Abstract
Little is known about the pathogenesis of cerebral sinovenous thrombosis (CSVT) in the neonate. Although thrombophilia has been described as increasing the risk of CSVT in adults, it remains controversial in pediatric patients, and prospective case–control studies regarding neonatal CSVT are lacking. From 2008 to 2017, all 26 consecutive newborn infants ≥35 weeks of gestation diagnosed with neonatal CSVT, and their mothers, were tested for factor V Leiden (FV) G1691A, FII G20210A, and methylenetetrahydrofolate reductase C677T (MTHFR C677T) mutations. Eighty-five mother–infant pairs were recruited as controls. All infants except 1 with CSVT were suspected due to clinical symptoms, mainly seizures (22/25). Magnetic resonance imaging was performed in 24/26 infants. Heterozygous FV G1691A, FII G20210A, and homozygous MTHFR C677T mutations were present in 1/26, 3/26, and 3/20 infants with CSVT, respectively. FII (odds ratio: 10.96; 95% confidence interval [CI]: 1.09-110.35) and male sex (3.93; 95% CI: 1.43-10.76) were associated with CSVT. When FII G20210A analysis was adjusted for sex, the OR for FII G20210A was 6.70 (95% CI: 0.65-69.22). No differences were found for FV G1691A or homozygous MTHFR mutations between neonates with CSVT and their mothers, compared to controls.
Keywords: stroke, pediatric thrombosis, thrombophilia, gene polymorphisms
Introduction
Neonatal cerebral sinovenous thrombosis (CSVT) is reported as a rare condition in the neonatal period, but the exact incidence in neonates remains unknown and is likely to be underestimated, as clinical presentation is nonspecific. Diagnosis is on the increase due to improved neuroimaging techniques.1
Although several risk factors have been associated with neonatal CSVT, little is known about the exact pathophysiological mechanisms responsible for most cases, suggesting that thrombophilia, described as a predisposition to thrombosis, may play a role in the pathogenesis of CSVT. Factor V Leiden (FV G1691A) and prothrombin G20210A (FII G20210A) mutations have been associated with CSVT, though evidence in the pediatric patients remains controversial.1–5
Thrombophilia in the mother could increase the hypercoagulable or prethrombotic state of pregnancy, generating a prethrombotic vasculopathy at the placental level, giving rise to thrombi and fetal–neonatal emboli.6 So far, there have been no prospective case–control studies to consider FV G1691A, FII G20210A, and methylenetetrahydrofolate reductase C677T (MTHFR C677T) mutations specifically in a cohort of infant–mother pairs with CSVT.
The aim of this multicenter prospective case–control study of mother–infant pairs was to determine the impact of FV G1691A, FII G20210A, and homozygous MTHFR C677T mutations on neonatal CSVT.
Methods
From January 2008 to December 2017, all consecutive infants ≥35 weeks of gestation <28 days of life with CSVT were prospectively recruited in 4 tertiary university neonatal units. Two of the hospitals admitted infants with congenital heart disease and 1 of them had extracorporeal membrane oxygenation therapy.
The suspicion of CSVT was based upon (1) neurological symptomatology (seizures, encephalopathy, or acute neurological deficit); (2) symptomatology not explained by another cause (eg, recurrent apnea); and/or (3) alterations in ultrasound and/or venous Doppler. Patients were included in the study if CSVT was confirmed in the magnetic resonance imaging (MRI) study or in the necropsy when an MRI could not be performed. Diagnosis was reached by consensus among the neuroradiologist and 2 of the authors (J.A. and A.G.A.) who reviewed the images; doubtful cases were excluded.
All MRI studies were performed as soon as the patient was stable enough to be transferred to the MRI unit. A 1.5 Tesla unit was used and sequences including sagittal and axial T1-weighted, coronal and axial T2-weighted, and diffusion-weighted axial images were performed in all cases, and time-of-flight sequences in most of the cases.
