Abstract
Venous thromboembolism (VTE) is associated with high recurrence, mortality, and cost burden. Direct oral anticoagulants (DOACs) are currently used for VTE treatment, and they offer more benefits over warfarin, despite being more expensive. There is no consensus on the most cost-effective DOAC agent, especially in VTE. This systematic review aims to summarize the comparative cost-effectiveness studies and their impact among DOACs in the treatment of VTE. Literature systematic review of PubMed, Embase, and EconLit was conducted in February 2018 to identify all cost-effectiveness studies of DOAC for the treatment and prevention of VTE. Two independent investigators systematically collected search results and assessed the quality of the studies. The search identified 7 articles, all of which had dabigatran and rivaroxaban as comparators, 6 of which also included apixaban, and 2 of which also had edoxaban. Results of 3 articles concluded that apixaban is a dominant strategy compared to other DOACs in terms of Incremental Cost-Effectiveness Ratio (ICER) in the treatment and prevention of recurrent VTE. One article compared rivaroxaban and dabigatran, with the latter dominating rivaroxaban in terms of ICER. Compared to other DOACs, 2 articles reported apixaban being associated with highest annual total medical cost avoidance of US$4244 and US$4440 per patient-year (ppy), respectively. One article reported that apixaban had the highest annual total medical cost differences of US$918 ppy compared to other DOACs. This systematic review demonstrates that apixaban is considered a cost-effective strategy for VTE treatment and prevention of recurrent VTE.
Keywords: cost-effectiveness, DOAC, venous thromboembolism, systematic review, NOAC, VTE
Background
Venous thromboembolism (VTE) is considered a serious and potentially life-threatening medical condition.1 The annual incidence of VTE (deep venous thrombosis [DVT] and pulmonary embolism [PE]), in Europeans as an example, is estimated to be 104 to 183 per 100 000 person-years.2 Incidence rates of DVT (without PE) and PE (without DVT) are 45 to 117 and 29 to 78 per 100 000 person-years, respectively.3–6 These rates are higher among the African American population and lower among the Native American population, Asian, and Asian American populations.2
Risk factors for VTE include advanced age, overweight, hospitalization, immobility, especially after total hip replacement (THR) and total knee replacement (TKR) surgeries, active cancer, trauma, fractures, and superficial vein thrombosis.7
Before 2010, the mainstay pharmacologic prophylaxis and treatment of VTE were warfarin, heparin, low-molecular-weight heparin (LMWH), and fondaparinux.8 Since the introduction of the first direct oral anticoagulant (DOAC), that is, dabigatran, in 2010, the anticoagulation landscape has started to change. Since then, several other DOACs have been approved, including rivaroxaban, apixaban, edoxaban, and betrixaban. Direct oral anticoagulants offer potential advantages compared to warfarin including fixed dosing, lack of food and drug interactions, minimal need for monitoring, and superior safety profile. Direct oral anticoagulants, however, are associated with an increased risk of gastrointestinal adverse drug reactions (dabigatran and rivaroxaban), lack of an easily monitored surrogate marker, and higher cost.9
Numerous studies and systematic reviews have compared DOACs with warfarin in terms of their cost-effectiveness and cost benefit.10–18 Apart from 1 report that was conducted on studies published till 2014,19 there have been no updated systematic reviews comparing among DOACs (either directly or indirectly) in the prevention and treatment of VTE. In this systematic review, we aim to explore studies comparing the cost-effectiveness of DOACs in the treatment and prevention of VTE.
The objective of this systematic review is to summarize and compare the main cost-effectiveness outcomes in studies comparing DOAC agents in the prevention and treatment of VTE. This will answer the question about which of the DOACs is the most cost-effective in the prevention and treatment of VTE.
Methods
The Literature Search
A systematic search of the literature was conducted via the following databases: PubMed, Embase, and EconLit. The search strategy followed the PICO format. As an example, within the PubMed database, the population was: venous thromboembolism, VTE, deep venous thrombosis, DVT, pulmonary embolism, PE, atrial fibrillation, AF, stroke; the intervention/comparator was: anticoagulants, rivaroxaban, dabigatran, apixaban, edoxaban, direct oral anticoagulant, novel oral anticoagulant, DOAC, NOAC; the outcome was cost-effectiveness, cost-benefit, cost analysis, economics, cost of illness, cost savings, cost control. A similar search strategy was used with the other search databases. Key words were customized to database-specific indexing terms, for example, the use of MeSH terms. As appropriate, the terms and their alternatives were combined with Boolean connectors (AND/OR/NOT). In addition to the electronic search, we performed a manual search of bibliographies and references of identified articles and cost-related specific issues in journals. The gray literature search also included preliminary progress and advanced reports, theses, conference proceeding, technical reports, and guidelines, in addition to searching indexing terms via the Google search engine. A search protocol of the systematic review was developed and registered in PROSPERO (ID# CRD42018098705).
Study Types
Any cost-effectiveness study comparing DOACs in the treatment and/or prevention of VTE.
Participants
Patients treated with DOACs for the treatment and/or prevention of VTE.
Eligibility Criteria
Studies were considered eligible for this review if they were pharmacoeconomic studies comparing more than 1 DOAC in adults (>18 years old) for the prevention or treatment of VTE, including DVT and/or PE. We included only comparative studies in the English language, of human species, and in journal articles with full-text availability from January 1, 2010 (the year of the first approved DOAC]dabigatran[) to February, 2018 (the date this review was conducted). Exclusion criteria include reviews, noncomparative studies, and studies with a single DOAC alone as a comparator or against warfarin. The selection of articles was conducted via 2 independent reviewers by the initial screening of titles/abstracts of articles, before a follow-up screening of the full text. When disagreements occurred, articles were discussed with a third reviewer until consensus.
