Abstract
Inflammation has been implicated in the pathogenesis of endothelial dysfunction, atherosclerosis, and microvascular coronary dysfunction. In this context, it is thought that fibrinogen, high-sensitive C-reactive protein (hsCRP), and albumin may be associated with the pathogenesis of coronary slow flow (CSF). We aimed to evaluate the ratios of fibrinogen-to-albumin and hsCRP-to-albumin in patients with CSF compared to patients with angiographically normal coronary arteries and stable coronary artery disease (CAD). In all, 65 patients with CSF, 65 patients with newly diagnosed stable CAD, and 65 control participants with angiographically normal coronary arteries were included. The coronary flow rates of all patients were determined by the Thrombolysis in Myocardial Infarction frame count method. Fibrinogen, hsCRP, and albumin levels were analyzed in all patients, and the fibrinogen-to-albumin and hsCRP-to-albumin ratios were calculated. The baseline characteristics of the 3 groups were similar. The plasma albumin level was significantly lower, whereas the fibrinogen and the hsCRP levels were significantly higher, in the CSF and CAD groups compared to the controls. The fibrinogen-to-albumin and hsCRP-to-albumin ratios were significantly higher in both the CSF and the CAD groups compared to the control group. The hsCRP-to-albumin ratio was positively correlated with the mean Thrombolysis in Myocardial Infarction frame count in the whole study population. According to the receiver–operating characteristic analysis, the efficacies of the fibrinogen-to-albumin and hsCRP-to-albumin ratios in predicting CSF were significant. The fibrinogen-to-albumin and hsCRP-to-albumin ratios, which were increased by a reciprocal change, suggest that inflammation may play a role in the pathogenesis of CSF.
Keywords: coronary slow flow, fibrinogen-to-albumin ratio, hsCRP-to-albumin ratio
Introduction
Coronary slow flow (CSF) is a coronary disorder characterized angiographically by delayed contrast opacification of the distal coronary arteries in the absence of obstructive coronary artery disease (CAD).1,2 The prevalence of CSF is 1% to 3% among patients undergoing coronary angiography. Histopathological studies have shown the presence of coronary microvascular dysfunction; however, the underlying cause remains unclear.2,3 Endothelial dysfunction, thrombocyte dysfunction, oxidative stress, vasomotor dysfunction, systemic/local inflammation, and/or combination all of them may play direct and/or indirect role in pathogenesis of CSF.4,5 Since vascular inflammatory changes may not always be evaluated using cardiac imaging methods, the importance of measuring inflammation-related biomarkers in peripheral blood is increasing.
Albumin, the major blood protein found in the extracellular liquid compartment, plays multiple significant physiological functions.6 It has been proposed that the plasma albumin concentration is related to inflammatory and hemostatic processes.7,8 Albumin is a significant inhibitor of platelet activation and aggregation and a significant mediator of platelet-induced coronary artery vasoconstriction.8 Low serum albumin levels have been associated with increased cardiovascular mortality and morbidity in several studies conducted at different times.9,10 Moreover, a recent study found low albumin levels in patients with CSF.11
Fibrinogen is a plasma protein produced in the liver which serves as an indicator of thrombotic status and plays a role in inflammatory processes at various levels.12 The baseline plasma fibrinogen level may predict cardiovascular events in the general population.13 Additionally, a recent study showed that an elevated fibrinogen level is related to the presence and severity of CAD.14
High-sensitive C-reactive protein (hsCRP) is the most well-investigated inflammatory biomarker in cardiovascular diseases. Numerous prospective studies have revealed a relationship between increased hsCRP levels and the incidence of cardiovascular disease in individuals at risk of atherosclerosis. In the event of chronic low-grade inflammation, CRP damages the glycocalyx of the vascular endothelium, causing its dysfunction and increasing its vulnerability to proatherogenic effects. Furthermore, the processes of endothelium-dependent vasodilation and endothelial stem cell adhesion and migration are disrupted, and endothelial apoptosis is induced, thereby resulting in endothelial dysfunction.15
Although many studies have investigated and focused on the relationships among hypoalbuminemia, high fibrinogen levels, high hsCRP levels, and CAD, there are insufficient data on CSF, a relatively common cause of nonobstructive CAD. Moreover, to our knowledge, there are no data on the reciprocal usage of these inflammation-related biomarkers in patients with CSF. From this point of view, we aimed to investigate whether the fibrinogen-to-albumin ratio (FAR) and hsCRP-to-albumin ratio (CAR) are associated with CSF compared to patients with angiographically normal coronary arteries and those with stable CAD.
Materials and Methods
Study Population
The present study was an observational, case–control, and comparative study. The relationship between the FAR and the presence of CSF was investigated. Approximately 2500 participants who underwent elective diagnostic coronary angiography at our institution were scanned to identify patients with apparent CSF. The indications for coronary angiography were either the presence of typical angina pectoris or positive or equivocal results from noninvasive screening tests for myocardial ischemia. We selected 65 consecutive patients (24 males and 39 females; mean age, 57.6 + 10.2 years) diagnosed with CSF without any obstructive atherosclerotic lesions (CSF group), 65 consecutive patients (27 males and 34 females; mean age, 55.1 + 10.7 years) with completely normal coronary arteries (control group), and 65 consecutive patients (44 males and 21 females; mean age, 59.6 + 10.5 years) who were catheterized during the same study period and who had ≥50% stenotic lesions on coronary angiogram (CAD group).
The following patients were excluded: those who developed secondary CSF following percutaneous coronary angioplasty, those who experienced myocardial infarction or had coronary artery bypass grafting surgery, those with significant organic valvular heart disease, congestive heart failure, congenital heart disease, atrial fibrillation, hypo/hyperthyroidism, any known collagen vascular disease, any known hematologic disease including anemia (hemoglobin level <12 g/dL in women or <13 g/dL in men according to World Health Organization criteria), any autoimmune or neoplastic disease, chronic kidney (creatinine >1.5 mg/dL) or hepatic failure (aspartate transaminase or alanine transaminase levels more than 3 times the normal value) or actively infectious diseases (history of any acute infection in the last 10 days), and those receiving active treatment with any antithrombocyte drugs except for acetylsalicylic acid, anticoagulants, or steroids. Patients with plasma d-dimer levels above the normal range were also excluded.
