Abstract
Hemophilic arthropathy from joint bleeding remains a complication with major morbidity in the increasingly aging patients with hemophilia. Prophylactic clotting factor infusions, based on pharmacokinetic dosing to reduce bleeding rates, are being explored more and more. However, there is little evidence on the benefits of pharmacokinetic dosing in direct association with bleeding events. Here, we prospectively followed a cohort of adult patients with hemophilia A and B (n = 26) and arthropathic joints on various clotting factor products over a period of 2 years with clinical and radiographic joint health assessments, frequent joint ultrasound, and pharmacokinetic studies. Joint bleeds and synovitis with synovial vascularity changes were objectively diagnosed by musculoskeletal ultrasound and power Doppler and analyzed in relation to pharmacokinetic, joint- and patient-specific parameters. Results revealed that, contrary to common beliefs, bleeding episodes were not readily explained by pharmacokinetic features, as they were not associated with more time spent below certain clotting factor thresholds. Joint bleeding was found to be associated with prominent vascularity changes, suggesting that vascular remodeling and leakiness may contribute to joint bleeding that cannot be prevented by clotting factor replacement alone.
Keywords: pharmacokinetics, hemophilia, hemarthrosis, vascular remodeling, ultrasound
Introduction
Pharmacokinetic (PK) features in patients with hemophilia (PWH) have potential significant implications in clinical practice to reduce joint bleeds and halt progression of hemophilic arthropathy. Although studies have shown the benefit of prophylactic factor infusion in decreasing the number of bleeds,1–3 the ideal trough level for optimal protection is not yet clear. Although increased time spent with factor VIII (FVIII) below 1 IU·dL−1 is associated with more breakthrough bleeding episodes, bleeding tendencies vary widely between patients and are likely influenced by many variables.4–6 Recently, FVIII trough levels of ≥12 to 15 IU·dL−1 have been suggested for best protection.1–2,7 Suggestions for such relatively high trough levels are derived from a few studies demonstrating a steep decline in bleeding for every 1% increase in residual FVIII activity and almost no joint bleeding when factor activity exceeds 12 to 15 IU·dL−1.2,7
Consequently, individualizing schedules and dosing of clotting factor preparations is increasingly adopted in specialized centers with some evidence that a PK-based approach to dosing may improve bleed rates.8 However, in clinical practice, it can be a complex and challenging process to create individual factor infusion schedules based on a patient’s PK profile that includes compartmental factor distribution,9 age, body composition, “third spacing,” bleed frequency, product features, and half-life. Moreover, the importance of patient- and joint-specific parameters that are less amenable to pure adjustments in clotting factor dosing schedules may be underappreciated. In particular, vascular changes and remodeling have been associated with hemophilic arthropathy as a potential contributor in the propagation of repeated bleeding events.10–13 This may be the case especially in aging patients with advanced arthropathies, mechanical joint instability, and a higher proportion of subclinical bleeding associated with abnormal, leaky intra-articular vasculature.10–13
In this study, we therefore aimed to elucidate how PK profiles in a cohort of adult PWH with advanced stages of hemophilic arthropathy affect joint bleeding in relation to joint- and patient-specific characteristics, mostly in the context of prophylactic factor use. First, we prospectively examined fluctuations in clotting factor activity levels, time spent below certain factor thresholds, area under the curve (AUC), and factor consumption in relation to bleeding patterns. Going beyond PK features, we analyzed factors such as body mass index (BMI) and the degree of arthropathy or joint vascularity changes by musculoskeletal ultrasound (MSKUS) and power Doppler (PD) that may propagate breakthrough bleeding events—the clinical implications of which would then extend beyond simply clotting factor replacement.
Methods
Patient Characteristics
Patients aged ≥21 years were recruited at the Hemophilia and Thrombosis Treatment Center, University of California, San Diego (UCSD). The inclusion criterion was severe, moderate, and mild congenital FVIII or FIX deficiency, denoted as intrinsic factor activity levels <1%, 1% to 4%, or 5% to 40%, respectively. Patients had to have at least one arthropathic joint, which was defined either by radiographic Pettersson scores or by Hemophilia Joint Health Scores (HJHS).14,15 Based on published correlations between the 2 joint outcome measures, Pettersson scores or HJHSs had to demonstrate a score of at least ≥1 or ≥3, respectively, to suggest arthropathic changes.16,17 Patients were followed prospectively for 30 months and subjected to joint health assessments at baseline, at regular intervals (6-12 months) thereafter, and when experiencing acute painful joints. Scheduled joint health assessments comprised a pain assessment by Visual Analog Scale (VAS), HJHS,14 and Pettersson score,15 as well as joint examination with MSKUS/PD. Patients were asked to maintain an infusion log throughout the study and record the date and time of each infusion, product name, and dosage. Joint health assessments during acute painful episodes (knee, ankle, and elbow) comprised VAS pain score and MSKUS/PD for the presence of joint bleeding. Patient demographic information, type and severity of hemophilia, BMI, and age were also collected. All participants signed an informed consent and the study was approved by the UCSD Human Research Protection Program.
Joint Health Assessment
Pain, Pettersson, and HJHS
For each patient, the total HJHS and Pettersson scores at study entry were taken individually per joint as well as a total score by the sum of 6 joints (knee, ankle, and elbow) at baseline. The total possible HJHS score is 124 and included gait analysis. The total Pettersson score is 78 or 13 points per joint. The VAS ranged from 0 (no pain) to 10 (worst pain).