Outcome Measures
The outcome measure of interest is the observed trends in relation to the comparative economic outcomes of the DOAC agents, dabigatran, rivaroxaban, apixaban, edoxaban, and betrixaban, including total cost, cost avoidance, and the incremental cost-effectiveness ratio (ICER). Also a comparative outcome of interest is when a DOAC is both more effective and less costly than another (ie, dominant DOAC), or the converse (ie, dominated DOAC), in which case the ICER becomes meaningless.
Data Extraction and Synthesis
A data extraction tool was developed and pilot tested using a sample of the eligible studies (n = 3). The extracted data includes the DOACs compared, country, disease states, eligibility criteria, primary and secondary outcomes (efficacy and pharmacoeconomic), funding, the comparative model used, type of cost-effectiveness analysis, uncertainty tests, and summary of findings. If any of the information was missing, the corresponding author of a particular article was contacted. Two reviewers independently extracted data from included articles, ensuring data reliability and trustworthiness. A consensus was reached whenever differences occurred.
Assessment of Quality of Studies
As with the study inclusion and data extraction, the quality of articles was assessed by 2 independent reviewers who critically appraised the included articles to assess the risk of bias and methodological quality. For the purpose, the Quality of Health Economic Studies (QHES) tool20 was utilized. The QHES includes 16 questions, and each question has a different score ranging from “0” to “9,” with the total of all questions scores adding to 100 points. The interpretation of a QHES score was as per 1 of 4 categories of quality, that is, good, fair, poor, and extremely poor, associated with the scores 75 to 100, 50 to 74, 25 to 49, and 0-24, respectively.21–24 A third independent reviewer would contribute whenever a disagreement occurs.
Only articles with fair or good methodological quality were included in this review.22 The systematic review followed the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guideline, including a 27-item checklist of essential items to be reported in a systematic review.25
Results
Study Selection
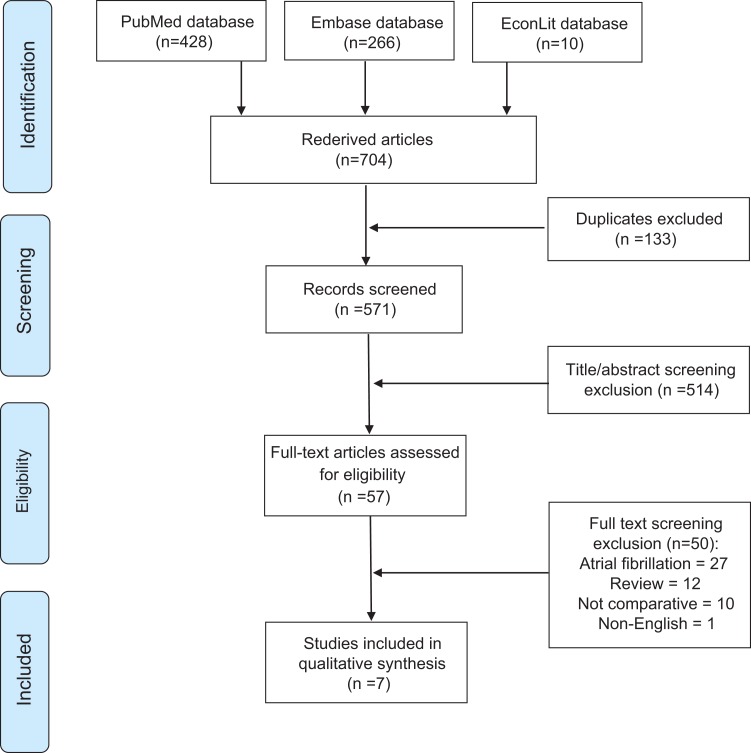
Of a total of 704 retrieved articles, 7 articles met the inclusion/exclusion criteria. Search results are illustrated in Figure 1.
Figure 1.
Flow diagram.10
Characteristics of the Included Articles
All studies in this review included dabigatran and rivaroxaban as part of the DOACs compared.26–32 Apixaban was included in 6 studies and edoxaban was included in 2 studies only. Furthermore, because of how recently approved it is, betrixaban was not included in any of the studies. The majority of the articles used Markov modeling that follows cohorts of patients over follow-up periods of 3, 6, and 12 months as well as the lifetime of patients, with 3- or 6-month transitional model cycles. The only exception was the study by Amin et al, where a non-Markov simulation was used to follow-up patients over 1 year from having recurrent VTE.
In general, all studies used pivotal trials versus warfarin as primary sources of clinical inputs for dabigatran (RE-COVER, RE-COVER II, and RE-MED),33–35 rivaroxaban (EINSTEIN, EINSTEIN-DVT, and EINSTEIN-PE),36–38 apixaban (AMPLIFY and AMPLIFY-EXT)39,40 and edoxaban (Hokusai-VTE).41 The modeled clinical events in included cost-effectiveness studies were therefore fairly consistent. Clinical efficacy and safety events included recurrent VTE and VTE-related death, minor bleeding, major bleeding, and clinically relevant non-major bleeding (CRNMB). Other events were chronic thromboembolic pulmonary hypertension, post thromboembolic syndrome, and intracranial bleed. These clinical events were evaluated over patient lifetime,26,27,29,32 except for Amin et al where the events were evaluated on annual basis.28,30,31
Among the 7 included studies, 3 were conducted in the United States,28,30,31 2 in the United Kingdom,26,32 and 2 in Canada.27,29 The most common measure for the cost-effectiveness evaluation was cost/ICAR (QALY), and it was used in 4 studies.26,27,29,32 Annual total medical cost avoidance was used in 2 studies28,30 and annual total medical cost differences in 1 study.31 In regard to funding, 6 studies were funded by drug sponsors,26–28,30–32 while no funding was received by Al Saleh et al. Table 1 summarizes the general characteristics of the included articles.