All cases with CSF included in the study were patients with primary CSF. Patients with secondary CSF were excluded from the study. In this context, cases with slow flow due to Percutaneous Coronary Intervention (PCI) (including no-reflow cases), cases with CSF due to coronary thromboembolism (including cases with microbubble during coronary angiography), cases with CSF due to coronary ectasia, patients with visible coronary atherosclerotic plaques, and patients with a history of substance use that may cause coronary spasm (eg, cocaine) were excluded from the study.16 In addition, cases with secondary CSF associated with bradycardia and / or hypotension were excluded from the study. All patients who were hypotensive or bradycardic during coronary angiography were excluded from the study.
The study protocol was approved by the local ethics committee, and written informed consent was obtained from all patients. The study was conducted in accordance with the Declaration of Helsinki, Good Clinical Practice and International Conference on Harmonization guidelines.
Cardiac Catheterization and Documentation of Participants
Coronary angiograms were performed by a femoral approach using the Judkins technique without the use of nitroglycerin, adenosine, or a calcium-channel blocker. All angiography procedures were performed by 2 experienced interventional cardiologists blinded to the clinical characteristics of the patients. Iohexol was used as a nonionic contrast agent during coronary angiography in all patients and controls. During coronary angiography, approximately 6 to 10 mL of contrast agent was manually injected at each position. Coronary arteries were visualized in standard planes. The coronary flow rates of all participants were documented using the Thrombolysis in Myocardial Infarction (TIMI) frame count (TFC) method described by Gibson et al.15 The TFCs of the left anterior descending (LAD) and circumflex (Cx) arteries were assessed in either the right anterior oblique projection with caudal angulations or the left anterior oblique projection with cranial angulations, and the right coronary artery (RCA) was usually assessed in the straight left anterior oblique projection. The initial frame was defined as the frame in which concentrated contrast medium occupied the full width of the proximal coronary artery lumen, touching both borders of the lumen, and forward motion down the artery. The final frame was designated when the leading edge of the contrast column initially arrived at the distal end. The last frames used for the LAD artery, Cx artery, and RCA were those in which the contrast medium first entered the mustache segment, distal bifurcation segment, and first branch of the posterolateral artery, respectively. The final count was then subtracted from the initial count, and the exact TFC was calculated for the given artery. The TFC of the LAD artery was corrected by dividing the final count by 1.7. Due to the different durations required for normal visualization of coronary arteries, the corrected cutoff values were 36.2 ± 2.6 frames for the LAD artery, 22.2 ± 4.1 frames for the Cx artery, and 20.4 ± 3.0 frames for the RCA, as reported previously.17 Patients with a TFC greater than 2 standard deviations (SDs) from the normal published range for any 1 of the 3 vessels were diagnosed with CSF. The mean TFC for each patient and control was calculated by adding the TFCs for the LAD artery, Cx artery, and RCA and then dividing the obtained value by 3. The evaluation of all coronary angiograms and TFC counting were performed by 2 other interventional cardiologists blinded to the clinical status and laboratory measurements. The intra- and interobserver variabilities for TFC were 0.975 and 0.966, respectively.
Coronary angiograms were evaluated for smooth appearance, luminal wall irregularities, epicardial local or diffuse caliber reduction, and stenosis. Coronary arteries were classified as normal in the absence of any lumen irregularity on visual evaluation.
Patients with coronary lesions with a stenosis diameter ≥ 50% in ≥1.5-mm vessels were diagnosed with CAD. The complexity of CAD was evaluated using the SYNergy between percutaneous coronary intervention with TAXus and cardiac surgery (SYNTAX) score. Coronary lesions with a stenosis diameter ≥50% in ≥1.5-mm vessels were scored separately and added together to provide the cumulative SYNTAX score, which was prospectively calculated using the SYNTAX score algorithm on the baseline diagnostic angiogram.18
Two experienced interventional cardiologists analyzed the SYNTAX score, and the final evaluation was determined by consensus in cases of disagreement. The final score was calculated from the individual lesion scores by analysts who were blinded to the procedural data and clinical outcomes.
Blood Sampling and Biochemical Measurements
Blood samples for biochemical analyses were taken from the antecubital vein of all individuals following an overnight fast just after coronary angiography. All biochemical tests and complete blood counts were performed using an automatic hematology analyzer (Beckman Coulter AU5800, Brea, California) within 1 hour after venous puncture. The plasma albumin level was measured by the bromocresol method, and the plasma hsCRP level by the nephelometric method using commercially available kits. Plasma fibrinogen levels were measured by the Clauss method with Stago Compact (Diagnostica Stago, Asnieres sur Seine, France) and a coagulation autoanalyzer (Diagnostica Stago Inc, Paris, France). Fasting blood glucose, total cholesterol, high-density lipoprotein, triglyceride, and low-density lipoprotein levels were measured by the hexokinase method, enzymatic method, accelerator selective detergent method, glycerol phosphate oxidase method, and Friedewald formula, respectively, using the Beckman Coulter AU5800 autoanalyzer. Urea and creatinine levels were measured by the spectrophotometric method. The estimated glomerular filtration rate was calculated according to the modification of diet in renal disease formula.19
Statistical Analyses
Variables were investigated for a normal distribution using visual (histograms and probability plots) and analytical (Shapiro-Wilk test) methods. Descriptive analyses are presented as means (SD) for normally distributed variables. Means, SDs, lowest median value, highest frequency value, and ratios were used. The Kolmogorov-Smirnov test was used to assess data distribution. Analysis of variance followed by Tukey post hoc method, Kruskal-Wallis test, and Mann-Whitney U test were used to analyze quantitative data. The χ2 test was used to analyze qualitative data. A P value <.05 was considered significant. The ability to predict the presence of CSF based on the FAR was analyzed using receiver–operating characteristic analysis. Sensitivity and specificity values were determined if a significant cutoff value was observed. A 5% type 1 error level was significantly predictive of the test variables when evaluating the area under the curve. Statistical analyses were performed using SPSS software version 20 (SPSS Inc., Chicago, Illinois).
Results
Baseline Characteristics
A total of 195 patients were included in the study. The demographic, clinical, and angiographic data of the study population are summarized in Tables 1 and 2. No differences in age or body mass index were observed among the 3 groups (all P > .05). However, there were significantly more males in the CAD group than in the control and CSF groups (means: 44, 27, and 24, respectively; P = .002). There was no significant difference between the CSF and control groups according to sex (P > .05).
Table 1.