Joint bleed detection by MSKUS
The MSKUS/PD imaging of joints was performed using a GE Logiq S8 ultrasound machine (General Electrics, Fairfield, Connecticut) with real-time spatial compound imaging, speckle reduction capabilities, and an 8 to 15 MHz high frequency transducer. Grayscale (B-mode) and PD settings were used in accordance with the manufacturer’s recommendations and images were acquired with a standardized, validated scanning protocol (Joint Tissue Activity and Damage Exam).18 Power Doppler was used to assess vascular abnormalities. The MSKUS also included sonopalpation to evaluate compressibility and displacement of intra-articular material to distinguish between simple and complex (bloody) effusions.19 All MSKUS studies were performed or supervised by a hematologist (A.v.D.) who was formally trained in MSKUS, is certified through the American Registry for Diagnostic Medical Sonography, and maintains certification aligned with requirements by the Institute of Ultrasound in Medicine. The hematologist was blinded to all other outcome measures.
Quantification of vascularity changes
The PD signal was scored semi-quantitatively in 3 anatomical locations in the knee and ankle (knee: medial and lateral recesses and medial meniscal area; ankle: tibiotalar axial, longitudinal, and lateral sinus tarsi) and 2 anatomical locations in the elbow (humeroradial joint anterior longitudinal and posterior olecranon fossa) as previously described.10 Scores from each location were combined to give a total for each joint (knee and ankle: 0-9; elbow: 0-6). The total score for each joint was divided by the maximum possible score to give the “MSKUS/PD score proportion,” reducing variability and enabling comparisons between different joints.
Cohort Separation According to Joint Bleed Patterns
This was an exploratory analysis, and while the study progressed, it became evident that 3 different groups formed based on joint bleed patterns. Since this separation determined the direction of analyses, we mention the groups a priori in the Methods section with further characterization in the Results section. First, episodes separated into 3 main groups: (1) acute painful episodes with bleeding (the “bleed group”), (2) acute painful episode without bleeding (the “no bleed group”), and (3) no acute painful episodes (the “control group”). In other words, the “control group” was defined post hoc. Second, for the characterization of painful episodes, the main unit of analysis was the acute painful episode rather than the individual patient since some patients had 2 or 3 painful episodes. If a patient had a painful episode with a bleed and another episode without a bleed, their episodes were allocated to the respective different groups. The date of a reported painful episode was named “day of interest.” Thus, the episodes were not independent of each other because some patients experienced multiple episodes. The lack of independence means that statistical tests with estimation of P values would be invalid. For the “control group,” we selected a random date as “the day of interest” to determine the PK parameters preceding this day. If patients in the “control group” changed clotting factor products (n = 3), the periods on different products were treated separately and random dates were selected as “day of interest” in each of these periods.
Determination of Individual PK Parameters
The PK parameters were analyzed during the 15 days preceding painful episodes and random dates during pain-free intervals. At first, we considered a shorter period preceding the day of interest (ie, 3 days). However, patients frequently reported the onset of pain not as an exact time point, but rather as a window of several days. A longer period of 30 days was felt to be too far from the painful episode. It therefore was ascertained that a 15-day period provided a better estimate of differences in PKs leading up to “the day of interest.”
For each episode, the AUC was calculated using the median dose, the half-life, and the volume of distribution (Vd).20 Half-lives and Vd were estimated by the Web Accessible Population Pharmacokinetic Service–Hemophilia (WAPPS-Hemo) project using the NONMEN (ICON Development Solutions modeling software) program.21 Some patients had breaks in their infusion logs or forgot to note the time of the infusion preceding a blood draw. In these instances, estimates of half-life or Vd were taken from the respective pharmaceutical companies’ drug inserts.
We used patients’ infusion logs to build a simulation model to estimate the time trend of each patient’s factor concentration. The model calculated the patient’s plasma factor concentration hour by hour and summed the total concentration hours (Supplementary Figure 1). A detailed depiction and description of the model is provided in Supplementary Figure 2.
For each group, median values of each PK parameter were calculated. For some analyses, patients with hemophilia B were separated from patients with hemophilia A, or omitted altogether, since their pharmacodynamics were different due to the longer half-lives and larger volumes of distribution of their clotting factor products. These omissions are specified in the relevant following sections. The main unit of analysis was, again, the acute painful episode.
Comparison of PK Parameters in Association With Painful Episodes
Comparison of half-life, Vd, factor consumption, and AUC
This analysis comprised episodes from patients with hemophilia A only, with hemophilia B patients omitted due to reasons noted above. The following PK parameters were compared among the 3 groups (acute painful episodes with and without bleeding and “control group”): half-life, Vd, factor consumption, AUC, and total concentration hours of episodes during the 15 days preceding the “day of interest.”
Comparison of time spent below clotting factor threshold between bleeding and nonbleeding patients
This analysis included episodes from patients with hemophilia A and B and evaluated time spent below the clotting factor thresholds of 5 and 15 IU·dL−1 in episodes from the 3 groups. The PK parameters were calculated for the 15 days preceding painful episodes or 15 days preceding randomly selected days for the “control group.”
Intraindividual PK Parameters in Relation to Painful Episodes With Bleeding in Comparison to Pain-Free Intervals
This analysis focused on the group with painful episodes associated with bleeding in all patients, both hemophilia A and B. Dose, total factor use and AUC, total concentration hours, and time spent below 5 and 15 IU·dL−1 clotting factor thresholds were determined during the 15 day periods immediately preceding a bleeding episode in comparison to 4 randomly selected pain-free intervals (15-day control periods). “Control periods” were defined as 2 randomly selected dates in the months before the bleed designated as “precontrol” periods. In addition, 2 randomly selected dates in the months after the bleed were designated as “postcontrol” periods (Supplementary Figure 1). For each episode, the 2 “control periods” before the bleeding event were averaged and the 2 after the bleeding event were averaged. The median of the “precontrol” averages and the median of the “postcontrol” averages were then calculated.
Association of Patient and Joint Characteristics With Joint Bleeding Episodes
Patient and joint characteristics were evaluated in association with painful joint episodes. Characteristics included age, weight, BMI, type of hemophilia (A or B), and baseline joint health assessment by HJHS and Pettersson scores, and baseline MSKUS/PD signal. In addition, vascularity changes by MSKUS/PD were quantified during painful episodes. For each of the 3 groups, we calculated the median values for each of these variables.
