Abstract
D-dimer might be correlated with prognosis in pulmonary embolism (PE). The predictive value of plasma D-dimer for disease severity and survival was investigated in the lowest and highest D-dimer quartile among 200 patients with PE. Patients with high D-dimers were significantly more often hypotensive (P = .001), tachycardic (P = .016), or hypoxemic (P = .001). Pulmonary arterial obstruction index (PAOI) values were significantly higher in the high D-dimer quartile (P < .001). Elevated troponin I (TNI) levels (P < .001), simplified PE severity indices ≥1 (P < .001), right-to-left ventricular (RV/LV) diameter ratios ≥1 (P < .001), and thrombolysis (P = .001) were more frequent in the high D-dimer quartile. D-dimer was associated with RV/LV ratios ≥1 (P = .021), elevated PAOI (P < .001) or TNI levels (P < .001), hypotension (P < .001), tachycardia (P = .003), and hypoxemia (P < .001), but not with long-term all-cause mortality. D-dimer predicts disease severity but not long-term prognosis in acute PE, possibly due to a more aggressive treatment strategy in severely affected patients.
Keywords: pulmonary embolism, D-dimer, disease severity, prognosis, risk stratification
Introduction
Plasma D-dimer, a degradation product of cross-linked fibrin, is elevated in the presence of thrombotic clots by simultaneous activation of coagulation and fibrinolysis.1 Elevated D-dimer levels are found in patients with venous thromboembolism (VTE), but also in other conditions associated with fibrin formation such as pregnancy, inflammation, cancer, or surgery. Plasma D-dimer has been proven to be a valuable tool in the diagnosis of acute pulmonary embolism (PE). Normal D-dimer levels virtually exclude VTE in most patients presenting with clinical signs suggestive of acute PE.2,3 On the other hand, elevated D-dimer values are predictive in detecting PE.4 Furthermore, elevated D-dimer levels may also predict PE recurrence after discontinuation of anticoagulant treatment.5,6
Acute PE is among the most common causes for cardiovascular-related deaths, with a short-term mortality rate reaching up to 16%.7 The disease severity varies between patients presenting with mild symptoms, being eligible for early discharge and further outpatient treatment, and patients presenting with life-threatening disease patterns and the need for urgent life support. Current guidelines developed a detailed risk stratification model in order to provide recommendations for patients with the ideal treatment focused on personal conditions and needs.4 Various clinical parameters, such as arterial hypotension, tachycardia, and oxygen desaturation, have been shown to be helpful in the prognostic assessment of patients with acute PE and are associated with a worse prognosis.8,9 Together with other parameters, these clinical signs account for the simplified Pulmonary Embolism Severity Index (sPESI) score, which has been proven to be reliable in identifying patients with an adverse 30-day outcome.10 Combined with troponin testing, the sPESI score provides additional prognostic information, especially for the identification of patients who are at low risk of early death.11 Moreover, biomarkers and imaging tests indicating acute right ventricular dysfunction (RVD) have been identified as independent predictors for an adverse outcome. For instance, elevated troponin levels on admission have been reported to be associated with a worse prognosis.12,13 Four-chamber views of the heart by computed tomographic pulmonary angiography (CTPA) can detect RV enlargement as an indicator of RVD.14 A right-to-left ventricular (RV/LV) diameter ratio ≥1 on CTPA, for example, has been reported to be associated with an increased short-term mortality in acute PE.7,15,16
In earlier studies, it has been shown that the extent of D-dimer elevation might be correlated with several surrogate parameters of prognosis in acute PE. At least, it has been demonstrated that D-dimer might be associated with the extent of pulmonary obstruction, RVD, troponin values, and several clinical parameters such as Pao 2.17–21 Thus, it has been speculated whether the plasma D-dimer concentration could serve as another prognostic marker. However, data are limited to very few studies and evidence is still lacking that D-dimer levels are associated with long-term prognosis. Therefore, we sought to investigate the predictive value of plasma D-dimer levels for disease severity and long-term survival in acute PE.