Demographic and Clinical Characteristics of the Study Population.
| Normal Coronary Artery, n = 65 | Coronary Slow Flow, n = 65 | Coronary Artery Disease, n = 65 | P Value | ||
|---|---|---|---|---|---|
| Age, years, mean (SD) | 55.1 (10.7) | 57.6 (10.2) | 59.6 ± 10.5 | .056 (NS) | |
| Gender | Female | 34 (55.7%) | 39 (61.9%) | 21 (32.3%) | .002 |
| Male | 27 (44.3%) | 24 (38.1%) | 44 (67.7%) | ||
| BMI, kg/m2, mean (SD) | 24.4 (3.7) | 28.5 (3.8) | 27.5 (3.8) | .091 (NS) | |
| Hypertension, n | 30 (49.2%) | 31 (49.2%) | 37 (56.9%) | .600 (NS) | |
| Diabetes Mellitus | 11 (18.0%) | 16 (25.4%) | 21 (32.3%) | .184 (NS) | |
| Dyslipidemia | 9 (14.8%) | 10 (15.9%) | 19 (29.2%) | .076 (NS) | |
| Smoker | 19 (31.1%) | 32 (50.8%) | 28 (58.2%) | .007 | |
| Family History of CAD | 12 (19.7%) | 7 (11.1%) | 15 (23.1%) | .194 (NS) | |
Abbreviations: BMI, body mass index; CAD, coronary artery disease
Bold-italic values in tables signifies p value of <0.05.
Table 2.
Angiographic Characteristics of the Study Population.
| Normal Coronary Artery, n = 65 | Coronary Slow Flow, n = 65 | Coronary Artery Disease, n = 65 | P Value | |
|---|---|---|---|---|
| TFC of Coronary Arteries | ||||
| LAD-TFC | 20.0 ± 7.5 | 45.6 ± 14.9 | 20.1 ± 6.9 | .000 |
| Cx-TFC | 16.2 ± 5.2 | 31.0 ± 10.1 | 16.5 ± 5.5 | .000 |
| RCA-TFC | 15.8 ± 5.6 | 37.8 ± 9.3 | 15.0 ± 4.3 | .000 |
| Mean-TFC | 17.5 ± 5.4 | 38.1 ± 9.3 | 17.3 ± 5.1 | .000 |
| CSF distribution | ||||
| LAD | – | 44 (32.3%) | – | – |
| Cx | – | 36 (26.4%) | – | – |
| RCA | – | 56 (41.1%) | – | – |
| Number of Coronary Artery Affected by CSF |
||||
| 1 coronary | – | 20 (30.7%) | – | – |
| 2 coronaries | – | 17 (26.1%) | – | – |
| 3 coronaries | – | 28 (43.07% | – | – |
| SYNTAX Score | – | – | 14.8 ± 9.9 | – |
Abbreviations: CSF, coronary slow flow; Cx, circumflex artery; LAD, anterior descending artery; RCA, right coronary artery; TFC, TIMI frame counts.
Bold-italic values in tables signifies p value of <0.05.
Clinical data are also summarized in Table 1. No differences in cardiovascular risk factors, such as hyperlipidemia, hypertension, diabetes mellitus, and family history, were detected among the groups (all P > .05). However, there were significantly more smokers in the CSF and CAD groups than in the control group (means: 32, 28 and 19, respectively; P = .007), but no significant difference between the CSF and CAD groups (P > .05).
The angiographic data are summarized in Table 2. The TFCs for all coronary arteries of the patients were significantly higher in the CSF group than in the CAD and control groups (all P < .001 for each artery; Table 2). The mean TFC was also significantly higher in the CSF group than in the control and CAD groups (all P < .001). The CSF involved the LAD artery in 44 (32.3%), left Cx artery in 36 (26.4%), and RCA in 56 (41.1%) patients. Of the patients with CSF, 20 (30.7%) were affected by a single coronary artery, 17 (26.1%) were affected by 2 coronary arteries, and 28 (43.07%) were affected by 3 coronary arteries. In the CAD group, the average SYNTAX score was 14.8 ± 9.9.
The biochemical and hematologic measurements are summarized in Table 3. There was no significant difference in any biochemical or hematologic measurement among the groups (all P > .05).
Table 3.
Laboratory Findings of the Study Population, Mean (Standard Deviation).
| Normal Coronary Artery, n = 65 | Coronary Slow Flow, n = 65 | Coronary Artery Disease, n = 65 | P Value | |
|---|---|---|---|---|
| WBC, 103/ μL | 6.3 (1.5) | 6.7 (1.6) | 6.3 (1.3) | .309 (NS) |
| Hemaglobulin, 103/ μL | 13.1 (1.2) | 13.2 (1.7) | 13.6 (1.5) | .792 (NS) |
| Hematocrit | 40.0 (4.0) | 40.3 (4.7) | 40.8 (4.8) | .055 (NS) |
| Platelet, 103/ μL | 237.2 (64.6) | 225.4 (66.7) | 264.3 (57.0) | .064 (NS) |
| Urea, mg/dL | 23.4 (10.1) | 22.4 (11.9) | 24.7 (12.3) | .745 (NS) |
| Creatinine, mg/dL | 0.81 (0.35) | 0.78 (1.2) | 0.91 (1.1) | .167 (NS) |
| eGFR, mL/dk/1.73 m2 | 100.1 (12.8) | 96.9 (12.4) | 94.7 (15.6) | .098 (NS) |
| FBG, mg/dL | 104.5 (37.5) | 103.3 (29.4) | 109.4 (41.1) | .106 (NS) |
| HgbA1c, % | 6.3 (1.0) | 6.2 (0.9) | 6.3 (1.6) | .399 (NS) |
| AST, U/L | 24.2 (1.7) | 24.9 (2.2) | 23.1 (1.7) | .758 (NS) |
| ALT, U/L | 21.2 (7.8) | 21.8 (8.3) | 22.4 (9.4) | .289 (NS) |
| GGT, U/L | 23.3 (10.6) | 27.3 (14.5) | 29.1 (11.5) | .160 (NS) |
| Total-C, mg/dL | 186.3 (43.13) | 176.3 (37.1) | 193 (43.2) | .052 (NS) |
| LDL-C, mg/dL | 113.9 (35.9) | 104.3 (32.2) | 114.5 (37.6) | .079 (NS) |
| HDL-C, mg/dL | 45.5 (12.1) | 42.3 (9.2) | 44.7 (9.7) | .312 (NS) |
| Triglyceride, mg/dL | 139.1 (64.5) | 147.5 (99.3) | 158 (80.9) | .070 (NS) |
Abbreviations: ALT, alanine transaminase; AST, aspartate transaminase; eGFR, estimation glomerular filtration rate; FBG, fasting blood glucose; HbA1c, hemoglobin A1c; HDL-C, high-density lipoprotein cholesterol; hs-CRP, high-sensitive C-reactive protein; LDL-C, low-density lipoprotein cholesterol; NS, not significant; Total-C, total cholesterol; WBC, white blood cell.
