Abstract
Bleeding has been reported in patients with chronic myeloid leukemia (CML) using tyrosine kinase inhibitors (TKIs). In this study, we aimed to evaluate platelet functions and associated bleeding symptoms in patients with CML using TKIs. A standardized questionnaire that was developed for inherited bleeding disorders (ISTH/SSC Bleeding Assessment Tool) was used to score bleeding symptoms in 68 chronic phase patients with CML receiving imatinib (n = 47), dasatinib (n = 15), or nilotinib (n = 6). Light transmission aggregometry was used for platelet function testing. None of the patients had major bleeding (score > 3). Minor bleeding was observed in 25.6% and 20% of the patients in imatinib and dasatinib treatment groups. Impaired/decreased platelet aggregation was observed in 29.8% of imatinib treatment group, 50% of nilotinib group, and 40% of dasatinib group. A secondary aggregation abnormality compatible with the release defect was observed in 26% of patients with CML; 25.5%, 33.3%, and 16.7% of patients receiving imatinib, dasatinib, and nilotinib, respectively. No correlation was found between bleeding symptoms and the impaired platelet function. We can conclude that TKIs may impair in vitro platelet aggregation but this impairment is not associated with bleeding diathesis.
Keywords: platelet aggregation, dasatinib, imatinib mesylate, chronic-phase myeloid leukemia
Introduction
The initial, chronic phase (CP) of chronic myeloid leukemia (CML) has a median duration of 4 to 6 years and is characterized by overproduction of immature myeloid cells and mature granulocytes.1 Philadelphia chromosome which results from a reciprocal translocation between the long arms of chromosome 9 and 22 [t(9;22)] is characteristic for CML. The resulting fusion gene is BCR-ABL oncogene encoding BCR-ABL oncoprotein which leads to constitutively active tyrosine kinase signaling inducing increased cellular proliferation and diminished apoptosis.2–4
Tyrosine kinase inhibitors (TKIs) have been developed for CML treatment. The first generation BCR-ABL TKI, imatinib mesylate, and the second generation TKIs, dasatinib and nilotinib, have improved the prognosis of CML dramatically. Tyrosine kinase inhibitors are to be used life-long in the majority of patients.
Bleeding has been reported in patients with CML using TKIs. Hemorrhage frequency was reported as 20.9% in imatinib arm of the International Randomized Study of Interferon and STI571 (IRIS) trial, however this frequency was similar to the control interferon-alpha plus cytarabine arm.5 Forty percent bleeding frequency was found in patients with CML using dasatinib.6 Bleeding may occur due to multiple mechanisms in CML including thrombocytopenia, dysfunction of CML platelets, and TKI-related bleeding events. Hemorrhagic colitis under dasatinib treatment is probably the most specific TKI-related bleeding event.7 The mechanism for TKI-induced hemorrhage is not clear. It has been suggested that TKIs can disrupt platelet functions and cause bleeding.8 Impaired platelet aggregation was observed on dasatinib, imatinib, and bosutinib treatment groups in a previous study.8 In this study, we aimed to evaluate bleeding symptoms and platelet functions in patients with CML using TKIs and to find out whether there is a correlation between them.
Materials and Methods
Patients with CP-CML on imatinib, dasatinib, or nilotinib for at least 2 months and at least on complete hematological response were included in this study. This study was supported by Hacettepe Research Council (Project Number: THD-2015-6345). All patients provided informed consent in accordance with the Declaration of Helsinki. The conduct of this study was approved by the institutional review board at Hacettepe University (GO 15/221).
Bleeding surveys were performed on the patients by using a questionnaire developed for type 1 von Willebrand disease (Table 1).9 The type of the bleeding (epistaxis, minor wounds, cutaneous symptoms, oral cavity bleeding, gastrointestinal system bleeding, muscle hematomas or hemarthrosis, tooth extraction, menorrhagia, and other), as well as frequency and severity of the bleeding were evaluated in the questionnaire. The scores of individual bleeding symptoms ranged between 0 and 4; when the bleeding score was ≤3, it was accepted as minor bleeding, and major bleeding when the bleeding score was >3.
