Abstract
Optimizing diagnostic criteria to detect specific patients likely to benefit from anticoagulants is warranted. A cutoff of 5 points for the International Society on Thrombosis and Haemostasis overt disseminated intravascular coagulation (DIC) scoring system was determined in the original article, but its validity was not evaluated. This study aimed to explore the optimal cutoff points of DIC scoring systems and evaluate the effectiveness of early intervention with anticoagulants. We used a nationwide retrospective registry of consecutive adult patients with sepsis in Japan to develop simulated survival data, assuming anticoagulants were conducted strictly according to each cutoff point. Estimated treatment effects of anticoagulants for in-hospital mortality and risk of bleeding were calculated by logistic regression analysis with inverse probability of treatment weighting using propensity scoring. Of 2663 patients with sepsis, 1247 patients received anticoagulants and 1416 none. The simulation model showed no increase in estimated mortality between 0 and 3 cutoff points, whereas at ≥4 cutoff points, mortality increased linearly. The estimated bleeding tended to decrease in accordance with the increase in cutoff points. The optimal cutoff for determining anticoagulant therapy may be 3 points to minimize nonsurvival with acceptable bleeding complications. The findings of the present study suggested a beneficial association of early intervention with anticoagulant therapy and mortality in the patients with sepsis-induced DIC. Present cutoff points of DIC scoring systems may be suboptimal for determining the start of anticoagulant therapy and delay its initiation.
Keywords: anticoagulants, coagulopathy, critically ill, diagnostic criteria, DIC, septic shock
Background
In the pathophysiology of sepsis, hemostatic abnormalities arise through the activation of inflammatory responses and vascular endothelial cell injury, which play critical roles in inducing organ dysfunctions and subsequent death.1–4 According to these theoretical mechanisms, anticoagulant therapies are expected to be beneficial in the treatment of sepsis, but most have failed in the past randomized control trials.5–7 However, several exploratory analyses reported that anticoagulant therapies might be beneficial when focused on certain specific subpopulations in sepsis.8–11 We previously reported that disseminated intravascular coagulation (DIC) is one of the most appropriate targets for anticoagulant therapies as determined by a meta-analysis12 and observational studies.13 Thus, if we consider the adequate target patients for anticoagulant therapy, optimization of the diagnostic criteria to detect patients with sepsis likely to have DIC is warranted.
Several organizations have proposed DIC scoring systems. Most classically, the Japanese Ministry of Health and Welfare proposed criteria for the diagnosis of DIC in 1983.14 Thereafter, the subcommittee of the International Society on Thrombosis and Haemostasis (ISTH) proposed a scoring system for overt and non-overt DIC in 2001,15 and the Japanese Association for Acute Medicine (JAAM) proposed another DIC scoring system in 2006 that was aimed at making an early diagnosis of DIC.16 In the JAAM DIC criteria, detection of the early stage of DIC was emphasized to maximize the treatment effects of anticoagulant therapy. However, little is known about whether early intervention with anticoagulant therapy is preferable to late intervention in sepsis.
Actually, the cutoff point values for the initiation of anticoagulant therapies of the ISTH overt and JAAM DIC scoring systems were determined by the original papers; 5 points for patients with sepsis in the ISTH overt DIC and 4 points in the JAAM DIC scoring system. In the present analysis, we hypothesized that other cutoff points for each set of DIC criteria might be optimal for minimizing nonsurvivors and bleeding complications. Therefore, this study aimed to establish the optimal cutoff values of the ISTH overt and JAAM DIC scoring systems via a simulation method using a nationwide sepsis data set.
Materials and Methods
Study Population
This investigation was performed using a data set from a multicenter nationwide retrospective cohort study (the Japan Septic Disseminated Intravascular Coagulation [J-Septic DIC] registry17) conducted in 42 intensive care units (ICUs) in Japan between January 2011 and December 2013. Patients were eligible for the registry if they were diagnosed as having severe sepsis or septic shock according to the conventional criteria proposed by the American College of Chest Physicians/Society of Critical Care Medicine consensus conference in 1991 and were 18 years of age or older.18 The exclusion criteria were use of warfarin/acetylsalicylic acid/thrombolytic therapy before study entry; a history of fulminant hepatitis, decompensated liver cirrhosis, or other serious liver disorder; a history of hematologic malignant disease; other conditions increasing the risk of bleeding; treatment with any chemotherapy at study entry; treatment with warfarin before/after study entry; and patients with missing data for the main evaluation.