Methods
Study Design, Imaging and Laboratory Tests, and Clinical Data
This study was conducted at a tertiary university hospital. Two hundred fifty-three patients were prospectively enrolled between August 2011 and March 2016 in order to evaluate the impact of sleep-disordered breathing on the disease severity of acute PE. The study design, methods, and sleep study results have been previously published.22 In 211 patients, D-dimer tests were performed the same day acute PE was diagnosed by multidetector CTPA. In 200 out of these 211 patients, long-term survival data could be provided; 11 patients had to be excluded due to missing follow-up data. From the remaining 200 patients, the final cohort for the present study was extracted, consisting of 100 patients, representing the lowest (≤3876 ng/mL) and highest (≥11065 ng/mL) quartile of D-dimer levels. In 2 patients from the low D-dimer group, CTPA data did not meet the requirements for accurate pulmonary thrombus load analysis. All patients provided written informed consent for study participation and the trial was approved by our institutional review board.
Pulmonary embolism was diagnosed by multidetector CTPA, using the routine scan protocol of our institution. Right ventricular dysfunction was assessed by computing the RV/LV diameter ratio on CTPA images. Calculation of the RV/LV ratios was performed in consensus by 2 experienced radiologists. Maximum RV and LV diameters were specified manually as the maximum distance from the corresponding endocardial border of the RV or LV to the interventricular septum. Here, the largest RV and LV diameters were normally observed at different sagittal positions of the heart. Right ventricular dilatation was defined as a RV/LV ratio of ≥1.0. Pulmonary thrombus load was quantified on CTPA using the protocol suggested by Qanadli et al.23 The Qanadli score considers the degree of obstruction (1 point for partial obstruction, 2 points for complete obstruction) and the number of affected branches (maximum of 10 branches for each lung). Consequently, the maximum possible score is 20 × 2 = 40 points.23 The Qanadli scores were independently analyzed by 2 experienced radiologists. The results showed a very strong interobservational correlation (r = .978, P < .001). Therefore, we calculated the average values and used them for further analysis. Pulmonary artery obstruction index is calculated as percentage of Qanadli Scores (patient’s score in points/40 × 100).
Biomarkers indicating ventricular pressure overload or myocardial injury (N-terminal pro-brain-type natriuretic peptide [NT-proBNP], troponin I [TNI], and D-dimers) were measured on admission. The D-dimer testing is done using citrated platelet-poor plasma on a SIEMENS BCS XP coagulation analyzer and the SIEMENS lNNOVANCE D-dimer test kit, a particle-enhanced, immunoturbidimetric assay for the quantitative determination of cross-linked fibrin degradation products in human plasma. Due to cross-linkage between D-domains in the fibrin clot, the action of plasmin releases fibrin degradation products with cross-linked D-domains. The analysis is based on polystyrene particles that are covalently coated with a monoclonal antibody (8D3)14 fitting on the D-dimer cross-linkage region. Consequently, 1 antibody suffices in order to trigger an aggregation reaction when mixed with samples containing D-dimer. The aggregation reaction results in an increase in turbidity that can be detected by the coagulation analyzer. An age-adjusted cutoff for D-dimer values using the formula (10 × patient’s age in years) ng/mL was applied.
Patients’ histories were taken by study nurses who were blinded for radiological and laboratory results. The anamnestic survey was focused on comorbidity including concomitant lung diseases such as uncontrolled asthma, chronic obstructive pulmonary disease, lung fibrosis or other interstitial lung diseases, cerebrovascular diseases, and history of active cancer. All study participants received transthoracic echocardiography before being discharged from hospital. For clinical risk stratification, the sPESI score was calculated.10 Therapy was applied corresponding to the international guidelines and internal standards by physicians who were blinded for study results. Survival data were collected by regular telephone contacts.
