscope assay utilizes fluorogenic substrate Z-Gly-Gly-Arg-AMC to monitor thrombin activity chromogenically. This fluorogenic substrate produces fluorescence at a wavelength of 460 nm.5,6 The parameter ETP, which corresponds to the area under the curve, represents the total enzymatic activity of thrombin produced. The thrombin generation curve reflects all 3 phases of coagulation (initiation, propagation, and termination). The ETP is considered the most predictive parameter for bleeding and thrombosis risk.7,8 Thrombinoscope-CAT assay has been used to assess the severity of the bleeding phenotype of hemophilia,9–12 the risk of venous thromboembolism,13 and monitoring oral anticoagulants.14 Innovance ETP assay is a commercially available chromogenic assay and reported automatically by the BCS XP System. This assay uses the H-β-Ala-Gly-Arg-pNA as a chromogenic substrate. Thrombin generation is recorded by monitoring the generation of the chromogenic substrate at a wavelength of 405 nm. The parameter ETP-the area under the curve (AUC), derived from the corrected substrate conversion curve, has been proved to correlate with the hemostatic state.15,16
The performance characteristics of these global assays are incompletely defined including their responses to FVIII inhibitors. It is also unclear how these assays perform in comparison to each other. The aim of our study was to perform a parallel comparison of these 3 global assays to assess their responses to FVIII inhibitors.
Materials and Methods
The study was approved by the Institutional Review Board of Johns Hopkins University (IRB00097630). Plasma samples from 20 healthy individuals (10 males and 10 females) and 7 patients with hemophilia A with different FVIII inhibitor levels (1091BU, 128BU, 75BU, 58BU, 20BU, 8BU, and<1BU) as well as 5 commercial samples with different levels of FVIII Inhibitors (6345-F8 inhibitor: <1BU, 6100-F8 inhibitor: 1BU, 6089-F8 inhibitor: 54BU, 6091-F8 inhibitor: 88BU and 6017-F8 inhibitor: 111BU) were used in the study. These 5 commercial plasmas are citrated human plasma derived from a congenital FVIII-deficient donor who has developed an antibody to FVIII. All Bethesda unit titers were measured using a standard 1-stage aPTT assay and the Nijmegen modification of the Bethesda assay on a Siemens XP coagulation analyzer. The commercial samples were purchased from George King Bio-Medical, Inc (Overland Park, Kansas). Normal pool plasmas were purchased from Precision Biological, Dartmouth, Nova Scotia, Canada.
Blood samples were collected into Vacutainer tubes (BD, Franklin Lakes, New Jersey) containing sodium citrate (3.2%) and were centrifuged twice at 2000 × g for 10 minutes at room temperature to obtain platelet-poor plasma. Plasma was stored at −80°C until the time of assay.
Plasmas from 5 commercial samples and 7 patients with hemophilia A with different FVIII inhibitor were diluted serially until plasma inhibitor levels were <1BU. The dilution was performed with normal plasma to keep FVIII level constant, and the only variable is different levels of specific FVIII inhibitors. After 2 hours incubation, thrombin generation potential was measured by 3 global assays.
Thromboelastography
The TEG assay was performed on the TEG 5000 Thrombelastograph Hemostasis Analyzer (Haemonetics Corporation, Braintree, Massachusetts) with TEG Analytical Software. According to the instruction manual of the instrument, after adding 20 μL of 0.2 mol/L calcium chloride (Haemonetics Corporation), all samples were activated by 340 µL kaolin. The TTG was calculated from the first derivative of the TEG waveform. Other parameters included the maximum rate of thrombus generation and time to maximum rate of thrombus generation.
Calibrated Automated Thrombography
The Thrombinoscope assay was performed on a microtiter plate using the Calibrated Automated Thrombogram assay (Thrombinoscope BV, Maastricht, the Netherlands). The CAT reagents (Thrombinoscope BV) contain PPP-reagent (a mixture of 4 pmol/L phospholipids and 5 pmol/L Tissue Factor), PPP-LOW reagent (a mixture of 4 pmol/L phospholipids and 1 pmol/L Tissue Factor), thrombin calibrator and FluCa-kit (a mixture of Fluo-Substrate and Fluo-Buffer). Plasma of 80 µL was added to reagents consisting of thrombin calibrator (20 µL) in one set of wells and a trigger (PPP-Reagent or PPP-Reagent LOW: 20 µL) in another set of wells to activate coagulation. All samples were run in triplicate. Thrombin generation was measured with the FluCa-kit. Several parameters can be derived from the thrombin generation curve, including endogenous thrombin potential (nmol/L/min; the area under the thrombin generation curve), lag time (the time from thrombin generation to reach one-sixth of the peak concentration), peak thrombin (nmol/L; the maximal height of the thrombogram), and the ttpeak (min; time to peak thrombin generation).