Cases and controls were tested for FV G1691A, FII G20210A, and MTHFR C677T genotypes. Regarding MTHFR C677T genotype, only homozygosis was considered abnormal. Other associated comorbidities were collected from the clinical history. Eighty-five healthy neonates and their mothers published elsewhere served as controls.7 Controls were randomly recruited during the study period in the well-baby nurseries of the participating hospitals. Thrombophilia investigations were performed in healthy neonates and their parents between 48 and 72 hours of life, together with standard metabolic screening tests.
The study is part of an extensive perinatal brain infarction project (PI15/00846 and PI08/1366) and was approved by the research ethics committees of the participating hospitals. Informed written consent was obtained from the parents.
Dichotomous variables were compared with χ2 test or Fisher exact test depending on the number of samples analyzed in each group and expressed as odds ratios (ORs) with 95% confidence intervals (95% CIs). A P value of <.05 was considered statistically significant. An initial sample calculation based on clinically significant effect size of 20% incidence of thrombophilia compared to 2% in controls, considering a case-to-control ratio of 1:3, power of 80%, and type 1 error of 0.05 suggested a sample of 26 infants in the case group and 76 infants as controls. Statistical analysis was performed using the statistics software SPSS version 18.0 (SPSS Inc, Chicago, Illinois).
Results
The study cohort was 26 patients with CSVT, after excluding 5 patients without thrombophilia workup and 2 patients with high suspicion from Doppler ultrasound who did not undergo MRI. Maternal and infant characteristics are summarized in Table 1. There were no differences in gestational age, weight, or ethnic origin between the 2 cohorts, but there were more males among the cases (OR: 3.93, 95% CI: 1.43-10.76, P = .006).
Table 1.
Maternal and Infant Characteristics of 26 Infants With Neonatal Cerebral Sinovenous Thrombosis Compared With 85 Controls.a
| Maternal and Infant Characteristics | CSVT, N = 26 | Controls, N = 85 | P Value | OR (95% CI) |
|---|---|---|---|---|
| Maternal characteristics | ||||
| Ethnicity, n (%) | ||||
| Caucasian | 23 (92) | 79 (94) | .659 | 0.73 (0.13-4.00) |
| Arab | 2 (8) | 4 (5) | ||
| Black | 0 (0) | 1 (1) | ||
| Age ≥35 years | 11/26 (42) | 24/84 (29) | .189 | 1.83 (0.74-4.56) |
| Primiparity | 11/17 (65) | 52/85 (61) | .785 | 1.16 (0.39-3.45) |
| Gestational diabetes | 3/26 (12) | 8/85 (9) | .717 | 1.26 (0.31-5.12) |
| Preeclampsia | 4/26 (15) | 6/85 (7) | .240 | 2.39 (0.62-9.24) |
| Type of delivery | ||||
| Eutocic | 10/26 (38) | 42/85 (49) | .328 | 1.56 (0.64-3.83) |
| Instrumental | 8/26 (31) | 14/85 (16) | ||
| Cesarean | 8/26 (31) | 29/85 (34) | ||
| Neonatal and perinatal characteristics | ||||
| Male-to-female ratio | 20:6 | 39:44 | .006 | 3.93 (1.43-10.76) |
| Gestational age, weeks; median (IQR) | 40 (38-41) | 40 (39-40) | .930 | |
| Birth weight, g; mean (SD) | 3276 (489) | 3337 (415) | .530 | |
| Birth weightb | 2/26 (8) | 0/85 (0) | .053 | |
| Meconium | 6/25 (23) | 13/85 (15) | .368 | 1.7 (0.59-5.21) |
| Arterial cord pH ≤7.10 | 3/16 (12) | 3/70 (4) | .075 | 5.2 (0.94-28.4) |
| Apgar score ≤5 at 5 minutes | 2/26 (8) | 1/84 (1) | .136 | 7 (0.61-80.55) |
| Advanced neonatal resuscitationc | 3/26 (12) | 0/84 (0) | .012 | |
| Infectiond | 10/26 (38) | NA | ||
| Dehydratione | 1/26 (4) | NA | ||
| Polycythemiaf | 0/26 (0) | NA | ||
| Hypoglycemiag | 2/26 (8) | NA | ||
| Central catheter | 8/26 (31) | NA | ||
| Congenital heart disease | 3/26 (12) | NA | ||
| ECMO | 2/26 (8) | NA | ||
| Hypoxic ischemic encephalopathy | 4/26 (15) | NA |
Abbreviations: CI, confidence interval; CSVT, cerebral sinovenous thrombosis; ECMO, extracorporeal membrane oxygenation; IQR, interquartile range; NA, not applicable; OR, odds ratio; SD, standard deviation.