This study followed the principles of the Declaration of Helsinki and was approved by the institutional review board of each participating hospital (#25-2050 for Osaka General Medical Center). Because of the anonymous and retrospective nature of this study, the board of each hospital waived the need for informed consent.
Data Collection and Definitions
Patients were followed up until hospital discharge or death. A dedicated case report form was developed for use in this study, and the following information was obtained: age, sex, multiple illness severity scores on the day of ICU admission, source of ICU admission, preexisting comorbidities, primary source of infection, new organ dysfunction, and concomitant therapeutic interventions against sepsis. The severity of illness was evaluated according to the Acute Physiology and Chronic Health Evaluation (APACHE) II score19 and systemic inflammatory response syndrome (SIRS) score. Organ dysfunction, defined as a Sequential Organ Failure Assessment (SOFA) subscore for each organ of ≥2, was assessed according to the SOFA score.20
Disseminated intravascular coagulation was also evaluated based on the criteria of the ISTH overt DIC and JAAM DIC scoring systems at the time of ICU admission (Table 1). The ISTH overt DIC scoring system was adopted as proposed by the Scientific Subcommittee on DIC of the ISTH for platelet counts, prothrombin time (PT), fibrin/fibrinogen degradation product (FDP), and fibrinogen level.15 Fibrin/fibrinogen degradation product values were chosen as the fibrin-related marker and scored according to the cutoff levels and ranges previously published by Gando et al.21 The JAAM DIC scoring system consists of the SIRS score and global coagulation tests, including platelet counts, PT, and FDP levels.16
Table 1.
ISTH Overt and JAAM DIC Scoring Systems.
| Factors | Points | ISTH Overt DIC | JAAM DIC |
|---|---|---|---|
| Platelet counts | 3 | – | <80 ×109/L or > 50% decrease/24 hours |
| 2 | <50 × 109/L | – | |
| 1 | ≥50, <100 × 109/L | ≥80, <120 ×109/L or 30%-50% decrease/24 hours | |
| FDP | 3 | Strong increase | ≥25 μg/mL |
| 2 | Moderate increase | – | |
| 1 | – | ≥10, <25 μg/mL | |
| Prothrombin timea | 2 | ≥6 seconds | – |
| 1 | ≥3, <6 seconds | ≥1.2 | |
| Fibrinogen | 1 | <100 g/mL | – |
| SIRS score | 1 | – | ≥3 |
| Points required to be criteria positive | 5 points | 4 points |
Abbreviations: DIC, disseminated intravascular coagulation; FDP, fibrin/fibrinogen degradation product; ISTH, International Society on Thrombosis and Haemostasis; JAAM, Japanese Association for Acute Medicine; SIRS, systemic inflammatory response syndrome.
a Prothrombin time was assessed in seconds above normal value for ISTH overt DIC and by international normalized ratio for JAAM DIC.
The primary outcome measure was all-cause in-hospital mortality. We also recorded as secondary outcomes any bleeding complications, including the occurrence of intracranial hemorrhage, transfusion requirements related to bleeding, and bleeding requiring surgical intervention.
Developing the Simulation Models
In this study, an optimal threshold for initiating anticoagulant therapies was investigated according to the scoring systems of both the ISTH overt DIC and the JAAM DIC criteria. Participants were stratified based on the score from each DIC scoring system and then categorized into 1 of 2 groups: the anticoagulant group, comprising patients who received any anticoagulant therapies such as antithrombin, recombinant human thrombomodulin, heparin/heparinoid, and serine protease inhibitors (nafamostat mesilate and gabexate mesilate), and the non-anticoagulant group, comprising patients who received no anticoagulant therapies. Because of the retrospective nature of this study, there was no predefined protocol for these interventions.