Results
Patient and Joint Characteristics
Among 29 prospectively recruited patients, 26 patients completed the study. The median age was 38 years (interquartile range [IQR]: 29-54). Most patients had severe hemophilia A and were on prophylactic clotting factor replacement (“prophylaxis”). One patient had an active inhibitor and is denoted by a specific symbol in Figures 1 and 2. One patient had a history of an inhibitor. Median total Pettersson and HJHS scores were 23 and 10 (IQR: 10-47 and 5-29), respectively (Table 1). Two patients were missing baseline Pettersson scores, whereas HJHS scores were available for all patients. The median observation period from enrollment to final study visit was 664 days. As recombinant human FVIII or FIX Fc fusion proteins (Eloctate or Alprolix; Bioverativ, Cambridge, Massachusetts) became available for half-life extension in the United States approximately 1 year after study start, patients were allowed to switch as desired.
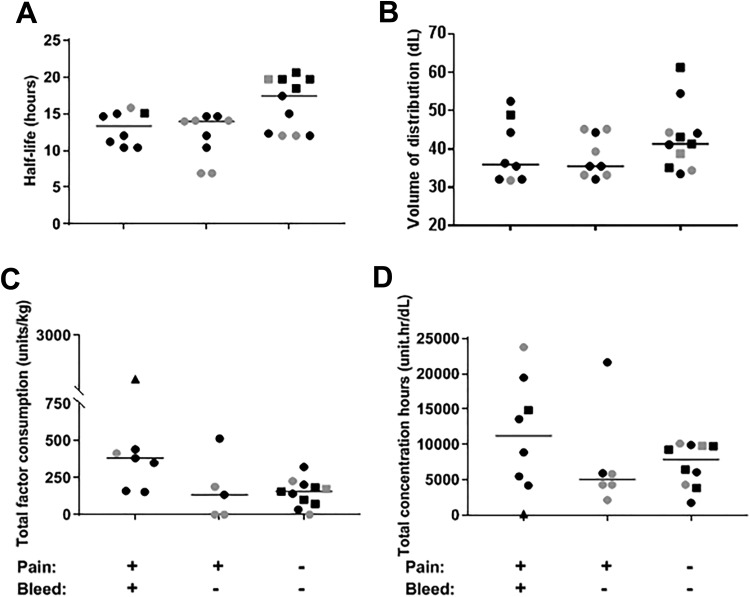
Figure 1.
Comparison of half-life, volume of distribution, total factor consumption, and total concentration hours in association with painful episodes in patients with hemophilia A: (A) half-life and (B) volume of recombinant human (rh) factor VIII (FVIII) distribution, (C) total factor use per kg body weight, and (D) total concentration hours of rhFVIII in advance of painful joint episodes with and without bleeding and “control” episodes encompassing 15 days prior to a randomly selected day, respectively. Black symbols denote episodes in patients with severe hemophilia and gray symbols denote episodes in patients with mild or moderate hemophilia. Filled squares denote episodes in patients on extended half-life FVIII Fc fusion protein. Triangle symbol denotes the episode in the patient with an active inhibitor. For each group, the median is shown as a horizontal bar. As there were gaps in the infusion logs of a few patients, some variables could not be calculated for those patients and explain a few absent data points in the figure.
Figure 2.
Comparison of time spent below factor concentration thresholds of 5 and 15 IU·dL−1 in association with painful episodes in patients with hemophilia A and B. Painful episodes with and without bleeding were compared to the “control group” without painful episodes. Time spent below (A) 5 IU·dL−1 and (B) 15 IU·dL−1 plasma factor activity in each group was analyzed during the 15-day period preceding each episode. For the “control group,” the day prior to a 15-day interval was selected randomly. Black and gray symbols denote episodes in patients with severe and mild/moderate hemophilia A, respectively. Red symbols denote episodes in patients with hemophilia B. Square-shaped symbols denote episodes on Fc fusion factor VIII (FVIII) or FIX. Triangle symbol denotes the episode in the patient with an active inhibitor. For each group, the median is shown as a horizontal bar. As there were gaps in the infusion logs of a few patients, some variables could not be calculated for those patients and explain a few absent data points in the figure.
Table 1.
Demographic Patient Characteristics.
| Characteristic | n (%) | Median | IQR |
|---|---|---|---|
| Hemophilia type | |||
| A | 21 (81) | ||
| B | 5 (19) | ||
| Severity | |||
| Mild | 4 (15) | ||
| Moderate | 4 (15) | ||
| Severe | 18 (69) | ||
| Factor use | |||
| Prophylaxis | 21 (81) | ||
| On demand | 5 (19) | ||
| Age (years) | 38 | 29-54 | |
| Weight (kg) | 83 | 74-98 | |
| BMI (kg/m2) | 27.7 | 24.5-29.4 | |
| Number of observation days | 664 | 608-751 | |
| Total Pettersson scorea | 23 | 10-47 | |
| Total HJHS | 10 | 5-29 |
Abbreviations: BMI, body mass index; HJHS, Hemophilia Joint Health Score; IQR, interquartile range; N, number of patients.
aTwo patients were missing for baseline Pettersson scores.
The 3 groups were defined at the end of the data collection phase. Seventeen patients experienced 29 painful episodes, of which 18 episodes were associated with bleeding confirmed by MSKUS, and 11 episodes were not, that is, pain only (Table 2). Six of those patients experienced painful episodes with bleeds and also painful episodes without bleeds. The number and type of painful episodes separated by joint is shown in Table 2. Nine patients experienced no painful episodes and were allocated to the “control group.” Eight patients had hemophilia A; 3 switched from conventional to extended half-life FVIII (Eloctate) during the study. Only 1 patient had hemophilia B using half-life extended FIX (Alprolix) prophylactically. In total, there were 12 control time periods in the “control group,” of which 6 were on extended half-life products (1 on extended half-life FIX and 5 on extended half-life FVIII products).
Table 2.
