Abstract
Background and Aim:
Hyperglycemia in type 1 diabetes (T1D) is accompanied by endothelial cell dysfunction which is known to contribute to the pathogenesis of cardiovascular disorders. The aim of the current study was to explore the profile of circulating endothelial progenitor cells (EPCs), circulating endothelial cells (CECs), endothelial and platelet derived micropaticles (EMPs, PMPs) and total microparticles (TMPs), in T1D children in relation to each other and to the metabolic disorders accompanying T1D.
Patients and Methods:
Thirty T1D patients and 20 age and sex matched healthy volunteers were assessed for HbA1c level and lipid profile. Quantification of CECs, EPCs, TMPs, EMPs and PMPs was done by flow cytometry.
Results:
The mean levels of EMPs, PMPs, TMPs and CECs were significantly higher in diabetic children compared to controls. Meanwhile, the levels of EPCs were significantly lower in diabetic children compared to controls. Both PMPs and CECs showed the highest significant differences between patients and controls and their levels were directly related to HbA1c, total cholesterol, LDL and triglycerides. A moderate correlation was observed between the frequency of PMPs and CECs. EPCs revealed negative correlations with both LDL and triglycerides. TMPs were only related to LDL, while EMPs were only related to HbA1c.
Conclusion:
Although there is disturbance in the levels of EMPs, PMPs, TMPs, CECs and EPCs in type 1 diabetic children compared to the controls, only the levels of PMPs and CECs were closely affected by the poor glycemic control and dyslipidemia occurring in T1D; thus may contribute to a higher risk of cardiovascular diseases.
Keywords: T1D, microparticles, PMPs, EMPs, CECs, EPCs
Introduction
Diabetes mellitus is a chronic metabolic disease characterized by hyperglycemia with disorders in carbohydrate, protein, and lipid metabolism ultimately inducing chronic progressive damage of eyes, nerves, kidneys, heart, and blood vessels.1
Hyperglycemia is associated with endothelial cell dysfunction and impaired neovascularization in response to tissue ischemia,2 which are known to contribute to the pathogenesis of cardiovascular disorders (CVDs).3 Thereby, investigating endothelium and searching for endothelial-derived noninvasive biomarkers of vascular dysfunction, including endothelial-derived microparticles (EMPs), circulating endothelial cells (CECs), and circulating endothelial progenitors cells (EPCs), have gained growing interest.4
Circulating endothelial cells are mature cells shed from the lining of blood vessels and are present in very low numbers in healthy individuals but are increased intensely in a variety of diseases with vascular damage in which their frequency are closely linked to the severity of vascular lesions.5 Likewise, some elements such as vascular endothelial cells and circulating platelets release microsized particles (MPs) from the outward budding of plasma membranes in response to injurious stimuli and during cell apoptosis or activation.6 These microparticles were found to be increased in a number of diseases associated with endothelial dysfunction and CVD.7 On the other hand, circulating EPCs are bone marrow–derived cells that play important role in the vascular regeneration and neoangiogenesis. In addition, lower number of circulating EPCs was reported to be associated with increased risk of CVD.8
Few studies have investigated the CECs, EPCs, EMPs, and platelet derived microparticles (PMPs) individually in type 1 diabetes (T1D)9–11; therefore, the present work aims to explore the profile of these potential biomarkers in children with T1D in relation to each other and to the metabolic disorders accompanying T1D and posing vascular risk.
Patients and Methods
Study Population
This study was done in Assiut University Hospitals on 30 patients with T1D, diagnosed according to the criteria of American Diabetes Association for diagnosis of T1D,12 presented to Pediatric Emergency Unit, Children Hospital, Faculty of Medicine, Assiut University, in the period from June 2017 to April 2018. Pediatric diabetic patients (age ≤18 years) were included. Patients with infection, other concurrent diseases, different age range or previously diagnosed as having type 2 diabetes (T2D) were excluded from the study. Additionally, 20 age- and sex-matched healthy volunteers were included as controls. The study was approved by the institutional review board of Assiut University, and informed written consents were taken from all guardians of cases and controls.