Innovance ETP
Innovance ETP assay was performed on a BCS XP (Siemens Healthcare Diagnostics Products GmbH, Marburg/Germany) according to the instruction manual of the instrument. Reagents include INNOVANCE ETP reagent (chromogenic substrate, fibrin aggregation inhibitor, salts, and stabilizers in aqueous solution), Innovance ETP calcium chloride solution, and Innovance ETP buffer. The first derivative of the corrected substrate conversion curve is obtained, which corresponds to the thrombin generation curve. The important parameters of the assay include ETP-AUC (the area under the thrombin generation curve), lag time (the time from starting the reaction to thrombin generation), ETP-AUC (endogenous thrombin potential-the area under the curve), and maximum thrombin generation (Cmax).
Results
Measuring Thrombin Generation With Healthy Individuals in 3 Global Assays
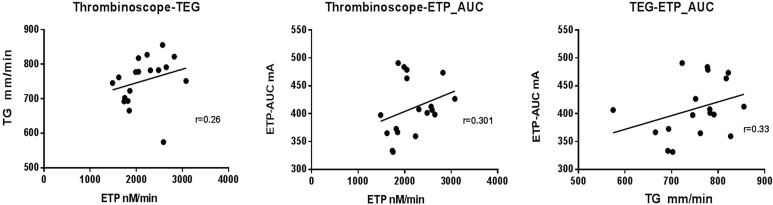
We compared thrombin generation in samples from 20 healthy individuals in 3 global assays: TEG, Thrombinoscope (PPP-reagent), and Innovance ETP. The corresponding parameters of each method are shown in Table 1. We tested whether there are correlations in thrombin generation measurements. As shown in Figure 1, although there appears to be a modestly positive correlation trend in thrombin generation potential, they are not statistically significant among ETP (nmol/L/min) by Thrombinoscope, TG (mm/min) by TEG, and ETP-AUC (mA) by Innovance ETP. We also ran thrombin generation potential by Thrombinoscope in normal pool plasmas 37 times to assess the Thrombinoscope assay’s precision. The means (standard deviations) of ETP were 1142.16 (±128.30) (nmol/L/min) with a coefficient of variation (CV) of 11.2%.
Table 1.
Thrombin Generation in Healthy Individuals With 3 Global Methods.
| TEG | Thrombinoscope | ETP-AUC | |||||||
|---|---|---|---|---|---|---|---|---|---|
| MTG, mm/min | TMG, min | TTG, mm/min | Lag time, min | ETP, nmol/L/min | Peak thrombin, nmol/L | ttpeak, min | ETP-AUC, mA | Lag time, sec | ETP-Cmax, mA/min |
| 14.5 ± 3.9 | 7.1 ± 1.4 | 761.2 ± 70.3 | 3.0 ± 0.6 | 2126.4 ± 441.6 | 397.2 ± 84.6 | 5.7 ± 0.9 | 411.0 ± 48.4 | 23.0 ± 2.5 | 114.3 ± 13.4 |
Abbreviations: Cmax, maximum thrombin generation; ETP, endogenous thrombin potential; ETP-AUC, endogenous thrombin potential–the area under the curve; MTG, maximum rate of thrombus generation; TEG, thromboelastography; TMG, time to maximum rate of thrombus generation; TTG, the total thrombus generation; ttpeak, time to peak thrombin generation.
Figure 1.
The correlations of thrombin generation between Thrombinoscope parameter endogenous thrombin potential (ETP; nmol/L/min), thromboelastography (TEG) parameter thrombus generation (TG; mm/min), and Innovance ETP parameter ETP-the area under the curve (AUC).