Number of Painful Episodes.
| Number of Painful Episodes by Type of Hemophilia | ||
|---|---|---|
| Painful Episodes With Bleed | Painful Episodes Without Bleed | |
| Hemophilia A | 11 | 11 |
| Hemophilia B | 7 | 0 |
| Total | 18 | 11 |
| Number of Painful Episodes by Joints | ||
| Type of Episode | Joints | |
| Painful episodes with bleeds (n = 18) | Knee | 4 |
| Elbow | 9 | |
| Ankle | 5 | |
| Painful episodes without bleeds (n = 11) | Knee | 4 |
| Elbow | 1 | |
| Ankle | 6 | |
| Control: periods without pain or bleeding (n = 12) | n/a | |
| Total | 29 | |
No patients in the cohort had repeat bleeding in the same joint within a short period of time. There were 4 patients who had repeat episodes in the same joint, the shortest of these intervals was 20 days and the median was 48 days. Overall, the median interval between all painful episodes was 155 days.
Comparison of PK Parameters in Association With Painful Episodes
Comparison of half-life, Vd, factor consumption, and AUC
This analysis comprised painful episodes (n = 17) from 10 patients with hemophilia A who had adequate infusion recordings for analyses. Of those episodes, 10 were in patients with severe hemophilia while on prophylaxis with conventional recombinant half-life products and one episode in a patient on extended half-life product Eloctate. Four painful episodes occurred in patients with moderate or mild hemophilia on prophylaxis with conventional factor products. Two episodes were in a single patient with mild hemophilia A on “on-demand” therapy with a conventional factor product. As there were gaps in the infusion logs of a few patients, some variables could not be calculated for those patients and explain a few absent data points in Figures 1 and 2.
Median half-life and volume of FVIII distribution were longer and higher in the “control group” compared to the “bleed” and “no bleed groups.” Among the 6 patients on Eloctate, 5 were in the “control group.” The median half-life and median Vd for episodes from the “bleed group” were 13.4 hours and 35.9 dL, for the “no bleed group” 14.0 hours and 35.5 dL, and for the “control group” 17.4 hours and 41.3 dL, respectively (Figure 1A and B).
However, in the 15 days preceding “the day of interest,” the “bleed group” used approximately twice as much factor as the other groups: 382 U/kg compared to 135 U/kg (“no bleed group”) and 157 U/kg (“control group”; Figure 1C). Aligned with factor consumption, the total concentration hours for the “bleed group” were twice as high (11259 unit.hr/dL) than for the “no bleed group” (5085 unit.hr/dL) and 43% more than the “control group” (7876 unit.hr/dL; Figure 1D).
In summary, these observations showed higher half-life and Vd in the “control group” compared to the “no bleed” and “bleed groups,” but factor consumption and concentration hours were highest in the “bleed group.” If factor concentrate PK was the sole determinant of bleeding, one would have expected less factor consumption and lower concentration hours during the 15 days leading up to a bleeding episode (without reporting any increase or variation in physical activity levels). Therefore, differences in clotting factor use or variation in time below threshold did not explain the differences in bleeding phenotypes. A possible explanation for our findings could be that over the lifetime of bleeders their treatment dose was progressively increased in response to a suboptimal control of bleeding.
Comparison of time spent below clotting factor threshold between bleeding and nonbleeding patients
This analysis included episodes from all 26 patients (hemophilia A and B). Contrary to expected results, time spent below thresholds was similar or less for the “bleed group” when compared to the “no bleed” and “control groups” (Figure 2). Specifically, median time spent <5 IU·dL−1 thresholds was 24, 0.25, and 104 hours (Figure 2A) and median time spent <15 IU·dL−1 thresholds was 164, 306, and 200 hours (Figure 2B) for the “bleed,” “no bleed,” and “control” groups, respectively.
When sufficient data points were available (n = 11), the estimates of half-life and Vd were calculated from the patients’ individual data by the WAPPS-Hemo system. Values for the remainder were taken from the respective pharmaceutical companies’ prescribing information inserts. To ensure that the use of published half-lives yielded reasonably similar results, we compared the output from the simulation model calculating time spent below factor thresholds of 5 and 15 IU·dL−1 for participants who had their half-lives and Vd estimated by the WAPPS system (using the NONMEM program) compared to estimates published by the drug company. There appeared to be a reasonable correlation (Supplementary Figure 4), suggesting no major impact on results using published half-life data.
Intraindividual PK Parameters in Relation to Painful Episodes With Bleeding in Comparison to Pain-Free Intervals
Since interindividual comparisons of PK parameters failed to uncover a clear pattern of bleeding tendencies, this analysis focused on the “bleed group” to evaluate intraindividual PK parameters in relation to bleeding. Various parameters preceding the bleed were compared to 4 random periods during pain-free intervals, the “pre- or postcontrol(s).” Four patients who had experienced joint bleeding early or late in the study lacked either “pre- or postcontrol(s).” Results are summarized in Table 3, separated by type of hemophilia, as the units used for FVIII and FIX replacement are different. There were 18 bleeding episodes analyzed, and for 6, the necessary infusion data were not available. The median amount of clotting factor used (a function of dose and frequency of administration), the median AUC (a function of dose, half-life, and Vd), and time spent below 5 or 15 IU·dL−1 were similar during the 15 days preceding joint bleeding and the random periods (Table 3).
Table 3.