Associations of Inflammation-Related Biomarker Levels With CSF and CAD
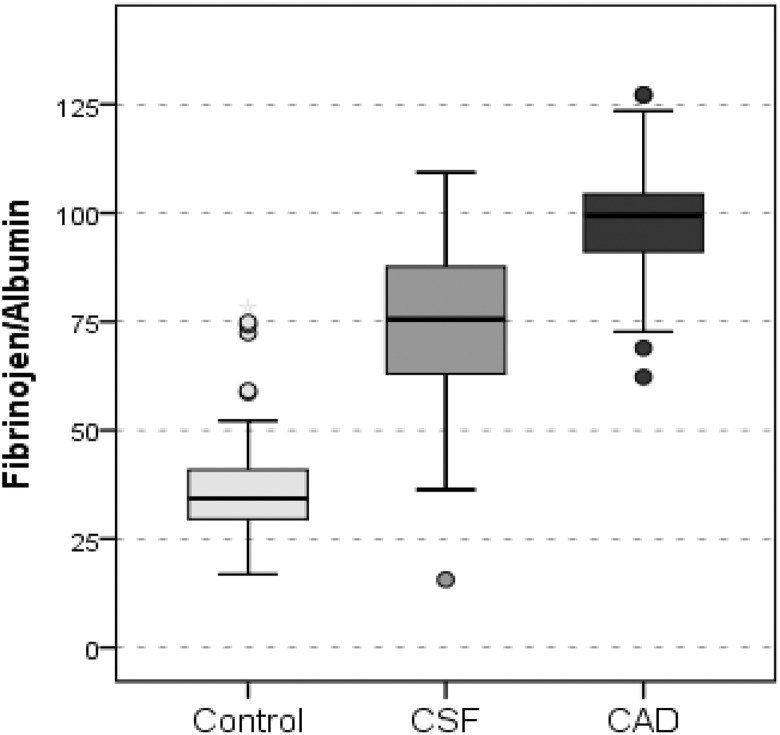
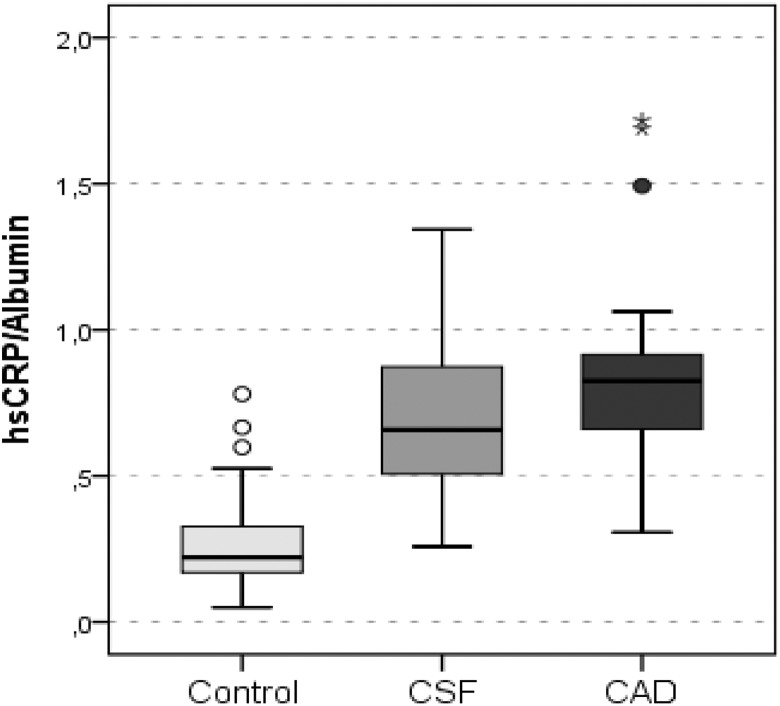
Inflammation-related biomarker levels and their reciprocal exchanges are summarized in Table 4. The hsCRP and fibrinogen levels were significantly higher in the patients with CSF and CAD than in the controls (all P < .001) and also significantly higher in patients with CAD than patients with CSF (all P < .001). As shown in Figures 1 and 2, the FAR and CAR were significantly higher in patients with CSF and CAD than controls. In addition, both the FAR and the CAR were significantly higher in patients with CAD patients than patients with CSF. The albumin level was significantly lower in the CSF and CAD groups than in the control group. However, there was no significant difference in the albumin level between the CSF and CAD groups.
Table 4.
Plasma Inflammation-Related Biomarker Levels of the Study Population, Mean (Standard Deviation).
| Normal Coronary Artery, n = 65 | Coronary Slow Flow, n = 65 | Coronary Artery Disease, n = 65 | P Value | |
|---|---|---|---|---|
| Fibrinogen, mg/dL | 162.9 (57.2) | 283.0 (69.3) | 375.9 (49.8) | .000 |
| Albumin, g/dL | 4.4 (0.3) | 3.9 (0.2) | 3.9 (0.2) | .000 |
| hs-CRP, mg/dL | 1.2 (0.6) | 2.7 (0.9) | 3.2 (0.9) | .000 |
| Fibrinogen–Albumin Ratio | 37.0 (13.4) | 73.6 (18.7) | 97.3 (13.5) | 0.000 |
| hs-CRP–Albumin Ratio | 0.27 (0.14) | 0.70 (0.26) | 0.80 (0.27) | .000 |
Abbreviation: hsCRP, high sensitive C-reactive protein.
Bold-italic values in tables signifies p value of <0.05.
Figure 1.
Graph showing fibrinogen–albumin ratio values of the groups. Control indicates angiographically normal patients; CSF, patients with coronary slow flow; CAD, patients with obstructive coronary artery disease.
Figure 2.
Graph showing hsCRP–albumin ratio values of the groups. hsCRP indicates high-sensitive C-reactive protein; Control, angiographically normal patients; CSF, patients with coronary slow flow; CAD, patients with obstructive coronary artery disease.