Comparison of PPP and PPP-LOW Reagents in Thrombinoscope Method
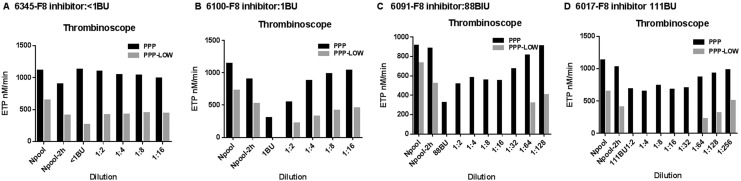
The PPP-reagent and PPP-LOW reagent are commonly used for measuring thrombin generation with Thrombinoscope in platelet-poor plasmas. The PPP-LOW reagent is particularly recommended for use with hemophilia plasma due to high sensitivity to deficiencies of FVIII, IX, and XI. We compared thrombin generation in 4 commercial plasma samples with a FVIII inhibitor (6345-F8 inhibitor: <1BU, 6100-F8 inhibitor: 1BU, 6091-F8 inhibitor: 88BU, and 6017-F8 inhibitor: 111BU) with PPP and PPP-LOW reagent by Thrombinoscope. As shown in Figure 2, the PPP-LOW reagent is more sensitive to inhibition of thrombin generation potential by FVIII inhibitors (Figure 2B–D). An exception is 6345 (Figure 2A), which has factor VIII inhibitor titer <1 BU.
Figure 2.
Comparison of different reagents in Thrombinoscope: Comparison of thrombin generation with different dilution in 4 commercial samples of factor VIII (FVIII)-deficient plasma with inhibitors (6345-F8 inhibitor: <1BU, 6100-F8 inhibitor: 1BU, 6091-F8 inhibitor: 88BU and 6017-F8 inhibitor: 111BU) after 2 hours of incubation with PPP and PPP-LOW reagent in the Thrombinoscope method.
Comparison of the Sensitivity of the 3 Global Assays to FVIII Inhibitors
Five commercial plasmas with different levels of plasma FVIII inhibitor (6345-F8 inhibitor: <1BU, 6100-F8 inhibitor: 1BU, 6089-F8 inhibitor: 54BU, 6091-F8 inhibitor: 88BU, and 6017-F8 inhibitor: 111BU) were used to compare the 3 global assays for their sensitivity to FVIII alloantibodies. We measured FVIII levels and confirmed inhibitor titers in these plasma samples. With different dilutions of the samples, thrombin generation potential was measured using all 3 global assays. As shown in Figure 3, TEG appears to be more sensitive than Thrombinoscope or Innovance ETP for detecting inhibition of thrombin generation potential by FVIII inhibitors. For example, in sample 6345 (Factor VIII inhibitor <1 BU), a dose-dependent inhibition (inhibition of thrombin generation potential is dependent on the concentration of FVIII inhibitors) of the thrombin generation potential can be seen with TEG but not with the other 2 assays (Figure 3 B–D). Other commercially available plasma samples with FVIII inhibitors completely abolish the thrombin generation potential as measured by TEG but not with the other assays. Only after serial dilutions of the samples were there detectable thrombin generation potentials (Figure 3 F, J, N, and R) by TEG. On the other hand, Innovance ETP method appears insensitive to FVIII inhibitors. No dose-dependent changes were seen in thrombin generation with serial dilution of the samples using the Innovance ETP assay (Figure 3H, L, P and T). With the Thrombinoscope method, thrombin generation potentials can be detected in all dilutions with all the specimens with a dose-dependent relationship of the FVIII inhibitors plasma (Figure 3 G, K, O, and S, and Figure 4B, C, D, and E).
Figure 3.
Comparison of 3 global assays in their sensitivity to factor VIII (FVIII) inhibitors with 5 commercial samples of FVIII-deficient plasma with inhibitor: FVIII levels in 5 commercial samples before incubation and after 2hours incubation (A, E, I, M, and Q); thrombin generation potential after 2 hours incubation with different dilution in thromboelastography (TEG; B, F, J, N, and R), Thrombinoscope(C, G, K, O, and S), and Innovance endogenous thrombin potential (ETP; D, H, L, P, and T) methods. *not tested.
