Abstract
Pulmonary embolism (PE) is a life-threatening condition and a leading cause of morbidity and mortality. There have been many advances in the field of PE in the last few years, requiring a careful assessment of their impact on patient care. However, variations in recommendations by different clinical guidelines, as well as lack of robust clinical trials, make clinical decisions challenging. The Pulmonary Embolism Response Team Consortium is an international association created to advance the diagnosis, treatment, and outcomes of patients with PE. In this consensus practice document, we provide a comprehensive review of the diagnosis, treatment, and follow-up of acute PE, including both clinical data and consensus opinion to provide guidance for clinicians caring for these patients.
Keywords: acute pulmonary embolism, venous thromboembolism, pulmonary embolism response team, systemic thrombolysis, catheter-directed thrombolysis, embolectomy, inferior vena cava filter, chronic thromboembolic pulmonary hypertension
Introduction
There are an estimated 900 000 cases of venous thromboembolism (VTE) every year in the United States, 150 000 to 250 000 pulmonary embolism (PE)-related hospitalizations and 60 000 to 100 000 deaths, making it the third most common cause of cardiovascular death.1 Once a PE is diagnosed, risk stratification is necessary to define appropriate management. Treatments can range from anticoagulation alone, catheter-directed thrombolysis, full-dose systemic thrombolysis (ST), reduced-dose ST, catheter embolectomy, surgical embolectomy, and/or mechanical circulatory support such as extracorporeal membrane oxygenation (ECMO). It has been recognized that advanced treatment options used for PE vary by institution, medical specialty, and operator experience.2 Variations or ambiguity in treatment recommendations in clinical guidelines published by societies such as the American College of Chest Physicians (ACCP),3 American Heart Association (AHA),4 and/or European Society of Cardiology (ESC),5 as well as lack of robust clinical trials make advanced treatment decisions challenging.
To aid physicians caring for patients with acute PE, consensus diagnosis, treatment, and follow-up algorithms were developed by the Pulmonary Embolism Response Team (PERT) consortium. A writing group was established by the PERT Clinical Practice and Protocols Development Committee. The writing group was divided into the topics focused in this manuscript based on each member content expertise. Each group systematically reviewed and summarized the relevant published literature and incorporated this information into a manuscript. In areas where high-quality evidence was lacking, the committee carefully consolidated algorithms from multiple institutions with PERT programs and administered a practice survey (Supplemental Material) in our PERT annual meeting and incorporated them into the manuscript. Differences in opinion were dealt with face-to-face meetings and subsequently through electronic and telephone communications. The final document and algorithms reflect the consensus opinion of the entire committee.
The purpose of these algorithms is to provide practical, evidence-based, and expert recommendations from across disciplines and institutions, for the management of PE that can be applied in the real world (Table 1). We present the structure of the PERT activation (Figure 1), the diagnosis (Figure 2), treatment (Figure 3), and follow-up (Figure 4) algorithms of the PERT Consortium as well as outlining the rationale and evidence or expert opinion to support each decision.
Table 1.
Executive Summary of the Diagnosis, Treatment, and Follow-Up Recommendations From the Pulmonary Embolism Response Team Consortium.
| Pulmonary Embolism Response Team (PERT) |
|
| Diagnosis, Imaging, and Risk Stratification of Pulmonary Embolism |
|
| Treatment of Pulmonary Embolism |
Anticoagulation
|
Systemic Thrombolysis (ST)
|
Catheter-Directed Therapy
|
Surgical Embolectomy (SPE)
|
Mechanical Hemodynamic Support
|
Inferior Vena Cava (IVC) Filters
|
| Pulmonary Embolism Follow-Up |
|
Abbreviations: BNP, brain natriuretic peptide; CDL, catheter directed thrombolysis; CTA, chest computed tomographic angiography; CTEPH, chronic thromboembolic pulmonary hypertension; DVT, deep venous thrombosis; IVC, inferior vena cava; PE, pulmonary embolism; PERT, pulmonary embolism response team; PESI, pulmonary embolism severity index; sPESI, simplified pulmonary embolism severity index; RV, right ventricular; SPE, surgical pulmonary embolectomy; ST, systemic thrombolysis; V/Q, ventilation/perfusion scan; VTE, venous thromboembolism.
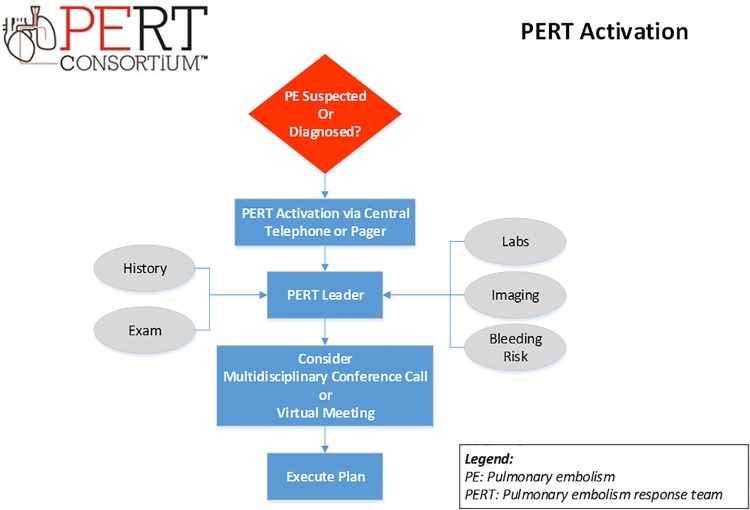
Figure 1.
PERT activation. PERT indicates Pulmonary Embolism Response Team.
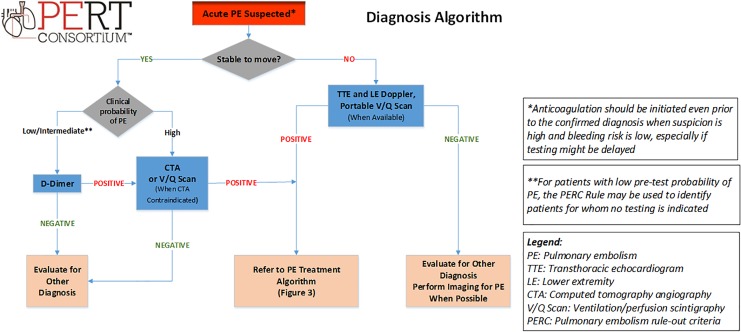
Figure 2.
Pulmonary embolism diagnosis algorithm.
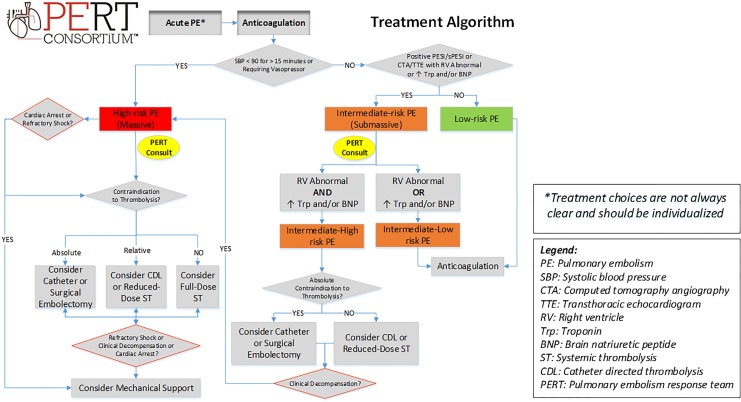
Figure 3.
Pulmonary embolism treatment algorithm.
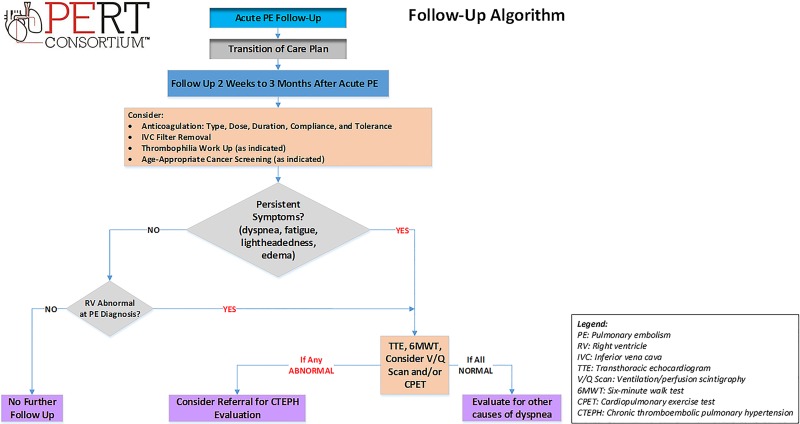
Figure 4.
Pulmonary embolism follow-up algorithm.
Pulmonary Embolism Response Team
The concept of multidisciplinary team collaboration for serious diseases has been implemented successfully for the last decade in the “Heart Team” approach in complex percutaneous coronary intervention and transcatheter aortic valve replacement and it is supported by the AHA and American College of Cardiology guidelines.6-9 In response to increasing patient complexity and increasing therapeutic alternatives, there has been a rise in the development of multidisciplinary groups of clinicians with expertise in the diagnosis and medical, surgical, and interventional management of PE who collaborate in a novel way to improve patient care. This multidisciplinary team approach is termed the PERT.