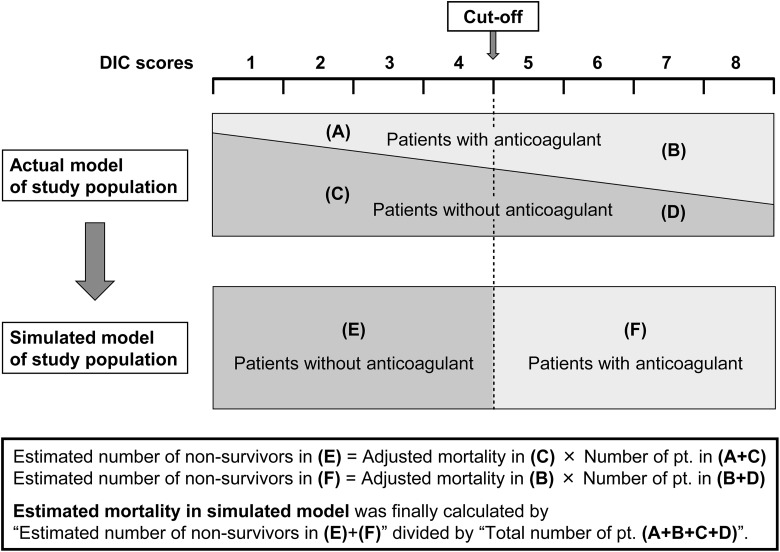
To determine the optimal cutoff points of each scoring system for initiating anticoagulant therapies, we developed simulated survival data, assuming that the anticoagulant therapies were conducted strictly according to each cutoff point (Figure 1). For instance, if we set the cutoff to equal 5 points in the ISTH overt DIC scoring system, we assumed that all patients with a score of 5 points or more received anticoagulant therapies and that all patients with a score of 4 or less did not. First, the estimated total number of nonsurvivors was calculated as the sum of the following 2 patient numbers: (1) “the adjusted mortality rate of patients with a score <5 and not receiving any anticoagulant therapies (mortality in population C in Figure 1)” × “the actual number of patients with a score <5 (sum of populations A + C)” and (2) “the adjusted mortality of patients with a score ≥5 who received anticoagulant therapies (mortality in population B)” × “the actual number of patients with a score ≥5 (sum of populations B + D).” Then, the estimated mortality was calculated as the estimated number of nonsurvivors mentioned above divided by the total population (sum of populations A + B + C + D). We conducted this estimation for all point values of the ISTH overt DIC score and the JAAM DIC score to evaluate the best cutoff point for initiating anticoagulant therapy.
Figure 1.
Schema of the simulation algorithm used in this study. DIC indicates disseminated intravascular coagulation.
Statistical Analysis
Because this was a retrospective study, imbalances were present between the different patient groups at baseline. To account for this imbalance, propensity scoring was used in the adjusted mortality analysis. The propensity score for the likelihood of receiving anticoagulant therapies was calculated using multivariable logistic regression and included several possible confounding variables such as age, sex, severity of illness, preexisting comorbidities, low-dose heparin for prophylaxis against venous thromboembolism, and other concomitant therapeutic interventions as covariates. We also included the types and volume of ICUs for logistic regression to estimate the propensity score. The detailed combinations of the variables are described in Table S1. The adjusted mortality and risk of bleeding complications for patients with and without anticoagulant therapy was calculated by logistic regression analysis with inverse probability of treatment weighting using the propensity score.
Descriptive statistics were calculated as medians with interquartile range or proportions, as appropriate. Univariate differences between groups were assessed using the Mann-Whitney U test, Kruskal-Wallis test, χ2 test, or Fisher exact test. A P value of <.05 indicated statistical significance. All statistical analyses were performed using STATA Data Analysis and Statistical Software version 14.0 (StataCorp, College Station, Texas).
Results
Baseline Characteristics
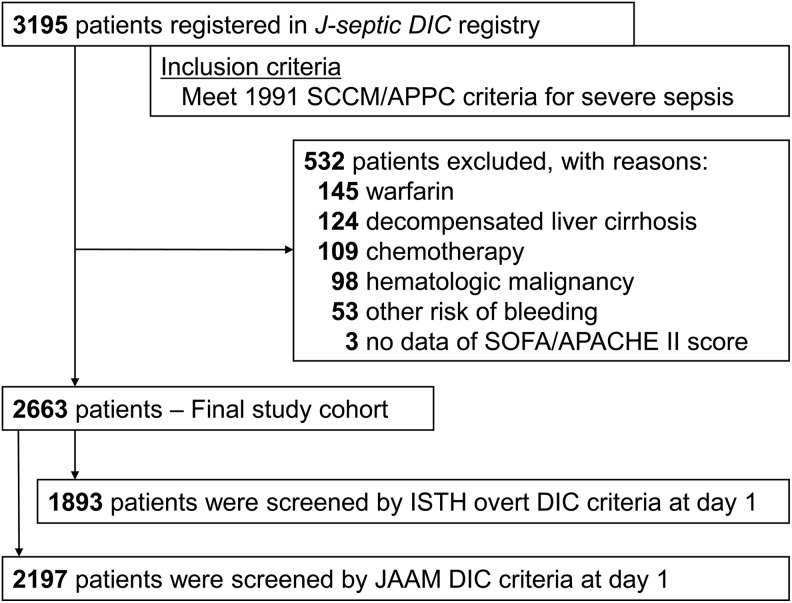
The patient flow diagram is shown in Figure 2. During the study period, 3195 consecutive patients were registered to the J-Septic DIC registry database. After excluding 532 patients who met at least 1 exclusion criterion, we analyzed 2663 patients as the study cohort. Among them, 1893 (71.1%) patients had sufficient information in their records in the registry database to calculate the exact score using the ISTH overt DIC criteria, and 2197 (82.5%) patients had that to calculate the exact score using the JAAM DIC criteria. Table 2 and Table S2 show the baseline characteristics of the study patients stratified by the scores of the ISTH overt and JAAM DIC criteria, respectively.