Baseline Investigations
All patients and controls were subjected to detailed history taking and physical examination. Evaluation of HbA1c was performed by turbidimetric inhibition immunoassay using Hitachi autoanalyzer (Roch, Germany), and lipid profile was performed using Cobas Integra 400, automated chemical analyzer (S.N.: 500558; Roche Diagnostics GmbH, Mannheim, Germany). In addition, flow cytometry (FACSCalibur, Becton Dickinson [BD], San Jose, California) was used for characterization and quantification of MPs and detection of CECs and EPCs.
Flow Cytometric Analysis
Microparticles isolation and characterization
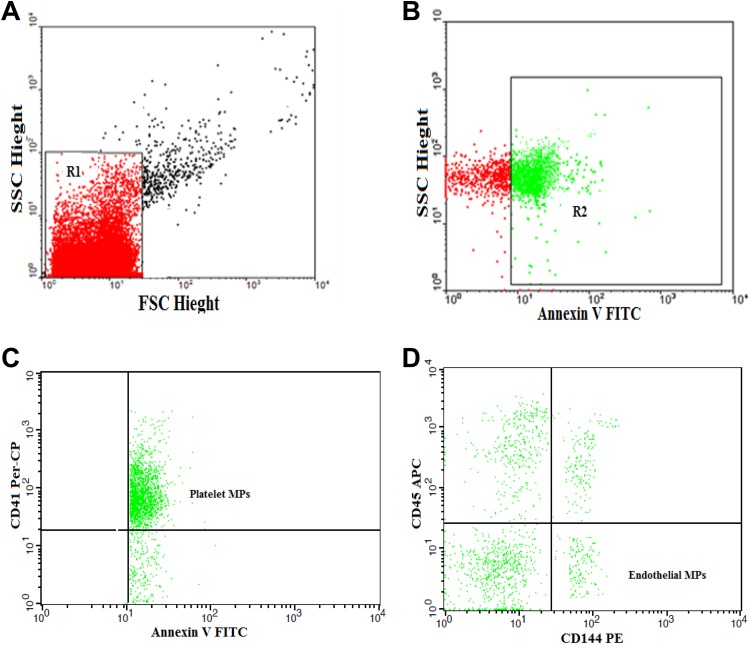
Citrated blood samples were used to isolate MPs within 15 minutes after collection. After centrifugation at 1550g for 20 minutes at 20°C, the cells were separated and 250 µL of plasma were centrifuged twice for 30 minutes at 18 800g at 20°C. The supernatant was discarded again and MPs pellet was resuspended in phosphate-buffered saline (PBS). Five microliter of MPs sample were diluted in 35-µL PBS containing 2.5 mM CaCl2 and incubated for 20 minutes with 5 µL of fluoroisothiocyanate (FITC)-conjugated annexin V (IQ products, the Netherlands), peridinin-chlorophyll-protein (Per-CP)-conjugated CD41, phycoerythrin (PE)-conjugated CD144, and allophycocyanin (APC)-conjugated CD45 (BD Biosciences). FACSCaliber flow cytometry with Cell Quest software (BD Biosciences) was used to quantify and characterize MPs. Fifty thousand events were analyzed. Isotype-matched antihuman immunoglobulin G (IgG) negative controls were used with each sample. Total MPs (TMPs) were identified on the basis of their size compared to calibrate reference beads of 1.0 µm (Latex beads, amine-modified polystyrene, fluorescent red aqueous suspension, 1.0-μm mean particle size; Sigma-Aldrich Chemie Gmbh Munich, Germany) and their positivity for annexin V. The TMPs were reported as a percentage of the total events. Endothelial derived MPs were detected as CD45− CD144+ MPs. The PMPs were detected as CD41+ MPs. The EMPs and PMPs were expressed as percentage of TMPs (Figure 1).
Figure 1.
Flow cytometric analysis of microparticles. A, Forward and side scatter histogram was used to define the MPs (R1) compared with the size of the reference calibrate bead. B, Events defined as MPs were then assessed for their expression of annexin V. C and D, Then annexin V-positive MPs (total MPs; R2) were further examined for the expression of cell-specific antibodies as CD41, CD144, and CD45.