Statistical Analysis
The statistical analysis was performed using IBM SPSS Statistics version 25. The Shapiro-Wilk test was used to test for the normality of distribution in continuous variables. Continuous variables were given as medians (with interquartile ranges) as they were not normally distributed. Differences in continuous variables were analyzed by the Mann-Whitney test. Categorical variables were specified as amounts (with percentages of total) and their distribution was further analyzed by the χ2 test or by the Fisher test in case of small frequencies. The influence of independent variables was tested by performing a covariate-adjusted linear regression analysis for continuous variables, whereas binary parameters were tested by logistic regression. Survival data were depicted in Kaplan-Meier curves, which were compared by the log-rank test. Adjusting survival data for potential confounders, a Cox regression analysis was performed. The level of significance was chosen at α = .05 and all probability values were given 2-tailed.
Results
The clinical characteristics of the entire study population are summarized in Table 1. In comparison with the low D-dimer subgroup (≤3876 ng/mL), patients from the high D-dimer cohort (≥11065 ng/mL) were significantly older (70 vs 56 years, P = .023), hypotensive (20.0% vs 0.0%, P = .001), tachycardic (42.0% vs 18.8%, P = .016), and hypoxemic (52.0% vs 18.8%, P = .001) when acute PE was diagnosed (Table 2). Concomitant lung disease was less frequent (4.0% vs 16.7%, P = .049) and elevated TNI levels were more frequent in the high D-dimer group (72.0% vs 25.0%, P < .001). The sPESI scores were significantly more often ≥1 in the high D-dimer cohort (86.0% vs 45.8%, P < .001). Pulmonary arterial obstruction index (PAOI) values and Qanadli scores were significantly higher in the high D-dimer group (66% vs 33%, P < .001 and 26 points vs 13 points, P < .001, respectively). Right ventricular dysfunction, defined as a RV/LV ratio ≥1 on CTPA, was more frequent in the cohort with high D-dimer levels (74.0% vs 33.3%, P < .001). Although NT-proBNP values tended to be considerably higher in this group, the difference was not statistically significant (P = .074). Thrombolysis was significantly more often applied in patients with a high D-dimer level (42% vs 12.5%, P = .001).
Table 1.
Clinical Characteristics of the Entire Study Cohort.a
| Demographics | |
| Age (range), years | 63 (47-78) |
| Female, n (%) | 53 (54.1) |
| Clinical findings at baseline | |
| Hypotension, n (%) | 10 (10.2) |
| Heart rate ≥110/min, n (%) | 30 (30.6) |
| Oxygen saturation <90%, n (%) | 35 (35.7) |
| Comorbidity | |
| Concomitant lung disease, n (%) | 10 (10.2) |
| LV ejection fraction <40%, n (%) | 3 (3.1) |
| Cerebrovascular disease, n (%) | 7 (7.1) |
| Active cancer, n (%) | 8 (8.2) |
| Laboratory parameters | |
| NT-proBNP (range), pg/mL | 1669 (233-4554) |
| Elevated TNI, n (%) | 48 (49.0) |
| D-dimer (range), ng/mL | 11 100 (2400-15 200) |
| Risk stratification | |
| sPESI ≥ 1, n (%) | 65 (66.3) |
| Imaging findings | |
| RV/LV ratio ≥1, n (%) | 53 (54.1) |
| Qanadli score (range), points | 22 (12-28) |
| PAOI (range), % | 55 (30-70) |
| Therapy | |
| Thrombolysis, n (%) | 27 (27.6) |
Abbreviations: LV, left ventricular; NT-proBNP, N-terminal pro-brain-type natriuretic peptide; PAOI, Pulmonary arterial obstruction index; RV, right ventricular; sPESI, simplified Pulmonary Embolism Severity Index; TNI, troponin I.
an = 98.
Table 2.