aCategorical variables are expressed as n/N (%).
bBirth weight less than 3th percentile for the population norms on the growth charts for gestational age.
cAdvanced neonatal resuscitation: intubation ± chest compressions ± adrenaline.
dClinical or laboratory suspicion of infection ± bacteriological confirmation. None of the infants had meningitis.
eWeight loss greater than 10% with respect to birth.
fHematocrit greater than 65%.
gBlood glucose less than 40 mg/dL.
In 25 patients, the diagnosis of CSVT was made from clinical suspicion, where seizures were the most common presentation in 22 (88%) cases. In 1 patient, the diagnostic suspicion was after cerebral Doppler ultrasound performed to severe pulmonary hypertension in a sedated neonate.
Cerebral MRI was performed as soon as the patient was stabilized for transfer to the MRI unit, at a median time point of 6 days (interquartile range [IQR]: 3-12) after the diagnostic suspicion. The diagnosis of CSVT was made at a median age of 11 days of life (IQR: 6-21) using standard sequences (see “Methods” section); in 2 infants, contrast-enhanced MRV was performed. Of the 26 infants, 2 died before the MRI was performed, and the diagnosis was made by necropsy. Cerebral sinovenous thrombosis involved multiple sinuses in 21/26 (81%). Table 2 and Figure 1 summarize affected sinuses and associated lesions. The most frequently involved sinus was the transverse sinus and the superior longitudinal sinus, both in 14/26 (53%). The most frequent brain-associated injury were white matter lesions in 21/26 infants (81%) and intraventricular and/or thalamic hemorrhage in 15/26 infants (58%).
Table 2.
Location and Associated Lesions in 26 Neonates With Cerebral Sinovenous Thrombosis.a,b
| Thrombosis Location | Total | IVH | Thalamic H | WM Mild-Moderate/Severe |
Extraaxial H | Arterial Infarct |
|---|---|---|---|---|---|---|
| Total | 26 (100) | 12 (46) | 13 (50) | 17/4 (81) | 12 (46) | 4 (15) |
| Multiple sinuses | 21 (81) | 10 (48) | 10 (48) | 13/3 | 9 (43) | 3 (14) |
| Superior sagittal sinus | 12 | 6 | 5 | 6/3 | 3 | 1 |
| Transverse/sigmoid sinusc | 12 | 3 | 2 | 8/1 | 5 | 3 |
| Galen vein/internal cerebral vein/straight sinusd | 13 | 10 | 10 | 8/3 | 6 | 0 |
| Isolated sinus | 5 (19) | 2 (40) | 3 (60) | 4/1 | 3 (60) | 1 (20) |
| Superior sagittal sinus | 2 | 1 | 2 | 2/0 | 2 | 0 |
| Transverse sinus | 2 | 1 | 0 | 1/1 | 1 | 1 |
| Internal cerebral vein | 1 | 0 | 1 | 1/0 | 0 | 0 |
Abbreviations: H, hemorrhage; IVH, intraventricular hemorrhage; WM, white matter.
aData are expressed as n (%). Extraaxial hemorrhage included subdural or subarachnoid hemorrhage.
bWM lesions were classified in (1) mild-moderate injury including focal lesions (periventricular, subcortical, punctate, or infarct) and (2) extensive lesions (extensive loss of gray-WM differentiation, infarctions, and bleeding).
cAll 12 infants had transverse sinus thrombosis (6/12 had also sigmoid sinus thrombosis).
d Three of 13 infants had only straight sinus thrombosis; 10/13 had cerebral internal vein and/or Galen vein thrombosis (8/10 also had straight sinus thrombosis).