Detection of CECs and circulating EPCs
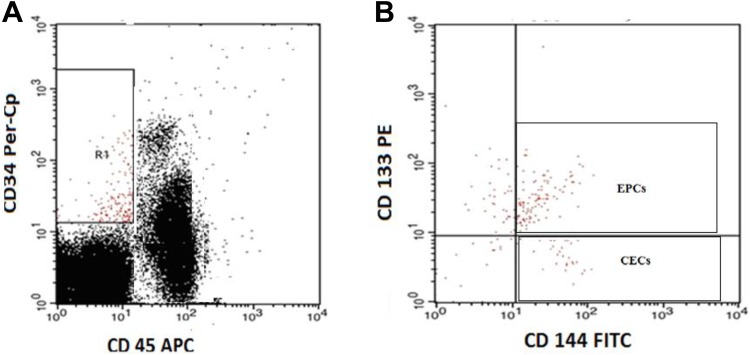
Blood samples were collected from freshly placed venous cannulas. Fifty microliters of blood sample were incubated with 5 µL of FITC-labeled CD144 (BD Biosciences), PE-conjugated CD133 (AC133; Miltenyi Biotec GmbH, Bergisch Gladbach, Germany), Per-CP-conjugated CD34 (BD Biosciences), and APC-conjugated CD45 (BD Biosciences) for 20 minutes. After incubation, RBC lysis and washing were done and the cells were suspended in PBS, and FACSCalibur flow cytometric analysis was done with Cell Quest software (BD Biosciences). Antihuman IgG was used as an isotype negative control and 50 000 events were analyzed. The EPCs are negative for CD45, positive for CD144, CD34, and CD133 (CD45– CD34+ CD144+ CD133+), while CECs are negative for CD45, positive for CD144 and CD34 and negative for CD133 (CD45– CD34+ CD144+ CD133–). The CECs and EPCs were expressed as absolute count per 50 000 cells (Figure 2).
Figure 2.
Flow cytometric detection of circulating endothelial cells (CECs) and circulating endothelial progenitor cells (EPCs). A, CD34+CD45− cells were gated (R1) for further analysis of the expression of CD144 and CD133. B, The expression of CD144 and CD133 on R1 gate was assessed to detect CECs that were identified as CD45−, CD34+, CD144+, and CD133− and EPCs which identified as CD45−, CD34+, CD144+, and CD133+.
Statistical Analysis
Statistical Package for Social Sciences, version 24.0 (IBM SPSS, Chicago, Illinois) was used for the statistical analysis. Results were expressed as mean (standard deviation). Student t test and χ2 test were used to compare continuous variables and categorical variables, respectively. Analysis of associations between the variables was done by the Pearson correlation coefficient. A P value was considered significant if less than .05.
Results
Clinical and Laboratory Characteristics of Study Population
In the study patients, the mean duration of T1D was 1.7 years. The mean levels of total cholesterol, low-density lipoprotein cholesterol (LDL), HDL cholesterol, and triglycerides were significantly higher in diabetic children compared to controls. Table 1 shows the clinical and laboratory characteristics of participants.
Table 1.
Baseline Clinical and Laboratory Characteristics of Children With T1D and Controls.a,b,c
| Parameters | T1D Cases (N = 30) | Controls (N = 20) | P Value |
|---|---|---|---|
| Age (years) | 8.9 (2.7) | 7.8 (2.1) | .2 NS |
| Sex | |||
| Male | 8 (40%) | 10 (50%) | – |
| Female | 12 (60%) | 10 (50%) | – |
| Duration of T1D (years) | 1.76 (1) | – | – |
| HbA1c (%) | 9.3 (1) | 5.4 (0.5) | <.0001 HS |
| Platelet count (×109/L) | 200.7 (70) | 218 (66) | .1 NS |
| WBCs (×109/L) | 7.8 (2) | 6.3 (2) | .2 NS |
| Total cholesterol (mg/dL) | 160.6 (9) | 148.3 (8) | <.0001 HS |
| LDL cholesterol (mg/dL) | 89.4 (9) | 76 (6.4) | <.0001 HS |
| HDL cholesterol (mg/dL) | 36.9 (3) | 37.6 (2) | .4 NS |
| Triglycerides (mg/dL) | 77.6 (6) | 64.8 (6) | <.0001 HS |
Abbreviations: HbA1c, glycated hemoglobin A1c; HDL, high-density lipoprotein cholesterol; HS, highly significant; LDL, low-density lipoprotein cholesterol; N, number; NS, not significant; T1D, type 1 diabetes.
aAll bold values are highly significant <0.01 (denoted by HS)
bResults expressed as mean (SD).
cStudent t test significant P value <.05.