Clinical Characteristics in the D-Dimer Subgroups.
| Low D-Dimer ≤ 3876 ng/mL, n = 48 | High D-Dimer ≥ 11 065 ng/mL, n = 50 | P Value | |
|---|---|---|---|
| Demographics | |||
| Age (range), years | 56 (41-78) | 70 (52-78) | .023 |
| Female, n (%) | 26 (54.2) | 27 (54.0) | 1.000 |
| Clinical findings at baseline | |||
| Hypotension, n (%) | 0 (0.0) | 10 (20.0) | .001 |
| Heart rate ≥110/minutes, n (%) | 9 (18.8) | 21 (42.0) | .016 |
| Oxygen saturation <90%, n (%) | 9 (18.8) | 26 (52.0) | .001 |
| Comorbidity | |||
| Concomitant lung disease, n (%) | 8 (16.7) | 2 (4.0) | .049 |
| LV ejection fraction <40%, n (%) | 0 (0.0) | 3 (6.0) | .241 |
| Cerebrovascular disease, n (%) | 1 (2.1) | 6 (12.0) | .111 |
| Active cancer, n (%) | 2 (4.2) | 6 (12.0) | .269 |
| Laboratory parameters | |||
| NT-proBNP (range), pg/mL | 525 (155-3575) | 2631 (291-4855) | .074 |
| Elevated TNI, n (%) | 12 (25.0) | 36 (72.0) | <.001 |
| D-dimer (range), ng/mL | 2400 (1400-2900) | 14 900 (12 200-22 600) | <.001 |
| Risk stratification | |||
| sPESI ≥ 1, n (%) | 22 (45.8) | 43 (86.0) | <.001 |
| Imaging findings | |||
| CTPA RV/LV ratio ≥1, n (%) | 16 (33.3) | 37 (74.0) | <.001 |
| Qanadli score (range), points | 13 (5-22) | 26 (22-30) | < .001 |
| PAOI (range), % | 33 (13-55) | 66 (55-75) | < .001 |
| Therapy | |||
| Thrombolysis, n (%) | 6 (12.5) | 21 (42.0) | .001 |
Abbreviations: LV, left ventricular; NT-proBNP, N-terminal pro-brain-type natriuretic peptide; PAOI, Pulmonary arterial obstruction index; RV, right ventricular; sPESI, simplified Pulmonary Embolism Severity Index; TNI, troponin I.
Regarding the impact of lung disease and age as possible confounders between the highest and lowest quartile, corrected comparisons in the form of comorbidity- and age-adjusted regression analyses were performed. For continuous variables, such as Qanadli scores and NT-proBNP levels, linear regression analyses were conducted, whereas binary parameters were tested by logistic regression. D-dimer levels and signs of RVD were the only independent predictors for the Qanadli score (β = .444, P < .001 and β = .529, P < .001, respectively; see also Table 3). No significant association was found between D-dimer and NT-proBNP levels (β = −.011, P = .928). Age and lung diseases showed no impact on the Qanadli scores (β = .043, P = .640 and β = .094, P = .307, respectively). Age- and comorbidity-adjusted logistic regression analysis is displayed in Table 4 and identified D-dimer levels as independent predictive parameters for the presence of RVD (odds ratio [OR] = 1.829, P = .021) and elevated TNI levels (OR = 4.416, P < .001). Age- and comorbidity-adjusted logistic regression further showed a significant association between D-dimer levels and clinical markers such as hypotension (OR = 4.910, P < .001), tachycardia (OR = 2.316, P = .003), and oxygen saturation <90% (OR = 2.770, P < .001).
Table 3.
Age- and Comorbidity-Adjusted Linear Regression Analysis: D-Dimer Predicts Qanadli Scores But not NT-proBNP.
| Independent Variable | Dependent Variable | β | Standard Error | P Value |
|---|---|---|---|---|
| D-dimer | Qanadli score | 0.444 | 0.000 | <.001 |
| D-dimer | NT-proBNP | −0.011 | 0.053 | .928 |
Abbreviations: NT-proBNP, N-terminal pro-brain-type natriuretic peptide.
Table 4.