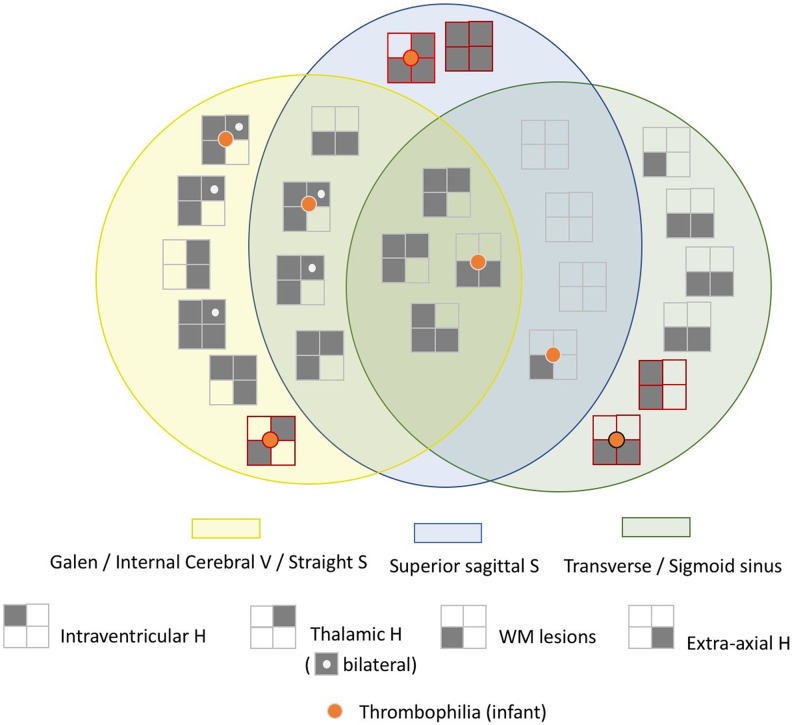
Figure 1.
Associated brain lesions in 26 infants with cerebral sinovenous thrombosis. Seven infants had thrombophilia: 4 with multiple sinus thrombosis (1 infant with FV G1691A mutation, 2 infants with FII G20210A mutation, and 1 infant with MTHFR C677T mutation) and 3 infants with isolated sinus thrombosis (1 infant with FII G20210A mutation and 2 infants with MTHFR C677T mutation). Five infants with isolated sinus thrombosis are drawn with line of red color (2 infants with superior sagittal sinus, 2 infants with transverse sinus, and 1 with internal cerebral vein). FV indicates factor V Leiden; H, hemorrhage; MTHFR C677T, methylenetetrahydrofolate reductase C677T; S, sinus; V, vein; WM, white matter.
Seven infants (27%) with CSVT had 1 of the 3 mutations: 4 with multiple sinus thrombosis (1 infant with FV G1691A mutation, 2 infants with FII G20210A mutation, and 1 infant with MTHFR C677T mutation), and 3 infants with isolated sinus thrombosis (1 infant with FII G20210A mutation and 2 infants with MTHFR C677T mutation). Infants with multiple sinus thrombosis did not present higher prevalence of thrombophilic mutations than those with isolated CSVT.
Though thrombophilia mutations were present more in infants with CSVT than in controls (30% vs 17%), the differences were not statistically significant. None of the case or control infants had more than 1 mutation.
The distribution of the results of the 3 mutations is presented in Table 3. Only FII G20210A mutation was more frequent in the infants with CSVT compared to controls (OR: 10.96, 95% CI: 1.09-110.35; P = .039).
Table 3.