Complications were recorded in 9 children with history of T1D more than 5 years and higher HbA1c levels than those with noncomplicated T1D. All were not on a regular insulin therapy. Hypertension was detected in 3 children, 2 had cerebrovascular stroke, 3 of them had impaired renal functions, and 1 had peripheral polyneuropathy.
Frequency of Microparticles, CECs, and Circulating EPCs
As shown in Table 2, the mean levels of EMPs, PMPs, TMPs, and CECs were significantly higher in diabetic children compared to controls. Meanwhile, the levels of EPCs were significantly lower in diabetic children compared to controls.
Table 2.
Microparticles, CECs, and Circulating EPCs in Children With T1D Compared to Controls.a,b,c
| Parameters | T1D Cases (N = 30) | Controls (N = 20) | P Value |
|---|---|---|---|
| PMPs (%) | 76.3 (8) | 56.8 (5) | <.0001 HS |
| EMPs (%) | 20.6 (6) | 15.2 (5) | .003 HS |
| TMPs (%) | 58.6 (12) | 49 (9) | .01 S |
| CECs (%) | 71.2 (17) | 21.5 (7) | <.0001 HS |
| EPCs (%) | 12.5 (3) | 28.3 (7) | .01 S |
Abbreviations: CECs, circulating endothelial cells; EMPs, endothelial-derived microparticles; EPCs, endothelial progenitors cells; HS, highly significant; N, number; PMPs, platelet microparticles; TMPs, total microparticles; S, significant; T1D, type 1 diabetes.
aBold values <0.05 are significant (denoted by S). Bold values <0.01 are highly significant (denoted by HS).
bResults expressed as mean (SD).
cStudent t test significant P value <.05.
Besides, several significant correlations were detected among the above tested parameters, as summarized in Table 3. The PMPs have shown correlations with all other tested parameters. Moderate correlations were observed between the frequency of PMPs and each of CECs (positive correlation) and EPCs (negative correlation). Furthermore, a moderate negative correlation was found between the frequencies of EPCs and CECs.
Table 3.
Correlations Among the Frequencies of Microparticles, CECs, and Circulating EPCs.a,b
| Parameters | PMPs | EMPs | TMPs | CECs | EPCs | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| PMPs | – | r = 0.4 | r = 0.3 | r = 0.6 | r = −0.5 | |||||
| P = .002 | HS | P = .04 | S | P < .0001 | HS | P = .001 | HS | |||
| EMPs | r = 0.4 | – | r = 0.3 | r = 0.2 | r = 0.03 | |||||
| P = .002 | HS | P = .02 | S | P = .01 | S | P = .4 | NS | |||
| TMPs | r = 0.3 | r = 0.3 | – | r = 0.3 | r = −0.2 | |||||
| P = .04 | S | P = .02 | S | P = .02 | S | P = .06 | NS | |||
| CECs | r = 0.6 | r = 0.2 | r = 0.3 | – | r = −0.6 | |||||
| P < .0001 | HS | P = .01 | S | P = .02 | S | P < .0001 | HS | |||
| EPCs | r = −0.5 | r = 0.03 | r = −0.2 | r = −0.6 | – | |||||
| P = .001 | HS | P = .4 | NS | P = .06 | NS | P < .0001 | HS | |||
Abbreviations: CECs, circulating endothelial cells; EMPs, endothelial-derived microparticles; EPCs, endothelial progenitors cells; HS, highly significant; NS, not significant; PMPs, platelet microparticles; r, Pearson correlation coefficient; S, significant; TMPs, total microparticles.
aBold values <0.05 are significant (denoted by S). Bold values <0.01 are highly significant (denoted by HS).
bSignificant P value <.05.
Correlations of the Frequencies of Microparticles, CECs, and Circulating EPCs With Some Laboratory Findings
Table 4 shows the relations between the frequencies of MPs, CECs, and circulating EPCs with some laboratory findings. Both PMPs and CECs showed significant positive correlations with HbA1c, total cholesterol, LDL, and triglycerides. The EPCs have only revealed negative correlations with both LDL and triglycerides. The TMPs level was only related to LDL whereas EMPs level was only related to HbA1c.
Table 4.