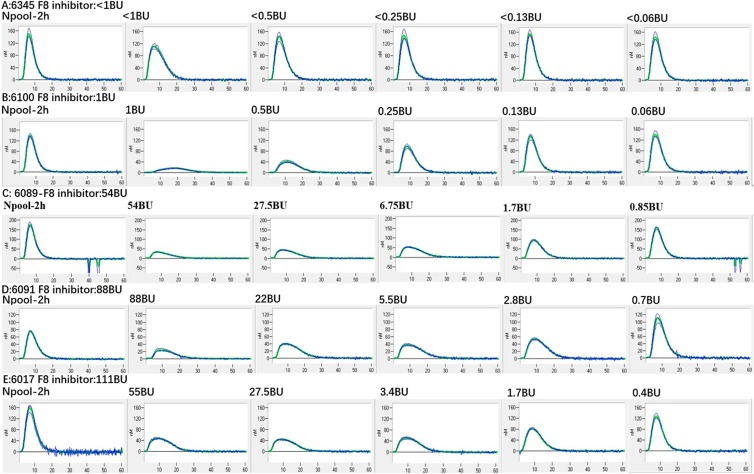
Figure 4.
Thrombin generation curve by Thrombinoscope displaying the parameter endogenous thrombin potential (ETP) in 5 commercial samples with different factor VIII (FVIII) inhibitor levels (A, 6345-F8 inhibitor: <1BU; B, 6100-F8 inhibitor: 1BU; C, 6089-F8 inhibitor: 54BU; D, 6091-F8 inhibitor: 88BU; E, 6017-F8 inhibitor: 111BU).
Assessing Thrombin Generation in Patients with Hemophilia A With Different FVIII Inhibitor Levels
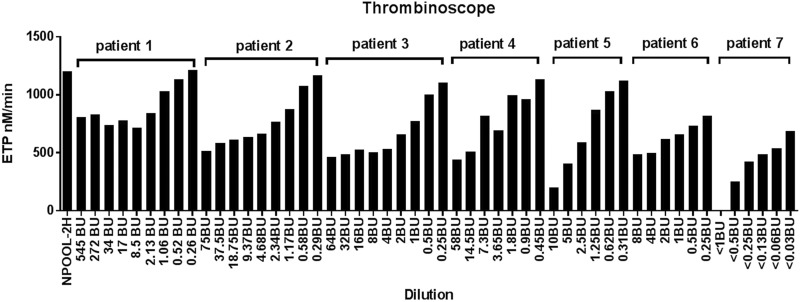
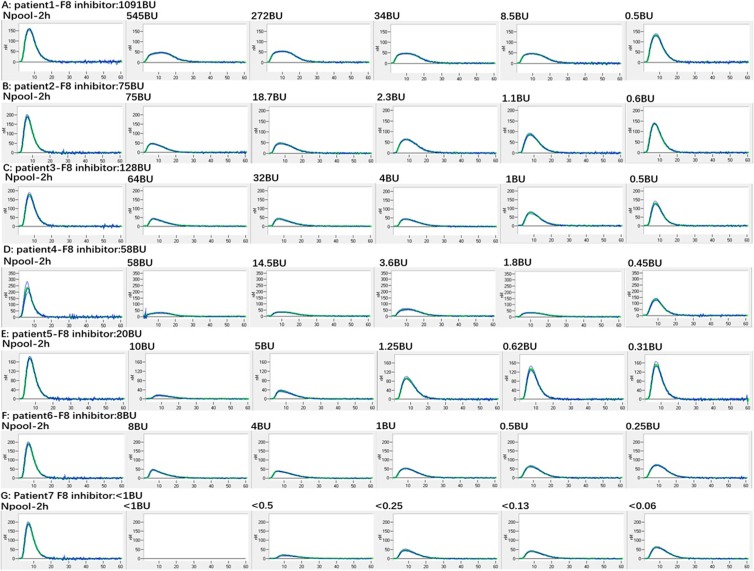
We next measured thrombin generation potential in 7 patients with hemophilia A (Table 2) with different plasma FVIII inhibitor levels (1091BU, 128BU, 75BU, 58BU, 20BU, 8BU, and<1BU) using Thrombinoscope. Serial dilutions were made before 2 hours of incubation (Figure 5 and Figure 6A–G). With all clinical specimens, a dose-dependent inhibition of thrombin generation potential was observed. Interestingly, the level of FVIII inhibitor did not always correlate with the level of inhibition of thrombin generation potential. For example, patient No. 1 had the highest inhibitor level (>500 BU), but the patient’s plasma only showed mild inhibition of thrombin generation potential, and clinically the patient had no bleeding symptoms. In contrast, patient No.5 with a 20 BU FVIII inhibitor displayed stronger inhibition than other patients’ plasma with higher titer FVIII inhibitors. Interestingly, patient No.7 with a <1 BU of FVIII inhibitor demonstrated the strongest inhibition of thrombin generation potential, which correlated with persistent bleeding clinically. The patient’s FVIII inhibitor was shown to be an autoantibody.