Table 1.
A Questionnaire for Bleeding Score (ISTH/SSC Bleeding Assessment Tool).
| Symptoms | Score | ||||
|---|---|---|---|---|---|
| 0a | 1a | 2 | 3 | 4 | |
| Epistaxis | No/trivial | >Five/year or more than 10 minutes | Consultation onlyb | Packing or cauterization or antifibrinolytic | Blood transfusion or replacement therapy (use of hemostatic blood components and rFVIIa) or desmopressin |
| Cutaneous | No/trivial | For bruises 5 or more (>1 cm) in exposed areas | Consultation onlyb | Extensive | Spontaneous hematoma requiring blood transfusion |
| Bleeding from minor wounds | No/trivial | >Five/year or more than 10 minutes | Consultation onlyb | Surgical hemostasis | Blood transfusion, replacement therapy, or desmopressin |
| Oral cavity | No/trivial | Present | Consultation onlyb | Surgical hemostasis or antifibrinolytic | Blood transfusion, replacement therapy or desmopressin |
| GI bleeding | No/trivial | Present (not associated with ulcer, portal hypertension, hemorrhoids, angiodysplasia) | Consultation onlyb | Surgical hemostasis, antifibrinolytic | Blood transfusion, replacement therapy, or desmopressin |
| Hematuria | No/trivial | Present (macroscopic) | Consultation onlyb | Surgical hemostasis, iron therapy | Blood transfusion, replacement therapy, or desmopressin |
| Tooth extraction | No/trivial or none done | Reported in ≤25% of all procedures, no interventionc | Reported in >25% of all procedures, no interventionc | Resuturing or packing | Blood transfusion, replacement therapy, or desmopressin |
| Surgery | No/trivial or none done | Reported in ≤25% of all procedures, no interventionc | Reported in >25% of all procedures, no interventionc | Surgical hemostasis or antifibrinolytic | Blood transfusion, replacement therapy or desmopressin |
| Menorrhagia | No/trivial | Consultation onlyb or changing pads more frequently than every 2 hours or clot and flooding or PBAC score>100d | Time off work/school >2/year or requiring antifibrinolytics or hormonal or iron therapy | Requiring combined treatment with antifibrinolytics and hormonal therapy or present since menarche and >12 months | Acute menorrhagia requiring hospital admission and emergency treatment or requiring blood transfusion, replacement therapy, desmopressin, or requiring dilatation and curettage or endometrial ablation or hysterectomy |
| Postpartum hemorrhage | No/trivial or no deliveries | Consultation onlyb or use of syntocin or lochia > 6 weeks | Iron therapy or antifibrinolytics | Requiring blood transfusion, replacement therapy, desmopressin, or requiring examination under anesthesia and/or the use of uterin balloon/package to tamponade the uterus | Any procedure requiring critical care or surgical intervention (eg, hysterectomy, internal iliac artery legation, uterine artery embolization, uterine brace sutures) |
| Muscle hematomas | Never | Post trauma, no therapy | Spontaneous, no therapy | Spontaneous or traumatic, requiring desmopressin or replacement therapy | Spontaneous or traumatic, requiring surgical intervention or blood transfusion |
| Hemarthrosis | Never | Post trauma, no therapy | Spontaneous, no therapy | Spontaneous or traumatic, requiring desmopressin or replacement therapy | Spontaneous or traumatic, requiring surgical intervention or blood transfusion |
| CNS bleeding | Never | - | - | Subdural, any intervention | Intracerebral, any intervention |
| Other bleedingse | No/trivial | Present | Consultation onlyb | Surgical hemostasis, antifibrinolytics | Blood transfusion or replacement therapy or desmopressin |
Abbreviations: CNS, central nervous system; GI, gastrointestinal; PBAC, pictorial blood loss assessment chart; rFVIIa, recombinant factor VIIa.