Figure 2.
Patient flow diagram. APACHE indicates Acute Physiology and Chronic Health Evaluation; DIC, disseminated intravascular coagulation; JAAM, Japanese Association for Acute Medicine; ISTH, International Society of Thrombosis and Hemostasis; SCCM/ACCP, Society of Critical Care Medicine/American College of Chest Physicians; SOFA, Sequential Organ Failure Assessment.
Table 2.
Baseline Characteristics in Patients With and Without Anticoagulant Therapy by Each Cutoff Point of the ISTH Overt DIC Scoring System.
| Characteristics | ISTH Overt DIC score | Total | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | ||
| n = 41 | n = 193 | n = 310 | n = 326 | n = 275 | n = 361 | n = 306 | n = 127 | n = 258 | n = 1893 | |
| Patient characteristics | ||||||||||
| Age (years) | 71 (61-79) | 70 (59-79) | 71 (60-81) | 73 (63-80) | 71 (63-80) | 72 (60-80) | 72 (64-81) | 74 (60-82) | 65 (44-71) | 72 (62-80) |
| Sex, male | 102 (63%) | 88 (67%) | 149 (61%) | 214 (58%) | 257 (59%) | 166 (57%) | 96 (55%) | 38 (52%) | 9 (56%) | 1119 (59%) |
| Illness severity | ||||||||||
| APACHE II score | 19 (14-23) | 19 (14-27) | 21 (15-26) | 22 (17-27) | 22 (17-28) | 23 (18-31) | 24 (19-31) | 26 (22-33) | 30 (22-37) | 22 (17-28) |
| SOFA score | 6 (4-9) | 8 (5-10) | 8 (5-11) | 8 (6-11) | 9 (7-12) | 11 (8-13) | 12 (10-15) | 13 (11-15) | 17 (14-19) | 9 (6-12) |
| Source of ICU admission | ||||||||||
| Emergency department | 99 (61%) | 56 (42%) | 129 (53%) | 167 (45%) | 177 (41%) | 125 (43%) | 64 (37%) | 33 (45%) | 9 (56%) | 859 (45%) |
| Ward | 44 (27%) | 50 (38%) | 61 (25%) | 104 (28%) | 145 (33%) | 84 (29%) | 53 (30%) | 20 (27%) | 5 (31%) | 566 (30%) |
| Other hospital | 18 (11%) | 26 (20%) | 53 (22%) | 99 (27%) | 111 (26%) | 82 (28%) | 57 (33%) | 20 (27%) | 2 (13%) | 468 (25%) |
| Preexisting comorbidities | ||||||||||
| Immunocompromised | 15 (9%) | 10 (8%) | 28 (12%) | 45 (12%) | 48 (11%) | 29 (10%) | 15 (9%) | 11 (15%) | 1 (6%) | 202 (11%) |
| Chronic kidney disease | 6 (4%) | 16 (12%) | 17 (7%) | 29 (8%) | 30 (7%) | 36 (12%) | 9 (5%) | 6 (8%) | 2 (13%) | 151 (8%) |
| Chronic heart failure | 16 (10%) | 3 (2%) | 18 (7%) | 15 (4%) | 21 (5%) | 14 (5%) | 14 (8%) | 1 (1%) | 1 (6%) | 103 (5%) |
| Chronic respiratory disorder | 7 (4%) | 4 (3%) | 9 (4%) | 12 (3%) | 21 (5%) | 12 (4%) | 5 (3%) | 3 (4%) | 0 (0%) | 73 (4%) |
| Liver insufficiency | 0 (0%) | 0 (0%) | 0 (0%) | 1 (0%) | 7 (2%) | 4 (1%) | 2 (1%) | 1 (1%) | 0 (0%) | 15 (1%) |
| Site of infection | ||||||||||
| Abdomen | 29 (18%) | 42 (32%) | 80 (33%) | 109 (29%) | 156 (36%) | 109 (37%) | 72 (41%) | 27 (37%) | 7 (44%) | 631 (33%) |