Table 2.
Clinical Information of 7 Patients With Hemophilia A With Different FVIII Inhibitor Levels.
| Diagnosis | Age Gender | FVIII Activity | Inhibitor | Bleeding Status | Treatment | Antibody Type | |
|---|---|---|---|---|---|---|---|
| Patient 1 | Hemophilia A | 2 yr Male | <1% | 1091BU | No bleeding | NovoSeven | Alloantibody |
| Patient 2 | Hemophilia A | 22 m.o Male | <1% | 75BU | Tripped 2 days ago and injured his knee; now no increasingly swollen, no limping, no mobility impact | Advate NovoSeven | Alloantibody |
| Patient 3 | Hemophilia A | 2 yr Male | <1% | 128BU | No signs of bleeding | NovoSeven | Alloantibody |
| Patient 4 | Hemophilia A | 14 m.o Male | <1% | 58BU | Knee hematoma a month ago | Advate | Alloantibody |
| Patient 5 | Hemophilia A | 13 m.o Male | <1% | 20BU | No breakthrough bleeds | NovoSeven | Alloantibody |
| Patient 6 | Hemophilia A | 19 m.o Male | <1% | 8BU | No breakthrough bleeds | Advate | |
| Patient 7 | Acquired FVIII deficiency | 73 yr Female | 3% | <1BU | Severe bleeding | NovoSeven prednisone cyclophosphamide | Autoantibody |
Abbreviations: FVIII, factor VIII; m.o., months old; yr, year.
Figure 5.
Thrombin generation in 7 patients with hemophilia A by Thrombinoscope with different factor VIII (FVIII) inhibitor levels (patient 1-F8 inhibitor: 1091BU; patient 2-F8 inhibitor: 75BU; patient 3-F8 inhibitor: 128BU; patient 4-F8 inhibitor: 58BU; patient 5-F8 inhibitor: 20BU; patient 6-F8 inhibitor: 8BU; patient 7-F8 inhibitor: <1BU): Inhibitor level does not always correlate with inhibition on thrombin generation potential with Thrombinoscope method. (Plasma samples of patients 2, 4, 6, and 7 were diluted from the beginning).
Figure 6.
Thrombin generation curve by Thrombinoscope showing the parameter endogenous thrombin potential (ETP) in 5 commercial samples with different factor VIII (FVIII) inhibitor levels (A: patient 1-F8 inhibitor: 1091BU; B: patient 2-F8 inhibitor: 75BU; C: patient 3-F8 inhibitor: 128BU; D: patient 4-F8 inhibitor: 58BU; E: patient 5-F8 inhibitor: 20BU; F: patient 6-F8 inhibitor: 8BU; G: patient 7-F8 inhibitor: <1BU).
Discussion
The FVIII inhibitors reduce the efficacy of factor concentrates, making it more difficult to treat bleeds. The FVIII activity and FVIII inhibitor titer are used to monitor patients with hemophilia, but they do not always correlate with bleeding risk.17 Therefore, widely available and standardized assays that are better measures of in vivo hemostasis in patients with factor inhibitors would be a significant advance. In this study, we performed parallel comparison of 3 global assays and assessed their potential clinical utility in monitoring patients with FVIII inhibitors.
In this study, we demonstrated the 3 global assays of thrombin generation do not correlate well with each other. We suspect this is at least partially explained by the large CV of precision for Thrombinoscope which was around 11%. This also suggests that for healthy individuals’ thrombin generation potential measured by the global assays should be interpreted with caution.
Thrombinoscope-CAT assay uses tissue factor as a trigger to activate the coagulation cascade. In general, PPP-reagent (5 pmol/L TF and 4 pmol/L phospholipids) is used for the measurement of thrombin generation, while PPP-LOW reagent (1 pmol/L TF and 4 pmol/L phospholipids) is recommended for use in hemophilia plasma due to increased sensitivity to FVIII, IX, and XI. It was shown that the sensitivity and specificity of the Thrombinoscope-CAT for individual coagulation factors depends on the TF concentration.18–21 In our study, it was found that Thrombinoscope with PPP-LOW reagent was highly sensitive to FVIII inhibitors and had narrow range of testing: Thrombin generation potential was abolished with even low level of FVIII inhibitors (Figure 2C and D). Lewis et al reported that using 1 pmol/L TF in some patients with severe hemophilia often resulted in incomplete thrombin generation curves with Thrombinoscope-CAT, but thrombin generation can be measured at 5 pmol/L TF22; Veen et al19 also compared thrombin generation at 1 and 5 pmol/L TF concentration in patients having hemophilia with Thrombinoscope-CAT and found that the assay can discriminate between patients with mild and severe hemophilia A at 5 pmol/L TF but not 1 pmol/L TF. Consistent with these studies, our findings support using regular PPP reagents by thrombinosope in monitoring patients having hemophilia with FVIII inhibitors.