Table 1.
General Characteristics of the Included Articles.
| Author | Comparators | Setting | Year | Analysis Type | Population |
|---|---|---|---|---|---|
| Al Saleh et al29 | Dabigatran, Rivaroxaban, Apixaban, Warfarin | Canada | 2017 | ICER (Cost/QALY) | Treatment of DVT and PE in outpatient settings |
| Amin et al30 | Dabigatran, Rivaroxaban, Apixaban (2.5-5 mg) | United States | 2014 | Annual total medical cost avoidance | Extended treatment of VTE |
| Amin et.al31 | Dabigatran, Rivaroxaban, Apixaban, Edoxaban | United States | 2014 | Annual total medical cost differences | Treatment of VTE |
| Amin et al28 | Dabigatran, Rivaroxaban, Apixaban, Edoxaban | United States | 2015 | Annual total medical cost avoidances | Treatment of VTE |
| Jurgin et al26 | Dabigatran, Rivaroxaban | United Kingdom | 2015 | ICER (Cost/QALY) | Treatment and extended treatment of VTE |
| Lanitis et al32 | Dabigatran, Rivaroxaban, Apixaban, Warfarin | United Kingdom | 2016 | ICER (Cost/QALY) | Initial treatment of VTE |
| Quon et al27 | Dabigatran, Rivaroxaban, Apixaban, Warfarin | Canada | 2016 | ICER (Cost/QALY) | Treatment and prevention of recurrence of VTE |
Abbreviations: DVT, deep venous thrombosis; ICER, incremental cost-effectiveness ratio; PE, pulmonary embolism; QALY, quality-adjusted life-years; VTE, venous thromboembolism.
Efficacy End Point Results
Different measures were used in different studies to assess the efficacy of each DOAC. Three studies reported differences in absolute clinical event rates versus placebo, control, and warfarin.28,30,31 Two studies reported results in terms of a number of events among a cohort of 10 000 patients.27,32 One study reported fatality rates and one study reported relative risk (RR) of recurrent VTE.26,29 Most articles conducted univariate (1-way) sensitivity analysis to determine the impact of every single parameter used in the analysis model on the total medical cost estimated through the model. Amin et al concluded in all of their 3 studies that variations in both VTE and major bleeding had the highest impact on medical cost differences in terms of total medical cost differences and avoidance between DOACs, standard therapy, and placebo.28,30,31 Al Saleh et al reported that fatality rates in the short run and pharmaceutical care were the highest determinants to uncertainty in the conducted analysis.29 Quon et al concluded that both major and CRNMB events were the main drivers for apixaban being the cost-effective choice among other DOACs.27 Lanitis et al reported that apixaban
would not be considered a dominant choice when the differential price between other DOACs and apixaban increased and when the relative risk of recurrent VTE is reduced for rivaroxaban versus apixaban from a baseline 1.08 to 0.69.32 Table 2 summarizes the efficacy end points for each DOAC in terms of recurrent VTE.
Table 2.
Recurrent VTE Efficacy End Point Results.
| Author | Measure Used | Clinical Events Results |
|---|---|---|
| Al Saleh et al29 | Fatality rates | Recurrent DVT/PE = 2.55%, 1.88%, 1.78%, and 2.14% for dabigatran + LMWH, rivaroxaban, apixaban, and VKA + LMWH, respectively |
| Amin et al30 | Differences in absolute clinical event rates | Recurrent VTE = −5.15% (−5.48% to −4.19%)a, −5.74% (−6.43% to −4.31%)a, −7.14% (−7.84% to −5.90%)a, −7.08% (−7.84% to −5.81%)a for dabigatran, rivaroxaban, apixaban 2.5 mg, and apixaban 5 mg, respectively (vs placebo) |
| Amin et al31 | Differences in absolute clinical event rates | Recurrent VTE/VTE related death = 0.20% (−0.52% to 1.22%)a, −0.23% (−0.78% to 0.44%)a, −0.43% (−1.08% to 0.49%), a and −0.34% (−0.78% to 0.27%)a for dabigatran, rivaroxaban, apixaban 2.5 mg and apixaban 5 mg, respectively (vs control) |
| Amin et al28 | Differences in absolute clinical event rates | Recurrent VTE among patients with VTE = 1.02% (−2.69 to 6.35%)a, −1.23% (−3.81% to 2.13%)a, −1.80% (−4.48% to 2.02%)a, and −2.02% (−4.48% to 1.57%)a for dabigatran, rivaroxaban, apixaban and edoxaban, respectively (vs warfarin) |
| Jurgin et al26 | Relative risk (RR) | Recurrent VTE (3, 6, 12 months) = RR for rivaroxaban vs dabigatran = 0.83 (0.46 to 1.49)a
Recurrent VTE (6 months) = RR for rivaroxaban vs dabigatran = 0.90 (0.53, 1.52)a |
| Lanitis et al32 | Number of events among (cohort of 10 000 patients) | Recurrent VTE/VTE-related death (6-month treatment over patient lifetime) = 604, 601, 600, and 602 for dabigatran/LMWH, rivaroxaban, apixaban, and VKA/LMWH, respectively |
| Quon et al27 | Number of events (cohort of 10 000 patients) | Recurrent VTE events = 520, 512, 521, and 607 for dabigatran, rivaroxaban, apixaban, and enoxaparin/VKA, respectively, for up to 18 months treatment over patient life time with DOACs or 6 months of enoxaparin/VKA |
Abbreviations: DVT, deep venous thrombosis; LMWH, low-molecular-weight heparin; PE, pulmonary embolism; RR, relative risk; VKA, vitamin K antagonist; VTE, venous thromboembolism.
a95% Confidence interval (CI).