| Lung | 71 (44%) | 45 (34%) | 79 (33%) | 109 (29%) | 92 (21%) | 43 (15%) | 21 (12%) | 8 (11%) | 3 (19%) | 471 (25%) |
| Urinary tract | 25 (16%) | 12 (9%) | 23 (9%) | 53 (14%) | 70 (16%) | 69 (24%) | 38 (22%) | 17 (23%) | 3 (19%) | 310 (16%) |
| Bone/soft tissue | 26 (16%) | 22 (17%) | 30 (12%) | 60 (16%) | 50 (12%) | 29 (10%) | 18 (10%) | 6 (8%) | 1 (6%) | 242 (13%) |
| Central nervous system | 4 (2%) | 4 (3%) | 5 (2%) | 6 (2%) | 11 (3%) | 9 (3%) | 7 (4%) | 3 (4%) | 0 (0%) | 49 (3%) |
| Other/unknown | 6 (4%) | 7 (5%) | 26 (11%) | 33 (9%) | 54 (12%) | 32 (11%) | 18 (10%) | 12 (16%) | 2 (13%) | 190 (10%) |
| Therapeutic interventions | ||||||||||
| Immunoglobulin | 36 (22%) | 35 (27%) | 79 (33%) | 109 (29%) | 150 (35%) | 111 (38%) | 67 (39%) | 33 (45%) | 6 (38%) | 626 (33%) |
| Low-dose steroid | 27 (17%) | 20 (15%) | 54 (22%) | 94 (25%) | 104 (24%) | 73 (25%) | 55 (32%) | 26 (36%) | 9 (56%) | 462 (24%) |
| Renal replacement therapy | 22 (14%) | 28 (21%) | 56 (23%) | 93 (25%) | 128 (30%) | 105 (36%) | 67 (39%) | 37 (51%) | 8 (50%) | 544 (29%) |
| Surgical intervention | 53 (33%) | 57 (43%) | 96 (40%) | 172 (46%) | 196 (45%) | 138 (47%) | 91 (52%) | 31 (42%) | 3 (19%) | 837 (44%) |
| Antithrombin | 19 (12%) | 30 (23%) | 59 (24%) | 106 (29%) | 165 (38%) | 141 (48%) | 90 (52%) | 39 (53%) | 10 (63%) | 659 (35%) |
| Recombinant thrombomodulin | 13 (8%) | 20 (15%) | 54 (22%) | 98 (26%) | 135 (31%) | 128 (44%) | 89 (51%) | 36 (49%) | 5 (31%) | 578 (31%) |
| Heparin/Heparinoid | 9 (6%) | 3 (2%) | 7 (3%) | 21 (6%) | 23 (5%) | 18 (6%) | 21 (12%) | 4 (5%) | 2 (13%) | 108 (6%) |
| Outcomes | ||||||||||
| In-hospital mortality | 25 (16%) | 27 (20%) | 61 (25%) | 90 (24%) | 124 (29%) | 99 (34%) | 71 (41%) | 31 (42%) | 12 (75%) | 540 (29%) |
| With anticoagulant | 5 (14%) | 8 (17%) | 30 (31%) | 43 (25%) | 75 (30%) | 61 (31%) | 54 (40%) | 20 (38%) | 7 (64%) | 303 (30%) |
| Without anticoagulant | 20 (16%) | 19 (22%) | 31 (21%) | 47 (23%) | 49 (27%) | 38 (40%) | 17 (44%) | 11 (52%) | 5 (100%) | 237 (26%) |
| Bleeding complications | 13 (8%) | 13 (10%) | 29 (12%) | 38 (10%) | 64 (15%) | 32 (11%) | 24 (14%) | 6 (8%) | 11 (69%) | 230 (12%) |
| With anticoagulant | 3 (8%) | 3 (6%) | 14 (15%) | 23 (14%) | 48 (19%) | 27 (14%) | 21 (16%) | 6 (12%) | 8 (73%) | 153 (15%) |
| Without anticoagulant | 10 (8%) | 10 (12%) | 15 (10%) | 15 (7%) | 16 (9%) | 5 (5%) | 3 (8%) | 0 (0%) | 3 (60%) | 77 (9%) |
Abbreviations: APACHE, Acute Physiology and Chronic Health Evaluation; DIC, disseminated intravascular coagulation; ICU, intensive care unit; ISTH, International Society of Thrombosis and Hemostasis; SOFA, Sequential Organ Failure Assessment.