Correlations Between Studied Parameters in Children With Type 1 Diabetes.a,b
| Parameters | HbA1c | Total Cholesterol | LDL Cholesterol | HDL Cholesterol | Triglycerides |
|---|---|---|---|---|---|
| PMPs | r = 0.6 | r = 0.4 | r = 0.5 | r = 0.01 | r = 0.6 |
| P < .0001 | P = .009 | P < .0001 | P = .9 | P < .0001 | |
| HS | HS | HS | NS | HS | |
| EMPs | r = 0.4 | r = 0.2 | r = 0.29 | r = 0.07 | r = 0.2 |
| P = .007 | P = .2 | P = .07 | P = .7 | P = .2 | |
| HS | NS | NS | NS | NS | |
| TMPs | r = 0.3 | r = 0.2 | r = 0.4 | r = 0.16 | r = 0.04 |
| P = .03 | P = .9 | P = .005 | P = .1 | P = .8 | |
| S | NS | HS | NS | NS | |
| CECs | r = 0.7 | r = 0.5 | r = 0.6 | r = 0.1 | r = 0.7 |
| P < .0001 | P < .0001 | P < .0001 | P = .5 | P < .0001 | |
| HS | HS | HS | NS | HS | |
| EPCs | r = −0.29 | r = −0.05 | r = −0.4 | r = −0.27 | r = −0.4 |
| P = .07 | P = .7 | P = .01 | P = .09 | P = .01 | |
| NS | NS | S | NS | S |
Abbreviations: CECs, circulating endothelial cells; EMPs, endothelial-derived microparticles; EPCs, endothelial progenitors cells; HbA1c, glycated hemoglobin A1c; HDL, high-density lipoprotein cholesterol; HS, highly significant; LDL, low-density lipoprotein cholesterol; NS, not significant; PMPs, platelet microparticles; r, Pearson correlation coefficient; S, significant; TMPs, total microparticles.
aBold values <0.05 are significant (denoted by S). Bold values <0.01 are highly significant (denoted by HS).
bSignificant P value <.05.
Discussion
Despite the higher risk of CVD observed in patients with T1D compared with T2D,13,14 few studies have individually investigated the CECs, EPCs, EMPs, and PMPs in T1D.9–11 The ability to explore the endothelium using noninvasive approaches will aid better understanding of the pathology of CVD. By using multiple markers in assessing vascular competence, it would be possible to detect endothelial dysfunction at early preclinical stages, evaluate the vascular risk at later stages of CVD, and evaluate available therapeutic options.4
Scarce data are available about the association of microparticles and T1D.9,15 Levels of EMPs, PMPs, and TMPs were significantly higher in our children with T1D compared to healthy children, which comes in line with earlier studies in T2D16,17 and T1D9,15 that considered these microparticles as early predictors of microvascular complications and subclinical atherosclerosis. The role played by MPs in the normal hemostasis in response to vascular injury and in CVD may be because they express procoagulant phospholipids, thereby increasing the risk of thromboembolic complications.18,19
Similar to previous studies, we found increased levels of CECs11 and decreased levels of EPCs10,20–24 in children with T1D compared to healthy controls. On the contrary, only Głowińska-Olszewska and colleagues25 have shown increased frequency of EPCs in children with T1D.
Both PMPs and CECs showed the highest significant differences between patients and controls compared to TMPS, EMPS, and EPCs. In addition, they have shown direct relations with HbA1c, total cholesterol, LDL, and triglycerides, unlike EPCs which have revealed negative correlations with both LDL and triglycerides. The TMPs were only related to LDL and EMPs were only related to HbA1c. A moderate correlation was also observed between the frequency of PMPs and CECs. These results may indicate that the levels of PMPs and CECs are closely affected by the poor glycemic control and dyslipidemia occurring in T1D and may contribute to a higher risk of CVD. Consequently, they together may represent good integrative potential biomarkers for endothelial dysfunction, vascular incompetence, and increased procoagulant activity. Worth mentioning, CECs showed stronger correlations with HbA1c and parameters of the lipid profile compared to PMPs.
Likewise, some previous studies reported positive correlations between HbA1c and each of CECs11 and microparticles.9,26 Taniyama and Griendling27 proposed that the mechanism by which the frequency of CECs increases in children with T1D may be related to hyperglycemia which enhances oxidative stress and increases sloughing of endothelial cells. On the contrary, an earlier study28 did not find the increase in CECs to be related to HbA1c in patients with T2D and suggested that even with the control of blood glucose levels, endothelial cell shedding will probably not decrease.