aDistinction between 0 and 1 is of critical importance. Score 1 means that the symptom is judged as present in the patient’s history by the interviewer but does not qualify for a score 2 or more.
bConsultation only: the patient sought medical evaluation and was either referred to a specialist or offered detailed laboratory investigation.
cExample: 1 extraction/surgery resulting in bleeding (100%): the score to be assigned is 2; 2 extractions/surgeries, 1 resulting in bleeding (50%): the score to be assigned is 2; 3 extractions/surgeries, 1 resulting in bleeding (33%): the score to be assigned is 2; 4 extractions/surgeries, 1 resulting in bleeding (25%): the score to be assigned is 1.
dIf already available at the time of collection.
eInclude: umbilical stump bleeding, cephalohematoma, cheek hematoma caused by sucking during breast/bottle feeding, conjunctival hemorrhage, or excessive bleeding following circumcision or venipuncture. Their presence in infancy requires detailed investigation independently from the overall score.
Venous blood samples were collected in sodium citrated tubes (1:9, 3.2%, Becton Dickinson, Meylan, France). Coagulation tests including prothrombin time, international normalized ratio, activated partial thromboplastin time (aPTT), thrombin time (TT), fibrinogen, von Willebrand factor antigen, and factor VIII were analyzed on BCS-XP coagulation analyzer (Siemens Healthcare Diagnostics, Erlanger, Germany). LH-780 hematology analyzer (Beckman Coulter, Havertown, Pennsylvania, USA) was used for complete blood count testing. For platelet function testing light transmission aggregometry (Chrono-Log, Diagnostica Stago, Havertown, Pennsylvania) was performed. The test was performed in the routine hemostasis laboratory of our university hospital. Test calibrations are done periodically in this setting using healthy control samples. The patients were warned not to take any drug (eg, nonsteroidal anti-inflammatory agents, antithrombotic drugs, or any other drug known to inhibit in vitro platelet functions) or substance (eg, alcohol, garlic) with possible interference with the tests for at least 7 days before the analyses. All tests were completed within 4 hours of blood sampling. Platelet rich plasma was prepared by centrifuging the citrate-anticoagulated blood sample at 170 to 200 g for 10 minutes. Autologous platelet poor plasma was prepared by centrifugation at 1500 g for at least 15 minutes. Platelet aggregation was triggered in vitro at 37°C by 2 and 6 µM of adenosine diphosphate (ADP), 1 mg/mL of collagen, 1 mM of epinephrine, and 0.6 mg/mL and 1.25 mg/mL of ristocetin under continuous stirring.10 The aggregation percentage/time graph was analyzed by an experienced hematologist (Y.B.). Both aggregation amplitudes and wave shapes were considered. An irreversible aggregation wave emerging after a normal lag time and having >70% amplitude was considered normal. A wave with a slightly diminished amplitude (generally between 50% and 70%) but otherwise normal shape was not considered as impairment. It was classified as decreased aggregation. Impairment meant an abnormal primary or secondary aggregation wave. An isolated prolonged lag time was classified separately. If a normal secondary wave of aggregation did not appear with ADP, a secretion defect was considered. If this abnormality was corrected with 6 µM ADP, the problem was classified as release defect.
Statistical Analysis
Numerical and categorical descriptive data were presented as median (minimum-maximum) and number (percentage), respectively. Comparison of numerical and categorical variables between TKI groups was performed by Kruskal-Wallis test and χ2 test, respectively. The correlations of platelet dysfunction on aggregometry with presence of bleeding symptoms and an elevated bleeding score (if present) were also evaluated with χ2 test. A P value <.05 was used as the criterion for statistical significance. SPSS statistics version 17 (SPSS Inc., Chicago, Illinois) was used for statistical analyses.