Cost-Effectiveness Results
Measures used to assess the cost-effectiveness of DOACs were not the same among the different studies. The majority of studies (n = 4) mostly used the cost/QALY measure, including the total costs calculations.26,27,29,32 Jurgin et al reported that for 6-month therapy with dabigatran compared to 3-, 6-, and 12-month treatment with rivaroxaban for VTE treatment and extended anticoagulation and index DVT and PE treatment among a cohort of 10 000 patients, dabigatran is dominant over rivaroxaban, having lower cost and higher QALY, in all these settings. A similar trend was observed in their study evaluating the VTE treatment and extended anticoagulation indication, and for index DVT and PE treatment in 6-month therapy among a cohort of 10 000 patients for both dabigatran and rivaroxaban, where dabigatran also dominates rivaroxaban in all these settings. In a 6-month evaluation of VTE treatment over a patient’s lifetime, Lanitis et al reported apixaban to be dominant over both rivaroxaban and LMWH/dabigatran, with total costs of £4696, £4731, and £4792 with each, respectively. Apixaban did not dominate LMWH/warfarin, with apixaban costing £2520 over the latter per QALY. In overall, the per-patient treatment, administration, and monitoring costs were lower with apixaban by £11 and £45 compared to rivaroxaban and LMWH/dabigatran, respectively. In the study by Quon et al, the total lifetime costs per patient with up to 18 months of DOACs or 6 months of enoxaparin/warfarin were reported. Apixaban had lower costs and longer survival or higher QALYs compared to enoxaparin/warfarin, rivaroxaban, and dabigatran. Al Saleh et al reported the comparative cost/QALY among the therapies LMWH/VKA, LMWH/dabigatran, rivaroxaban, and apixaban. Apixaban dominated other DOACs with an ICER of US$84.08 relative to LMWH/VKA. Furthermore, at a discount rate of 0%, apixaban dominates other strategies and with 3% discount rate, apixaban dominates other DOACs with an ICER of US$36.79 relative to LMWH/VKA. In a different analysis of 3 months of therapy and for lifetime duration of the anticoagulation therapy, apixaban dominated other DOACs with an ICER relative to LMWH/VKA of US$7379.66 and US$174 614.23, respectively. However, with a 12-month therapy, apixaban dominated all other treatments.
Two studies reported the annual total medical cost avoidance as the primary measure.28,30 Amin et al reported the annual total medical cost avoidance associated with DOAC use compared to placebo as US$2794, US$2948, US$4249, and US$4244 ppy for patients with VTE treated with dabigatran, rivaroxaban, apixaban 2.5 mg, and apixaban 5 mg, respectively, with the highest cost avoidance associated with apixaban 2.5 mg followed by apixaban 5 mg. A similar trend was also observed in a different study of theirs, where they reported annual total medical cost avoidance for VTE treatment with DOACs versus warfarin ppy as follows: US$572, US$2971, US$4440, and US$1957 with dabigatran, rivaroxaban, apixaban, and edoxaban, respectively.
Reporting the total medical cost differences as the outcome measure, in a third study of theirs, Amin et al reported that the use of DOACs in comparison to standard therapy was associated with overall medical cost differences of US$146, US$482, US$918, and US$344 for patient with VTE treated with dabigatran, rivaroxaban, apixaban, and edoxaban, respectively, with the highest cost differences associated with apixaban. When treatment duration was normalized, the annual total medical cost differences were US$153, US$454, US$1108, and US$261 for a patient with VTE treated with dabigatran, rivaroxaban, apixaban, and edoxaban, respectively, also with the highest cost differences associated with apixaban. Table 3 summarizes the time horizon, event of interest, comparators, outcome measures, and results of the cost-effectiveness analysis conducted in each article.
Table 3.
General View of the Model Structure and Events Used in the Included Articles.