Identified Mutations in Infant–Mother Pairs Involving 26 Infants With Neonatal Cerebral Sinovenous Thrombosis Compared With 85 Controls.a
| Mutation | CSVT | Controls | OR (95% CI) | P Value |
|---|---|---|---|---|
| FV G1691A | ||||
| Infant | 1/26 (4) | 2/85 (2) | 1.66 (0.14-19.08) | 0.555 |
| Mother | 1/21 (5) | 1/85 (1) | 4.20 (0.25-70.07) | 0.358 |
| FII G20210A | ||||
| Infant | 3/26 (12) | 1/85 (1) | 10.96 (1.09-110.35) | 0.039 |
| Mother | 0/21 (0) | 3/85 (4) | 1.000 | |
| MTHFR C677T (homozygous) | ||||
| Infant | 3/20 (15) | 11/81 (14) | 1.12 (0.28-4.47) | 1.000 |
| Mother | 1/15 (7) | 9/82 (11) | 0.58 (0.07-4.94) | 1.000 |
| FV G1691A or FII G20210A | ||||
| Infant and mother | 1/21 (5) | 1/85 (1) | 4.20 (0.25-70.07) | 0.358 |
| Infant or mother | 4/21 (19) | 6/85 (7) | 3.10 (0.79-12.18) | 0.107 |
| Any of the 3 mutations testedb | ||||
| Infant | 6/20 (30) | 14/81 (17) | 2.05 (0.67-6.26) | 0.219 |
| Mother | 1/15 (7) | 13/82 (16) | 0.38 (0.05-3.14) | 0.689 |
| Infant and mother | 1/14 (7) | 4/81 (5) | 1.48 (0.15-14.32) | 0.558 |
| Infant or mother | 2/14 (14) | 23/81 (28) | 0.42 (0.09-2.03) | 0.342 |
Abbreviations: CI, confidence interval; CSVT, cerebral sinovenous thrombosis; FV, factor V Leiden; MTHFR C677T, methylenetetrahydrofolate reductase C677T; OR, odds ratio.
aNo infant or mother had more than 1 mutation. There were no cases of F5 G1691A or F2 G20210A homozygous mutation.
bResults are shown for infants and mothers who were evaluated for the 3 genetic mutations.
All infants with FII G20210A mutation were male. When FII G20210A analysis was adjusted for sex, the OR for FII G20210A was 6.70 (95% CI: 0.65-69.22; P = .110) and OR for male sex was 3.43 (95% CI: 1.23-9.56; P = .018).
There were no differences in the distribution of the mutations of the mothers from the 2 cohorts. When analyzing the “thrombophilic environment” in mother–infant pairs defined by the presence of some mutation either in the mother or in her infant, no differences were observed between cases and controls (Table 3).
Regarding non-thrombophilic factors, infection, central catheter and hypoxic–ischemic encephalopathy were the factors most frequently present in 10, 8, and 4 infants, respectively (Table 1). Only 3 of 7 infants with thrombophilia had perinatal or neonatal comorbidities: 2 of them had homozygous MTHFR C677T mutation, one had an infection event and the other had congenital heart disease and underwent central catheterization. The third one had FII G20210A mutation and associated infection.
Thrombophilia in mothers was not associated with any of the neonatal or perinatal analyzed factors.
Discussion
As far as we know, this is the first prospective case–control study to evaluate thrombophilic mutations associated with CSVT in a neonatal population. To date, the available evidence of the role of these 3 mutations in the neonatal CSVT has been contradictory, for several reasons. First of all, the neonatal CSTV series are observational without control cohorts. In addition, few patients in each series have been tested for thrombophilic mutations performed. Of the 3 published series specifically aimed at a neonatal population with CSVT, 1 of them included very preterm infants,1 and the number of neonates with thrombophilic mutation studied remains low: 7/30 in Wu et al’s article2 and 24/42 in Fitzgerald et al’s.3 Only Berfelo et al, with a sample of 52 patients, analyzed FV G1691A in most of the patients (79%).1 Despite these limitations, the prevalence of these mutations in these series was 5% to 12% for FV G1691A and 0% to 11% for FII G20210A—the same percentages that we found.1, 3
There are 2 meta-analyses that examined the prevalence of FV G1691A and FII G20210A mutations in a pediatric population with CSVT. Kenet et al found a positive association of FV G1691A and FII G20210A with CSVT but did not separate the results for neonatal age, while the neonatal representation was very low in the included studies.5 The meta-analysis of Laugesaar et al considered the neonatal population separately, observing an association of FV G1691A with CSVT (OR: 5.5, 95% CI: 2.1-14.5, in a total sample of 39 neonates). This meta-analysis found no association with FII G20210A in neonatal patients, although there was an association for the rest of the pediatric age.4
The available results regarding the MTHFR mutation in neonatal series are even scarcer: 1/7 and 1/10 in the studies by Wu et al and Fitzgerald et al, respectively.2,3 Our results are in line with the lack of association found in the pediatric population.8
A second reason for these differences between studies is that they are not comparable in terms of ethnicity. It is known that the estimated prevalence of FV G1691A and FII G20210A in the general population is somewhat higher among Caucasians, around 4%, and less than 1% in Africans and Asians.9,10 In our series, Caucasian representation was the majority, unlike the other studies.4,5
A third reason for the differences between studies has to do with the particular characteristics of CSVT, especially in the neonatal period, during which symptoms may be nonspecific, even asymptomatic, while diagnostic techniques, both ultrasound and MRI, have limitations.11 Though color Doppler ultrasound is highly specific to rule out CSVT in neonates and holds potential for clinical application, contrast-enhanced MRV is recognized as the current “gold” standard for CSVT diagnosis.12,13 Only 2 of the 4 hospitals participating in the study had sufficient experience in carrying out venous Doppler ultrasound.