Results
The Patients and Basic Hemostasis Results
Sixty-eight patients with CP-CML with a median age of 47 (18-78) years receiving imatinib (n = 47), dasatinib (n = 15), and nilotinib (n = 6) were evaluated. Median CML duration was 115 (36-195), 122 (53-154), and 133 (85-174) months, respectively. Median durations on the respective TKI were 34 (2-147), 19 (2-66), and 13.5 (2-18) months, respectively. Platelet counts ranged between 103 000 and 456 000/µL. Prothrombin time, aPTT, and TT were minimally prolonged in 1.5%, 3%, and 1.5% of the patients, respectively. Demographical data and basic hemostatic test results are summarized in Table 2. There were no statistical differences between TKI groups for any of these parameters.
Table 2.
Demographical Data and Basic Hemostatic Test Results.
| İmatinib (n = 47) | Dasatinib (n = 15) | Nilotinib (n = 6) | P | |
|---|---|---|---|---|
| Age | 46 (18-68) | 48 (34-66) | 51.5 (30-59) | .98 |
| Gender (F/M) | 24/23 | 8/7 | 4/2 | .81 |
| Platelet (×103/µL), (159-388 × 103/µL) | 232 (103-387) | 267 (147-456) | 164 (145-353) | .37 |
| aPTT (22.8-32 sec) | 28 (20-32) | 27.5 (22-33) | 29.1 (24-33) | .58 |
| INR (0.8-1.2) | 1 (0.8-1.7) | 0.9 (0.8-1.1) | 1 (0.9-1) | .91 |
| vWF-Ag (50%-160%) | 134 (66-193) | 144.5 (104-196) | 129 (81-177) | .5 |
| TT (14-21 sec) | 16.4 (14.6-22.1) | 16.4 (10.2-20.2) | 15.3 (14.7-17.9) | .22 |
| Fibrinogen (180-350 mg/dL) | 308.6 (192-506) | 334.7 (254-506) | 328 (263-459) | .2 |
| Factor VIII (70%-150%) | 155 (81-287) | 175.5 (129-288) | 162 (82-202) | .2 |
Abbreviations: aPPT, activated partial thromboplastin time; INR, international normalized ratio; TT, thrombin time; vWF-Ag, von Willebrand factor antigen.
Aggregation Tests
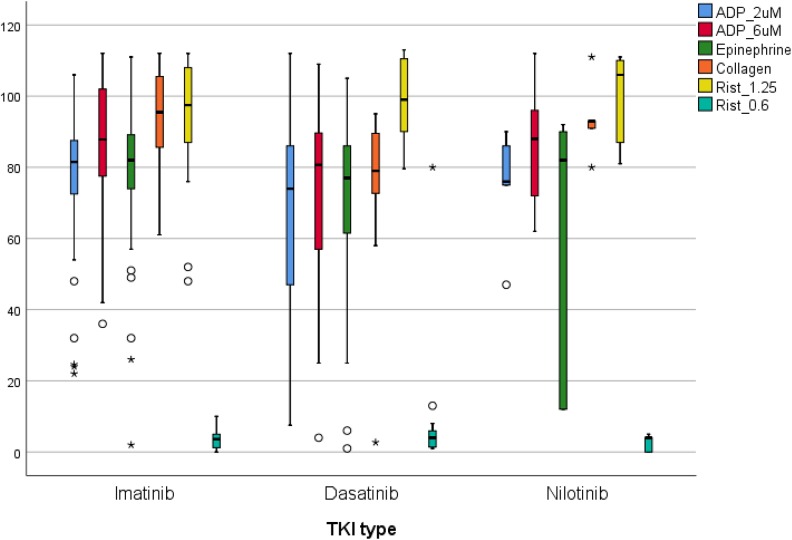
Impaired/decreased platelet aggregation was observed in 14 (29.8%) of imatinib treatment group, 3 (50%) of nilotinib group, and 6 (40%) of dasatinib group (P = .52). Aggregation amplitudes and the ratio of an impaired/decreased platelet aggregation with different reagents on different TKIs are summarized in Figure 1 and Table 3. Adenosine diphosphate and ristocetin-induced aggregation results were not different between TKI groups. But collagen-induced aggregation amplitudes were significantly lower in dasatinib compared to imatinib and nilotinib (P = .002). Epinephrine-induced aggregation results are also different between groups: impaired/decreased epinephrine-induced aggregation was observed more frequently in dasatinib (5 cases, 33.3%) and nilotinib (2 of 5 cases, 33.3%, 1 case was not tested) groups compared to imatinib (8 cases, 17%) group (P = .01). The abnormality with epinephrine was generally a prolonged lag time or diminished aggregation amplitude. Abnormal epinephrine-induced aggregation was an isolated abnormality in 4 cases (2 nilotinib, 1 dasatinib, 1 imatinib).