Pulmonary Embolism Response Team structure and approaches vary by institution and may involve members from cardiac surgery, cardiac imaging, interventional and noninterventional cardiology, critical care, emergency medicine, hematology, clinical pharmacy, pulmonary, diagnostic and interventional radiology, vascular medicine, and vascular surgery.2,10 A PERT is typically activated via a single contact to a hospital’s central call service or designated phone number (or pager) which triggers a prompt patient evaluation by the on-call clinician who gathers relevant clinical information (Figure 1). Following that initial evaluation, when the decision is not immediately clear, and in order to facilitate real-time discussion and generate shared decision-making recommendations, a virtual multidisciplinary meeting or conference call among all PERT members is often held. Finally, the consensus plan of action is rapidly presented to the referring clinician. Pulmonary Embolism Response Teams may also coordinate outpatient follow-up care and serve as platform for clinical research.
The National PERT Consortium was established in 2015 when different institutions across the United States met in Boston, Massachusetts, with the intent to collaborate, guide, and influence PE management and research. Currently, the concept of PERT has been adopted in more than 100 centers worldwide. A framework was created by establishing committees to structure the PERT Consortium: Governance, Research, Education, Clinical Practice and Protocol Development and Communication. One of the most important goals of the PERT Clinical Practice and Protocols Development Committee is to characterize consensus practice for clinical care. The algorithms include clinical evidence and expert recommendations from across disciplines and institutions. This manuscript details the rationale for these consensus algorithms.
Recommendation:
Utilize a multidisciplinary Pulmonary Embolism Response Team (PERT) in patients with high or intermediate-risk PE, as well as for PE patients in whom there is uncertainty regarding treatment.
Diagnosis, Imaging, and Risk Stratification of Pulmonary Embolism
A thorough history is the first step in suspected acute PE. Patients may experience chest discomfort, palpitations, dyspnea, dizziness or syncope, extremity pain, or swelling. Physical examination findings of PE may include tachypnea, tachycardia, jugular venous distension, parasternal heave, augmented second heart sound, right-sided S3, tricuspid regurgitation murmur, pulsatile liver, peripheral edema and chest wall, back, or flank tenderness (pulmonary infarction). Most clinical presentations are nonspecific and may lead to frequent misdiagnosis.11,12
Findings of PE are rarely detected on chest radiography but if present, include Hampton hump (wedge-shaped airspace opacity indicative of infarct) or Westermark sign (prominent proximal pulmonary artery [PA] with reduction in more peripheral blood vessel markings). On electrocardiography, the most common sign of PE is sinus tachycardia or atrial fibrillation, however some well-described though insensitive signs of right heart strain include S1Q3T3, anterior precordial T-wave inversions, and either inferior or anterior precordial ST-segment elevation.13
After a comprehensive history and physical examination, determining the pretest probability of PE is the next step in diagnosis. The 2 most utilized and validated clinical decision rules are the Geneva score and the Wells’ score.14,15 These classify pretest probability of PE into low-, intermediate- or high-probability (or PE likely or unlikely). D-dimer testing is highly sensitive and can rule out PE in patients with low or intermediate pretest probability.16 Age- and risk-adjusted D-dimer testing has higher specificity than the typical cutoff of 500 μg/L and may be useful to exclude PE in those patients with low-probability or PE-unlikely.17
For patients with low pretest probability of PE, the Pulmonary Embolism Rule-out Criteria (PERC) rule may be used to identify patients for whom no testing is indicated.18 Imaging is recommended for those patients with low- or intermediate-pretest probability and a positive D-dimer, or high pretest probability (Figure 2). Contrast-enhanced chest computed tomography angiography (CTA) is the gold standard for the diagnosis of PE due to its sensitivity and specificity profile, as well as its wide availability across hospitals.5 Ventilation/perfusion scintigraphy (V/Q scan) may be used for pregnant women, patients with contraindications to iodinated contrast, such as contrast-induced anaphylaxis or significant renal insufficiency, or when patients cannot be moved for CTA (when portable V/Q scan is available). Transthoracic echocardiography should also be considered in higher risk patients with PE, including patients with high pretest probability of PE but with hemodynamic instability and inability to travel for CTA. Transthoracic echocardiography provides an assessment of right ventricular (RV) structure and function as well as an assessment for intracardiac thrombus-in-transit. Right ventricular “abnormal” may include right heart dilation, interventricular septal compression, increased tricuspid regurgitation, RV hypokinesis or presence of McConnell’s sign, which refers to a regional pattern of RV free wall dysfunction with sparing of the apex.19,20 Duplex leg ultrasonography may be a complimentary method to investigate for evidence of VTE when PE is suspected and pulmonary vascular imaging cannot be performed.
Once a PE has been confirmed, risk stratification is necessary to determine appropriate therapy. Risk stratification is primarily based on the ability of the RV to overcome the afterload caused by the thrombus. Acute PE in the presence of hypotension (defined as systolic blood pressure [SBP] <90 mm Hg for >15 minutes, a drop in SBP of 40 mm Hg or more from baseline, or requiring hemodynamic support) is considered high-risk (massive) PE.4 In the absence of hypotension, further stratification by use of biomarkers, imaging (CTA or echocardiography), and the Pulmonary Embolism Severity Index (PESI) or simplified PESI (sPESI) can be performed.5 As none of these factors or scores definitively determines prognosis, clinician gestalt also plays an important role.21 The PESI and the sPESI represent a validated clinical scoring system that predict 30-day mortality, with sPESI being a simpler, more practical clinical approach.22 Intermediate-risk (submassive) PE patients have PESI class III-V or sPESI ≥1 or RV dilation or dysfunction by CTA or echocardiography, or the presence of positive biomarkers suggestive of myocardial injury (troponin) or myocardial distention (brain natriuretic peptide [BNP] or N-terminal pro-BNP).5 Further classification by the ESC of intermediate-risk PE into intermediate-high risk (presence of both RV dysfunction on imaging and biomarker elevation) or intermediate-low risk (either RV dysfunction or biomarker elevation)5 may help gauge risk of decompensation and suggest the possible need for more aggressive PE treatment. Hemodynamic decompensation may be evidenced by clinical deterioration based on vital signs, worsening of RV dysfunction, tissue perfusion, and/or gas exchange. The rationale for sub-dividing intermediate-risk patients into 2 categories is patients with both RV dysfunction and abnormal biomarkers have much higher in-hospital mortality compared to either alone which may aid in the selection of patients in whom more advance therapies could be considered.23 In addition, adding lactate levels to risk stratification may identify an even higher risk group for early decompensation. In a study of 496 normotensive patients with acute PE, the combination of elevated lactic acid, RV dysfunction, and elevated troponin was associated with a 17.9% incidence of in-hospital mortality or nonfatal hemodynamic collapse.24
Finally, low-risk PE patients are defined as hemodynamically stable with the absence of clinical markers of adverse prognosis that define intermediate or high-risk PE. In patients who are hemodynamically stable at diagnosis, no individual clinical, imaging, or laboratory finding have been shown to be superior in predicting risk of decompensation. However, combinations of clinical findings with imaging and laboratory tests have been proposed and tested in registries and cohort studies in attempt to provide enhanced risk stratification.5,25,26
Many low-risk PE patients may be safe for early discharge without admission to the hospital. In a meta-analysis of 1657 low-risk patients with acute PE from 13 studies, there was similar mortality, recurrent VTE and bleeding with early hospital discharge (<24 hours) compared to routine hospitalization. However, most of these trials were small in size and included different methods for identification of risk.27 In the recently presented HoT-PE trial, 525 patients were discharged home within 24 hours if they had low risk based on modified Hestia criteria, including hemodynamic stability, free of significant comorbidities, normal RV function, and absence of thrombus-in-transit on CTA or echocardiogram.28 The ESC and ACCP guidelines recommend selected low-risk patients are safe for early discharge.3,5 In addition, selected low risk patients with sub-segmental PE and no DVT may be at so low risk for death or recurrent PE that the risk of anticoagulation outweighs any benefit. While the quality of data is low, it may be reasonable to consider omitting anticoagulation in patients with a single sub-segmental PE, especially if seen only on only one image, without DVT, no active cancer, and no symptoms.3
Recommendations:
2. Use a combination of low- or intermediate-pretest probability, the PERC rule and D-dimer testing to rule out PE without imaging.
3. When possible, use CTA to diagnose acute PE in patients with low or intermediate pretest probability and a positive D-dimer, or high pretest probability.
4. Echocardiography and/or portable V/Q scan, when available, should be considered when there are contraindications to or inability to obtain CTA. Additionally, duplex ultrasonography should be considered to confirm the presence, acuity, and extent of VTE.
5. Once PE is diagnosed, risk stratification is recommended using a composite of clinical appearance, systolic blood pressure, heart rate, respiratory rate, oxygen requirement, PESI or sPESI, imaging for RV dysfunction (CTA or echocardiography) and/or biomarkers (troponin, BNP, or NT-pro-BNP).
Treatment of Pulmonary Embolism
The treatment options for PE are wide ranging and include anticoagulation alone, catheter-directed thrombolysis, full-dose ST, reduced-dose ST, catheter embolectomy, surgical embolectomy, and/or mechanical circulatory support devices.