Age- and Comorbidity-Adjusted Logistic Regression Analysis: D-Dimer Predicts Markers for Disease Severity and RVD.
| Independent Variable | Dependent Variable | OR (95% CI) | Standard Error | P Value |
|---|---|---|---|---|
| D-dimer | Hypotension | 4.910 (2.054-11.733) | 0.445 | <.001 |
| D-dimer | Tachycardia | 2.316 (1.337-4.011) | 0.280 | .003 |
| D-dimer | SO2 < 90% | 2.770 (1.574-4.876) | 0.289 | <.001 |
| D-dimer | RV/LV ratio ≥ 1 | 1.829 (1.096-3.052) | 0.261 | .021 |
| D-dimer | Elevated TNI | 4.416 (2.181-8.942) | 0.360 | <.001 |
| D-dimer | sPESI ≥ 1 | 4.578 (2.087-10.043) | 0.401 | <.001 |
Abbreviations: CI, confidence interval; LV, left ventricular; OR, odds ratio; RV, right ventricular; RVD, right ventricular dysfunction; sPESI, simplified Pulmonary Embolism Severity Index; TNI, troponin I.
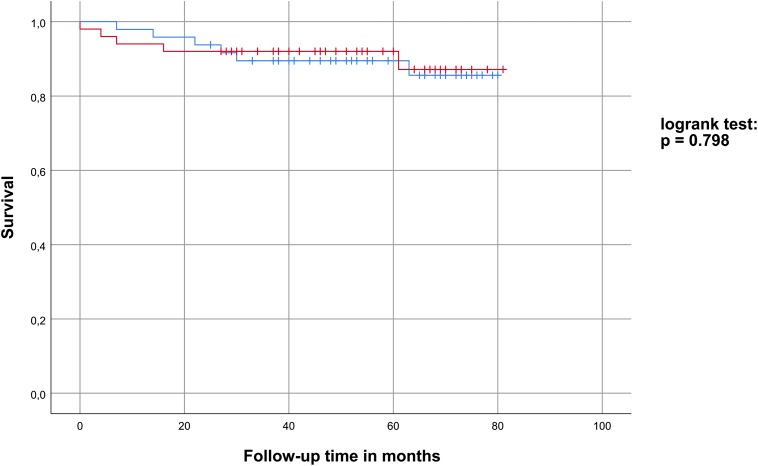
Survival data were analyzed by Kaplan-Meier curves, which are depicted in Figure 1. There were no deaths observed during hospitalization. During the median follow-up time of 55 (38-70) months, 11 patients died (resulting in a mortality rate of 11.2%). The mortality rate did not differ significantly in both D-dimer subgroups (P = .798). No other parameters tested (age ≥65 years, sPESI scores, RV/LV diameter ratios) were found to significantly influence survival. A gender-, age-, and comorbidity-adjusted Cox regression analysis describing the impact of D-dimer levels on the long-term survival showed a hazard ratio of 0.536 (0.162-1.776) with a P value of .308.
Figure 1.
Survival analysis: Kaplan-Meier plot for the low D-dimer (blue) and high D-dimer (red) subgroup.
Discussion
In the present study, we investigated the association of plasma D-dimer concentrations with disease severity and long-term survival in patients with acute PE. We found that plasma D-dimer is significantly associated with disease severity in acute PE assessed by several well-established clinical, laboratory, and radiological parameters. D-dimer was significantly associated with clinical surrogate markers of disease severity such as arterial hypotension, tachycardia, and hypoxemia in our study. Consequently, the sPESI score, a combination of 6 prognostic relevant clinical markers, was significantly increased in the high D-dimer subgroup. In addition, regression analysis demonstrated that D-dimer levels are significantly associated with elevated TNI values as markers of myocardial injury. Furthermore, multivariate analysis revealed that D-dimer values predict RVD, defined as a RV/LV ratio ≥1 on CTPA in our study. Additionally, we found that patients with elevated D-dimer levels had a significantly higher pulmonary thrombus load. Even age- and comorbidity-adjusted linear regression analysis revealed a significant dose–response relation between D-dimer concentration and PAOI. Although not being included in the current risk stratification model, the extent of pulmonary thrombus load has been proven to predict short-term mortality. For example, it has been demonstrated that a PAOI above 40% was associated with an 11.2-fold risk of death during 3-month follow-up.24 Pulmonary clot burden may lead to increased pulmonary artery pressure, which causes RV pressure overload and finally may result in RVD. As a consequence, LV preload and cardiac output are lowered, leading to arterial hypotension and cardiac shock.4
These findings are, at least in part, in accordance with results of earlier studies in which a significant association between the plasma D-dimer concentration and parameters such as hypoxemia, myocardial injury, right heart function, or pulmonary clot burden could be found.17,18,20,21 Based on these observations, it has been concluded that D-dimer levels may predict survival in patients with acute PE as they are highly correlated with surrogate markers of prognosis.