Although MRI was performed in our study in the event of diagnostic suspicion, we cannot rule out the possibility of a neonate with CSVT in whom repermeabilization of the thrombosis vessel might have occurred at the time of MRI, thereby excluding the patient. Nor can we rule out the progression of the thrombosis since MRI was not repeated in the following days.
Furthermore, we cannot exclude the possibility of false-positive CSVT, as we did not systematically perform sequences such as contrast-enhanced MRV that might help to differentiate slow flow of the venous system from thrombosis.13
Nevertheless, the most frequent false positives are described at the level of the posterior third of the superior sagittal sinus and transverse sinuses.14 In our 2 patients with isolated transverse sinus thrombosis, the thrombosed sinus was the right one. Frequently, the right sinus has the highest caliber and dominance in the published series15 and flow gaps are rarely on the dominant side.16 Regarding the risk of a false positive in the superior sagittal sinus, one of the cases with isolated thrombosis of this sinus had associated thrombosed cortical veins, punctate lesions, and thalamic hemorrhage. The other infant had extraaxial, intraventricular, and thalamic hemorrhages. These findings have been described in association with CSVT.17,18
In any case, we think that in our study CSVT was more likely to have been underdiagnosed than the reverse. We did not include doubtful cases in which the MRI findings were not conclusive, mainly when the thrombosis was confined to a single location and there were no symptoms and/or no alterations in the Doppler ultrasound. All 5 children with isolated thrombosis (2 with superior sagittal sinus thrombosis, 2 with transverse sinus thrombosis, and 1 with internal cerebral vein thrombosis) presented neurological symptoms, and all of them showed associated lesions.
Although the percentage of multiple sinus involvement is variable in the literature, our results are consistent with those of other neonatal series.19,20 We also found the superior sagittal sinus and transverse sinus to be the most frequently thrombosed sites,3,20,21 although the number of infants with thrombosis of the deep venous system was also high.19
As in other neonatal CSVT series, the most frequent associated findings in our cohort were white matter lesions and intraventricular and/or thalamic hemorrhage.1,19,21 As previously reported by others, we also found that thrombosis of the deep venous system was associated with intraventricular and/or thalamic hemorrhage, as was the case in 86% infants with Galen vein, internal cerebral vein, and/or straight sinus thrombosis.1,17,19 Importantly, we found that although these findings may also be present in superior sagittal sinus thrombosis, they are rarely seen when transverse or sigmoid sinus thrombosis is involved.1 In addition, intraventricular hemorrhage and thalamic hemorrhage were also linked in our cohort, as they occurred in 67% of the 15 infants with either of the 2 findings.18
Thus, if either of the 2 findings is seen on an ultrasound scan, one may rule out CSVT of the deep venous system and/or superior sagittal sinus. Likewise, it may be ruled out when white matter injury is found, especially if there is extraaxial hemorrhage, regardless of whether there is intraventricular or thalamic hemorrhage.
The majority of neonates with CSVT did not present genetic thrombophilia, but when it is present it was distributed equally among children with multiple or isolated CSVT. Infants with genetic thrombophilia did not have more brain-associated lesions than those without thrombophilia, so we cannot say that children with isolated thrombosis or associated minimal lesions are exempt from thrombophilic mutation.