Anticoagulation
The benefit of early therapeutic anticoagulation to improve mortality and decrease recurrence in acute PE is well proven.29 Anticoagulation should be initiated even prior to the confirmed diagnosis when there is high clinical suspicion of acute PE and the bleeding risk is low, particularly if results of diagnostic tests are expected to be delayed.4 Even when additional therapeutic modalities are considered, anticoagulation should not be delayed unless contraindicated.4,5
Selection of the initial anticoagulation agent is dependent on many factors, including risk stratification, patient clinical factors (eg, hepatic and renal function and bleeding risk), and clinician judgment. In the case of intermediate- and high-risk PE in which advanced therapies such as thrombolysis, catheter-based interventions, surgical pulmonary embolectomy (SPE) or mechanical circulatory support are being considered, unfractionated heparin (UFH) may be the preferred agent as it allows for interventional and surgical teams optimal management flexibility. The dosing and monitoring of UFH should be based on institution-specific protocols designed to achieve therapeutic anticoagulation as quickly as possible. Aggressive monitoring and dose adjustment are often required to ensure rapid therapeutic anticoagulation with UFH. Low-molecular weight heparin with its rapid and reliable bioavailability and excellent safety profile may also be considered.30 Frontline clinicians are advised to discuss these options with their consultants (ie, their PERT team). For low-risk PE, especially when outpatient management is contemplated, multiple factors should be considered including but not limited to patient comorbidities, patient compliance, affordability and insurance coverage, and drug characteristics and benefits and should also include shared decision-making with the patient.31 The direct oral anticoagulants offer many advantages and are now considered first-line therapy for low-risk PE3 and should be considered in intermediate and high-risk PE patients after clinical stability has been achieved and/or treatment with advanced therapies is completed.32 Direct oral anticoagulants do not require routine monitoring of international normalized ratio for safety or efficacy, have a rapid onset of action, short half-life, predictable pharmacokinetics and pharmacodynamics, fixed dosing, and fewer drug and food interactions, making them convenient for both patient and providers. A detailed description of all of the various anticoagulants is beyond the scope of this article but is widely available in the literature.33
Recommendations:
6. Anticoagulation should be initiated as soon as PE is diagnosed unless contraindicated.
7. Anticoagulation should be initiated even prior to the confirmed diagnosis when the clinical suspicion of acute PE is high and the bleeding risk is low.
8. Utilize evidence-based institution-specific anticoagulation guidelines to assist in anticoagulant choice, dosing, administration, and appropriate laboratory monitoring strategies to achieve therapeutic anticoagulation as quickly as possible.
Systemic thrombolysis
Systemic thrombolysis is given to achieve rapid clot resolution and restoration of pulmonary perfusion thereby improving ventilation/perfusion matching, and importantly relieving RV afterload, reducing pulmonary vascular resistance, and thereby improving hemodynamics.5,34,35 Systemic thrombolysis however, is associated with an increase in hemorrhagic complications including intracranial hemorrhage (ICH).3-5,36,37
The decision to administer ST therapy in patients with PE should be based on the delicate balance between a patient’s clinical and hemodynamic status and individualized risks of bleeding. There are several populations in which this therapy may be considered: cardiac arrest with known or suspected PE, right-heart thrombus (RHT) or thrombus-in-transit, high-risk PE, and selected intermediate-high-risk PE cases with low risk of bleeding (Figure 3).
When confirmed or highly suspected PE is felt to be the precipitant of cardiac arrest, ST with tissue plasminogen activator (tPA) via bolus or rapid infusion, or tenecteplase via bolus, have been reported to increase the rate of return of spontaneous circulation and survival in nonrandomized observational trials.38-42 In some reports, resuscitative measures have continued for up to 30 minutes after thrombolytic administration with successful outcomes.39,43
In high-risk PE, ST is recommended when there are no contraindications (Table 2),3-5 as it has been shown to reduce total and PE-related mortality and PE recurrence when compared to UFH alone.36,44 The Food and Drug Administration (FDA) recommended dose of tPA is 100 mg over 2 hours. In patients with relative contraindications to ST, a reduced-dose of 50 mg over 2 hours has been suggested as an alternative to full-dose ST, with similar improvements in obstruction, perfusion, PA pressure, and RV size with fewer bleeding complications, although data supporting this approach is limited.45 There is no standardized approach on anticoagulation management during thrombolysis, but a reasonable approach is to hold UFH during the thrombolytic infusion and resume without a bolus once the partial thromboplastin time is less than twice control (Supplemental Material).46
Table 2.
Absolute and Relative Contraindications to Thrombolysis.
| Absolute contraindications to systemic thrombolysis |
| Active bleeding |
| Prior intracranial hemorrhage |
| Ischemic stroke within 3 months |
| Suspected or confirmed aortic dissection |
| Recent brain or spinal surgery |
| Recent head or facial trauma |
| Intracranial neoplasm, vascular malformation, aneurysm, or any other structural brain disease |
| Relative contraindications to systemic thrombolysis |
| Age > 75 |
| Total body weight < 60 kg |
| Known bleeding diathesis or acquired coagulopathy |
| Platelet count < 100 000 |
| Coagulopathy (INR > 1.7) |
| Uncontrolled hypertension (SBP > 180 mm Hg /DBP > 110 mm Hg) |
| Recent significant nonintracranial bleeding (within 1 month) |
| Recent major surgery, invasive procedure, and/or trauma (within 1 month) |
| Current pregnancy or childbirth (within 1 week) |
| History of remote ischemic stroke (>3 months) |
Abbreviations: DBP, diastolic blood pressure; INR, international normalized ratio; kg, kilograms; SBP, systolic blood pressure.
Successful use of ST has been reported in a subset of patients with RHT.47 Also in retrospective studies of thombus-in-transit, ST was associated with improved survival when compared to either anticoagulation therapy or surgery.48,49
In intermediate-risk PE, the use of ST is more controversial. Various reports have suggested that ST reduces the risk of clinical decompensation compared to anticoagulation alone.37,50 However, in the largest trial to date, the PEITHO trial, the decreased risk of clinical decompensation was not accompanied by a decrease in mortality, and was associated with an increased risk of hemorrhagic stroke. A randomized trial comparing full-dose to reduced-dose ST in patients with intermediate or high-risk PE demonstrated similar improvements in a host of surrogate end points including metrics of RV dysfunction or PA pressure change with small differences in bleeding favoring the reduced-dose strategy.45 Reduced-dose ST may be considered in select patients with intermediate-high risk PE and evidence of clinical decompensation after starting anticoagulation based on their symptoms, vital signs, tissue perfusion, or gas exchange indicators, and who have low risk of bleeding.3–5,51 The impact of ST approaches on long-term mortality, dyspnea, RV dysfunction, and/or incidence of chronic thromboembolic pulmonary hypertension (CTEPH) in intermediate-risk PE patients is controversial.51,52 Full-dose ST showed a decreased composite outcome of functional capacity, self-perception of physical health and quality of life at 90-days (Treatment of submassive PE with tenecteplase or placebo: cardiopulmonary outcomes at 3 months: multicenter double-blind, placebo-controlled randomized trial [TOPCOAT] trial)53 and a reduced-dose ST showed a sustained reduction in PA pressure at 28 months, but this was by echocardiography and thus must be interpreted with caution (Moderate PE treated with thrombolysis [MOPETT] trial).51 However, in a long-term study, with a 37-month median follow up time, ST did not affect mortality rates, residual dyspnea or RV dysfunction in intermediate-risk PE.52 Thus, the decision to use this approach in an intermediate-risk PE patient should be highly individualized. Systemic thrombolysis is not recommended for clinically stable patients with minor RV dysfunction or minor myocardial necrosis.4
Recommendations:
-
9. Consider full-dose ST in:
9.1 High-risk PE without contraindications to ST
-
10. Consider reduced-dose ST in:
10.1 High-risk PE with relative contraindications to thrombolysis
10.2 Selected intermediate-high risk PE with evidence of or risk of clinical deterioration based on vital signs, severity of RV dysfunction, tissue perfusion, and/or gas exchange, and presence of low bleeding risk.
11. Consider ST in patients with cardiac arrest and suspected PE
12. Consider ST in selected patients with intermediate or high-risk PE with thrombus-in-transit.
Catheter-Directed Therapy
Catheter-directed thrombolysis
Catheter-directed thrombolysis (CDL) is performed using dedicated 4-6F multi-side-hole infusion catheters to deliver low-dose thrombolytics directly into the PE. The EkoSonic (EKOS/BTG, Bothell, Washington) ultrasound-assisted catheter-directed thrombolysis (UCDL) system is FDA approved for treatment of acute PE.54 This device consists of a conventional infusion catheter that contains an inner cable, which transmits high-frequency, low-power ultrasound waves, with the hypothesis that this will separate fibrin strands, enhancing the penetration of the thrombolytic agent into the emboli. In the 59 intermediate-risk PE patients who were randomized in the Ultrasound Accelerated Thrombolysis of PE trial, UCDL together with UFH reversed RV dilation faster than anticoagulation alone in the first 24 hours, without an increase in bleeding.55 In the SEATTLE II registry, 150 patients with high or intermediate-risk PE treated with UCDL had low in-hospital mortality and significant early reductions in pulmonary pressures, RV size, and obstruction index, with a low bleeding rate and no ICH.56 Similar findings were noted in 101 consecutive patients treated with CDL in the PERFECT registry, and no difference was found between CDL and UCDL (EKOS).57 In a meta-analysis of 860 patients undergoing CDL for PE, there were very low rates of ICH (0.35%) and major vascular complications (4.65%) with consistent early reductions in RV/left ventricular (LV) ratio and RV systolic pressure.58 These studies underscore the relative safety and efficacy of CDL, and this strategy may be considered in addition to anticoagulation in select patients with intermediate-high risk and high-risk PE, particularly those with evidence of clinical deterioration based on symptoms, vital signs, severity of RV dysfunction, tissue perfusion or gas exchange, and high risk of bleeding. Clearly availability of both expertise and rapid mobilization of catheterization laboratory or operating room facilities are vital in the decision-making process. (Figure 3) It is unknown how the safety and efficacy of CDL compares to reduced dose ST for patients with intermediate and high-risk PE.