However, the comparison of the high and low D-dimer quartile did not reveal significant differences in the long-term, all-cause mortality rate in our study. To the best of our knowledge, no similar long-term survival data have been published yet. Therefore, it is difficult to compare our data with the existing, short-term focused analyses. Concerning these studies, their results do not warrant a significant trend, as some studies showed an association between D-dimer level and prognosis,25,26 whereas others did not.27,28 In general, a possible association between D-dimer and survival could be confounded by comorbidity, such as cancer.29 Regarding our study, in which active cancer was rare, several other reasons might be responsible for the similar mortality rates in both D-dimer groups: first, the missing statistical significance could be due to the relatively small size of the cohort. Second, the observed mortality rate in our trial was lower compared to earlier studies.7,15,26 This might be, at least in part, due to a selection bias. All our study participants were recruited from a trial where patients had to qualify for nocturnal polysomnography. Thus, these subjects might be less severely affected by acute PE. However, the observed rates of hypotensive, tachycardic, or hypoxemic patients in our cohort were similar to other studies.30 Therefore, it seems unlikely that a potential selection bias might have a relevant impact on our survival analysis. Another explanation for the favorable outcome of our study cohort might be that thrombolysis was significantly more often applied in patients with high D-dimer levels, as they were significantly more often hemodynamically instable. No observed deaths during hospitalization might be a consequence of successful thrombolytic therapy. Previous studies revealed a significant reduction in short-term mortality by thrombolysis in hemodynamically instable patients.31,32 Consequently, very recent research reported a decrease in PE-related mortality rates in the last decade, possibly due to a more aggressive treatment strategy.33 Hence, we postulate that thrombolytic therapy might have prevented early deaths among severely affected patients and, therefore, led to a lower than expected mortality rate in the high D-dimer group.
We admit that our study has some limitations. First, we did not enroll consecutive patients, as all our study participants had to qualify for sleep studies during hospitalization. Second, the aforementioned selection bias could account for the low mortality rate in our study cohort. Third, survival analysis did not focus on PE-related deaths. Despite these constraints, our study has several strengths. To the best of our knowledge, this study is the first to examine the association of D-dimer levels and long-term survival data in patients with acute PE. The prospective study design and the low dropout rate in the follow-up are further strengths of the present trial.
Conclusions
Despite the limitations of the study, we conclude that plasma D-dimer is associated with disease severity, but not with long-term prognosis in acute PE, possibly due to a higher rate of thrombolysis in patients with elevated D-dimer levels.
Footnotes
Authors’ Note: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was conducted with the approval of the Institution’s Ethics Committee (project number 080-11 with an amendment BKF 2017-2).