Comparison of PK Parameters During Control Periods and the 15-Day Period Preceding Joint Bleeding.a
| Episodes From Patients With Hemophilia A | |||
|---|---|---|---|
| PK Parameter | Median (Range) | ||
| Control Period Prior to Bleed, n = 7 | Bleed, n = 8 | Control Period Post Bleed, n = 8 | |
| Dose (U) | 3700 (3207-15260) | 3814 (2755-15260) | 3787 (2824-15260) |
| Total factor consumed (U/kg) | 350 (207-2802) | 367 (155-2802) | 317 (121-2802) |
| AUC (U·h/dL) | 1855 (1141-3023) | 2010 (1166-2761) | 1953 (1166-2722) |
| Total concentration-hours (U·h/dL) | 9359 (180-18259) | 11 259 (180-23797) | 7039 (180-20547) |
| Time below 5 IU·dL−1 (hours) | 31 (0-360) | 77 (0-360) | 77 (0-360) |
| Time below 15 IU·dL−1 (hours) | 145 (23-360) | 163 (50-360) | 182-360) |
| Episodes From Patients With Hemophilia B | |||
| Median (Range) | |||
| PK Parameter | Control Period Prior to Bleed, n = 2 | Bleed, n = 4 | Control Period Post Bleed, n = 3 |
| Dose (U) | 5310 (5082-5538) | 5023 (5000-15995) | 7621 5087-7621) |
| Total factor consumed (U/kg) | 168 (83-252) | 224 (97-1785) | 114 (99-662) |
| AUC (U·h/dL) | 2273 (1383-3164) | 2364 (1361-5139) | 2907 (2074-3794) |
| Total concentration hours (U·h/dL) | 4851 (2980-6723) | 6458 (3962-46016) | 4587 (2163-17292) |
| Time below 5 IU·dL−1 (hours) | 93 (8-179) | 0 (0-59) | 92 (0-249) |
| Time below 15 IU·dL−1 (hours) | 184 (82-286) | 155 (0-282) | 210 (0-311) |
Abbreviations: AUC, area under the curve; PK, pharmacokinetic.
aPK parameters were estimated during two 15-day control periods randomly selected before the bleed and two 15-day control periods randomly selected after the bleed. For each episode, the 2 control periods before the bleed were averaged, as were the 2 control periods afterward. The median values for the averaged precontrol, bleed and averaged postcontrol periods are shown. Episodes from patients with hemophilia A and B are separated as the units used for FVIII and FIX replacement are different.
These observations indicate that determinants of bleeding are more complex than simple PK of factor concentrates, since joint bleeding episodes were not associated with variation in factor consumption, lower total concentration hours, or longer time spent below clotting factor activity thresholds compared to randomly selected control periods.
Association of Patient and Joint Characteristics With Joint Bleeding Episodes
Since inter- and intraindividual PK parameters and clotting factor consumption patterns could not explain joint bleed phenotypes, we examined additional elements that might play a role such as intra-articular vascular changes, Pettersson and HJHS scores, age, and BMI.
Vascularity changes by MSKUS/PD
For patients with an acute painful episode, the VAS pain scores and MSKUS/PD score proportions for the patients who were to experience future painful episodes with and without bleeding were similar at baseline. Median VAS scores were 1.80 and 1.63, and median MSKUS/PD score proportions at baseline were 0.20 and 0.33, respectively (Figure 3). During painful episodes, characterized by an approximately 3.2-fold increase in VAS score for both groups, median MSKUS/PD score proportion was relatively unaltered in the absence of joint bleeding with a median value of 0.33 (n = 11). However, in the group with joint bleeding, the median MSKUS/PD score proportion rose significantly with an approximately 3.0-fold increase from median value of 0.22 at baseline to 0.67 during the painful episode (n = 18; Figure 3). This suggests that vascularity changes may have a role in the propagation of bleeding events.
Figure 3.
Vascular flow abnormalities in joints at baseline and at time of acute episodes with and without joint bleeding (“bleed” and “no bleed”). A, Vascularity changes by power Doppler (PD) signal was scored semi-quantitatively in certain anatomical locations in the knee, ankle, and elbow. The scores from each location were combined to give a total for each joint (knee and ankle: 0-9; elbow: 0-6). The total score for acute painful joint was divided by the maximum possible score to give the “PD score proportion.” B, Pain assessment Visual Analog Scale (VAS) was obtained at baseline and during an acute painful episode associated with no bleed and bleeding. BL indicates baseline.
Other variables such as age, BMI, Pettersson, and HJHS scores
Baseline characteristics including HJHS, Pettersson scores at baseline, and age are shown among the 3 groups in Table 4. Episodes with bleeding joints had the highest HJHS and Pettersson scores at baseline and occurred in older patients. The groups had similar BMI values. These findings suggest that more pronounced arthropathy, particularly in the afflicted joints, in association with aging may influence joint bleeding.
Table 4.
Baseline Characteristics: Comparison of Continuous Potential Predictors of Bleeding in Joint Episodes Between the 3 Groups: Acute Painful Episode With Bleeding, Without Bleeding, and “Control Group.”a
| Variable | Median (Range: Min, Max) | ||
|---|---|---|---|
| Bleed | No Bleed | Control | |
| Age (years) | 41 (24, 70) | 31 (27, 70) | 32.5 (21, 66) |
| Weight (kg) | 81 (62, 100) | 85 (67, 96) | 82 (71, 124) |
| BMI (kg/m2) | 25.6 (20.2, 34.5) | 26.3 (24.0, 36.0) | 28.7 (23.8, 38.2) |
| HJHS for all joints at baseline | 15 (0, 45) | 13 (3, 15) | 8 (0, 43) |
| HJHS for afflicted joint at baseline | 5 (0, 8) | 1 (0, 9) | n/a |
| Pettersson score for all joints at baseline | 31 (8, 63) | 15 (5, 29) | 23 (0, 69) |
| Pettersson score for afflicted joint at baseline | 8 (0, 13) | 5 (1, 10) | n/a |
Abbreviation: BMI, Body Mass Index; HJHS, Hemophilia Joint Health Scores.
aControl group experienced no painful episodes; thus, HJHS and Pettersson analysis for afflicted joint was not applicable (n/a).