| Author | Time Horizon | Event of Interest | Comparators | Outcome Measure | Results |
|---|---|---|---|---|---|
| Al Saleh et al29 | Each cycle = 6 m Follow-up = 6 m, 12 m Life-time |
Recurrent DVT and PE and major bleeding | Dabigatran, rivaroxaban, apixaban, warfarin | ICER (Cost/QALY) | Apixaban dominates other DOACs in 3, 6, aand 12 months and lifetime treatment duration |
| Amin, et.al30 | 1 year | Recurrent VTE, major bleeding and CRNMB | Dabigatran, rivaroxaban, apixaban (2.5-5 mg) | Annual total medical cost avoidance | Apixaban 2.5 mg dominates other DOACs with US$4249 cost avoidance compared to placebo |
| Amin, et.al31 | 1 year | Recurrent VTE, major bleeding, CRNMB and other minor bleeding | Dabigatran, rivaroxaban, apixaban, edoxaban | Annual total medical cost differences | Apixaban dominates other DOACs with US$918 cost difference compared to standard therapy |
| Amin, et.al28 | 1 year | Recurrent VTE and major bleeding | Dabigatran, rivaroxaban, apixaban, edoxaban | Annual total medical cost avoidances | Apixaban dominates other DOACs with cost avoidance of US$2971 per patient year (ppy), compared to warfarin |
| Jurgin et al26 | Each cycle = N/A Follow-up= 3 m, 6 m, 12 m and Life-time |
Recurrent VTE, MCRBE, CTEPH, and PTS | Dabigatran, rivaroxaban | ICER (Cost/QALY) | Dabigatran dominates rivaroxaban in all treatment settings |
| Lanitis, et.al32 | Each cycle = 3 m Follow-up = 3 m, 6 m, 12 m Life-time |
Recurrent VTE, major bleeding, CTEPH, CRNM, Death | Dabigatran, rivaroxaban, apixaban, warfarin | ICER (Cost/QALY) | Apixaban dominates rivaroxaban and LMWH/dabigatran in 6 months treatment duration and with ICER of US$2520 relative to LMWH/VKA |
| Quon et al27 | Cycle = 3 m Follow-up= 3 m, 6 m, 12 m Life-time |
Recurrent PE and DVT, IC, non-IC major bleed, CRNM, treatment discontinuation, CTEPH, PTS, death, or no event | Dabigatran, rivaroxaban, apixaban, warfarin | ICER (Cost/QALY) |
Apixaban dominates dabigatran, rivaroxaban with treatment duration up to 18 months and with ICER of US$4827.78 relative to enoxaparin/VKA |
Abbreviations: CRNMB, clinically relevant non-major bleeding; CTEPH, chronic thromboembolic pulmonary hypertension; DOAC, Direct oral anticoagulants; DVT, deep venous thrombosis; IC, intracranial bleed; ICER, incremental cost-effectiveness ratio; MCRBE, major and clinically relevant bleeding event; PE, pulmonary embolism; PTS, post thromboembolic syndrome; QALY, quality-adjusted life-years; VTE, venous thromboembolism.
All studies performed probabilistic sensitivity analysis to ensure the robustness of their results. Jurgin et al reported that at £20 000 willingness to pay threshold (WTP), dabigatran therapy compared to 3-, 6-, and 12-month therapy of rivaroxaban had 61% and 88% probability of being good value for money in the treatment and extended anticoagulation of VTE, respectively, and 62% and 62% probability in DVT and PE, respectively. Furthermore, Lanitis et al reported that apixaban was also a dominant choice in 87% of the trials compared to rivaroxaban and in 98% of the trials compared to LMWH/dabigatran. Furthermore, in comparison to LMWH/VKA, apixaban was found to be the cost-effective choice in 100% of trials with an ICER of < £20 000 per QALY. Moreover, Quon et al reported that at WTP of US$5000 per QALY, apixaban had the highest probability of being cost-effective compared to dabigatran, rivaroxaban, and warfarin. At WTP of US$10 000 and US$50 000 for each additional QALY, apixaban had 93.5% and 97.7% probability of being the most cost-effective choice compared to other treatments. Amin et al reported both univariate and multivariate sensitivity analyses in all of their 3 studies. The results were also in favor of the original results with apixaban being associated with the highest cost avoidance and differences compared to dabigatran, rivaroxaban, and edoxaban.
Quality Assessment Results
The majority of studies were fair in quality, with none of the studies performing poorly. Table 4 represents the results of the quality assessment of the included articles.
Table 4.
Quality Assessment, QHES Tool.
| Author | Score | Overall Assessment | Grading Criteria |
|---|---|---|---|
| Al Saleh et al29 | 82 | Good Quality | Good Quality = 76-100 points Fair Quality = 51-75 points Poor Quality = 0-50 points |
| Amin et al30 | 74 | Fair Quality | |
| Amin et al31 | 71 | Fair Quality | |
| Amin et al(2015)28 | 71 | Fair Quality | |
| Jurgin et al26 | 89 | Good Quality | |
| Lanitis et al32 | 88 | Good Quality | |
| Quon et.al27 | 71 | Good Quality |
Discussion
To our best knowledge, this is the first systematic review that summarizes cost-effectiveness studies comparing among DOACs in VTE, either directly or indirectly. There is lack of standardization on how systematic reviews of cost-effectiveness studies are to be conducted, and the current study achieves its objectives of comparatively summarizing the cost-effectiveness evaluations among DOACs for the purpose of health-care providers and decision makers in practices, including formulary decisions. It seems that the higher cost of DOACs was dominated by the value of their advantages of the minimized need for monitoring and the superior efficacy and safety profiles.
In relation to the results of efficacy end points, as seen in Table 2, the majority of the studies (n = 5) concluded that apixaban was associated with the least number of clinical events in terms of recurrent VTE, compared to the other DOACs. Amin et al, however, reported that edoxaban was superior to apixaban in terms of the reduced recurrent VTE rate in the general population, with both showing superiorities in efficacy over the dabigatran and rivaroxaban. In the study by Quon et al, only investigating dabigatran and rivaroxaban, the latter was associated with superior efficacy compared to dabigatran. However, in all other included studies (n = 6), dabigatran had superior efficacy over rivaroxaban. In summary, edoxaban appears to be second to apixaban in efficacy, followed by dabigatran and then rivaroxaban. In relation to the efficacy against initial treatment and extended treatment of VTE, apixaban was demonstrated in this review to be the most efficacious, with a superior safety and efficacy profile, compared to other DOACs. These results come in line with previous studies evaluating the safety and efficacy of DOACs in different disease conditions.42–44 Important to note is that results have to be interpreted cautiously giving that all comparative data are not based on head-to-head study sources and were all performed using clinical events reported in the literature. These events were extracted from studies that compared DOACs to the gold standard warfarin/LMWH. And to the best of our knowledge, there are no real-world head-to-head data in the VTE treatment/prophylaxis that compares DOACs in terms of effectiveness and/or safety.