Although an association among several risk factors has been suggested as a cause of CSVT,2,3 in our series, 4 of 7 infants with thrombophilic mutations did not present other perinatal or neonatal factors. Perhaps, these mutations were sufficient for CSVT thrombosis or other unidentified associated risk factors. In the rest of the infants with CSVT, described risk factors such as dehydration, polycythemia, and meningitis were rare in our series, whereas infection and the use of central catheter were relatively frequent. Although vascular catheters are the most commonly identified pediatric risk factor for thrombosis,22,23 they have not been described before in neonatal CSVT.1–3,20 However, the presence of a central catheter tends to be associated with severe pathologies that could condition the prothrombotic risk.
Despite its inclusion of a control population and a lengthy period—10 years—the main limitation of our study is that the cohort of neonates with CSVT was not large. Larger, well-designed studies are needed to find smaller differences than those initially estimated in our study. These studies will help clarify whether these mutations, as well as other thrombophilic alterations, contribute alone or in the presence of other perinatal and neonatal prothrombotic factors in the etiopathogenesis of neonatal CSVT. The influence of male gender at this age also needs to be explored.
Until we have more evidence at hand on the pathogenesis of neonatal CSVT and are able to rule out thrombophilia as a risk factor, we believe a full thrombophilic screening should continue to be carried out. It is also unknown whether thrombophilia may play a role in the progression of thrombosis in some infants and whether it might have implications in therapeutic therapies. If thrombophilia is involved in this first event as a neonate, recommendations for prevention can help these patients in the future.
Acknowledgments
The authors would like to thank all the families of the infants participated in this study.
Authors’ Note: The study is included in an extensive perinatal brain infarction project (PI15/00846 and PI08/1366) and was approved by the research ethics committees of the participating hospitals and informed written consent was obtained from the parents. Verbal informed consent was obtained from a legally authorized representative(s) for anonymized patient information to be published in this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by the “Plan Nacional de I+D+I and Instituto de Salud Carlos III- Subdirección General de Evaluación y Fomento de la Investigación Sanitaria,” Projects PI15/00846 e PI08/1366, and the European Regional Development Fund (FEDER).
ORCID iD: Juan Arnaez  https://orcid.org/0000-0001-8883-5181
https://orcid.org/0000-0001-8883-5181
References
- 1. Berfelo FJ, Kersbergen KJ, van Ommen CH, et al. Neonatal cerebral sinovenous thrombosis from symptom to outcome. Stroke. 2010;41(7):1382–1388. [DOI] [PubMed] [Google Scholar]
- 2. Wu YW, Miller SP, Chin K, et al. Multiple risk factors in neonatal sinovenous thrombosis. Neurology. 2002;59(3):438–440. [DOI] [PubMed] [Google Scholar]
- 3. Fitzgerald KC, Williams LS, Garg BP, Carvalho KS, Golomb MR. Cerebral sinovenous thrombosis in the neonate. Arch Neurol. 2006;63(3):405–409. [DOI] [PubMed] [Google Scholar]
- 4. Laugesaar R, Kahre T, Kolk A, Uustalu U, Kool P, Talvik T. Factor V Leiden and prothrombin 20210G>A [corrected] mutation and paediatric ischaemic stroke: a case–control study and two meta-analyses. Acta Paediatr (Oslo, Norway: 1992). 2010;99(8):1168–1174. [DOI] [PubMed] [Google Scholar]