Figure 1.
Aggregation amplitudes with different reagents on different TKIs. Collagen and epinephrine induced aggregation amplitudes were significantly different in dasatinib compared to imatinib and nilotinib (P = .002 and P = .01). TKIs indicates tyrosine kinase inhibitors.
Table 3.
The Numbers (Ratios) of Impaired/Decreased Platelet Aggregation on Different TKI Treatments.
| İmatinib, n = 47 | Dasatinib, n = 15 | Nilotinib, n = 6 | P | |
|---|---|---|---|---|
| ADP (2 µM) | 12 (25.5%) | 5 (33.3%) | 1 (16.6%) | .71 |
| ADP (6 µM) | 3 (6.4%) | 4 (26.6%) | 1 (16.6%) | .09 |
| Epinephrine | 8 (17%) | 5 (33.3%) | 2 (33.3%) | .01 |
| Collagen | 2 (4.2%) | 2 (13.3%) | 0 | .002 |
| Ristocetin (1.25 mg/mL) | 2 (4.2%) | 0 | 0 | .63 |
| Ristocetin (0.6 mg/mL) | 47 (100%) | 14 (93.3%) | 6 (100%) | .55 |
Abbreviations: ADP, adenosine diphosphate; TKIs, tyrosine kinase inhibitors.
When all aggregation test results were considered, a platelet secretion defect was observed in 18 (26%) of patients with CML; 12 (25.5%) in imatinib, 5 (33.3%) in dasatinib, and 1 (16.7%) in nilotinib group (P = .71).
Bleeding Questionnaire
According to survey results, bleeding symptoms were observed only in 15 (22%) of 68 patients with CML consisting 2 epistaxis, 4 cutaneous symptoms, 2 minor wound bleeding, 2 bleeding after tooth extraction, and 5 menorrhagia. The bleeding score was less than 3 in all of the patients and accepted as minor bleeding.
When the relation between bleeding scores and TKI treatment was evaluated, minor bleeding was observed in 25.6% and 20% of the patients in imatinib and dasatinib treatment group, respectively. No bleeding was observed in nilotinib treatment group as shown in Table 4 (P = .65).
Table 4.
Bleeding Score in the Treatment Groups.a
| Bleeding Score | Total | |||
|---|---|---|---|---|
| 0 | 1 | 2 | ||
| Imatinib | 35 (74.5%), (11b) | 10 (21.3%), (0b) | 2 (4.3%), (1b) | 47 (100%), (12b) |
| Dasatinib | 12 (80%), (4b) | 2 (13.3%), (1b) | 1 (6.7%), (0b) | 15 (100%), (5b) |
| Nilotinib | 6 (100%), (1b) | 0, (0b) | 0, (0b) | 6 (100%), (1b) |
aThere was no significant difference (P = .65).
bThe patients with a secretion defect on platelet aggregometry.