Results of the cost-effectiveness analyses are based on studies from 3 different countries (United States, United Kingdom, and Canada), and they differ in the used economic model, study perspective (ie, the adopted viewpoint of the analysis regarding the type of included costs and effects; eg, society, payer, provider, and patient), comparators, acquisition costs, willingness-to-pay threshold, and presentation of results as well as the financial year of results. The variability in such important methodological aspects of studies makes the generation of cumulative quantitative evidence or summative cost values nonfeasible for DAOCs. Descriptive study results, nevertheless, and based on the currently available evidence, show that apixaban was the most cost-effective (dominant) option in terms of annual total medical cost avoidance, savings ppy and cost/QALY. In terms of VTE initial treatment, apixaban showed favorable prevention of VTE recurrence and reduction in bleeding events after 6 months of treatment, at a lower cost. The major and CRNM bleeding events were also lower with apixaban, compared to other DOACs, resulting in apixaban being a more cost-effective treatment option compared with other alternatives, including dabigatran and rivaroxaban. Rivaroxaban was inferior to apixaban but superior (cost-effective) over edoxaban and dabigatran. Edoxaban was inferior to both apixaban and rivaroxaban according to Amin et al, but it was superior to dabigatran in terms of annual total medical cost differences and avoidance per patient-year. Dabigatran was dominated in almost all cost-effectiveness studies, except in that by Jurgin et al, where it dominates rivaroxaban in terms of cost/QALY.
More importantly, results in this review are consistent with other reports in the literature, including systematic reviews, cost-effectiveness analyses and meta-analyses that also compared the cost-effectiveness among DOACs, and for stroke prevention in patients with atrial fibrillation.11,12,14–16 An additional example of consistent results is a Monte-Carlo cost-effectiveness simulation of rivaroxaban against apixaban, where the former had the lowest cost compared to other DOACs, while the latter had the highest QALYs and was considered the most cost-effective.45
This review includes several limitations. First, while the search strategy did include gray literature, this did not include nonpublished articles, which could have been of relevance. Also, the search was language restricted, where relevant articles could have been missed. Resources to translate non-English articles, however, are not available to authors. Additional articles could have been identified in the literature with the use of additional search engines. Here, nonetheless, it is noted that the PubMed and Embase databases cover almost 80% of the literature, and with the utilization of EconLit as well, the authors believe to have covered a representative sample of literature.46 Additional articles could have also been found with other key search terms and/or new combinations of them. Here, of relevance, it is important to note that we included “atrial fibrillation” and “stroke” in the search terms, in case of having studies looking at VTE as a secondary underlying indication to the stroke and atrial fibrillation. This would help ensure the comprehensiveness of our search and reduce the possibility of missing any potential articles. Moreover, 6 of the included studies in this review were industry funded. A final limitation in the study is that the quality of journals and their editorial requirements were not weighed into the quality assessment.
Conclusion
Apixaban dominates other DOACs for the prevention and treatment of VTE. In VTE extended treatment, apixaban was associated with the highest cost avoidance mainly due to the reduced rates of recurrent VTE and major bleeding compared to other DOACs. The cost-effectiveness of apixaban is followed by that of rivaroxaban, edoxaban, and then dabigatran.
Recommendation
All economic studies in this review were not based on head-to-head clinical sources of data. They were based on the data obtained from the phase 3 clinical trials for each DOAC, when compared against warfarin. Thus, future head-to-head clinical studies among DOACs are recommended.
Footnotes
Authors’ Note: Mohammad Al Mukdad, B.Pharm, Daoud Al-Badriyeh, PhD, and Hazem Fathy Elewa, PhD contributed equally to this work.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This report was made possible by Qatar University grant # (QUST-1-CPH-2019-3). The statements made herein are solely the responsibility of the authors.
ORCID iD: Mohammad Al Mukdad  https://orcid.org/0000-0003-0273-6882
https://orcid.org/0000-0003-0273-6882
References
- 1. Blann AD, Lip GYH. Clinical review Venous thromboembolism. BMJ. 2006;332(7535):215–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Heit JA. Epidemiology of venous thromboembolism. Nat Rev Cardiol. 2015;12(8):464–474. doi: 10.1038/nrcardio.2015.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Spencer FA, Emery C, Lessard D, et al. The Worcester Venous Thromboembolism study: a population-based study of the clinical epidemiology of venous thromboembolism. J Gen Intern Med. 2006;21(7):722–727. doi: 10.1111/j.1525-1497.2006.00458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Silverstein MD, Heit JA, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ., 3rd Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25-year population-based study. Arch Intern Med. 1998;158(6):585–593. [DOI] [PubMed] [Google Scholar]