The results of correlation analyses are summarized in Table 5. There was a significant positive correlation between mean TFC and hsCRP level (r = .144, P = .048; Table 5) and a significant negative correlation between mean TFC and albumin level (r = −.260, P < .001; Table 5). Furthermore, there were significant positive correlations between the CAR and mean TFC (r = .173, P = .017; Table 5), between the number of epicardial coronary arteries affected by slow flow and both the fibrinogen level and the FAR (r = .305, P = .015 and r = .047, P = .047, respectively; Table 5), and between the SYNTAX score and both the fibrinogen level and FAR (mean: r = .307, P = .013 and r = .291, P = .019, respectively).
Table 5.
Correlation Analysis.
| hsCRP, mg/dL | Fibrinogen, mg/dL | Albumin, g/dL | Fibrinogen-to- Albumin Ratio | hsCRP-to- Albumin Ratio | ||
|---|---|---|---|---|---|---|
| Mean-TFC | r | .144 | .005 | −.260 | .031 | .173 |
| P | .048 | .944 | .000 | .671 | .017 | |
| The number of epicardialcoronary arteries affected by slow flow | r | .286 | .305 | .147 | .251 | .243 |
| P | .023 | .015 | .249 | .047 | .055 | |
| SYNTAX score | r | .013 | .307 | .121 | .291 | .046 |
| P | .917 | .013 | .338 | .019 | .719 |
Abbreviation: TFC, Thrombolysis in Myocardial Infarction frame count
Bold-italic values in tables signifies p value of <0.05.
Predictors of CSF
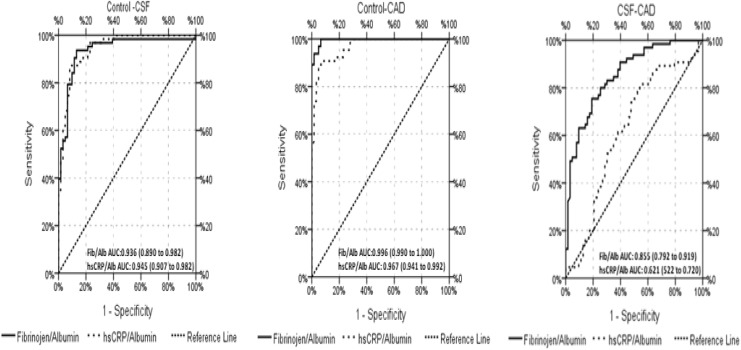
Receiver–operating characteristic analysis was performed in the CSF, CAD, and control groups to determine the value of the reciprocal exchange of inflammation-related biomarkers in predicting CSF. The results are presented in Figure 3 and Table 6.
Figure 3.
Receiver–operating characteristic curve analysis of fibrinogen–albumin ratio and hsCRP–albumin ratio for predicting CSF and CAD. hsCRP indicates high-sensitive C-reactive protein; CAD, coronary artery disease; CSF, coronary slow flow.
Table 6.
ROC Curve Analysis Results.
| AUC | AUC (%95 CI) | P Value | |
|---|---|---|---|
| Control—CSF | |||
| Fibrinogen/Albumin ratio | 0.936 | 0.890-0.982 | .000 |
| hsCRP/Albumin ratio | 0.945 | 0.907-0.982 | .000 |
| Control—CAD | |||
| Fibrinogen/Albumin ratio | 0.996 | 0.990-1.000 | .000 |
| hsCRP/Albumin ratio | 0.967 | 0.941-0.992 | .000 |
| CSF—CAD | |||
| Fibrinogen/Albumin ratio | 0.855 | 0.792-0.919 | .000 |
| hsCRP/Albumin ratio | 0.621 | 0.522-0.720 | .018 |
Abbreviations: AUC, area of under curve; CI, confidence interval; ROC, receiver–operating characteristic.
Bold-italic values in tables signifies p value of <0.05.
There FAR and CAR showed significant efficacy in predicting CSF between the control and the patients with CSF (mean area under the curve: 0.936 [0.890-0.982] and 0.945 [0907-0.982], respectively) and between the control and the patients with CAD (mean area under the curve: 0.996 [0.990-1.000] and 0.967 [0.941-0.992], respectively).
On the other hand, according to univariate logistic regression analyses, male sex, smoking, LAD-TFC, Cx-TFC, RCA-TFC, mean-TFC, hsCRP, fibrinogen, albumin, CAR, and FAR were all significantly associated with CSF. When these 11 parameters were included in multivariate logistic regression analyses, CAR and RCA-TFC were independently and significantly associated with CSF. Results from the multivariate logistic regression analysis are presented in Table 7. In the multivariate model, the CAR had significant and independent efficacy in predicting CSF between control and patients with CSF and the FAR in predicting CSF between control and patients with CAD.
Table 7.
Multiple Logistic Regression Analysis Showing Significant Independent Predictors of Coronary Slow Flow and Coronary Artery Disease.
| Variable | P Value | Odds Ratio | 95% Confidence Interval | |
|---|---|---|---|---|
| Lower | Upper | |||
| Control—CSF | ||||
| hsCRP–Albumin ratio | .036 | >100 | 4.99 | >100 |
| RCA TFC | .027 | 2.32 | 1.10 | 4.90 |
| Control—CAD | ||||
| Fibrinogen–Albumin ratio | .000 | 1.22 | 1.09 | 1.36 |
| CSF–CAD | ||||
| RCA TFC | .001 | 0.496 | 0.330 | 0.745 |
Abbreviations: CAD, obstructive coronary artery disease patients; Control, angiographically normal patients; CSF, coronary slow flow patients; RCA, right coronary artery; TFC, TIMI frame counts.
Bold-italic values in tables signifies p value of <0.05.
Discussion
To our knowledge, this study is the first to show that CSF is associated with FAR and CAR. Patients with CSF had a significantly higher FAR and CAR than patients with angiographically normal coronary arteries. In addition, the FAR and CAR were similar between patients with CSF and those with CAD. We also found that FAR and CAR were significantly related to the TIMI frame count of CSF severity.