Prior clinical studies have shown conflicting results regarding the relation between the levels of EPCs and HbA1c. While Arcangeli et al,10 in agreement with our results, didn’t find a relation between their levels, other studies20,21 found the level of EPCs to be inversely associated with HbA1c.
In this study, the positive correlations detected between PMPs and CECs in relation to LDL and triglycerides levels may explain the role of LDL and triglycerides in initiating atherosclerosis, through increasing the level of microparticles and CECs that promotes endothelial dysfunction and inflammation of arterial wall.29,30 Also, the finding of negative correlations between the level of EPCs and each of LDL and triglycerides was in line with a previous study,31 which concluded that LDL impairs EPCs function, at least partly, by causing oxidative stress and activating NF-κB pathway and hence ruins host repair of endothelial injury.
Conclusion
Although there is disturbance in the levels of EMPs, PMPs, TMPs, CECs, and EPCs in T1 diabetic children compared to controls, only the levels of PMPs and CECs are closely affected by the poor glycemic control and dyslipidemia occurring in T1D and thus may contribute to a higher risk of CVD.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2009;32(suppl 1):S62–S67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bakker W, Eringa EC, Sipkema P, van Hinsbergh VW. Endothelial dysfunction and diabetes: roles of hyperglycemia, impaired insulin signaling and obesity. Cell Tissue Res. 2009;335(1):165–189. [DOI] [PubMed] [Google Scholar]
- 3. Landmesser U, Drexler H. Endothelial function and hypertension. Curr Opin Cardiol. 2007;22(4):316–320. [DOI] [PubMed] [Google Scholar]
- 4. Sabatier F, Camoin-Jau L, Anfosso F, Sampol J, Dignat-George F. Circulating endothelial cells, microparticles and progenitors: key players towards the definition of vascular competence. J Cell Mol Med. 2009;13(3):454–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Woywodt A, Streiber F, de Groot K, Regelsberger H, Haller H, Haubitz M. Circulating endothelial cells as markers for ANCA-associated small-vessel vasculitis. Lancet. 2003;361(9353):206–210. [DOI] [PubMed] [Google Scholar]
- 6. György B, Szabó TG, Pásztói M, et al. Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell Mol Life Sci. 2011;68(16):2667–2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Diamant M, Tushuizen ME, Sturk A, Nieuwland R. Cellular microparticles: new players in the field of vascular disease? Eur J Clin Invest. 2004;34(6):392–401. [DOI] [PubMed] [Google Scholar]
- 8. Hill JM, Zalos G, Halcox JP, et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348(7):593–600. [DOI] [PubMed] [Google Scholar]
- 9. Sabatier F, Darmon P, Hugel B, et al. Type 1 and type 2 diabetic patients display different patterns of cellular microparticles. Diabetes. 2002;51(9):2840–2845. [DOI] [PubMed] [Google Scholar]
- 10. Arcangeli A, Lastraioli E, Piccini B, et al. Circulating endothelial progenitor cells in type 1 diabetic patients: relation with patients’ age and disease duration. Front Endocrinol. 2017;8:278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Asicioglu E, Yavuz DG, Koc M, et al. Circulating endothelial cells are elevated in patients with type 1 diabetes mellitus. Eur J Endocrinol. 2010;162(4):711–717. [DOI] [PubMed] [Google Scholar]
- 12. American Diabetes Association. 2. Classification and diagnosis of diabetes. Diabetes Care. 2015;38(suppl 1):S8–S16. [DOI] [PubMed] [Google Scholar]
- 13. De Ferranti SD, De Boer IH, Fonseca V, et al. Type 1 diabetes mellitus and cardiovascular disease: a scientific statement from the American heart association and American Diabetes Association. Circulation. 2014;130(13):1110–1130. [DOI] [PubMed] [Google Scholar]
- 14. Orchard TJ, Costacou T, Kretowski A, Nesto RW. Type 1 diabetes and coronary artery disease. Diabetes Care. 2006;29(11):2528–2538. [DOI] [PubMed] [Google Scholar]