- 5. Cushman M, Tsai AW, White RH, et al. Deep vein thrombosis and pulmonary embolism in two cohorts: the longitudinal investigation of thromboembolism etiology. Am J Med. 2004;117(1):19–25. doi: 10.1016/j.amjmed.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 6. Huang W, Goldberg RJ, Anderson FA, Kiefe CI, Spencer FA. Secular trends in occurrence of acute venous thromboembolism: the Worcester VTE study (1985-2009). Am J Med. 2014;127(9):829–839.e5. doi: 10.1016/j.amjmed.2014.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Heit JA. Venous thromboembolism: disease burden, outcomes and risk factors. J Thromb Haemost. 2005;3(8):1611–1617. doi: 10.1111/j.1538-7836.2005.01415.x. [DOI] [PubMed] [Google Scholar]
- 8. Hill J, Treasure T. Reducing the risk of venous thromboembolism (deep vein thrombosis and pulmonary embolism) in patients admitted to hospital: summary of the NICE guideline. Heart. 2010;96(11):879–882. [DOI] [PubMed] [Google Scholar]
- 9. Sterne JAC, Bodalia PN, Bryden PA, et al. Oral anticoagulants for primary prevention, treatment and secondary prevention of venous thromboembolic disease, and for prevention of stroke in atrial fibrillation: Systematic review, network meta-analysis and cost-effectiveness analysis. Health Technol Assess (Rockv). 2017;21(9):1–385. doi: 10.3310/hta21090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brockbank J, Wolowacz S. Economic Evaluations of New Oral Anticoagulants for the Prevention of Venous Thromboembolism After Total Hip or Knee Replacement: A Systematic Review. Pharmacoeconomics. 2017;35(5):517–535. doi: 10.1007/s40273-017-0486-4. [DOI] [PubMed] [Google Scholar]
- 11. Kasmeridis C, Apostolakis S, Ehlers L, Rasmussen LH, Boriani G, Lip GYH. Cost effectiveness of treatments for stroke prevention in atrial fibrillation: focus on the novel oral anticoagulants. Pharmacoeconomics. 2013;31(11):971–980. doi: 10.1007/s40273-013-0090 -1. [DOI] [PubMed] [Google Scholar]
- 12. Liberato NL, Marchetti M. Cost-effectiveness of non-vitamin K antagonist oral anticoagulants for stroke prevention in non-valvular atrial fibrillation: a systematic and qualitative review. Expert Rev Pharmacoecon Outcomes Res. 2016;16(2):221–235. doi: 10.1586/14737167.2016.1147351. [DOI] [PubMed] [Google Scholar]
- 13. McKeage K. Dabigatran etexilate: a pharmacoeconomic review of its use in the prevention of stroke and systemic embolism in patients with atrial fibrillation. Pharmacoeconomics. 2012;30(9):841–855. doi: 10.2165/11209130-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 14. Singh SM, Wijeysundera HC. Cost-Effectiveness of Novel Oral Anticoagulants for Stroke Prevention in Non-Valvular Atrial Fibrillation. Curr Cardiol Rep. 2015;17(8):61 doi: 10.1007/s11886-015-0618-4. [DOI] [PubMed] [Google Scholar]
- 15. Ferreira J, Mirco A. Systematic review of cost-effectiveness analyses of novel oral anticoagulants for stroke prevention in atrial fibrillation. Rev Port Cardiol. 2015;34(3):179–191. doi: 10.1016/j.repc.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 16. López-López JA, Sterne JAC, Thom HHZ, et al. Oral anticoagulants for prevention of stroke in atrial fibrillation: systematic review, network meta-analysis, and cost effectiveness analysis. BMJ. 2017;359:j5058 doi: 10.1136/bmj.j5058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Marshall S, Fearon P, Dawson J, Quinn TJ. Stop the clots, but at what cost? Pharmacoeconomics of dabigatran etexilate for the prevention of stroke in subjects with atrial fibrillation: A systematic literature review. Expert Rev Pharmacoeconomics Outcomes Res. 2013;13(1):29–42. doi: 10.1586/erp.12.79. [DOI] [PubMed] [Google Scholar]
- 18. Kansal AR, Zheng Y, Pokora T, Sorensen SV. Cost-effectiveness of new oral anticoagulants in the prevention of stroke in patients with atrial fibrillation. Best Pract Res Clin Haematol. 2013;26(2):225–237. doi: 10.1016/j.beha.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 19. Sterne JA, Bodalia PN, Bryden PA, et al. Oral anticoagulants for primary prevention, treatment and secondary prevention of venous thromboembolic disease, and for prevention of stroke in atrial fibrillation: systematic review, network meta-analysis and cost-effectiveness analysis. Health Technol Assess. 2017;21(9):1–386. doi: 10.3310/hta21090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ofman JJ, Sullivan SD, Neumann PJ, et al. Examining the value and quality of health economic analyses: implications of utilizing the QHES. J Manag Care Pharm. 2003;9(1):53–61. doi: 2003(9)1: 53-61 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Marshall DA, Donald F, Lacny S, et al. Assessing the quality of economic evaluations of clinical nurse specialists and nurse practitioners: A systematic review of cost-effectiveness. NursingPlus Open. 2015;1:11–17. doi: 10.1016/j.npls.2015.07.001. [Google Scholar]
- 22. Al-Badriyeh D, Alameri M, Al-Okka R. Cost-effectiveness research in cancer therapy: a systematic review of literature trends, methods and the influence of funding. BMJ Open. 2017;7(1):1–11. doi: 10.1136/bmjopen-2016-012648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mishra D, Nair SR. Systematic literature review to evaluate and characterize the health economics and outcomes research studies in India. Perspect Clin Res. 2015;6(1):20–33. doi: 10.4103/2229-3485.148802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tran BX, Nong VM, Maher RM, Nguyen PK, Luu HN. A systematic review of scope and quality of health economic evaluation studies in Vietnam. PLoS One. 2014;9(8):e103825 doi: 10.1371/journal.pone.0103825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA - preferred reporting items for systematic reviews and meta-analyses - checklist. PLoS Med. 2009;6(6):e1000097 doi: 10.1371/journal.pmed1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jugrin AV, Hösel V, Ustyugova A, De Francesco M, Lamotte M, Sunderland T. Indirect comparison and cost-utility of dabigatran etexilate and rivaroxaban in the treatment and extended anticoagulation of venous thromboembolism in a UK setting. J Med Econ. 2016;19(1):1–10. doi: 10.3111/13696998.2015.1078340. [DOI] [PubMed] [Google Scholar]