We compared the sensitivity to FVIII inhibitors by 3 global assays using 5 commercial samples. Thrombinoscope-CAT with PPP reagent (5 pmol/L TF) invariably showed dose-dependent response to different levels of FVIII inhibitors from the same samples (Figure 3G, K, O, and S). However, Innovance ETP showed virtually no response to FVIII inhibitors regardless of inhibitor levels (Figure 3D, H, L, P, and T). Both the Innovance ETP and the Thrombinoscope methods use the term endogenous thrombin potential to describe the area under the curve for thrombin generation. The main difference between these 2 methods are the application of different substrates and different data-processing processes.23 We found in our study that no thrombin generation potential was detected in some of the serial diluted samples by TEG (Figure 3F, J, N and R), and a moderate level of FVIII inhibitor often abolished thrombin generation, suggesting that TEG is more sensitive to FVIII inhibitors with a narrow range of detection. The TEG has been found to be highly variable in patients with hemophilia and is considered too unreliable for routine monitoring.22,24 It was also shown that TEG parameters were unreliable to predict clinical phenotypes.25 Thrombinoscope assay showed more relevance to the hemostatic state of patients having hemophilia with inhibitor,26–29 but there is still some controversy.25,30,31.Our study suggested that Thrombinoscope with PPP-reagent (5pM TF) has appropriate sensitivity for monitoring patients having hemophilia with inhibitors.
With 7 well-characterized clinical samples with different levels of FVIII inhibitor (1091BU, 128BU, 75BU, 58BU, 20BU, 8BU, and<1BU), we measured the thrombin generation potential using Thrombinoscope (PPP-reagent). It was found that the same levels of FVIII inhibitor from different patients have different levels of inhibition for thrombin generation potential (Figure 5), which is consistent with the findings of others.26,27 The No. 1 patient with >1000 BU FVIII inhibitor showed only mild thrombin generation inhibition (Figure 5, No.1 patient) and no bleeding clinically, which potentially explains the phenotypic heterogeneity of patients with same levels of FVIII inhibition. On the other hand, the No. 7 patient with <1 BU FVIII inhibitor showed unexplained severe bleeding with very low FVIII activity of 3%, and the levels of other factor, von Willebrand antigen, serum immunoglobulins and Ristocetin cofactor, were all normal; ANA screen and hepatitis studies were negative. When she received an infusion of FVIII, her FVIII levels initially had 62% recovery and subsequent drops, suggesting the presence of an inhibitor. On Thrombinoscope (PPP-reagent), this patient’s thrombin generation was significantly inhibited (Figure 5, patient 7 and Figure 6G), correlating well with clinical bleeding phenotype. Inhibition of thrombin generation was more significant by patient No.5’s plasma at 10BU than by patient No. 4’s plasma at 58 BU (Figure 5), again indicating that the inhibition level of thrombin generation potential measured by Thrombinoscope (PPP-reagent) is not entirely dictated by the level of FVIII inhibitor and suggesting that thrombin generation potential by Thrombinoscope is a better marker to monitor patients having hemophilia with inhibitors than FVIII inhibitor levels.
In this proof-of-principle study, we have shown that of 3 global assays, Thrombinoscope with PPP reagent show appropriate sensitivity to FVIII inhibition, and thrombin generation potential could be a valuable marker in monitoring patients with FVIII inhibitors. Future studies are warranted to validate the clinical utility.
Ethical approval to report this case series was obtained from Institutional Review Board of Johns Hopkins University (IRB00097630). Informed consent for patient information to be published in this article was not obtained because this is a retrospective study with existing leftover samples, and specimens were not individually identifiable. Moreover, the results of the study will not in any way affect management of patients involved in the study. Thus, the research involves no more than minimal risk to participants.