The optimal catheter and dose of thrombolytic agent and UFH for CDL remains unknown. No significant differences have been shown between UCDL (EKOS) and CDL, but no randomized head-to-head studies have been conducted.57-61 As UCDL is significantly more expensive than CDL, the ongoing randomized standard vs. ultrasound-assisted catheter thrombolysis for submassive PE (SUNSET sPE) trial (NCT02758574) is investigating the value of ultrasound-facilitated strategies in CDL.62 Traditionally, the most common dose of tPA has been 0.5 to 1 mg/hour per catheter for a total dose of 12 to 24 mg delivered over a 24 hours infusion.55 More recently, the optimum duration and dose of r-tPA with the acoustic pulse thrombolysis procedure for submassive PE (OPTALYSE PE) trial included even smaller doses of tPA (8-24 mg) with shorter infusions times, suggesting similar improvements in RV/LV ratio to higher dose EKOS regimen, but larger confirmatory studies should be considered.63 In addition, the optimal UFH dose during CDL is unknown, but the above studies typically used either a fixed dose of 500 to 1000 units/hour or a low intensity protocol targeting anti-Xa therapeutic range 0.2 to 0.5 units/mL or PTT 40 to 60 seconds during tPA infusions (note that the range may vary between assays). These 2 approaches are used by the majority of clinicians in the PERT consortium (Supplemental Material). Utilization of CDL in intermediate-high risk PE patients currently varies at different institutions; this remains an area in need of additional research.
Catheter embolectomy
Percutaneous pulmonary embolectomy is largely reserved for selected patients with contraindications to thrombolysis and/or failure of thrombolysis, when surgical embolectomy is not available, and if the percutaneous interventional equipment and expertise is available at the treatment facility (Figure 3).4,5 Given the lack of data and the absence of a standard approach to catheter embolectomy, selection of patients for such treatment is best undertaken after multidisciplinary discussions among specialists with expertise in PE treatment and in centers familiar with its techniques.64
The Flowtriever system (Inari Medical, Irvine, California) consists of a large aspiration catheter with 3 self-expanding nitinol disks that are unsheathed to engage the emboli once inside the desired PA branch.65 Simultaneous manual aspiration and withdrawal of the disks through the 20Fr guide catheter allow for partial clot extraction. The recently completed prospective Flowtriever Pulmonary Embolectomy Clinical Study (FLARE trial), showed a significant reduction in RV/LV ratio in a series of 106 patients, of whom 104 were treated with catheter embolectomy alone without CDL as an adjunctive technique (unpublished data, presented at Society for Cardiovascular Angiography and Interventions, Scientific Sessions 2018). There was an adverse event rate of 3.8% using this large device, and no cases of ICH or access site bleeding, and only one bleeding event. Though limited by its single-arm nature and its lack of long-term outcome data, the FLARE trial demonstrates mechanical thrombectomy is feasible and safe. How this will impact long-term outcomes such as mortality or the development of CTEPH is unknown.
The Penumbra Indigo embolectomy system (Penumbra Inc, Alameda, California) works on the principle of thrombus aspiration and consists of an 8Fr angled or straight catheter that is connected to a suction pump. The data to support the use of the Penumbra catheter in acute PE are limited to case reports,66 but a prospective multicenter trial investigating the safety and efficacy of this catheter is ongoing (NCT03218566).
The AngioVac catheter (Angiodynamics, Latham, New York) is a 22F catheter that removes embolic material through a centrifugal pump with blood returned via a venous reinfusion cannula such as is used in cardiopulmonary bypass. There are no large case series or randomized trials, and published experience is limited to case reports and small case series. AngioVac is best utilized with inferior vena cava or intracardiac thrombus, as accessing the pulmonary arterial tree using currently available devices is technically difficult and may incur in increased complications.
The Angiojet rheolytic thrombectomy system has been extensively reported upon for the treatment of acute PE. Despite a large global experience, there have been numerous adverse events reported with its use including arrhythmias, hemodynamic compromise, and even death. At present there is currently a black label warning from the FDA regarding its use in the pulmonary circulation.67,68
Utilization of catheter embolectomy in intermediate-high risk PE patients currently varies at different institutions; this remains an area in need of additional research.
Recommendations:
-
13. Consider CDL in:
13.1 Intermediate-high risk PE with risk for clinical deterioration based on vital signs, severity of RV dysfunction, tissue perfusion, and/or gas exchange, and without absolute contraindication to thrombolysis.
13.2 High-risk PE with relative contraindications to ST.
-
14. Consider catheter embolectomy in:
14.1 Intermediate-high risk PE with risk for clinical deterioration based on vital signs, severity of RV dysfunction, tissue perfusion, and/or gas exchange, with absolute or relative contraindications to thrombolysis.
14.2 High-risk PE with absolute contraindications to ST.
14.3 After failed ST or CDL.
14.4 Thombus-in-transit in the right atrium or right ventricle (AngioVac system).
Surgical Embolectomy
Current consensus clinical guidelines and scientific statements from the AHA,4 ACCP,3 and ESC5 recommend SPE in patients with high-risk and intermediate-high risk PE with absolute contraindications to thrombolysis, failed thrombolysis or cardiogenic shock that can lead to death before thrombolysis can take effect. The PERT treatment algorithms are in line with these society recommendations (Figure 3).
In the past, SPE was associated with high perioperative mortality, although in more recent case series mortality was reported at 4% to 11%, likely reflecting a change in patients selected for surgery and advances in technique.69 -71 Preoperative thrombolysis increases the risk of surgical bleeding but does not pose an absolute contraindication to SPE. Survival depends on patient comorbidities, hemodynamic compromise prior to surgery and the center’s experience in performing the procedure.72-74 Patients with central emboli, such as a saddle PE or those with right atrial thrombus, are considered appropriate candidates for SPE, although such patients should be compromised enough to require more aggressive therapy than anticoagulation or catheter embolectomy.75,76 There are no randomized trials comparing ST to SPE, although both improve RV function and PA systolic pressures.77 Surgical pulmonary embolectomy is associated with a decreased risk of major bleeding compared to ST.70
Right heart thrombi or thrombus-in-transit has been reported in 2.6% to 4% of PE patients and has been associated with 2 to 3 fold higher mortality than acute PE without RHT.78-82 While the optimal treatment for RHT is unknown, patients treated with anticoagulation alone appear to have a higher mortality than patients treated with ST or SPE.49,79,81 Given the high short-term mortality especially within the initial 24 hours, consultation with multidisciplinary PERT is important when considering surgical, medical, and endovascular options for these patients. The size of the RHT and the embolic burden and reserve should be considered; very small RHT may be best treated conservatively particularly when the overall clot burden is low. Thrombus-in-transit across a patent foramen ovale (PFO) represents a unique subgroup of RHT. While the optimal treatment for these patients is unknown, SPE is probably the preferred treatment given the high risk for stroke and/or ICH with thrombolysis, endovascular therapies, and/or anticoagulation alone.
Recommendation:
-
15. Consider SPE in:
15.1 High-risk PE with contraindications to, or failure of ST or CDL.
15.2 Intermediate-high risk PE, with contraindications to, or failure of ST or CDL, with risk for clinical deterioration based on vital signs, severity of RV dysfunction, tissue perfusion, and/or gas exchange.
15.3 Right-heart thrombi, especially with large thromboembolic burden.
15.4 Thrombus-in-transit across a patent foramen ovale (PFO).
Mechanical Hemodynamic Support
Mechanical circulatory support should be considered for patients with high-risk PE and cardiogenic shock with cardiac arrest or shock refractory to low-dose inotropes or with failure of thrombolysis (Figure 3). When the acute increase in RV afterload exceeds the contractile reserve of the thin-walled right ventricle, this can result in decreased cardiac output and hemodynamic collapse. The acute RV volume/pressure overload also impairs LV output, increasing the potential for hypotension. While there are no published randomized trials examining the role of mechanical circulatory support in PE, multiple uncontrolled case series have demonstrated favorable outcomes with its use.83-85 Mechanical support may be used in conjunction with ST, catheter-directed therapy, or SPE. There are 3 primary devices available utilizing 2 general modes of support.
Veno-arterial extracorporeal membrane oxygenation (VA-ECMO)
Veno-arterial extracorporeal membrane oxygenation (VA-ECMO) is effective when used for high-risk PE associated with higher survival rates than those achieved when VA-ECMO is used for other common causes of cardiogenic shock.86,87 Survival rates range from 36% to 95% in various case series.84,87-91 In experienced centers, VA-ECMO can be initiated rapidly via the percutaneous insertion of a 25 to 29F venous cannula and a 15 to 17F arterial cannula. An oxygenator is present in the circuit providing oxygenation independent of pulmonary blood flow or RV function. Rates of access site complications, including limb ischemia and hemorrhage, vary widely but may occur in up to 33% of patients.84,91-94 Veno-arterial extracorporeal membrane oxygenation has been successfully used in combination with multiple treatment modalities, including thrombolysis, and surgical or catheter embolectomy.84,88,95-102 The median duration of ECMO in these series is 4 to 6 days, and together with other PE therapy, allow time for RV recovery.84,91
Right ventricular assistance to augment antegrade flow
The Impella RP (Abiomed, Danvers, Massachusetts) is an axial flow pump placed percutaneously across the pulmonary valve via a 23Fr sheath in the femoral vein. The pump is capable of delivering 4 L of flow through the right heart. There is however, no oxygenator in this circuit, which limits its use in severely hypoxic patients. The TandemHeart Protek Duo (CardiacAssist, Pittsburgh, Pennsylvania) is an extracorporeal centrifugal pump utilizing a 29Fr dual lumen catheter placed percutaneously through the internal jugular vein into the PA. An oxygenator may be added to the circuit if hypoxia is present. There are limited single center case reports describing the use of these devices (Impella RP or TandemHeart Protek Duo) in patients with high-risk PE.103
The choice of mechanical circulatory support should be based on the center experience and device-specific characteristics.