The Correlation Between Aggregation Results and Bleeding Symptoms
There was no correlation between presence of any bleeding or total bleeding score and platelet secretion defect or any aggregation abnormality. In the imatinib group, platelet functions were normal in 11 (91.7%) of patients with minor bleeding. Secretion type release defect was observed only in 1 (8.3%) of the patients. Eleven (31.4%) of 35 imatinib patients who had no bleeding also showed secretion type platelet dysfunction. There was no association between the minor bleeding symptoms and platelet function test results in the imatinib group.
In the dasatinib treatment group, platelet functions were normal in 2 out of 3 patients with minor bleeding symptoms, secretion type defect was observed in the remaining patient with minor bleeding symptoms. In the group without bleeding (n = 12), 4 patients had platelet dysfunction (33%), whereas 8 (66%) have normal platelet functions. There was no association between the minor bleeding symptoms and platelet function test results in the dasatinib group, too. Platelet secretion defect was observed in 1 patient in the nilotinib treatment group. This patient had no bleeding symptoms at the time of aggregation testing (Table 4).
Discussion
This is the study evaluating the correlation between TKI-induced platelet dysfunction and bleeding diathesis in patients with CP-CML. We report that TKIs may cause inhibition of platelet aggregation in vitro, but this is not associated with bleeding diathesis in patients with CP-CML. The superiority of our study is the administration of an established bleeding survey. According to the survey results, minor bleeding symptoms were observed in 15 (22%) of 68 patients with CML. Minor bleeding was observed in 25.6% and 20% of the patients in imatinib and dasatinib treatment group, respectively. No bleeding was observed in nilotinib treatment group; however, there were a limited number of patients in this group. Secretion type release defect was observed in 26% (18/68) of patients with CML; 25.5% (n = 12) of patients using imatinib, 33.3% (n = 5) in dasatinib group, and 16.7% (n = 1) in nilotinib group. No correlation was observed between presence of any bleeding or total bleeding scores and platelet dysfunction in our study.
Previously, some investigators showed impaired platelet aggregation due to TKIs in CML. The mechanism of TKI-induced platelet dysfunction is unclear. Quintás-Cardama et al8 tested platelet aggregation in 87 patients with CML in CP receiving dasatinib (n = 27), bosutinib (n = 32), imatinib (n = 19), or nilotinib (n = 9). Impaired platelet aggregation on stimulation with arachidonic acid (AA), epinephrine, or both was observed in 70%, 85%, and 59% of patients on dasatinib, respectively. In imatinib treatment group, 66% had impaired AA-induced platelet aggregation. In bosutinib group, only 15% of the patients exhibited decreased aggregation to AA or epinephrine. All patients on nilotinib therapy had normal platelet aggregation. It is impossible to compare our results with this study because of different patient profiles and methodological differences in reporting of platelet function results. Their patient cohort included some patients with no hematological response. Some cases were receiving aspirin. Most importantly, Quintás-Cardama et al8 defined impaired platelet aggregation only by evaluating aggregation wave amplitudes. Abnormality was defined by decreased amplitude. This approach is not standard. All of these wave characteristics must be considered during reporting: lag phase, maximal amplitude, primary aggregation slope, and disaggregation.10