Footnotes
Authors’ Note: GZ, JJ, and TSK were involved in study design. PC analyzed the data. GZ, TSK, and MBS provided advice on all aspects of the study. PC wrote the manuscript, and all authors edited and approved the final version. Gang Zheng, MD, PhD, and Thomas S Kickler, MD, contributed equally.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Walshi PN, Rainsford S, Biggs R. Platelet coagulant activities and clinical severity in haemophilia. Thromb Diath Haemorrh. 1973;29(01):722–729. [PubMed] [Google Scholar]
- 2. Brummel KE, Paradis SG, Butenas S, Mann KG. Thrombin functions during tissue factor–induced blood coagulation. Blood. 2002;100(1):148–152. [DOI] [PubMed] [Google Scholar]
- 3. Spahn DR, Bouillon B, Cerny V, et al. Management of bleeding and coagulopathy following major trauma: an updated European guideline. Crit care. 2013;17(2):R76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wasowicz M, Srinivas C, Meineri M, Banks B, McCluskey SA, Karkouti K. Technical report: analysis of citrated blood with thromboelastography: comparison with fresh blood samples. Can J Anaesth. 2008;55(5):284–289. [DOI] [PubMed] [Google Scholar]
- 5. Chandler WL, Roshal M. Optimization of plasma fluorogenic thrombin-generation assays. Am J Clin Pathol. 2009;132(2):169–179. [DOI] [PubMed] [Google Scholar]
- 6. De Smedt E, Al Dieri R, Spronk HM, Hamulyak K, Ten Cate H, Hemker HC. The technique of measuring thrombin generation with fluorogenic substrates: 1. Necessity of adequate calibration. Thromb Haemost. 2008;99(02):343–349. [PubMed] [Google Scholar]
- 7. Dargaud Y, Béguin S, Lienhart A, et al. Evaluation of thrombin generating capacity in plasma from patients with haemophilia A and B. Thromb Haemost. 2005;93(3):475–480. [DOI] [PubMed] [Google Scholar]
- 8. Besser M, Baglin C, Luddington R, van Hylckama Vlieg A, Baglin T. High rate of unprovoked recurrent venous thrombosis is associated with high thrombin-generating potential in a prospective cohort study. J Thromb Haemost. 2008;6(10):1720–1725. [DOI] [PubMed] [Google Scholar]
- 9. Trossaert M, Regnault V, Sigaud M, Boisseau P, Fressinaud E, Lecompte T. Mild hemophilia A with factor VIII assay discrepancy: using thrombin generation assay to assess the bleeding phenotype. J Thromb Haemost. 2008;6(3):486–493. [DOI] [PubMed] [Google Scholar]
- 10. Al Dieri R, de Laat B, Hemker HC. Thrombin generation: what have we learned? Blood Rev. 2012;26(5):197–203. [DOI] [PubMed] [Google Scholar]
- 11. Santagostino E, Mancuso M, Tripodi A, et al. Severe hemophilia with mild bleeding phenotype: molecular characterization and global coagulation profile. J Thromb Haemost. 2010;8(4):737–743. [DOI] [PubMed] [Google Scholar]
- 12. Beltran−Miranda C, Khan A, Jaloma−Cruz A, Laffan M. Thrombin generation and phenotypic correlation in haemophilia a. Haemophilia. 2005;11(4):326–334. [DOI] [PubMed] [Google Scholar]
- 13. Espitia O, Fouassier M, Hardouin JB, et al. Thrombin generation assay in hospitalized nonsurgical patients: a new tool to assess venous thromboembolism risk? Clin Appl Thromb/Hemost. 2017;23(1):45–51. [DOI] [PubMed] [Google Scholar]
- 14. Herrmann R, Thom J, Wood A, Phillips M, Muhammad S, Baker R. Thrombin generation using the calibrated automated thrombinoscope to assess reversibility of dabigatran and rivaroxaban. Thromb Haemost. 2014;111(05):989–995. [DOI] [PubMed] [Google Scholar]
- 15. Eichinger S, Hron G, Kollars M, Kyrle PA. Prediction of recurrent venous thromboembolism by endogenous thrombin potential and D-dimer. Clin Chem. 2008;54(12):2042–2048. [DOI] [PubMed] [Google Scholar]
- 16. Harder S, Merz M, Klinkhardt U, Lorenz H, Koster A. Influence of argatroban on coagulation parameters in heparin-induced thrombocytopenia patients after cardiothoracic surgery. J Thromb Haemost. 2007;5(9):1982–1984. [DOI] [PubMed] [Google Scholar]