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Thomas M. Berghaus  https://orcid.org/0000-0002-8551-6190
https://orcid.org/0000-0002-8551-6190
References
- 1. Cesarman-Maus G, Hajjar KA. Molecular mechanisms of fibrinolysis. Br J Haematol. 2005;129(3):307–321. [DOI] [PubMed] [Google Scholar]
- 2. Kruip MJHA, Slob MJ, Schijen JHEM, van der Heul C, Büller HR. Use of a clinical decision rule in combination with D-dimer concentration in diagnostic workup of patients with suspected pulmonary embolism: a prospective management study. Arch Intern Med. 2002;162(14):1631–1635. [DOI] [PubMed] [Google Scholar]
- 3. Carrier M, Righini M, Djurabi RK, et al. VIDAS D-dimer in combination with clinical pre-test probability to rule out pulmonary embolism: a systematic review of management outcome studies. Thromb Haemost. 2009;101(5):886–892. [PubMed] [Google Scholar]
- 4. Konstantinides SV, Torbicki A, Agnelli G, et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014;35(43):3033–3069, 3069a-3069k. [DOI] [PubMed] [Google Scholar]
- 5. Poli D, Cenci C, Antonucci E, et al. Risk of recurrence in patients with pulmonary embolism: predictive role of D-dimer and of residual perfusion defects on lung scintigraphy. Thromb Haemost. 2013;109(2):181–186. [DOI] [PubMed] [Google Scholar]
- 6. van Hylckama Vlieg A, Baglin CA, Luddington R, et al. The risk of a first and a recurrent venous thrombosis associated with an elevated D-dimer level and an elevated thrombin potential: results of the THE-VTE study. J Thromb Haemost. 2015;13(9):1642–1652. [DOI] [PubMed] [Google Scholar]
- 7. Furlan A, Aghayev A, Chang CCH, et al. Short-term mortality in acute pulmonary embolism: clot burden and signs of right heart dysfunction at CT pulmonary angiography. Radiology. 2012;265(1):283–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Goldhaber SZ, Visani L, de RM. Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER). Lancet. 1999;353(9162):1386–1389. [DOI] [PubMed] [Google Scholar]
- 9. Aujesky D, Obrosky DS, Stone RA, et al. Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med. 2005;172(8):1041–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jiménez D, Aujesky D, Moores L, et al. Simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism. Arch Intern Med. 2010;170(15):1383–1389. [DOI] [PubMed] [Google Scholar]
- 11. Lankeit M, Jiménez D, Kostrubiec M, et al. Predictive value of the high-sensitivity troponin T assay and the simplified pulmonary embolism severity index in hemodynamically stable patients with acute pulmonary embolism: a prospective validation study. Circulation. 2011;124(24):2716–2724. [DOI] [PubMed] [Google Scholar]
- 12. Apfaltrer P, Walter T, Gruettner J, et al. Prediction of adverse clinical outcome in patients with acute pulmonary embolism: evaluation of high-sensitivity troponin I and quantitative CT parameters. Eur J Radiol. 2013;82(3):563–567. [DOI] [PubMed] [Google Scholar]
- 13. Keller K, Beule J, Schulz A, Coldewey M, Dippold W, Balzer JO. Cardiac troponin I for predicting right ventricular dysfunction and intermediate risk in patients with normotensive pulmonary embolism. Neth Heart J. 2015;23(1):55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Henzler T, Roeger S, Meyer M, et al. Pulmonary embolism: CT signs and cardiac biomarkers for predicting right ventricular dysfunction. Eur Respir J. 2012;39(4):919–926. [DOI] [PubMed] [Google Scholar]
- 15. Schoepf UJ, Kucher N, Kipfmueller F, Quiroz R, Costello P, Goldhaber SZ. Right ventricular enlargement on chest computed tomography: a predictor of early death in acute pulmonary embolism. Circulation. 2004;110(20):3276–3280. [DOI] [PubMed] [Google Scholar]
- 16. Meinel FG, Nance JW, Schoepf UJ, et al. Predictive value of computed tomography in acute pulmonary embolism: systematic review and meta-analysis. Am J Med. 2015;128(7):747–759.e2. [DOI] [PubMed] [Google Scholar]