- 27. Quon P, Le HH, Raymond V, Mtibaa M, Moshyk A. Clinical and economic benefits of extended treatment with apixaban for the treatment and prevention of recurrent venous thromboembolism in Canada. J Med Econ. 2016;19(6):557–567. doi: 10.3111/13696998.2016.1141780. [DOI] [PubMed] [Google Scholar]
- 28. Amin A, Bruno A, Trocio J, Lin J, Lingohr-Smith M. Real-world medical cost avoidance when new oral anticoagulants are used versus warfarin for venous Thromboembolism in the United States. Clin Appl Thromb. 2016;22(1):5–11. doi: 10.1177/1076029615585991. [DOI] [PubMed] [Google Scholar]
- 29. Al Saleh AS, Berrigan P, Anderson D, Shivakumar S. Direct oral anticoagulants and vitamin K antagonists for treatment of deep venous thrombosis and pulmonary embolism in the outpatient setting: comparative economic evaluation. Can J Hosp Pharm. 2017;70(3):188–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Amin A, Jing Y, Trocio J, Lin J, Lingohr-Smith M, Graham J. Evaluation of medical costs avoided when new oral anticoagulants are used for extended treatment of venous thromboembolism based on clinical trial results. J Thromb Thrombolysis. 2015;40(2):131–138. doi: 10.1007/s11239-014-1158-2. [DOI] [PubMed] [Google Scholar]
- 31. Amin A, Jing Y, Trocio J, Lin J, Lingohr-Smith M, Graham J. Evaluation of medical costs associated with use of new oral anticoagulants compared with standard therapy among venous thromboembolism patients. J Med Econ. 2014;17(11):763–770. doi: 10.3111/13696998.2014.950670. [DOI] [PubMed] [Google Scholar]
- 32. Lanitis T, Leipold R, Hamilton M, et al. Cost-effectiveness of apixaban versus other oral anticoagulants for the initial treatment of venous thromboembolism and prevention of recurrence. Clin Ther. 2016;38(3):416–478. doi: 10.1016/j.clinthera.2016.01.020. [DOI] [PubMed] [Google Scholar]
- 33. Schulman S, Kearon C, Kakkar AK, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med. 2009;361(24):2342–2352. doi: 10.1056/NEJMoa0906598. [DOI] [PubMed] [Google Scholar]
- 34. Schulman S, Kakkar AK, Goldhaber SZ, et al. Treatment of acute venous thromboembolism with dabigatran or warfarin and pooled analysis. Circulation. 2014;129(7):764–772. doi: 10.1161/CIRCULATIONAHA.113.004450. [DOI] [PubMed] [Google Scholar]
- 35. Schulman S, Kearon C, Kakkar AK, et al. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N Engl J Med. 2013;368(8):709–718. doi:10.1056/NEJMoa1113697. [DOI] [PubMed] [Google Scholar]
- 36. Bauersachs R, Berkowitz SD, Brenner B, et al. ; EINSTEIN Investigators. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363(26):2499–2510. doi: 10.1056/NEJMoa1007903. [DOI] [PubMed] [Google Scholar]
- 37. Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 38. Buller HR, Prins MH, Lensin AW, et al. ; EINSTEIN–PE Investigators. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med. 2012;366(14):1287–1297. doi: 10.1056/NEJMoa1113572. [DOI] [PubMed] [Google Scholar]
- 39. Agnelli G, Buller HR, Cohen A, et al. apixaban for extended treatment of venous thromboembolism. N Engl J Med. 2012;368(8):699–708. doi: 10.1056/NEJMoa1207541. [DOI] [PubMed] [Google Scholar]
- 40. Agnelli G, Buller HR, Cohen A, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. 2013;369(9):799–808. doi: 10.1056/NEJMoa1302507. [DOI] [PubMed] [Google Scholar]
- 41. Büller HR, Décousus H, Grosso MA, et al. ; Hokusai-VTE Investigators. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med. 2013;369(15):1406–1415. doi: 10.1056/NEJMoa1306638. [DOI] [PubMed] [Google Scholar]
- 42. Vinogradova Y, Coupland C, Hill T, Hippisley-Cox J. Risks and benefits of direct oral anticoagulants versus warfarin in a real world setting: cohort study in primary care. BMJ. 2018:k2505 doi: 10.1136/bmj.k2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shah S, Norby FL, Datta YH, et al. Comparative effectiveness of direct oral anticoagulants and warfarin in patients with cancer and atrial fibrillation. Blood Adv. 2018;2(3):200 LP–209. http://www.bloodadvances.org/content/2/3/200.abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cohen AT, Hamilton M, Mitchell SA, et al. Comparison of the Novel Oral Anticoagulants Apixaban, Dabigatran, Edoxaban, and Rivaroxaban in the Initial and Long-Term Treatment and Prevention of Venous Thromboembolism: Systematic Review and Network Meta-Analysis. PLoS One. 2015;10(12):e0144856 doi: 10.1371/journal.pone.0144856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Harrington AR, Armstrong EP, Nolan PE, Malone DC. Cost-effectiveness of apixaban, dabigatran, rivaroxaban, and warfarin for stroke prevention in atrial fibrillation. Stroke. 2013;44(6):1676–1681. doi: 10.1161/STROKEAHA.111.000402. [DOI] [PubMed] [Google Scholar]
- 46. Falagas ME, Pitsouni EI, Malietzis GA, Pappas G. Comparison of PubMed, Scopus, Web of Science, and Google Scholar: strengths and weaknesses. FASEB J Off Publ Fed Am Soc Exp Biol. 2008;22(2):338–342. doi: 10.1096/fj.07-9492LSF. [DOI] [PubMed] [Google Scholar]