In 4 single-arm multicenter studies including 445 patients, the efficacy and safety of dasatinib was tested. Common toxicities with dasatinib included myelosuppression, bleeding, and fluid retention. Forty percent of the safety population experienced bleeding events of any type and 10% experienced grade 3 or 4 bleeding. Epistaxis was the most common event, occurring in 11% of patients, followed by gastrointestinal bleeding in 14%.6 Quintás-Cardama et al11 observed bleeding in 23% of patients with CML under dasatinib therapy and it was much more frequent in blastic (35%) and accelerated (31%) phase of the disease. Basic coagulation tests were normal in 97% of patients who developed bleeding complications. The platelet count was < 100 × 109/L in 63% of the patients. Gastrointestinal bleeding was by far the most common hemorrhage (81%). They reported that especially thrombocytopenia and advanced phase CML were associated with increased risk of bleeding. Significant (not all) bleeding was observed in 5.4% of nilotinib (400 mg) versus 1.8% of imatinib group in the Evaluating Nilotinib Efficacy and Safety in Clinical Trials–Newly Diagnosed Patients (ENESTnd) study comparing these 2 drugs in the first-line setting.12
Chronic myeloid leukemia may be associated with bleeding manifestations due to disease characteristics. Therefore, one should be careful when ascribing bleeding symptoms to TKI treatment: several studies on patients with CML before TKI era have reported hemorrhagic manifestations (20%-40%), including gastrointestinal bleeding, probably due to platelet dysfunction, thrombocytopenia, acquired von Willebrand disease, and peptic ulceration induced by hyperhistaminemia.13–16 Bleeding was observed at diagnosis in 21.3% of 430 CML cases seen at a referral center between 1981 and 1995.16 Most bleeding patients had normal or elevated platelet counts, suggesting that platelet dysfunction was the primary cause of hemorrhage. Bleeding events occurred in 52% and 44% of patients in blastic and accelerated phase CML on imatinib therapy, respectively, including 8% and 5% gastrointestinal hemorrhage.17 Gastrointestinal bleeding is especially frequent during transformation phases of CML. In a retrospective study which was performed when TKIs were not available, we found highest frequency of gastrointestinal hemorrhage between hematologic neoplasms in CML cases, especially during blastic phase (26%).14
In accordance with this observation, frequent gastrointestinal hemorrhage has been reported in accelerated (5%-11%) and blastic (8%) phases CML imatinib and dasatinib studies,17,18 respectively, but not in CP CML or even Philadelphia chromosome-positive acute lymphoblastic leukemia treated with these agents.5,6,19
The main limitation of this study is the relatively low number of cases taking nilotinib. This factor was due to later emergence of Nilotinib in the market and reimbursement strategies of TKIs in our country. Second generation agents cannot be used before imatinib in this country.
In conclusion, we showed that impaired platelet aggregation with tyrosine kinase inhibitors is just an in vitro finding without important clinical consequences just like the platelet dysfunctions observed on different antihypertensive drugs, antidepressants, antibiotics, and so on. We had no patients who underwent surgery during TKI treatment. To the best of knowledge, there is no CML treatment guideline recommending stopping TKI for any surgical/invasive intervention. We recommend continuing TKI therapy preoperatively. The diagnosis of bleeding diathesis should be based on clinical manifestations; laboratory findings without clinical correlation should not be considered as clues for bleeding diathesis.
Footnotes
Author Contributions: Y.S., F.A., Z.G.D., and Y.B. contributed to design, S.A., Y.S., and M.O. contributed to data collection, Y.S., M.O., and Y.B. wrote the main manuscript text. M.O. and Y.B. prepared Tables 1 to 4 and Figure 1. All authors reviewed the manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by Hacettepe Research Council (Project Number: THD-2015-6345).