- 17. Aledort L, Haschmeyer RH, Pettersson H, Group OOS. A longitudinal study of orthopaedic outcomes for severe factor-VIII-deficient haemophiliacs. J Intern Med. 1994;236(4):391–399. [DOI] [PubMed] [Google Scholar]
- 18. Van Veen JJ, Gatt A, Cooper PC, Kitchen S, Bowyer AE, Makris M. Corn trypsin inhibitor in fluorogenic thrombin-generation measurements is only necessary at low tissue factor concentrations and influences the relationship between factor VIII coagulant activity and thrombogram parameters. Blood Coagul Fibrinolysis. 2008;19(3):183–189. [DOI] [PubMed] [Google Scholar]
- 19. Van Veen J, Gatt A, Bowyer A, Cooper P, Kitchen S, Makris M. The effect of tissue factor concentration on calibrated automated thrombography in the presence of inhibitor bypass agents. Int J Lab Hematol. 2009;31(2):189–198. [DOI] [PubMed] [Google Scholar]
- 20. Duchemin J, Pan-Petesch B, Arnaud B, Blouch MT, Abgrall JF. Influence of coagulation factors and tissue factor concentration on the thrombin generation test in plasma. Thromb Haemost. 2008;99(04):767–773. [DOI] [PubMed] [Google Scholar]
- 21. Bagot C, Leishman E. Establishing a reference range for thrombin generation using a standard plasma significantly improves assay precision. Thromb Res. 2015;136(1):139–143. [DOI] [PubMed] [Google Scholar]
- 22. Quarterman C, Shaw M, Johnson I, Agarwal S. Intra-and inter-centre standardisation of thromboelastography (TEG®). Anaesthesia. 2014;69(8):883–890. [DOI] [PubMed] [Google Scholar]
- 23. Kintigh J, Monagle P, Ignjatovic V. A review of commercially available thrombin generation assays. Res and Practice in Thromb Haemost. 2018;2(1):42–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kenet G, Stenmo C, Blemings A, Wegert W, Goudemand J, Krause M. Intra-subject variability of thromboelastographic parameters following rFVIIa in vivo administration in haemophilia patients: a multi-centre, randomized trial. J Thromb Haemost. 2007;103(2):351–359. [DOI] [PubMed] [Google Scholar]
- 25. Ay Y, Balkan C, Karapinar DY, Akin M, Bilenoğlu B, Kavakli K. Feasibility of using thrombin generation assay (TGA) for monitoring bypassing agent therapy in patients with hemophilia having inhibitors. Clin Appl Thromb/Hemost. 2013;19(4):389–394. [DOI] [PubMed] [Google Scholar]
- 26. Barrowcliffe TW, ed. Monitoring inhibitor patients with the right assays. Seminars in hematology. Amsterdam, Netherlands: Elsevier; 2008. [DOI] [PubMed] [Google Scholar]
- 27. Dargaud Y, Lienhart A, Meunier S, et al. Major surgery in a severe haemophilia A patient with high titre inhibitor: use of the thrombin generation test in the therapeutic decision. Haemophilia. 2005;11(5):552–558. [DOI] [PubMed] [Google Scholar]
- 28. Dargaud Y, Lienhart A, Negrier C. Prospective assessment of thrombin generation test for dose monitoring of bypassing therapy in hemophilia patients with inhibitors undergoing elective surgery. Blood. 2010;116(25):5734–5737. [DOI] [PubMed] [Google Scholar]
- 29. Dargaud Y, Lienhart A, Janbain M, LeQuellec S, Enjolras N, Negrier C. Use of thrombin generation assay to personalize treatment of breakthrough bleeds in a patient with hemophilia and inhibitors receiving prophylaxis with emicizumab. Haematologica. 2018;103(4):e181–e183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Young G, Sørensen B, Dargaud Y, Negrier C, Brummel-Ziedins K, Key NS. Thrombin generation and whole blood viscoelastic assays in the management of hemophilia: current state-of-art and future perspectives. Blood. 2013;121(11):1944–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tripodi A. Thrombin generation assay and its application in the clinical laboratory. Clin Chem. 2016;62(5):699–707. [DOI] [PubMed] [Google Scholar]