Microvascular dysfunction has been associated with the pathophysiology of CSF, but the causes leading to this have not been fully elucidated.1,2 Potential mechanisms, such as vasoactive autacoids, diffuse atherosclerosis, platelet dysfunction, and impaired endothelial function, have been suggested in the development of CSF. These can all lead to microvascular coronary dysfunction and thus require further consideration. In addition, numerous studies have reported that inflammation is involved in the pathophysiology of CSF and manifests differently depending on the conditions.1,2,4 Moreover, CSF may be a systemic phenomenon rather than limited to coronary arteries and is caused by the interplay between local features of coronary arteries and systemic pathophysiological factors.2,4
There is evidence that inflammation plays a role in the pathophysiology of CSF as in many other cardiovascular diseases. Turhan et al20 evaluated plasma-soluble adhesion molecules, intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), and E-selectin, as potential indicators of endothelial activation or inflammation in patients with CSF with angiographically proven normal coronary arteries. The authors showed that serum ICAM-1, VCAM-1, and E-selectin levels were significantly higher in patients with CSF than in controls with normal coronary flow, suggesting the presence of more severe and extensive systemic chronic inflammation in the coronary circulation of these patients. Additionally, Doğan et al21 showed the distribution of leukocyte count changes in favor of neutrophils in relation to systemic inflammation in patients with CSF. The present study supports the results of these previous studies and demonstrated a relationship between CSF and inflammation. Moreover, we evaluated the relationship between systemic inflammatory load and CSF. For this purpose, we used inflammatory biomarkers that are easily accessible, relatively low cost, and frequently used in routine daily clinical practice. Unlike previous studies, we used these biomarkers by combining them instead of using them alone. Our aim in doing so was to use the reciprocal relationship between these biomarkers to examine the relationship between systemic inflammation and CSF and to demonstrate this relationship more significantly and more powerfully. We also assessed whether we could achieve better predictive values using a combination of these biomarkers.
Albumin is not only the basic protein regulating plasma oncotic pressure but also a transporter of various substances and involved in both acute and chronic inflammatory processes. In cases of malabsorption or malnutrition, increased capillary loss (especially renal), decreased hepatic synthesis, inflammatory processes (acute or chronic), and increased plasma volume can lead to hypoalbuminemia.22 Normal physiological levels of serum albumin selectively inhibit TNFα-induced VCAM-1 expression, nuclear factor-κB activation, and monocyte cell adhesion in aortic endothelial cells, suggesting albumin to be an anti-inflammatory and antiatherogenic biomarker.23 Decreased serum albumin levels are associated with increased systemic inflammatory load. A decreased albumin level has been ascribed to the presence of proinflammatory cytokines. Inflammatory processes have been associated with increased albumin catabolism and decreased albumin synthesis.22 In a recent study, Çetin et al found lower serum albumin levels in patients with CSF than in those with normal coronary arteries.11 In support of these results, we found that serum albumin levels were lower in patients with CSF than in those with normal coronary arteries. Furthermore, serum albumin levels were statistically similar between patients with CSF and patients with CAD. We believe that lower serum albumin levels in patients with CSF may be associated with inflammatory load. We know from previous studies that albumin is an important inhibitor of platelet aggregation that enhances the production of PGD2, an effective antiaggregant, from cyclic endoperoxides.8,24 Furthermore, platelet dysfunction is one possible cause of CSF.25 An alternative cause of CSF could be that a systemic proinflammatory status activates platelets and enhances aggregation, which impairs the coronary microcirculation, thereby causing CSF. Another explanation for this complex relationship between serum albumin levels and CSF may be related to endothelial dysfunction. Hypoalbuminemia may increase blood viscosity and disturb endothelial functions because of an increased level of free lysophosphatidylcholine.26 In the current study, decreased serum albumin levels due to systemic inflammatory load may have caused CSF with endothelial dysfunction or increased platelet activity and aggregation.
Fibrinogen represents an inflammatory marker implicated in the pathophysiology, presence, severity, and prognosis of CAD. Its presence contributes to the development and progression of CAD and to the formation of acute coronary syndrome via its interaction with other inflammatory cells, the endothelium, and prothrombotic molecules.14,27 In the current study, the fibrinogen level was higher in patients with CSF than in those with angiographically normal coronary arteries. To our knowledge, this is the first study to evaluate the association between fibrinogen and CSF. Several potential pathophysiological mechanisms may explain the association between fibrinogen and CSF. Fibrinogen and its metabolites may lead to endothelial dysfunction. Fibrinogen adhering to the endothelial surface leads to the release of vasoactive molecules and disturbs endothelial permeability, leading to fibrinogen deposition in the subendothelial area, which provides a predisposing endothelial surface for the extracellular accumulation of atherosclerotic aggregates.12 Additionally, fibrinogen may stimulate endothelial cell deterioration and disorganization and increase the release of endothelial cell-derived growth factors.28 All of these processes suggest that fibrinogen plays a role in the stimulation of vascular inflammation and is responsible for endothelial dysfunction. Increased levels of fibrinogen, similar to decreased albumin levels, may be associated with endothelial dysfunction. Independent of an acute condition, as a consequence of a reciprocal interaction, the high fibrinogen-to-albumin ratio appears to indicate a chronic inflammatory load.
The CRP is a uniquely sensitive biomarker of systemic inflammation. The hsCRP analysis has enabled the detection of even low-grade chronic inflammation, which was previously regarded as clinically not significant. Previous studies have clearly indicated the predictive and prognostic roles of CRP in cardiovascular diseases.29,30 Li et al31 and Barutcu et al32 found that a higher serum level of hsCRP is associated with CSF. An association between hsCRP and endothelial dysfunction was also demonstrated.33 Hein et al33 showed that CRP reduces endothelial NO synthase activity and inhibits endothelium-dependent NO-mediated vasodilatation. Additionally, Devaraj et al34 suggested that CRP impairs the endothelial cell glycocalyx (ie, the endothelial surface), which provides crucial protection of the endothelium, resulting in endothelial dysfunction. All of these findings suggest that the increased hsCRP level in patients with CSF may be another biomarker of endothelial dysfunction and inflammation and is likely involved in the pathophysiological process resulting in CSF. In the current study, we evaluated the level of hsCRP, an essential inflammatory biomarker associated with inflammatory load, in patients with CSF. Moreover, in addition to previous studies, we also found that the CAR was higher in patients with CSF than in patients with angiographically normal coronary arteries. In the current study, an increased hsCRP level and CAR suggested the presence of inflammatory load and endothelial injury in the pathogenesis of CSF. An increased hsCRP level may also be an early indicator of slowed coronary blood flow.
In the present study, we compared the inflammatory load of patients with CSF to not only angiographically normal patients but also to patients with obstructive CAD. Plasma albumin levels were similar between patients with CSF and CAD but were significantly lower in these groups than in the control group. Moreover, both plasma hsCRP and plasma fibrinogen levels were significantly higher in patients with CAD than in patients with CSF. As expected, the FAR and CAR were also significantly higher in cases with CAD than in cases with CSF. These results suggest that systemic inflammatory load is associated with atherosclerotic obstructive CAD more strongly than with CSF. In the current study, the plasma fibrinogen level was a strong independent predictor of CAD, and the SYNTAX score was strongly and positively correlated with the plasma fibrinogen level and FAR, suggesting that the strong association between fibrinogen and CAD may be related to another physiopathologic mechanism other than systemic inflammatory load. Tabakçı et al recently showed a correlation between CAD and the plasma fibrinogen level,14 supporting the results of the current study.