We observed no notable difference in age, sex, source of the ICU admission, preexisting comorbidities, or site of infections according to the baseline DIC scores. However, there were stepwise deteriorations in the severity of illness, as indicated by the APACHE II and SOFA scores, and greater in-hospital mortality in accordance with increases in the ISTH overt and JAAM DIC scores. We also found that the percent of patients who received any anticoagulant therapies increased in accordance with the increases in both DIC scores. In the present data set, recombinant thrombomodulin was administered to patients with sepsis at an almost comparable frequency to that of antithrombin.
Estimated Mortality at Each Cutoff Point
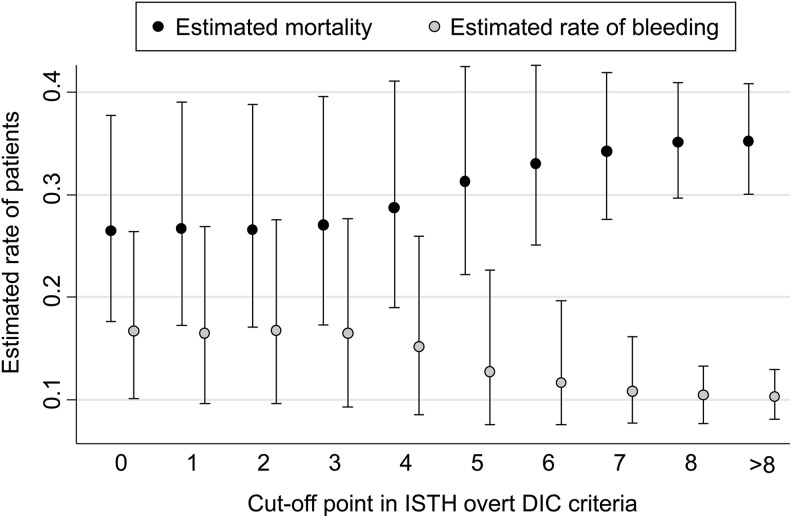
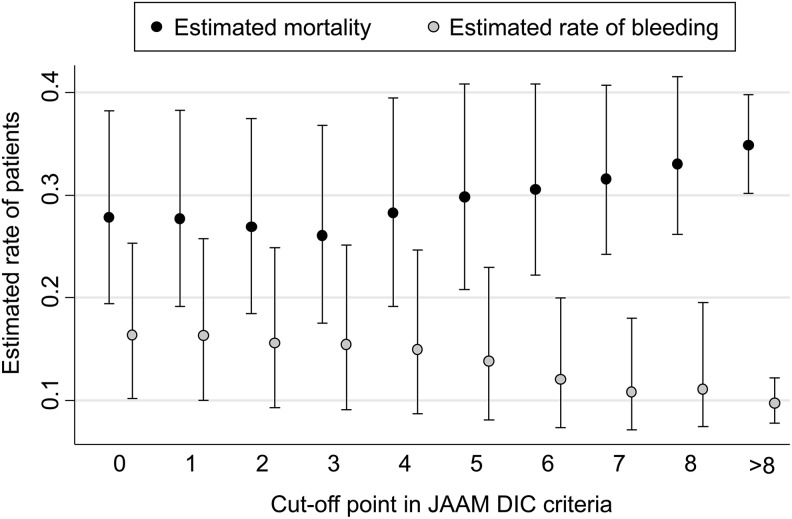
First, the sample size and estimated adjusted mortality in the anticoagulant and control groups above and below each cutoff point of the ISTH overt and JAAM DIC scoring systems were, respectively, analyzed (Tables S3 and S4). On the basis of these actual sample sizes and estimated mortality rates, we showed the estimated mortality, which was calculated as the estimated number of nonsurvivors divided by the total number of each population, for each cutoff point of the ISTH overt and JAAM DIC scoring systems according to the hypothesis that anticoagulant therapies were conducted strictly according to these cutoff points (Figure 3 for the ISTH overt and Figure 4 for the JAAM DIC scoring system).
Figure 3.
Estimated mortality and rate of bleeding complications at each cutoff point of the ISTH overt DIC scoring system. The y-axis shows the estimated mortality calculated by assuming that anticoagulant therapies were conducted strictly according to each cutoff value of ISTH overt DIC score on the x-axis. DIC indicates disseminated intravascular coagulation; ISTH, International Society of Thrombosis and Hemostasis.