- 5. Kenet G, Lutkhoff LK, Albisetti M, et al. Impact of thrombophilia on risk of arterial ischemic stroke or cerebral sinovenous thrombosis in neonates and children: a systematic review and meta-analysis of observational studies. Circulation. 2010;121(16):1838–1847. [DOI] [PubMed] [Google Scholar]
- 6. Kraus FT. Fetal thrombotic vasculopathy: perinatal stroke, growth restriction, and other sequelae. Surg Pathol Clin. 2013;6(1):87–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Arnaez J, Arca G, Martin-Ancel A, Garcia-Alix A. Coagulation factor V G1691A, factor II G20210A and methylenetetrahydrofolate reductase C677T gene mutations do not play a major role in symptomatic neonatal arterial ischaemic stroke. Br J Haematol. 2018;180(2):290–292. [DOI] [PubMed] [Google Scholar]
- 8. Heller C, Heinecke A, Junker R, et al. Cerebral venous thrombosis in children: a multifactorial origin. Circulation. 2003;108(11):1362–1367. [DOI] [PubMed] [Google Scholar]
- 9. Rees DC. The population genetics of factor V Leiden (Arg506Gln). Br J Haematol. 1996;95(4):579–586. [DOI] [PubMed] [Google Scholar]
- 10. Rosendaal FR, Doggen CJ, Zivelin A, et al. Geographic distribution of the 20210 G to A prothrombin variant. Thromb Haemost. 1998;79(4):706–708. [PubMed] [Google Scholar]
- 11. Yang JY, Chan AK, Callen DJ, Paes BA. Neonatal cerebral sinovenous thrombosis: sifting the evidence for a diagnostic plan and treatment strategy. Pediatrics. 2010;126(3):e693–e700. [DOI] [PubMed] [Google Scholar]
- 12. Miller E, Daneman A, Doria AS, et al. Color Doppler US of normal cerebral venous sinuses in neonates: a comparison with MR venography. Pediatr Radiol. 2012;42(9):1070–1079. [DOI] [PubMed] [Google Scholar]
- 13. Rollins N, Ison C, Reyes T, Chia J. Cerebral MR venography in children: comparison of 2D time-of-flight and gadolinium-enhanced 3D gradient-echo techniques. Radiology. 2005;235(3):1011–1017. [DOI] [PubMed] [Google Scholar]
- 14. Widjaja E, Shroff M, Blaser S, Laughlin S, Raybaud C. 2D time-of-flight MR venography in neonates: anatomy and pitfalls. Am J Neuroradiol. 2006;27(9):1913–1918. [PMC free article] [PubMed] [Google Scholar]
- 15. Rollins N, Ison C, Booth T, Chia J. MR venography in the pediatric patient. Am J Neuroradiol. 2005;26(1):50–55. [PMC free article] [PubMed] [Google Scholar]
- 16. Ayanzen RH, Bird CR, Keller PJ, McCully FJ, Theobald MR, Heiserman JE. Cerebral MR venography: normal anatomy and potential diagnostic pitfalls. Am J Neuroradiol. 2000;21(1):74–78. [PMC free article] [PubMed] [Google Scholar]
- 17. Wu YW, Hamrick SE, Miller SP, et al. Intraventricular hemorrhage in term neonates caused by sinovenous thrombosis. Ann Neurol. 2003;54(1):123–126. [DOI] [PubMed] [Google Scholar]
- 18. Dlamini N, Billinghurst L, Kirkham FJ. Cerebral venous sinus (sinovenous) thrombosis in children. Neurosurg Clin N Am. 2010;21(3):511–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kersbergen KJ, Groenendaal F, Benders MJ, et al. The spectrum of associated brain lesions in cerebral sinovenous thrombosis: relation to gestational age and outcome. Arch Dis Fetal Neonatal Ed. 2011;96(6):F404–F409. [DOI] [PubMed] [Google Scholar]
- 20. Nwosu ME, Williams LS, Edwards-Brown M, Eckert GJ, Golomb MR. Neonatal sinovenous thrombosis: presentation and association with imaging. Pediatr Neurol. 2008;39(3):155–161. [DOI] [PubMed] [Google Scholar]
- 21. deVeber G, Andrew M, Adams C, et al. Cerebral sinovenous thrombosis in children. N Engl J Med. 2001;345(6):417–423. [DOI] [PubMed] [Google Scholar]
- 22. Andrew M, David M, Adams M, et al. Venous thromboembolic complications (VTE) in children: first analyses of the Canadian Registry of VTE. Blood. 1994;83(5):1251–1257. [PubMed] [Google Scholar]
- 23. McCrory MC, Brady KM, Takemoto C, Tobias JD, Easley RB. Thrombotic disease in critically ill children. Pediatr Crit Care Med. 2011;12(1):80–89. [DOI] [PubMed] [Google Scholar]