Demographic data on CSF are generally limited in the literature. Some clinical studies have reported male gender as a predictor of CSF, while others have found no relationship between gender and CSF.35,36 However; CSF usually tends to be more common in young men.1 Additionally, previous clinical studies have clearly shown that within the age spectrum of 40 to 60 years, the incidence of atherosclerotic cardiovascular disease is at least twice as high in men compared to women (37). We observed male gender dominance in the CAD group that was compatible with current literature.
Smoking has long been established as a risk factor for atherosclerotic vascular disease.37 Smoking has a critical role in both the aggravation and the development of atherosclerotic vascular disease. Smoking is hypothesized to enforce its adverse effects by facilitating endothelial dysfunction through oxidative stress and proinflammatory effect, modifying the lipids and inducing a highly prothrombotic status.38 Coronary slow flow, which is thought to be associated with endothelial dysfunction, is more common in smokers.1 In the present study, the higher prevalence of smoking in both the CAD group and the CSF group may be related to these effects of tobacco.
Study Limitations
Our study had some limitations, including the small number of patients and the exclusively caucasian cohort. Second, the observational and cross-sectional design of our study made it difficult to interpret the causal relationships of inflammatory biomarker levels with CSF and CAD. Third, none of the patients underwent intravascular ultrasonography to detect subclinical atherosclerosis in coronary arteries because an intravascular ultrasound (IVUS) system is not available in our clinic. However, patients with CSF do not routinely undergo IVUS in clinical practice, and CSF is usually diagnosed only by coronary angiography. Although no patient had a suspicious image or coronary wall irregularity on angiographic evaluation, an atherosclerotic lesion or plaque that can be detected only by IVUS examination could have gone unobserved and potentially affected the plasma inflammatory biomarker levels.
Mangieri et al3 suggested that CSF normalizes after intracoronary dipyridamole infusion. Another limitation of our study is that we did not test whether CSF normalized after infusion of an intracoronary vasoactive agent, such as dipiridamol. However, if only the patients in who CSF persisted after dipyridamole infusion had been included, this would have been more supportive of a role for inflammation over other functional abnormalities. As a final limitation, some medical agents already in use by patients included in the study may have directly or indirectly affected the inflammatory status or clinical process of CSF.39,40 However, we do not know their effects on our results.
Conclusion
In conclusion, our study showed that a higher fibrinogen level, FAR, and CAR were significantly and independently related to the presence of CSF and CAD. In addition, hsCRP and CAR were positively correlated, and the serum albumin level negatively correlated with mean TIMI frame counts in patients with CSF. These results suggest that a higher FAR and CAR may be associated with impaired endothelial function and increased inflammatory load in patients with CSF.
Footnotes
Authors Note: The English in this document has been checked by at least 2 professional editors, both native speakers of English. For a certificate, please see: http://www.textcheck.com/certificate/LDNjcG
Declaration of Conflicting Interests: The author(s) declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Beltrame JF, Limaye SB, Horowitz JD. The coronary slow flow phenomenon: a new coronary microvascular disorder. Cardiology. 2002;97(4):197–202. [DOI] [PubMed] [Google Scholar]
- 2. Beltrame J, Ganz P. The coronary slow flow phenomenon In: Kaski J, Eslick G, Bairey Merz C. (eds). Chest Pain with Normal Coronary Arteries. London: Springer; 2013:101–117. [Google Scholar]
- 3. Mangieri E, Macchiarelli G, Ciavolella M, et al. Slow coronary flow: clinical and histopathological features in patients with otherwise normal epicardial coronary arteries. Cathet Cardiovasc Diagn. 1996;37(4):375–381. [DOI] [PubMed] [Google Scholar]
- 4. Wang X, Geng LL, Nie SP. Coronary slow flow phenomenon: a local or systemic disease? Med Hypotheses. 2010;75(3):334–337. [DOI] [PubMed] [Google Scholar]
- 5. Canpolat U, Çetin EH, Cetin S, et al. Association of monocyte-to-HDL cholesterol ratio with slow coronary flow is linked to systemic inflammation. Clin Appl Thromb Hemost. 2016;22(5):476–482. [DOI] [PubMed] [Google Scholar]
- 6. Peters T., Jr Serum albumin. Adv Protein Chem. 1985;37:161–245. [DOI] [PubMed] [Google Scholar]
- 7. Danesh J, Collins R, Appleby P, Peto R. Association of fibrinogen, C-reactive protein, albumin, or leukocyte count with coronary heart disease: meta-analyses of prospective studies. JAMA. 1998;279(18):1477–1482. [DOI] [PubMed] [Google Scholar]
- 8. Gresele P, Deckmyn H, Huybrechts E, Vermylen J. Serum albumin enhances the impairment of platelet aggregation with thromboxane synthase inhibition by increasing the formation of prostaglandin D2. Biochem Pharmacol. 1984;33(13):2083–2088. [DOI] [PubMed] [Google Scholar]
- 9. Phillips A, Shaper AG, Whincup PH. Association between serum albumin and mortality from cardiovascular disease, cancer, and other causes. Lancet. 1989;2(8677):1434–1436. [DOI] [PubMed] [Google Scholar]
- 10. Oduncu V, Erkol A, Karabay CY, et al. The prognostic value of serum albumin levels on admission in patients with acute STsegment elevation myocardial infarction undergoing a primary percutaneous coronary intervention. Coron Artery Dis. 2013;24(2):88–94. [DOI] [PubMed] [Google Scholar]
- 11. Cetin M, Zencir C, Tasolar H, Baysal E, Balli M, Akturk E. The association of serum albumin with coronary slow flow. Wien Klin Wochenschr. 2014;126(15-16):468–473. [DOI] [PubMed] [Google Scholar]