Figure 4.
Estimated mortality and rate of bleeding complications at each cutoff point of the JAAM DIC scoring system. The y-axis shows the estimated mortality calculated by assuming that anticoagulant therapies were conducted strictly according to each cutoff value of JAAM DIC score on the x-axis. DIC indicates disseminated intravascular coagulation; JAAM, Japanese Association for Acute Medicine.
Between the cutoff values of 0 to 3 points, the estimated number of nonsurvivors did not increase, whereas at a cutoff value of 4 points or more in the ISTH overt DIC scoring system, the rate began to increase linearly in accordance with the increase in cutoff points (Figure 3). This finding for ISTH overt DIC score was similar to that for the JAAM DIC score shown in Figure 4. The confidence intervals for each cutoff value of DIC scores did, however, partially overlap. Consequently, from the viewpoint of minimizing the estimated mortality, the optimal cutoff value for both the ISTH overt and the JAAM DIC scoring systems should be around 3 points.
Estimated Rate of Bleeding Complications at Each Cutoff Point
In the same manner described above, we simulated the rate of bleeding complications at each cutoff point of the ISTH overt and JAAM DIC scoring systems. In both systems, the estimated rate of bleeding complications tended to decrease in accordance with the increase in cutoff point value, which indicates a lower target population for anticoagulant therapies. These findings suggested that the optimal cutoff point for determining whether to initiate anticoagulant therapy should be as high as possible from the viewpoint of only decreasing the number of patients suffering from bleeding complications.
Discussion
Summary of Evidence
In this study, we analyzed the optimal cutoff point of the ISTH overt and JAAM DIC scoring systems using a simulation model. From the results of this model, if we administered anticoagulants to patients with sepsis with scores of 3 points for both DIC scoring systems, the mortality from sepsis would be minimized and the rate of bleeding complications would be acceptable. The present cutoff point values for both DIC scoring systems may be suboptimal for determining when to start anticoagulants and may delay the initiation of therapy, even when using the JAAM DIC criteria, which were originally designed for diagnosing DIC at an early phase of sepsis.
Current Status of DIC Diagnosis in Sepsis
Historically, numerous randomized trials of anticoagulant therapies in sepsis have been conducted over the past few decades.12 However, the evidence available from these trials remains insufficient. Although several reasons for the failure to demonstrate a survival benefit were given,22–24 we would like to reemphasize the importance of patient selection and limiting the target population in trials of anticoagulant therapy against sepsis.25 The first step in selecting patients with sepsis for anticoagulant therapy should be to accurately evaluate the DIC status of the patient.
Previously, we showed that DIC screening itself in the early phase after the onset of sepsis may be associated with decreased mortality.26 However, because of the lack of a gold standard for DIC diagnosis, we could not properly determine the DIC status in each patient, nor could we reach a consensus as to which system, the ISTH overt or the JAAM DIC scoring system was better. Furthermore, we could not determine which cutoff point values among these DIC criteria were the best for initiating anticoagulant therapies.
Significance for Early Initiation of Anticoagulant Therapy
Due to the rational beliefs mentioned above, accurate judgment and prompt action corresponding to rapid pathophysiological changes of sepsis-induced DIC can improve patient outcome. In 1995, Wada et al reported that greater efficacy of DIC treatment was achieved when treatment was begun when the patient’s DIC score was lower and concluded that early diagnosis and treatment were important.27 However, some concern could be raised about their analysis. One of the main biases possibly influencing the reported outcomes was selection bias. The patient selection criteria for each group were not balanced or adjusted for. It is likely that the greater efficacy of DIC treatment seen in the patient groups with lower DIC scores might have been caused not by the timing of the intervention, but simply by the lower baseline severity.
To avoid such biases, in the present study, we applied a simulation method using a large, non-real sepsis cohort to estimate the treatment effect of initiating DIC treatment at each cutoff point value of the DIC criteria. Consequently, we showed that early intervention to initiate DIC treatment at 3 points for both the ISTH overt and the JAAM DIC scoring systems might be the best timing for anticoagulant therapy, even though the tendency was not statistically significant. To our knowledge, this is the only study to show an association between early intervention with anticoagulant therapy and better outcome for sepsis-induced DIC. The establishment of randomized trials would be warranted to confirm the hypothesis derived from this study.