Recommendation:
16. Consider mechanical hemodynamic support in high-risk PE with cardiac arrest, refractory shock, and/or contraindications to or failure of ST.
Inferior Vena Cava Filters
Inferior vena cava (IVC) filters should be considered in patients with acute PE and contraindications to anticoagulation and considered in those with recurrent PE despite anticoagulation.3,5,104 For patients with acute PE who tolerate anticoagulation, the routine use of an IVC filter is not recommended.3,5 A randomized trial of 400 patients with PE found that placement of retrievable IVC filters in addition to anticoagulation for 3 months did not reduce recurrent PE or mortality.105 However, it is feasible that this trial was not enriched with potential candidates who could benefit, such as iliofemoral vein DVT. On the contrary, registry studies have suggested that patients after SPE106 and those with high-risk PE have lower rates of recurrent PE and mortality when an IVC filter is placed, but these findings have not been verified in prospective trials.3,107 Furthermore, in these registries it is unknown if findings reflect patient selection bias or true benefit from the filter.
Free-floating proximal end DVT are more likely to embolize prior to presentation than those with occlusive thrombi.108,109 Consideration for insertion of IVC filter should be given when large, free-floating, proximal DVT is identified.110
Nearly all IVC filters currently being placed are retrievable. When a retrievable filter is placed, patients must be assessed for removal of the filter at the earliest opportunity. Prolonged filter implantations have been associated with higher retrieval failure and other complications, including filter migration, tilting or deformation, penetration of the cava wall by filter limbs, fracturing of the filter, embolization of fragments, or thrombosis of the device.111,112
Recommendation:
17. Consider an IVC filter for patients with contraindications to or failure of therapeutic anticoagulation and for highly selected patients with intermediate or high-risk PE.
18. Consider an IVC filter in select patients when large, free-floating, proximal DVT is identified.
Pulmonary Embolism Follow-Up
An important component of a PERT program is assuring appropriate follow up care of patients following hospitalization for acute PE. Although, there are no formal guidelines on specific post-PE follow up, we propose a consensus-based approach (Figure 4). The primary goal of an outpatient follow-up clinic is to assess the patient for persistent or recurrent symptoms, decide on appropriate type, dosage and duration of anticoagulation, monitor medication compliance and tolerance, evaluate for underlying thrombophilia or age-appropriate cancer screening, assist with temporary IVC filter retrieval, and identify sequelae of PE, such as post-PE syndrome, chronic thromboembolic disease (CTED) or CTEPH. A detailed discussion regarding the optimal duration and type of anticoagulation as well as thrombophilia testing and cancer screening is beyond the scope of this manuscript. Timing of follow-up is based on the patient’s severity of illness at presentation, hospital course, and clinical condition on discharge. The ideal time for the initial visit is individualized, and generally ranges from 2 weeks to 3 months depending on patient characteristics and on local resources.
A large retrospective, insurance claims-based study of 7068 incident PE patients, found that most patients with persistent symptoms following a PE did not undergo any further imaging or diagnostic studies.113 Persistent symptoms, such as dyspnea, fatigue, lightheadedness or leg edema, merit follow up, particularly if present after 3 months of anticoagulation.
There are no clear guidelines on post-PE imaging but most agree that repeat lung imaging (with a CTA or V/Q scan) and echocardiography, and obtaining an objective assessment of the patient’s physiologic and functional capacity (with 6-minute walk testing or cardiopulmonary exercise test) should be considered in patients whose symptoms do not resolve. The timing of such testing is individualized; most would consider testing 3 months after the PE diagnosis.
Abnormal follow-up echocardiography or V/Q scan should raise concern for the development of CTED or CTEPH. Both of these entities can have clinical significance and are considered part of the post-PE syndrome, defined as any functional limitation following an acute PE.114,115 Chronic thromboembolic disease is characterized by presence of pulmonary vascular obstruction without pulmonary hypertension.115,116 Chronic thromboembolic pulmonary hypertension is pulmonary hypertension caused by chronic organized thrombi obstructing the pulmonary arteries. Chronic thromboembolic pulmonary hypertension is defined as mean PA pressure ≥25 mm Hg with a pulmonary capillary wedge pressure <15 mm Hg and at least one segmental perfusion defect detected by V/Q scan, CTA or pulmonary angiography after 3 months of effective anticoagulation. Although rare, CTEPH can lead to RV failure if not diagnosed and treated appropriately. If CTEPH is suspected or confirmed, the patient should be referred to an expert CTEPH center.
A dedicated outpatient follow-up clinic by the PERT may provide an opportunity for ensuring standardized follow-up testing and facilitate comprehensive long-term PE care.
Recommendations:
19. Pulmonary embolism patients should have a short interval follow up visit (2 weeks-3 months) post-PE, or sooner if symptoms or patient complexity suggests the need for this. Expert follow-up with the PERT team is recommended.
20. The initial post-discharge visit should focus on the patient’s clinical status, anticoagulation regimen (type, dose, duration, compliance, and tolerance), consideration for filter removal, evaluation of thrombophilia and age-appropriate cancer screening.
21. Patients with persistent or recurrent symptoms, particularly after 3 months, merit follow-up testing.
22. If CTEPH is highly suspected or confirmed, the patient should be referred to an expert CTEPH center
Conclusion
Although PE is a leading cause of death worldwide, controversies regarding treatment and follow-up persist. This document describes the consensus algorithms for the diagnosis, treatment, and follow-up of acute PE using the multidisciplinary approach of the PERT consortium and its members.
The treatment of acute PE is rapidly evolving and clinical practice will likely benefit from the input from numerous medical specialties. The consensus practices provided in this document represent the cohesive, collective, and collaborative recommendations of the PERT consortium. Though the PERT concept is relatively novel, research regarding how PERTs can impact both early and long-term outcomes of PE patients is ongoing.
Supplemental Material
Supplemental Material, Supp_Material for Diagnosis, Treatment and Follow Up of Acute Pulmonary Embolism: Consensus Practice from the PERT Consortium by Belinda Rivera-Lebron, Michael McDaniel, Kamran Ahrar, Abdulah Alrifai, David M. Dudzinski, Christina Fanola, Danielle Blais, David Janicke, Roman Melamed, Kerry Mohrien, Elizabeth Rozycki, Charles B. Ross, Andrew J. Klein, Parth Rali, Nicholas R. Teman, Leoara Yarboro, Eugene Ichinose, Aditya M. Sharma, Jason A. Bartos, Mahir Elder, Brent Keeling, Harold Palevsky, Soophia Naydenov, Parijat Sen, Nancy Amoroso, Josanna M. Rodriguez-Lopez, George A. Davis, Rachel Rosovsky, Kenneth Rosenfield, Christopher Kabrhel, James Horowitz, Jay S. Giri, Victor Tapson, Richard Channick and the PERT Consortium in Clinical and Applied Thrombosis/Hemostasis
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Belinda Rivera-Lebron  https://orcid.org/0000-0002-7842-671X
https://orcid.org/0000-0002-7842-671X
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Blood Clots: A Serious but Preventable Medical Condition. http://www.cdc.gov/ncbddd/dvt/documents/blood-clots-fact-sheet.pdf. Updated May 4, 2016. Accessed May 6, 2016.