- 15. Salem MAEK, Adly AAM, Ismail EAR, Darwish YW, Kamel HA. Platelets microparticles as a link between micro-and macro-angiopathy in young patients with type 1 diabetes. Platelets. 2015;26(7):682–688. [DOI] [PubMed] [Google Scholar]
- 16. Li S, Wei J, Zhang C, et al. Cell-derived microparticles in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Cell Physiol Biochem. 2016;39(6):2439–2450. [DOI] [PubMed] [Google Scholar]
- 17. Tramontano A, Lyubarova R, Tsiakos J, Palaia T, Deleon JR, Ragolia L. Circulating endothelial microparticles in diabetes mellitus. Mediators Inflamm. 2010;2010:250476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nomura S. Dynamic role of microparticles in type 2 diabetes mellitus. Curr Diabetes Rev. 2009;5(4):245–251. [DOI] [PubMed] [Google Scholar]
- 19. Enjeti AK, Seldon M. Microparticles: role in haemostasis and venous thromboembolism In: Abdelaal MA, eds. Pathophysiology and Clinical Aspects of Venous Thromboembolism in Neonates, Renal Disease and Cancer Patients. IntechOpen Limited; 2012. [Google Scholar]
- 20. Hörtenhuber T, Rami-Mehar B, Satler M, et al. Endothelial progenitor cells are related to glycemic control in children with type 1 diabetes mellitus over time. Diabetes Care. 2013;36(6):1647–1653. DC_121206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Loomans CJ, de Koning EJ, Staal FJ, et al. Endothelial progenitor cell dysfunction: a novel concept in the pathogenesis of vascular complications of type 1 diabetes. Diabetes. 2004;53(1):195–199. [DOI] [PubMed] [Google Scholar]
- 22. Sibal L, Aldibbiat A, Agarwal SC, et al. Circulating endothelial progenitor cells, endothelial function, carotid intima-media thickness and circulating markers of endothelial dysfunction in people with type 1 diabetes without macrovascular disease or microalbuminuria. Diabetologia. 2009;52(8):1464–1473. [DOI] [PubMed] [Google Scholar]
- 23. DiMeglio LA, Tosh A, Saha C, et al. Endothelial abnormalities in adolescents with type 1 diabetes: a biomarker for vascular sequelae? J Pediatr. 2010;157(4):540–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Palombo C, Kozakova M, Morizzo C, et al. Circulating endothelial progenitor cells and large artery structure and function in young subjects with uncomplicated type 1 diabetes. Cardiovasc Diabetol. 2011;10:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Glowinska-Olszewska B, Moniuszko M, Hryniewicz A, et al. Relationship between circulating endothelial progenitor cells and endothelial dysfunction in children with type 1 diabetes: a novel paradigm of early atherosclerosis in high-risk young patients. Eur J Endocrinol. 2013;168(2):153–161. [DOI] [PubMed] [Google Scholar]
- 26. Feng B, Chen Y, Luo Y, Chen M, Li X, Ni Y. Circulating level of microparticles and their correlation with arterial elasticity and endothelium-dependent dilation in patients with type 2 diabetes mellitus. Atherosclerosis. 2010;208(1):264–269. [DOI] [PubMed] [Google Scholar]
- 27. Taniyama Y, Griendling KK. Reactive oxygen species in the vasculature: molecular and cellular mechanisms. Hypertension. 2003;42(6):1075–1081. [DOI] [PubMed] [Google Scholar]
- 28. McClung JA, Naseer N, Saleem M, et al. Circulating endothelial cells are elevated in patients with type 2 diabetes mellitus independently of HbA(1)c. Diabetologia. 2005;48(2):345–350. [DOI] [PubMed] [Google Scholar]
- 29. Goon P, Boos CJ, Lip G. Circulating endothelial cells: markers of vascular dysfunction. Clin Lab. 2005;51(9-10):531–538. [PubMed] [Google Scholar]
- 30. VanWijk MJ, VanBavel E, Sturk A, Nieuwland R. Microparticles in cardiovascular diseases. Cardiovasc Res. 2003;59(2):277–287. [DOI] [PubMed] [Google Scholar]
- 31. Ji K-t, Qian L, Nan J-l, et al. Ox-LDL induces dysfunction of endothelial progenitor cells via activation of NF-κB. Biomed Res Int. 2015;2015:175291. [DOI] [PMC free article] [PubMed] [Google Scholar]