Discussion
In this study, we prospectively followed 26 adult hemophilia patients with arthropathic joints on various clotting factor products. We recorded infusion logs, PK features, and joint health and intra-articular vascularity evaluations for a median of 664 observation days. It was our goal to elucidate the relative importance of clotting factor plasma parameters and thresholds associated with bleeding events in a “real-world setting.” The objective diagnosis of hemarthrosis in association with joint pains was provided by MSKUS and is an important aspect of this study. Historically, the etiology of joint pains in hemophilia has mostly been determined by patient and/or physician perception only, which is increasingly being recognized as imprecise. Symptoms such as pain, swelling, or loss of range of motion are nonspecific, yielding inaccurate diagnoses in up to 30% to 50% of painful joint events in PWH.11,22-24
Based on conventional logic, we expected that acute painful episodes with bleeding would be associated with more time spent below specified clotting factor thresholds, lower clotting factor concentrations, or any combination of those elements in the days preceding the bleed. However, our analyses revealed that this was not the case. During periods of 15 days preceding bleeds, we found that: (1) factor consumption and concentration hours were both higher before painful episodes with bleeding compared to the groups without bleeding, (2) factor consumption and concentration hours in episodes of patients with joint bleeds were not less than randomly selected control periods in the months before and after the event, and (3) in episodes with and without bleeding, there was less time spent below 5 IU·dL−1 than random periods in the “control group.”
Overall, joint bleeding was not easily explained by PK features alone. It was noted that all hemophilia A patients, except one, on extended half-life products belonged to the “control group,” which had a longer half-life and higher Vd compared to the groups with painful episodes (Figure 1). A similar comparison for PWH B was not possible since there were too few patients (n = 5) and all but one was on extended half-life product. For hemophilia A patients, there was considerably less clotting factor consumption in the “control group” compared to clotting factor consumption in the “bleed group,” where the majority of patients were on conventional half-life products (Figure 1C and D). Initially, we assumed that the extended half-life feature of the Fc fusion would translate into better bleed protection. However, subsequent analyses using time spent below clotting factor thresholds (5 and 15 IU·dL−1) as well as individual patient comparisons of PK parameters in advance of bleeding episodes provided information independent of product use and half-lives. These analyses revealed that optimization of clotting factor exposure titrated to meet relatively high plasma activity thresholds may not necessarily convey optimal bleed protection. Furthermore, the results also implied that Fc-fused factor products may have advantages regarding bleed protection in association with other elements important for breakthrough bleeding, through mechanisms not yet clearly understood.
When delving beyond PK parameters, the analysis of joint-specific factors showed a higher propensity for bleeding in joints with a higher baseline arthropathy burden. Another noteworthy finding was the substantially increased vascularity changes revealed by MSKUS/PD in the joints with bleeds compared to baseline and nonbleeding joints. Vascular remodeling associated with hemophilic arthropathy has been shown to be a potential important contributor that propagates repeated bleeding events despite adequate clotting factor replacement.10–13 Since hypervascularity detected by PD in association with synovial hypertrophy is a hallmark of synovitis in arthritic conditions,25,26 it is conceivable that local inflammatory flares as described in osteoarthritis27,28 may disrupt the joint vasculature or local milieu in hypertrophied synovium, inciting bleeding and vascularity changes. Aging joints and joints with high arthropathy burden may be more affected than healthier joints in younger patients due to factors such as vascular fragility and mechanical joint instability. Therefore, preceding joint damage and synovial hypervascularity in the setting of synovitis, most likely more prominent in adults, appear to be important determinants of joint bleeding. However, while our observations further highlight the possibility of vascular contributions to bleeding, the triggers and timing of vascularity changes in association with bleeding events remain unclear. Joint bleeding may be a potential trigger to promote vascularity changes, which in turn may perpetuate further bleeding. Serial MSKUS/PD evaluations before, during, and after an acute joint bleed in a prospective setting would help elucidate this association.
Given that patients with hemophilia A on Eloctate (Fc-fused proteins) experienced fewer painful episodes in comparison to those on conventional products, it is worthwhile to consider the potential anti-inflammatory properties of the Fc component in this context. Fc receptors are implicated in the regulation of inflammatory processes with potential anti-inflammatory and immunomodulatory activities via interactions with the Fc neonatal receptor (FcRn) and Fcγ receptors.29–31 Fc fusion of molecules extends half-life through binding of the Fc portion of human IgG to FcRn on endothelial cells leading to uptake and recycling of the fusion protein back into circulation.32 However, Fc-fused clotting factor proteins may also impact inflammatory arthritic manifestations in hemophilia in a yet unknown fashion. Hypothetical support of this concept is provided by recent observations in a preclinical model, showing immune suppressive effects in respect to formation of inhibitory anti-FVIII antibodies, and clinical trials suggesting that prolonged use of FVIII Fc fusion protein may result in improved joint health.32–34
This study has several limitations. For one, this study has a limited patient size with a divergent phenotype, including a patient with a history of an inhibitor and an active inhibitor. There was also no objective measurement for lifestyle or physical activity characteristics among the groups. However, while such data were not formally collected, no patient reported a meaningful change in lifestyle or activity during the clinic visits. While the HJHS is widely accepted as a joint assessment tool for all ages in the clinical setting, it has been validated for use in children only. However, there is emerging evidence of its applicability in adults.16,35,36 Perhaps the most difficult challenge was to motivate patients to continuously record complete infusion logs over 2 years. Shorter observation periods on each product plus incomplete infusion logs with gaps of varying length necessitated the use of published rather than individual half-lives for some patients. However, comparisons of PK parameters derived from individual and published half-lives in patients with optimal infusion logs demonstrated reasonable correlations (Supplementary Figure 4). Another unavoidable challenge was posed by the fact that some patients fell into both groups with painful episodes and some had 2 episodes in the same group. These episodes were not independent of each other, thus precluding the use of statistical analyses and P values. Some patients switched factor products during the observation period and suffered painful episodes under different PK characteristics. Although switching products meant that the time patients spent on each factor product was shorter than anticipated, we believe that these observations can still provide insights that would remain obscure in shorter studies that use only one clotting factor product.