- 2. Barnes GD, Kabrhel C, Courtney DM, et al. Diversity in the pulmonary embolism response team model: an organizational survey of the national PERT consortium members. Chest. 2016;150(6):1414–1417. [DOI] [PubMed] [Google Scholar]
- 3. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149(2):315–352. [DOI] [PubMed] [Google Scholar]
- 4. Jaff MR, McMurtry MS, Archer SL, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation. 2011;123(16):1788–1830. [DOI] [PubMed] [Google Scholar]
- 5. Konstantinides SV, Torbicki A, Agnelli G, et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014;35(43):3033–3069, 3069a–3069 k. [DOI] [PubMed] [Google Scholar]
- 6. Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention. A report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines and the society for cardiovascular angiography and interventions. J Am Coll Cardiol. 2011;58(24):e44–e122. [DOI] [PubMed] [Google Scholar]
- 7. Hillis LD, Smith PK, Anderson JL, et al. 2011 ACCF/AHA guideline for coronary artery bypass graft surgery: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;124(23): e652–e735. [DOI] [PubMed] [Google Scholar]
- 8. Holmes DR, Jr, Rich JB, Zoghbi WA, Mack MJ. The heart team of cardiovascular care. J Am Coll Cardiol. 2013;61(9):903–907. [DOI] [PubMed] [Google Scholar]
- 9. Dudzinski DM, Horowitz JM. Start-up, organization and performance of a multidisciplinary pulmonary embolism response team for the diagnosis and treatment of acute pulmonary embolism. Rev Esp Cardiol (Engl Ed). 2017;70(1):9–13. [DOI] [PubMed] [Google Scholar]
- 10. Barnes G, Giri J, Courtney DM, et al. Nuts and bolts of running a pulmonary embolism response team: results from an organizational survey of the National PERT Consortium members. Hosp Pract (1995). 2017;45(3):76–80. [DOI] [PubMed] [Google Scholar]
- 11. Ancion A, Lopez R, D’Orio V, Ghuysen A, Zandona R. Acute pulmonary embolism: about paradox, judgments and evidences. Rev Med Liege. 2018;73(5-6):319–325. [PubMed] [Google Scholar]
- 12. Ilvan A, Celikdemir M, Ayrik C, et al. Misdiagnosis of pulmonary embolism and causes. Tuberk Toraks. 2015;63(1):13–21. [DOI] [PubMed] [Google Scholar]
- 13. Hariharan P, Dudzinski DM, Okechukwu I, Takayesu JK, Chang Y, Kabrhel C. Association between electrocardiographic findings, right heart strain, and short-term adverse clinical events in patients with acute pulmonary embolism. Clin Cardiol. 2015;38(4):236–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wells PS, Anderson DR, Rodger M, et al. Derivation of a simple clinical model to categorize patients probability of pulmonary embolism: increasing the models utility with the SimpliRED D-dimer. Thromb Haemost. 2000;83(3):416–420. [PubMed] [Google Scholar]
- 15. Le Gal G, Righini M, Roy PM, et al. Prediction of pulmonary embolism in the emergency department: the revised Geneva score. Ann Intern Med. 2006;144(3):165–171. [DOI] [PubMed] [Google Scholar]
- 16. Wells PS, Anderson DR, Rodger M, et al. Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and d-dimer. Ann Intern Med. 2001;135(2):98–107. [DOI] [PubMed] [Google Scholar]
- 17. Righini M, Van Es J, Den Exter PL, et al. Age-adjusted D-dimer cutoff levels to rule out pulmonary embolism: the ADJUST-PE study. JAMA. 2014;311(11):1117–1124. [DOI] [PubMed] [Google Scholar]
- 18. Kline JA, Courtney DM, Kabrhel C, et al. Prospective multicenter evaluation of the pulmonary embolism rule-out criteria. J Thromb Haemost. 2008;6(5):772–780. [DOI] [PubMed] [Google Scholar]
- 19. Casazza F, Bongarzoni A, Capozi A, Agostoni O. Regional right ventricular dysfunction in acute pulmonary embolism and right ventricular infarction. Eur J Echocardiogr. 2005;6(1):11–14. [DOI] [PubMed] [Google Scholar]
- 20. McConnell MV, Solomon SD, Rayan ME, Come PC, Goldhaber SZ, Lee RT. Regional right ventricular dysfunction detected by echocardiography in acute pulmonary embolism. Am J Cardiol. 1996;78(4):469–473. [DOI] [PubMed] [Google Scholar]
- 21. Penaloza A, Verschuren F, Meyer G, et al. Comparison of the unstructured clinician gestalt, the wells score, and the revised Geneva score to estimate pretest probability for suspected pulmonary embolism. Ann Emerg Med. 2013;62(2):117–124 e112. [DOI] [PubMed] [Google Scholar]
- 22. Jimenez D, Aujesky D, Moores L, et al. Simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism. Arch Intern Med. 2010;170(15):1383–1389. [DOI] [PubMed] [Google Scholar]
- 23. Stein PD, Matta F, Janjua M, Yaekoub AY, Jaweesh F, Alrifai A. Outcome in stable patients with acute pulmonary embolism who had right ventricular enlargement and/or elevated levels of troponin I. Am J Cardiol. 2010;106(4):558–563. [DOI] [PubMed] [Google Scholar]
- 24. Vanni S, Jimenez D, Nazerian P, et al. Short-term clinical outcome of normotensive patients with acute PE and high plasma lactate. Thorax. 2015;70(4):333–338. [DOI] [PubMed] [Google Scholar]
- 25. Jimenez D, Aujesky D, Moores L, et al. Combinations of prognostic tools for identification of high-risk normotensive patients with acute symptomatic pulmonary embolism. Thorax. 2011;66(1):75–81. [DOI] [PubMed] [Google Scholar]
- 26. Barco S, Mahmoudpour SH, Planquette B, Sanchez O, Konstantinides SV, Meyer G. Prognostic value of right ventricular dysfunction or elevated cardiac biomarkers in patients with low-risk pulmonary embolism: a systematic review and meta-analysis. Eur Heart J. 2019;40(11):902–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zondag W, Kooiman J, Klok FA, Dekkers OM, Huisman MV. Outpatient versus inpatient treatment in patients with pulmonary embolism: a meta-analysis. Eur Respir J. 2013;42(1):134–144. [DOI] [PubMed] [Google Scholar]
- 28. Konstantinides S. Outpatient management of patients with acute pulmonary embolism. the Home Treatment of Pulmonary Embolism (HoT-PE) trial. Presented March 16, 2019 at the American College of Cardiology 68th Annual Scientific Sessions (ACC19): Abstract 402-16. 2019. [Google Scholar]
- 29. Smith SB, Geske JB, Maguire JM, Zane NA, Carter RE, Morgenthaler TI. Early anticoagulation is associated with reduced mortality for acute pulmonary embolism. Chest. 2010;137(6):1382–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Robertson L, Jones LE. Fixed dose subcutaneous low molecular weight heparins versus adjusted dose unfractionated heparin for the initial treatment of venous thromboembolism. Cochrane Database Syst Rev. 2017;2: CD001100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Islam EA, Winn RE, Test V. Management of low-risk pulmonary embolism. Clin Chest Med. 2018; 39(3):561–568. [DOI] [PubMed] [Google Scholar]
- 32. Groetzinger LM, Miller TJ, Rivosecchi RM, Smith RE, Gladwin MT, Rivera-Lebron BN. Apixaban or rivaroxaban versus warfarin for treatment of submassive pulmonary embolism after catheter-directed thrombolysis. Clin Appl Thromb Hemost. 2018;24(6):908–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rosovsky R, Merli G. Anticoagulation in pulmonary embolism: update in the age of direct oral anticoagulants. Tech Vasc Interv Radiol. 2017;20(3):141–151. [DOI] [PubMed] [Google Scholar]
- 34. Konstantinides S, Tiede N, Geibel A, Olschewski M, Just H, Kasper W. Comparison of alteplase versus heparin for resolution of major pulmonary embolism. Am J Cardiol. 1998;82(8):966–970. [DOI] [PubMed] [Google Scholar]
- 35. Goldhaber SZ, Haire WD, Feldstein ML, et al. Alteplase versus heparin in acute pulmonary embolism: randomised trial assessing right-ventricular function and pulmonary perfusion. Lancet. 1993;341(8844):507–511. [DOI] [PubMed] [Google Scholar]
- 36. Wan S, Quinlan DJ, Agnelli G, Eikelboom JW. Thrombolysis compared with heparin for the initial treatment of pulmonary embolism: a meta-analysis of the randomized controlled trials. Circulation. 2004;110(6):744–749. [DOI] [PubMed] [Google Scholar]
- 37. Meyer G, Vicaut E, Danays T, et al. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med. 2014;370(15):1402–1411. [DOI] [PubMed] [Google Scholar]
- 38. Kurkciyan I, Meron G, Ster007A F, et al. Pulmonary embolism as a cause of cardiac arrest: presentation and outcome. Arch Intern Med. 2000;160(10):1529–1535. [DOI] [PubMed] [Google Scholar]
- 39. Sharifi M, Berger J, Beeston P, et al. Pulseless Electrical Activity in Pulmonary Embolism Treated With Thrombolysis (from the “PEAPETT” study). Am J Emerg Med. 2016;34(10):1963–1967. [DOI] [PubMed] [Google Scholar]
- 40. Janata K, Holzer M, Kurkciyan I, et al. Major bleeding complications in cardiopulmonary resuscitation: the place of thrombolytic therapy in cardiac arrest due to massive pulmonary embolism. Resuscitation. 2003;57(1):49–55. [DOI] [PubMed] [Google Scholar]
- 41. Bougouin W, Marijon E, Planquette B, et al. Pulmonary embolism related sudden cardiac arrest admitted alive at hospital: Management and outcomes. Resuscitation. 2017;115:135–140. [DOI] [PubMed] [Google Scholar]