- 12. Reinhart WH. Fibrinogen--marker or mediator of vascular disease? Vasc Med. 2003;8(3):211–216. [DOI] [PubMed] [Google Scholar]
- 13. Kaptoge S, Di Angelantonio E, Pennells L, et al. C-reactive protein, fibrinogen, and cardiovascular disease prediction. N Engl J Med. 2012;367(14):1310–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tabakcı MM, Gerin F, Sunbul M, et al. Relation of plasma fibrinogen level with the presence, severity, and complexity of coronary artery disease. Clin Appl Thromb Hemost. 2017;23(6):638–644. [DOI] [PubMed] [Google Scholar]
- 15. Adukauskienė D, Čiginskienė A, Adukauskaitė A, Pentiokinienė D, Šlapikas R, Čeponienė I. Clinical relevance of high sensitivity C-reactive protein in cardiology. Medicina (Kaunas). 2016;52(1):1–10. [DOI] [PubMed] [Google Scholar]
- 16. Beltrame JF. Defining the coronary slow flow phenomenon. Circ J. 2012;76(4):818–820. [DOI] [PubMed] [Google Scholar]
- 17. Gibson CM, Cannon CP, Daley WL, et al. TIMI frame count: a quantitative method of assessing coronary artery flow. Circulation. 1996;93(5):879–888. [DOI] [PubMed] [Google Scholar]
- 18. Sianos G, Morel MA, Kappetein AP, et al. The SYNTAX Score: an angiographic tool grading the complexity of coronary artery disease. EuroIntervention. 2005;1(2):219–227. Web site http://www.syntaxscore.com. [PubMed] [Google Scholar]
- 19. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461–470. [DOI] [PubMed] [Google Scholar]
- 20. Turhan H, Saydam GS, Erbay AR, et al. Increased plasma soluble adhesion molecules; ICAM-1, VCAM-1, and E-selectin levels in patients with slow coronary flow. Int J Cardiol 2006;108(2):224–230. [DOI] [PubMed] [Google Scholar]
- 21. Doğan M, Akyel A, Çimen T, et al. Relationship between neutrophil to lymphocyte ratio and slow coronary flow. Clin Appl Thromb Hemost. 2015;21(3):251–254. [DOI] [PubMed] [Google Scholar]
- 22. Don BR, Kaysen GA. Serum albumin: relationship to inflammation and nutrition. Semin Dial. 2004;17(6):432–437. [DOI] [PubMed] [Google Scholar]
- 23. Zhang WJ, Frei B. Albumin selectively inhibits TNF alpha-induced expression of vascular cell adhesion molecule-1 in human aortic endothelial cells. Cardiovasc Res. 2002;55(4):820–829. [DOI] [PubMed] [Google Scholar]
- 24. Maalej N, Albrecht R, Loscalzo J, Folts JD. The potent platelet inhibitory effects of S-nitrosated albumin coating of artificial surfaces. J Am Coll Cardiol. 1999;33(5):1408–1414. [DOI] [PubMed] [Google Scholar]
- 25. Celik T, Yuksel UC, Bugan B, et al. Increased platelet activation in patients with slow coronary flow. J Thromb Thrombolysis. 2010;29(3):310–315. [DOI] [PubMed] [Google Scholar]
- 26. Joles JA, Willekes-Koolschijn N, Koomans HA. Hypoalbuminemia causes high blood viscosity by increasing red cell lysophosphatidylcholine. Kidney Int. 1997;52(3):761–770. [DOI] [PubMed] [Google Scholar]
- 27. Papageorgiou N, Tousoulis D, Siasos G, Stefanadis C. Is fibrinogen a marker of inflammation in coronary artery disease? Hellenic J Cardiol. 2010;51(1):1–9. [PubMed] [Google Scholar]
- 28. Folsom AR, Wu KK, Rosamond WD, Sharrett AR, Chambless LE. Prospective study of hemostatic factors and incidence of coronary heart disease: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 1997;96(4):1102–1108. [DOI] [PubMed] [Google Scholar]
- 29. Haverkate F, Thombson SG, Pyke SD, Gallimore JR, Pepys MB. for the European Concerted Action on Thrombosis and Disabilities Angina Pectoris Study Group: Production of C-reactive protein and risk of coronary events in stable and unstable angina. Lancet. 1997;349(9050):462–466. [DOI] [PubMed] [Google Scholar]
- 30. Pepys MB, Thompson SG, Collins R, et al. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet. 2010;375(9709):132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li JJ, Qin XW, Li ZC, et al. Increased plasma C-reactive protein and interleukin-6 concentrations in patients with slow coronary flow. Clin Chim Acta. 2007;385(1-2):43–47. [DOI] [PubMed] [Google Scholar]
- 32. Barutçu I, Sezgin AT, Sezgin N, et al. Increased high sensitive CRP level and its significance in pathogenesis of slow coronary flow. Angiology. 2007;58(4):401–407. [DOI] [PubMed] [Google Scholar]
- 33. Hein TW, Singh U, Vasquez-Vivar J, Devaraj S, Kuo L, Jialal I. Human C-reactive protein induces endothelial dysfunction and uncoupling of eNOS in vivo. Atherosclerosis. 2009;206(1):61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Devaraj S, Yun JM, Adamson G, Galvez J, Jialal I. C-reactive protein impairs the endothelial glycocalyx resulting in endothelial dysfunction. Cardiovasc Res. 2009;84(3):479–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hawkins BM, Stavrakis S, Rousan TA, Abu-Fadel M, Schechter E. Coronary slow flow--prevalence and clinical correlations. Circ J. 2012;76(4):936–942. [DOI] [PubMed] [Google Scholar]
- 36. Fineschi M, Bravi A, Gori T. The “slow coronary flow” phenomenon: evidence of preserved coronary flow reserve despite increased resting microvascular resistances. Int J Cardiol. 2008;127(3):358–361. [DOI] [PubMed] [Google Scholar]
- 37. Tegos TJ, Kalodiki E, Sabetai MM, Nicolaides AN. The genesis of atherosclerosis and risk factors: a review. Angiology. 2001;52(2):89–98. [DOI] [PubMed] [Google Scholar]
- 38. Messner B, Bernhard D. Smoking and cardiovascular disease: mechanisms of endothelial dysfunction and early atherogenesis. Arterioscler Thromb Vasc Biol. 2014;34(3):509–515. [DOI] [PubMed] [Google Scholar]
- 39. Niu H, Wei Z, Zhang Y, He J, Jia D. Atorvastatin improves coronary flow and endothelial function in patients with coronary slow flow. Exp Ther Med. 2018;15(1):904–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Patarroyo Aponte MM, Francis GS. Effect of Angiotensin-converting enzyme inhibitors and Angiotensin receptor antagonists in atherosclerosis prevention. Curr Cardiol Rep. 2012;14(4):433–442. [DOI] [PubMed] [Google Scholar]