ORCID iD: Mufide Okay  https://orcid.org/0000-0001-5317-0597
https://orcid.org/0000-0001-5317-0597
References
- 1. Kantarjian HM, Dixon D, Keating MJ, et al. Characteristics of accelerated disease in chronic myelogenous leukemia. Cancer. 1988;61(7):1441–1446. [DOI] [PubMed] [Google Scholar]
- 2. Groffen J, Stephenson JR, Heisterkamp N, de Klein A, Bartram CR, Grosveld G. Philadelphia chromosomal breakpoints are clustered within a limited region, BCR, on chromosome 22. Cell. 1984;36(1):93–99. [DOI] [PubMed] [Google Scholar]
- 3. Heisterkamp N, Stephenson JR, Groffen J, et al. Localization of the c-abl oncogene adjacent to a translocation break point in chronic myelocytic leukaemia. Nature. 1983;306(5940):239–242. [DOI] [PubMed] [Google Scholar]
- 4. Lugo TG, Pendergast A-M, Muller AJ, Witte ON. Tyrosine kinase activity and transformation potency of bcr-abl oncogene products. Science. 1990;247(4946):1079–1082. [DOI] [PubMed] [Google Scholar]
- 5. O’brien SG, Guilhot F, Larson RA, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348(11):994–1004. [DOI] [PubMed] [Google Scholar]
- 6. Brave M, Goodman V, Kaminskas E, et al. Sprycel for chronic myeloid leukemia and Philadelphia chromosome-positive acute lymphoblastic leukemia resistant to or intolerant of imatinib mesylate. Clin Cancer Res. 2008;14(2):352–359. [DOI] [PubMed] [Google Scholar]
- 7. Nishiwaki S, Maeda M, Yamada M, et al. Clinical efficacy of fecal occult blood test and colonoscopy for dasatinib-induced hemorrhagic colitis in CML patients. Blood. 2017;129(1):126–128. doi:10.1182/blood-2016-08-734947. [DOI] [PubMed] [Google Scholar]
- 8. Quintás-Cardama A, Han X, Kantarjian H, Cortes J. Tyrosine kinase inhibitor-induced platelet dysfunction in patients with chronic myeloid leukemia. Blood. 2009;114(2):261–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rodeghiero F, Tosetto A, Abshire T, et al. ISTH/SSC bleeding assessment tool: a standardized questionnaire and a proposal for a new bleeding score for inherited bleeding disorders. J Thromb Haemost. 2010;8(9):2063–2065. [DOI] [PubMed] [Google Scholar]
- 10. Harrison P, Mackie I, Mumford A, et al. Guidelines for the laboratory investigation of heritable disorders of platelet function. Br J Haematol. 2011;155(1):30–44. [DOI] [PubMed] [Google Scholar]
- 11. Quintás-Cardama A, Kantarjian H, Ravandi F, et al. Bleeding diathesis in patients with chronic myelogenous leukemia receiving dasatinib therapy. Cancer. 2009;115(11):2482–2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hochhaus A, Saglio G, Hughes TP, et al. Long-term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5-year update of the randomized ENESTnd trial. Leukemia. 2016;30(5):1044–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wehmeier A, Daum I, Jamin H, Schneider W. Incidence and clinical risk factors for bleeding and thrombotic complications in myeloproliferative disorders. Ann Hematol. 1991;63(2):101–106. [DOI] [PubMed] [Google Scholar]
- 14. Soylu A, Buyukasik Y, Cetiner D, et al. Overt gastrointestinal bleeding in haematologic neoplasms. Dig Liver Dis. 2005;37(12):917–922. [DOI] [PubMed] [Google Scholar]
- 15. Baş B, Köksal A, Özatll D, et al. Thrombosis and hemorrhage in chronic myeloproliferative disorders. Clin Appl Thromb Hemost. 1999;5(4):282–284. [DOI] [PubMed] [Google Scholar]
- 16. Savage DG, Szydlo RM, Goldman JM. Clinical features at diagnosis in 430 patients with chronic myeloid leukaemia seen at a referral centre over a 16-year period. Br J Haematol. 1997;96(1):111–116. [DOI] [PubMed] [Google Scholar]
- 17. Drugs@FDA. Gleevec label information.
- 18. Guilhot F, Apperley J, Kim D-W, et al. Dasatinib induces significant hematologic and cytogenetic responses in patients with imatinib-resistant or -intolerant chronic myeloid leukemia in accelerated phase. Blood. 2007;109(10):4143–4150. [DOI] [PubMed] [Google Scholar]
- 19. Cortes J, Kim DW, Raffoux E, et al. Efficacy and safety of dasatinib in imatinib-resistant or -intolerant patients with chronic myeloid leukemia in blast phase. Leukemia. 2008;22(12):2176–2183. doi:10.1038/leu.2008.221. [DOI] [PubMed] [Google Scholar]