- 42. Lavonas EJ, Drennan IR, Gabrielli A, et al. Part 10: Special circumstances of resuscitation: 2015 American Heart Association Guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2015;132(18 Suppl 2):S501–S518. [DOI] [PubMed] [Google Scholar]
- 43. Ruiz-Bailen M, Aguayo-de-Hoyos E, Serrano-Corcoles MC, et al. Thrombolysis with recombinant tissue plasminogen activator during cardiopulmonary resuscitation in fulminant pulmonary embolism. A case series. Resuscitation. 2001;51(1):97–101. [DOI] [PubMed] [Google Scholar]
- 44. Stein PD, Matta F. Thrombolytic therapy in unstable patients with acute pulmonary embolism: saves lives but underused. Am J Med. 2012;125(5):465–470. [DOI] [PubMed] [Google Scholar]
- 45. Wang C, Zhai Z, Yang Y, et al. Efficacy and safety of low dose recombinant tissue-type plasminogen activator for the treatment of acute pulmonary thromboembolism: a randomized, multicenter, controlled trial. Chest. 2010;137(2):254–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Goldhaber SZ, Kessler CM, Heit J, et al. Randomised controlled trial of recombinant tissue plasminogen activator versus urokinase in the treatment of acute pulmonary embolism. Lancet. 1988;2(8606):293–298. [DOI] [PubMed] [Google Scholar]
- 47. Chartier L, Bera J, Delomez M, et al. Free-floating thrombi in the right heart: diagnosis, management, and prognostic indexes in 38 consecutive patients. Circulation. 1999;99(21):2779–2783. [DOI] [PubMed] [Google Scholar]
- 48. Rose PS, Punjabi NM, Pearse DB. Treatment of right heart thromboemboli. Chest. 2002;121(3):806–814. [DOI] [PubMed] [Google Scholar]
- 49. Athappan G, Sengodan P, Chacko P, Gandhi S. Comparative efficacy of different modalities for treatment of right heart thrombi in transit: a pooled analysis. Vasc Med. 2015;20(2):131–138. [DOI] [PubMed] [Google Scholar]
- 50. Konstantinides S, Geibel A, Heusel G, et al. Heparin plus alteplase compared with heparin alone in patients with submassive pulmonary embolism. N Engl J Med. 2002;347(15):1143–1150. [DOI] [PubMed] [Google Scholar]
- 51. Sharifi M, Bay C, Skrocki L, Rahimi F, Mehdipour M, Investigators M. Moderate Pulmonary Embolism Treated With Thrombolysis (from the “MOPETT” Trial). Am J Cardiol. 2013;111(2):273–277. [DOI] [PubMed] [Google Scholar]
- 52. Konstantinides SV, Vicaut E, Danays T, et al. Impact of thrombolytic therapy on the long-term outcome of intermediate-risk pulmonary embolism. J Am Coll Cardiol. 2017;69(12):1536–1544. [DOI] [PubMed] [Google Scholar]
- 53. Kline JA, Nordenholz KE, Courtney DM, et al. Treatment of submassive pulmonary embolism with tenecteplase or placebo: cardiopulmonary outcomes at 3 months: multicenter double-blind, placebo-controlled randomized trial. J Thromb Haemost. 2014;12(4):459–468. [DOI] [PubMed] [Google Scholar]
- 54. Garcia MJ. Endovascular management of acute pulmonary embolism using the ultrasound-enhanced ekosonic system. Semin Intervent Radiol. 2015;32(4):384–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kucher N, Boekstegers P, Muller OJ, et al. Randomized, controlled trial of ultrasound-assisted catheter-directed thrombolysis for acute intermediate-risk pulmonary embolism. Circulation. 2014;129(4):479–486. [DOI] [PubMed] [Google Scholar]
- 56. Piazza G, Hohlfelder B, Jaff MR, et al. A prospective, single-arm, multicenter trial of ultrasound-facilitated, catheter-directed, low-dose fibrinolysis for acute massive and submassive pulmonary embolism: the SEATTLE II study. JACC Cardiovasc Interv. 2015;8(10):1382–1392. [DOI] [PubMed] [Google Scholar]
- 57. Kuo WT, Banerjee A, Kim PS, et al. Pulmonary Embolism Response to Fragmentation, Embolectomy, and Catheter Thrombolysis (PERFECT): initial results from a prospective multicenter registry. Chest. 2015;148(3):667–673. [DOI] [PubMed] [Google Scholar]
- 58. Bloomer TL, El-Hayek GE, McDaniel MC, et al. Safety of catheter-directed thrombolysis for massive and submassive pulmonary embolism: Results of a multicenter registry and meta-analysis. Catheter Cardiovasc Interv. 2017;89(4):754–760. [DOI] [PubMed] [Google Scholar]
- 59. Lin PH, Annambhotla S, Bechara CF, et al. Comparison of percutaneous ultrasound-accelerated thrombolysis versus catheter-directed thrombolysis in patients with acute massive pulmonary embolism. Vascular. 2009;17(Suppl 3): S137–S147. [DOI] [PubMed] [Google Scholar]
- 60. Liang NL, Avgerinos ED, Marone LK, Singh MJ, Makaroun MS, Chaer RA. Comparative outcomes of ultrasound-assisted thrombolysis and standard catheter-directed thrombolysis in the treatment of acute pulmonary embolism. Vasc Endovascular Surg. 2016;50(6):405–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Graif A, Grilli CJ, Kimbiris G, et al. Comparison of ultrasound-accelerated versus pigtail catheter-directed thrombolysis for the treatment of acute massive and submassive pulmonary embolism. J Vasc Interv Radiol. 2017;28(10):1339–1347. [DOI] [PubMed] [Google Scholar]
- 62. Avgerinos ED, Mohapatra A, Rivera-Lebron B, et al. Design and rationale of a randomized trial comparing standard versus ultrasound-assisted thrombolysis for submassive pulmonary embolism. J Vasc Surg Venous Lymphat Disord. 2018;6(1):126–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Tapson VF, Sterling K, Jones N, et al. A randomized trial of the optimum duration of acoustic pulse thrombolysis procedure in acute intermediate-risk pulmonary embolism: the OPTALYSE PE trial. JACC Cardiovasc Interv. 2018;11(14):1401–1410. [DOI] [PubMed] [Google Scholar]
- 64. Jaber WA, Fong PP, Weisz G, et al. Acute pulmonary embolism: with an emphasis on an interventional approach. J Am Coll Cardiol. 2016;67(8):991–1002. [DOI] [PubMed] [Google Scholar]
- 65. Tukaye DN, McDaniel M, Liberman H, Burkin Y, Jaber W. Percutaneous pulmonary embolus mechanical thrombectomy. JACC Cardiovasc Interv. 2017;10(1):94–95. [DOI] [PubMed] [Google Scholar]
- 66. Kumar Bhatia N, Dickert NW, Samady H, Babaliaros V. The use of hemodynamic support in massive pulmonary embolism. Catheter Cardiovasc Interv. 2017;90(3):516–520. [DOI] [PubMed] [Google Scholar]
- 67. Nassiri N, Jain A, McPhee D, et al. Massive and submassive pulmonary embolism: experience with an algorithm for catheter-directed mechanical thrombectomy. Ann Vasc Surg. 2012;26(1):18–24. [DOI] [PubMed] [Google Scholar]
- 68. Bonvini RF, Righini M, Roffi M. Angiojet rheolytic thrombectomy in massive pulmonary embolism: locally efficacious but systemically deleterious? J Vasc Interv Radiol. 2010;21(11):1774–1776; author reply 1776-1777. [DOI] [PubMed] [Google Scholar]
- 69. Leacche M, Unic D, Goldhaber SZ, et al. Modern surgical treatment of massive pulmonary embolism: results in 47 consecutive patients after rapid diagnosis and aggressive surgical approach. J Thorac Cardiovasc Surg. 2005;129(5):1018–1023. [DOI] [PubMed] [Google Scholar]
- 70. Aymard T, Kadner A, Widmer A, et al. Massive pulmonary embolism: surgical embolectomy versus thrombolytic therapy—should surgical indications be revisited? Eur J Cardiothorac Surg. 2013;43(1):90–94; discussion 94. [DOI] [PubMed] [Google Scholar]
- 71. Keeling WB, Sundt T, Leacche M, et al. Outcomes after surgical pulmonary embolectomy for acute pulmonary embolus: a multi-institutional study. Ann Thorac Surg. 2016;102(5):1498–1502. [DOI] [PubMed] [Google Scholar]
- 72. Stein PD, Alnas M, Beemath A, Patel NR. Outcome of pulmonary embolectomy. Am J Cardiol. 2007; 99(3):421–423. [DOI] [PubMed] [Google Scholar]
- 73. Stein PD, Matta F. Pulmonary embolectomy in elderly patients. Am J Med. 2014;127(4):348–350. [DOI] [PubMed] [Google Scholar]
- 74. Takahashi H, Okada K, Matsumori M, Kano H, Kitagawa A, Okita Y. Aggressive surgical treatment of acute pulmonary embolism with circulatory collapse. Ann Thorac Surg. 2012;94(3):785–791. [DOI] [PubMed] [Google Scholar]
- 75. Neely RC, Byrne JG, Gosev I, et al. Surgical embolectomy for acute massive and submassive pulmonary embolism in a series of 115 patients. Ann Thorac Surg. 2015;100(4):1245–1251; discussion 1251-1242. [DOI] [PubMed] [Google Scholar]
- 76. Sareyyupoglu B, Greason KL, Suri RM, Keegan MT, Dearani JA, Sundt TM., III A more aggressive approach to emergency embolectomy for acute pulmonary embolism. Mayo Clin Proc. 2010;85(9):785–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Azari A, Beheshti AT, Moravvej Z, Bigdelu L, Salehi M. Surgical embolectomy versus thrombolytic therapy in the management of acute massive pulmonary embolism: Short and long-term prognosis. Heart Lung. 2015;44(4):335–339. [DOI] [PubMed] [Google Scholar]
- 78. Barrios D, Rosa-Salazar V, Jimenez D, et al. Right heart thrombi in pulmonary embolism. Eur Respir J. 2016;48(5):1377–1385. [DOI] [PubMed] [Google Scholar]
- 79. Torbicki A, Galie N, Covezzoli A, et al. Right heart thrombi in pulmonary embolism: results from the international cooperative pulmonary embolism registry. J Am Coll Cardiol. 2003;41(12):2245–2251. [DOI] [PubMed] [Google Scholar]
- 80. Barrios D, Rosa-Salazar V, Morillo R, et al. Prognostic significance of right heart thrombi in patients with acute symptomatic pulmonary embolism: systematic review and meta-analysis. Chest. 2017;151(2):409–416. [DOI] [PubMed] [Google Scholar]