Conclusion
In summary, to our knowledge, this is the first prospective study to shed light on the interplay of PK parameters in relation to objectively diagnosed joint bleeding events. Bleed propensity in arthropathic joints of hemophilic patients was not explained by PK parameters alone, but associated with pronounced vascular remodeling suggesting synovitis flares, possibly initiating or perpetuating bleeds. Therefore, the development of targeted interventions to control synovitis and vascularity changes deserves consideration for improving bleed control beyond simply clotting factor replacement therapy.
Supplemental Material
Supplemental Material, Supplementary_PK_Revised_1 for Joint Bleeding Tendencies in Adult Patients With Hemophilia: It’s Not All Pharmacokinetics by Jenny Y. Zhou, Richard F. W. Barnes, Gary Foster, Alfonso Iorio, Thomas J. Cramer and Annette von Drygalski in Clinical and Applied Thrombosis/Hemostasis
Footnotes
Authors’ Note: J.Y.Z. contributed to data analysis and interpretation of MSKUS and provided the first draft of the manuscript. R.F.W.B. performed data analyses and contributed to manuscript drafting. T.J.C. contributed to study coordination and patient data collection. A.I. and G.F. provided pharmacokinetic analyses. A.v.D. performed and interpreted MSKUS, designed the study, and provided study oversight regarding study coordination, data analysis, and manuscript writing. All authors critically reviewed the manuscript and approved it in its final version. Ethical approval to report this case was obtained from University of California San Diego Human Research Protection Program, IRB #120510. Written informed consent was obtained from the patients for their anonymized information to be published in this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study is supported by Bioverativ (now Sanofi) and by funding from the Health Resource and Service Agency (HRSA) H30MC24045. The WAPPS-Hemo team participation in this study was supported by intramoenia funds of the Health Information Research Unit, McMaster University. A.v.D. has received honoraria for participating in scientific advisory board panels, consulting, and speaking engagements for Baxalta/Shire, Bayer, Pfizer, Bioverativ (now Sanofi), CSL-Behring Novo Nordisk, Biomarin and Uniqure. Alfonso Iorio’s Institution has received in the last 5 years project-based funding via research or service agreements with Bayer, CSL, Grifols, NovoNordisk, Octapharma, Pfizer, Roche, Sobi, and Takeda/Shire (formerly Baxter and Baxalta).
ORCID iD: Alfonso Iorio  https://orcid.org/0000-0002-3331-8766
https://orcid.org/0000-0002-3331-8766
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Jimenez-Yuste V, Auerswald G, Benson G, et al. Achieving and maintaining an optimal trough level for prophylaxis in haemophilia: the past, the present and the future. Blood Transfus. 2014;12(3):314–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. den Uijl IE, Fischer K, Van Der Bom JG, Grobbee DE, Rosendaal FR, Plug I. Analysis of low frequency bleeding data: the association of joint bleeds according to baseline FVIII activity levels. Haemophilia. 2011;17(1):41–44. [DOI] [PubMed] [Google Scholar]
- 3. Manco-Johnson MJ, Abshire TC, Shapiro AD, et al. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N Engl J Med. 2007;357(6):535–544. [DOI] [PubMed] [Google Scholar]
- 4. Collins PW, Fischer K, Morfini M, Blanchette VS, Bjorkman S. Implications of coagulation factor VIII and IX pharmacokinetics in the prophylactic treatment of hameophilia. Haemophilia. 2011;17(1):2–10. [DOI] [PubMed] [Google Scholar]
- 5. Collins PW, Bjorkman S, Fischer K, et al. Factor VIII requirement to maintain a target plasma level in the prophylactic treatment of severe hemophilia A: influences of variance in pharmacokinetics and treatment regimens. J Thromb Haemost. 2010;8(2):269–275. [DOI] [PubMed] [Google Scholar]
- 6. Collins PW, Blanchette VS, Fischer K, et al. Break-through bleeding in relation to predicted factor VIII levels in patients receiving prophylactic treatment for severe hemophilia A. J Thromb Haemost. 2009;7(3):413–420. [DOI] [PubMed] [Google Scholar]
- 7. den Uijl IE, Mauser Bunschoten EP, Roosendaal G, et al. Clinical severity of haemophilia A: does the classification of the 1950s still stand? Haemophilia. 2011;17(6):849–853. [DOI] [PubMed] [Google Scholar]
- 8. Mingot-Castellano ME, Parra R, Nunez R, Martorell M. Improvement in clinical outcomes and replacement FVIII use in patients with haemophilia A after factor VIII pharmacokinetic-guided prophylaxis based on Bayesian models with myPKFIT®. Haemophilia. 2018;24(5): e338–343. [DOI] [PubMed] [Google Scholar]
- 9. Bjorkman S. Pharmacokinetics of plasma-derived and recombinant factor IX—implications for prophylaxis and on-demand therapy. Haemophilia. 2013;19(6):808–813. [DOI] [PubMed] [Google Scholar]
- 10. Bhat V, Olmer M, Joshi S, et al. Vascular remodeling underlies rebleeding in hemophilic arthropathy. Am J Hematol. 2015;90(11):1027–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kidder W, Nguyen S, Larios J, Bergstrom J, Ceponis A, von Drygalski A. Point-of-care musculoskeletal ultrasound is critical for the diagnosis of hemarthroses, inflammation and soft tissue abnormalities in adult patients with painful hemophilic arthropathy. Haemophilia. 2015;21(4):530–537. [DOI] [PubMed] [Google Scholar]
- 12. Melchiorre D, Linari S, Innocenti M, et al. Ultrasound detects joint damage and bleeding in haemophilic arthropathy: a proposal of a score. Haemophilia. 2011;17(1):112–117. [DOI] [PubMed] [Google Scholar]