- 17. Ghanima W, Abdelnoor M, Holmen LO, Nielssen BE, Ross S, Sandset PM. D-dimer level is associated with the extent of pulmonary embolism. Thromb Res. 2007;120(2):281–288. [DOI] [PubMed] [Google Scholar]
- 18. Keller K, Beule J, Balzer JO, Dippold W. D-Dimer and thrombus burden in acute pulmonary embolism. Am J Emerg Med. 2018;36(9):1613–1618. [DOI] [PubMed] [Google Scholar]
- 19. Masotti L, Antonelli F, Venturini E, Landini G. Cardiac troponin I and plasma D-dimer are related to proximal and bilateral extension of clots and right cardiac dysfunction in patients with pulmonary embolism. J Intern Med. 2007;262(5):588–589. [DOI] [PubMed] [Google Scholar]
- 20. Rydman R, Söderberg M, Larsen F, Alam M, Caidahl K. . d-Dimer and simplified pulmonary embolism severity index in relation to right ventricular function. Am J Emerg Med. 2013;31(3):482–486. [DOI] [PubMed] [Google Scholar]
- 21. Gutte H, Mortensen J, Jensen CV, et al. ANP, BNP and D-dimer predict right ventricular dysfunction in patients with acute pulmonary embolism. Clin Physiol Funct Imaging. 2010;30(6):466–472. [DOI] [PubMed] [Google Scholar]
- 22. Konnerth D, Schwarz F, Probst M, et al. Is acute pulmonary embolism more severe in the presence of obstructive sleep apnea? results from an observational cohort study. J Thromb Thrombolysis. 2018;46(2):253–259. [DOI] [PubMed] [Google Scholar]
- 23. Qanadli SD, El Hajjam M, Vieillard-Baron A, et al. New CT index to quantify arterial obstruction in pulmonary embolism: comparison with angiographic index and echocardiography. AJR Am J Roentgenol. 2001;176(6):1415–1420. [DOI] [PubMed] [Google Scholar]
- 24. van der Meer RW, Pattynama PMT, van Strijen MJL, et al. Right ventricular dysfunction and pulmonary obstruction index at helical CT: prediction of clinical outcome during 3-month follow-up in patients with acute pulmonary embolism. Radiology. 2005;235(3):798–803. [DOI] [PubMed] [Google Scholar]
- 25. Grau E, Tenías JM, Soto MJ, et al. D-dimer levels correlate with mortality in patients with acute pulmonary embolism: findings from the RIETE registry. Crit Care Med. 2007;35(8):1937–1941. [DOI] [PubMed] [Google Scholar]
- 26. Aujesky D, Roy P-M, Guy M, Cornuz J, Sanchez O, Perrier A. Prognostic value of D-dimer in patients with pulmonary embolism. Thromb Haemost. 2006;96(4):478–482. [PubMed] [Google Scholar]
- 27. Kabbara R, Labarere J, Pernod G, et al. D-dimer level is not a prognostic biomarker specific of pulmonary embolism. Crit Care Med. 2008;36(2):652–653; author reply 653. [DOI] [PubMed] [Google Scholar]
- 28. Stein PD, Janjua M, Matta F, Alrifai A, Jaweesh F, Chughtai HL. Prognostic value of D-dimer in stable patients with pulmonary embolism. Clin Appl Thromb Hemost. 2011;17(6):E183–E185. [DOI] [PubMed] [Google Scholar]
- 29. Polo Friz H, Pezzetti V, Orenti A, et al. Comorbidity burden conditions the prognostic performance of D-dimer in elderly patients with acute pulmonary embolism. Am J Emerg Med. 2019;37(5):799–804. [DOI] [PubMed] [Google Scholar]
- 30. Lobo JL, Zorrilla V, Aizpuru F, et al. Clinical syndromes and clinical outcome in patients with pulmonary embolism: findings from the RIETE registry. Chest. 2006;130(6):1817–1822. [DOI] [PubMed] [Google Scholar]
- 31. Becattini C, Agnelli G. Risk stratification and management of acute pulmonary embolism. Hematology Am Soc Hematol Educ Program. 2016;2016(1):404–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chatterjee S, Chakraborty A, Weinberg I, et al. Thrombolysis for pulmonary embolism and risk of all-cause mortality, major bleeding, and intracranial hemorrhage: a meta-analysis. JAMA. 2014;311(23):2414–2421. [DOI] [PubMed] [Google Scholar]
- 33. Lehnert P, Lange T, Møller CH, et al. Acute pulmonary embolism in a national danish cohort: increasing incidence and decreasing mortality. Thromb Haemost. 2018;118(3):539–546. [DOI] [PubMed] [Google Scholar]