- 81. Barrios D, Chavant J, Jimenez D, et al. Treatment of right heart thrombi associated with acute pulmonary embolism. Am J Med. 2017;130(5):588–595. [DOI] [PubMed] [Google Scholar]
- 82. Burgos LM, Costabel JP, Galizia Brito V, et al. Floating right heart thrombi: a pooled analysis of cases reported over the past 10 years. Am J Emerg Med. 2018;36(6):911–915. [DOI] [PubMed] [Google Scholar]
- 83. Ain DL, Albaghdadi M, Giri J, et al. Extra-corporeal membrane oxygenation and outcomes in massive pulmonary embolism: Two eras at an urban tertiary care hospital. Vasc Med. 2018;23(1):60–64. [DOI] [PubMed] [Google Scholar]
- 84. George B, Parazino M, Omar HR, et al. A retrospective comparison of survivors and non-survivors of massive pulmonary embolism receiving veno-arterial extracorporeal membrane oxygenation support. Resuscitation. 2018;122:1–5. [DOI] [PubMed] [Google Scholar]
- 85. Pasrija C, Shah A, George P, et al. Triage and optimization: A new paradigm in the treatment of massive pulmonary embolism. J Thorac Cardiovasc Surg. 2018;156(2):672–681. [DOI] [PubMed] [Google Scholar]
- 86. Carroll BJ, Shah RV, Murthy V, et al. Clinical Features and outcomes in adults with cardiogenic shock supported by extracorporeal membrane oxygenation. Am J Cardiol. 2015;116(10):1624–1630. [DOI] [PubMed] [Google Scholar]
- 87. Aso S, Matsui H, Fushimi K, Yasunaga H. In-hospital mortality and successful weaning from venoarterial extracorporeal membrane oxygenation: analysis of 5,263 patients using a national inpatient database in Japan. Crit Care. 2016;20:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Yusuff HO, Zochios V, Vuylsteke A. Extracorporeal membrane oxygenation in acute massive pulmonary embolism: a systematic review. Perfusion. 2015;30(8):611–616. [DOI] [PubMed] [Google Scholar]
- 89. Dolmatova EV, Moazzami K, Cocke TP, et al. Extracorporeal membrane oxygenation in massive pulmonary embolism. Heart Lung. 2017;46(2):106–109. [DOI] [PubMed] [Google Scholar]
- 90. Corsi F, Lebreton G, Brechot N, et al. Life-threatening massive pulmonary embolism rescued by venoarterial-extracorporeal membrane oxygenation. Crit Care. 2017;21(1):76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Pasrija C, Kronfli A, George P, et al. Utilization of veno-arterial extracorporeal membrane oxygenation for massive pulmonary embolism. Ann Thorac Surg. 2018;105(2):498–504. [DOI] [PubMed] [Google Scholar]
- 92. Yannopoulos D, Bartos JA, Raveendran G, et al. Coronary artery disease in patients with out-of-hospital refractory ventricular fibrillation cardiac arrest. J Am Coll Cardiol. 2017;70(9):1109–1117. [DOI] [PubMed] [Google Scholar]
- 93. Cheng R, Hachamovitch R, Kittleson M, et al. Complications of extracorporeal membrane oxygenation for treatment of cardiogenic shock and cardiac arrest: a meta-analysis of 1,866 adult patients. Ann Thorac Surg. 2014;97(2):610–616. [DOI] [PubMed] [Google Scholar]
- 94. Wong JK, Melvin AL, Joshi DJ, et al. Cannulation-Related complications on veno-arterial extracorporeal membrane oxygenation: prevalence and effect on mortality. Artif Organs. 2017;41(9):827–834. [DOI] [PubMed] [Google Scholar]
- 95. Arlt M, Philipp A, Iesalnieks I, Kobuch R, Graf BM. Successful use of a new hand-held ECMO system in cardiopulmonary failure and bleeding shock after thrombolysis in massive post-partal pulmonary embolism. Perfusion. 2009;24(1):49–50. [DOI] [PubMed] [Google Scholar]
- 96. Jeong WJ, Lee JW, Yoo YH, et al. Extracorporeal cardiopulmonary resuscitation in bedside echocardiography-diagnosed massive pulmonary embolism. Am J Emerg Med. 2015;33(10):1545 e1541–1542. [DOI] [PubMed] [Google Scholar]
- 97. Cao J, Liu Y, Wang Y, et al. Salvage thrombolysis and extracorporeal membrane oxygenation for massive pulmonary embolism during the distal femur fracture surgery. Am J Emerg Med. 2016;34(6):1189 e1183–1185. [DOI] [PubMed] [Google Scholar]
- 98. Sharma V, Goldberg HD, Zubkus D, Shears LL, Kaczorowski DJ. Successful management of cardiac arrest due to pulmonary embolus using extracorporeal membrane oxygenation and ultrasound-accelerated catheter-directed thrombolysis. Ann Thorac Surg. 2016;101(4):e107–109. [DOI] [PubMed] [Google Scholar]
- 99. Silvetti S, Pappalardo F, Melisurgo G, et al. Ultrasound-accelerated thrombolysis and extracorporeal membrane oxygenation in a patient with massive pulmonary embolism and cardiac arrest. Circ Cardiovasc Interv. 2013;6(3):e34–36. [DOI] [PubMed] [Google Scholar]
- 100. Chung JH, Yeo HJ, Cho HM, et al. Treatment of pulmonary tumor embolism from choriocarcinoma: extracorporeal membrane oxygenation as a bridge through chemotherapy. Cancer Res Treat. 2017;49(1):279–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Hsieh YK, Siao FY, Chiu CC, Yen HH, Chen YL. Massive pulmonary embolism mimicking acute myocardial infarction: successful use of extracorporeal membrane oxygenation support as bridge to diagnosis. Heart Lung Circ. 2016;25(7):e78–80. [DOI] [PubMed] [Google Scholar]
- 102. Kim YS, Choi W, Hwang J. Resuscitation of prolonged cardiac arrest from massive pulmonary embolism by extracorporeal membrane oxygenation. Eur J Cardiothorac Surg. 2017;51(6):1206–1207. [DOI] [PubMed] [Google Scholar]
- 103. Elder M, Blank N, Kaki A, et al. Mechanical circulatory support for acute right ventricular failure in the setting of pulmonary embolism. J Interv Cardiol. 2018;31(4):518–524. [DOI] [PubMed] [Google Scholar]
- 104. NCCN Guidelines Cancer Associated Venous Thromboembolic Disease. 2017. https://wwwnccnorg/professionals/physician_gls/pdf/vtepdf. Accessed December 1, 2018.
- 105. Mismetti P, Laporte S, Pellerin O, et al. Effect of a retrievable inferior vena cava filter plus anticoagulation vs anticoagulation alone on risk of recurrent pulmonary embolism: a randomized clinical trial. JAMA. 2015;313(16):1627–1635. [DOI] [PubMed] [Google Scholar]
- 106. Stein PD, Matta F, Lawrence FR, Hughes MJ. Usefulness of inferior vena cava filters in unstable patients with acute pulmonary embolism and patients who underwent pulmonary embolectomy. Am J Cardiol. 2018;121(4):495–500. [DOI] [PubMed] [Google Scholar]
- 107. Kucher N, Rossi E, De Rosa M, Goldhaber SZ. Massive pulmonary embolism.Circulation. 2006;113(4):577–582. [DOI] [PubMed] [Google Scholar]
- 108. Pacouret G, Alison D, Pottier JM, Bertrand P, Charbonnier B. Free-floating thrombus and embolic risk in patients with angiographically confirmed proximal deep venous thrombosis. A prospective study. Arch Intern Med. 1997;157(3):305–308. [PubMed] [Google Scholar]
- 109. Norris CS, Greenfield LJ, Herrmann JB. Free-floating iliofemoral thrombus. A risk of pulmonary embolism. Arch Surg. 1985;120(7):806–808. [DOI] [PubMed] [Google Scholar]
- 110. Baldridge ED, Martin MA, Welling RE. Clinical significance of free-floating venous thrombi. J Vasc Surg. 1990;11(1):62–67; discussion 68-69. [PubMed] [Google Scholar]
- 111. Desai KR, Pandhi MB, Seedial SM, et al. Retrievable IVC filters: comprehensive review of device-related complications and advanced retrieval techniques. Radiographics. 2017;37(4):1236–1245. [DOI] [PubMed] [Google Scholar]
- 112. Morales JP, Li X, Irony TZ, Ibrahim NG, Moynahan M, Cavanaugh KJ., Jr Decision analysis of retrievable inferior vena cava filters in patients without pulmonary embolism. J Vasc Surg Venous Lymphat Disord. 2013;1(4):376–384. [DOI] [PubMed] [Google Scholar]
- 113. Tapson VF, Platt DM, Xia F, et al. Monitoring for pulmonary hypertension following pulmonary embolism: the INFORM study. Am J Med. 2016;129(9):978–985 e972. [DOI] [PubMed] [Google Scholar]
- 114. Sista AK, Klok FA. Late outcomes of pulmonary embolism: The post-PE syndrome. Thromb Res. 2018;164:157–162. [DOI] [PubMed] [Google Scholar]
- 115. Klok FA, van der Hulle T, den Exter PL, Lankeit M, Huisman MV, Konstantinides S. The post-PE syndrome: a new concept for chronic complications of pulmonary embolism. Blood Rev. 2014;28(6):221–226. [DOI] [PubMed] [Google Scholar]
- 116. Rali PM, Criner GJ. Submassive pulmonary embolism. Am J Respir Crit Care Med. 2018;198(5):588–598. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, Supp_Material for Diagnosis, Treatment and Follow Up of Acute Pulmonary Embolism: Consensus Practice from the PERT Consortium by Belinda Rivera-Lebron, Michael McDaniel, Kamran Ahrar, Abdulah Alrifai, David M. Dudzinski, Christina Fanola, Danielle Blais, David Janicke, Roman Melamed, Kerry Mohrien, Elizabeth Rozycki, Charles B. Ross, Andrew J. Klein, Parth Rali, Nicholas R. Teman, Leoara Yarboro, Eugene Ichinose, Aditya M. Sharma, Jason A. Bartos, Mahir Elder, Brent Keeling, Harold Palevsky, Soophia Naydenov, Parijat Sen, Nancy Amoroso, Josanna M. Rodriguez-Lopez, George A. Davis, Rachel Rosovsky, Kenneth Rosenfield, Christopher Kabrhel, James Horowitz, Jay S. Giri, Victor Tapson, Richard Channick and the PERT Consortium in Clinical and Applied Thrombosis/Hemostasis