- 13. Cooke EJ, Zhou JY, Wyseure T, et al. Vascular permeability and remodeling coincide with inflammatory and reparative processes after joint bleeding in factor VIII-deficient mice. Thromb Haemost. 2018;118(6):1036–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hilliard P, Funk S, Zourikian N, et al. Hemophilia joint health score reliability study. Haemophilia. 2006;12(5):518–525. [DOI] [PubMed] [Google Scholar]
- 15. Pettersson H, Ahlberg A, Nilsson IM. A radiologic classification of hemophilic arthropathy. Clin Orthop Relat Res. 1980;149:153–159. [PubMed] [Google Scholar]
- 16. Fischer K, de Kleijn P. Using the Haemophilia Joint Health Score for assessment of teenagers and young adults: exploring reliability and validity. Haemophilia. 2013;19(6):944–950. [DOI] [PubMed] [Google Scholar]
- 17. Poonnoose PM, Hilliard P, Doria AS, et al. Correlating clinical and radiological assessment of joints in haemophilia: results of a cross sectional study. Haemophilia. 2016;22(6):925–933. [DOI] [PubMed] [Google Scholar]
- 18. Volland LM, Zhou JY, Barnes RFW, et al. Development and reliability of the joint tissue activity and damage examination for quantitation of structural abnormalities by musculoskeletal ultrasound in hemophilic joints. J Ultrasound Med. 2018;00:1–13. [DOI] [PubMed] [Google Scholar]
- 19. Nguyen S, Lu X, Ma Y, Du J, Chang EY, von Drygalski A. Musculoskeletal ultrasound for intra-articular bleed detection: a highly sensitive imaging modality compared with conventional magnetic resonance imaging. J Thromb Haemost. 2018;16(3):490–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bauer LA. Applied Clinical Pharmacokinetics. New York, NY, McGraw Hill Medical; 2014;3:868. [Google Scholar]
- 21. McEneny-King A, Foster G, Iorio A, Edginton AN. Data analysis protocol for the development and evaluation of population pharmacokinetic models for incorporation into the Web-Accessible Population Pharmacokinetic Service–Hemophilia (WAPPS-Hemo). JMIR Res Protoc. 2016;5(4): e232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mahlangu J, Powell JS, Ragni MV, et al. Phase 3 study of recombinant factor VIII Fc fusion protein in severe hemophilia A. Blood. 2014;123(3):317–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Berro M, Elichiry M, Wasen K, Insagaray J, Rodriguez I. Use of ultrasound for evaluation of painful joint episodes perceived as haemarthrosis in adult patients with severe haemophilia. Haemophilia. 2018;24(3):e124–125. [DOI] [PubMed] [Google Scholar]
- 24. Ceponis A, Wong-Sefidan I, Glass CS, von Drygalski A. Rapid musculoskeletal ultrasound for painful episodes in adult haemophilia patients. Haemophilia. 2013;19(5):790–798. [DOI] [PubMed] [Google Scholar]
- 25. D’Agostino MA, Terslev L, Aegerter P, et al. Scoring ultrasound synovitis in rheumatoid arthritis: a EURAL-OMERACT ultrasound taskforce- Part 1: definition and development of a standardised, consensus-based scoring system. RMD Open. 2017;3(1):e000428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Torp-Pedersen S, Christensen R, Szkudlarek M, et al. Power and color Doppler ultrasound settings for inflammatory flow: impact on scoring of disease activity in patients with rheumatoid arthritis. Arthritis Rheumatol. 2015;67(2):386–395. [DOI] [PubMed] [Google Scholar]
- 27. D’Agostino MA, Conaghan P, Le Bars M, et al. EULAR report on the use of ultrasonography in painful knee osteoarthritis. Part 1: prevalence of inflammation in osteoarthritis. Ann Rheum Dis. 2005;64(12):1703–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Esen S, Akarirmak U, Yildiz Aydin F, Unalan H. Clinical evaluation during acute exacerbation of knee osteoarthritis: the impact of diagnostic ultrasonography. Rheumatol Int. 2013;33(3):711–717. [DOI] [PubMed] [Google Scholar]
- 29. Gelfand EW. Intravenous immune globulin in autoimmune and inflammatory diseases. N Engl J Med. 2012;367:2015–2025. [DOI] [PubMed] [Google Scholar]
- 30. Blumberg R, Lillicrap D. Tolerogenic properties of Fc portion of IgG and its relevance to the treatment and management of hemophilia. Blood. 2007;131(20):2205–2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kis-Toth K, Manohar Rajani G, Simpson A, et al. Recombinant factor VIII Fc fusion protein drives regulatory macrophage polarization. Blood Adv. 2(21):2904–2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Roopenian DC, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol. 2007;7(9):715–725. [DOI] [PubMed] [Google Scholar]
- 33. Krishnamoorthy S, Liu T, Drager D, et al. Recombinant factor VIII Fc (rFVIIIFc) fusion protein reduces immunogenicity and induces tolerance in hemophilia a mice. Cell Immunol. 2016;301:30–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Oldenburg J, Kulkami R, Srivastava A, et al. Improved joint health in subjects with severe haemophilia A treated prophylactically with recombinant factor VIII Fc fusion protein. Haemophilia. 2018;24(1):77–84. [DOI] [PubMed] [Google Scholar]
- 35. Kuijlaars IAR, Timmer MA, de Kleijn P, Pisters MF, Fischer K. Monitoring joint health in haemophilia: factors associated with deterioration. Haemophilia. 2017; 23(6):934–940. [DOI] [PubMed] [Google Scholar]
- 36. Timmer MA, Foppen W, Schutgens RE, Pisters MF, Fischer K. Comparing findings of routine Haemophilia Joint Health Score and Haemophlia Early Arthropathy Detection with UltraSound assessments in adults with haemophilia. Haemophilia. 2017; 23(2):e141–e143. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, Supplementary_PK_Revised_1 for Joint Bleeding Tendencies in Adult Patients With Hemophilia: It’s Not All Pharmacokinetics by Jenny Y. Zhou, Richard F. W. Barnes, Gary Foster, Alfonso Iorio, Thomas J. Cramer and Annette von Drygalski in Clinical and Applied Thrombosis/Hemostasis