Perfect Selection of Candidates for Anticoagulant Therapy
In our very recent review,25 we proposed that the optimal target population for anticoagulant therapy in sepsis should be determined based on 2 conditions: DIC and high disease severity. In the present analysis, we clarified the importance of the early initiation of anticoagulants for patients with sepsis with a relatively less severe coagulation disorder. Because the downside of anticoagulant therapy, that is, complications from bleeding, must not be ignored in the clinical situation,12,28,29 we must strictly limit the target of the intervention and explore well-balanced timing of the intervention.
Therefore, we would like to propose a revised treatment strategy for anticoagulant therapy against sepsis. First, the status of the coagulation disorder should be assessed rapidly and repeatedly and the diagnosis of DIC made promptly.26 Second, in patients with sepsis fulfilling criteria for a score of 3 or more in the ISTH overt or JAAM DIC system, the initiation of anticoagulant therapy should be considered based on the main results of the present analysis. Third, systemic severity, such as disorders of circulatory or respiratory function, should be assessed and anticoagulants should be administered only to those patients with high disease severity, as recommended in previous studies.10,11,13
The next clinical question to address should be which criteria should we apply to assess disease severity, the SOFA score, APACHE II score, or simply the status of organ dysfunction or other assessments? Such current treatment concepts will need to be elucidated by high-quality evidence in the future.
Limitations
We acknowledge several limitations in our study. Due to its retrospective nature, the anticoagulant intervention was not standardized, and the baseline characteristics were different between the treatment groups. In estimating the intervention effect size, we developed a propensity score approach to cope with the nonrandomization. Also, this study involved subgroup analysis using a simulation cohort, and thus, we cannot deny the potential of accidental false-positive results. The simulation model used in this analysis has not been previously verified. Some exclusion criteria used in the analysis such as the previous use of warfarin and oral anticoagulants may limit the generalizability of the study findings to those kinds of patients. The exposure in the analysis (anticoagulant therapy) contains 4 heterogeneous components: antithrombin, recombinant human thrombomodulin, heparin/heparinoid, and serine protease inhibitors. Previously, a comparison of the treatment effects between several anticoagulants in the same data set used in the present study was reported elsewhere.30 A prospective interventional study should be performed to assess the optimal timing of intervention with anticoagulants in sepsis.
Conclusions
Our simulation analysis implied that by administering anticoagulants early to patients with sepsis with DIC scores of 3 points or more according to both the ISTH overt and the JAAM DIC scoring systems, mortality from sepsis could be minimized while maintaining an acceptable rate of bleeding complications. The findings in the present study suggested the beneficial association of early intervention with anticoagulant therapy and mortality in the patients with sepsis-induced DIC. The establishment of randomized trials is warranted to confirm the hypothesis derived from this study.
Supplemental Material
Supplmental_Tables for Optimal Timing and Early Intervention With Anticoagulant Therapy for Sepsis-Induced Disseminated Intravascular Coagulation by Kazuma Yamakawa, Yutaka Umemura, Shuhei Murao, Mineji Hayakawa, and Satoshi Fujimi in Clinical and Applied Thrombosis/Hemostasis
Acknowledgments
The authors wish to express our gratitude to all of the regional J-Septic DIC study coordinators, nurses, physicians, and patients who contributed to the success of this study.
Authors’ Note: KY and YU equally contributed to this work. KY conceived and designed this study, contributed to acquisition, analysis, and interpretation of the data and was responsible for drafting, editing, and submission of the manuscript. YU contributed to the design of the study, statistical analysis, and drafting of the manuscript. SM, MH, and SF assisted with the design of the study and interpretation of the data. All authors reviewed and revised the manuscript and approved the final version of the manuscript. The data sets supporting the conclusions of this article are available in the University Hospital Medical Information Network Individual Case Data Repository (UMIN000012543, http://www.umin.ac.jp/icdr/index-j.html). Please contact the corresponding author to access the data.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Kazuma Yamakawa, MD, PhD  https://orcid.org/0000-0003-2999-4021
https://orcid.org/0000-0003-2999-4021
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369:840–851. [DOI] [PubMed] [Google Scholar]
- 2. Iba T, Levy JH. Inflammation and thrombosis: roles of neutrophils, platelets and endothelial cells and their interactions in thrombus formation during sepsis. J Thromb Haemost. 2018;16(2):231–241. [DOI] [PubMed] [Google Scholar]
- 3. Levi M, Schultz M, van der Poll T. Sepsis and thrombosis. Semin Thromb Hemost. 2013;39(5):559–566. [DOI] [PubMed] [Google Scholar]
- 4. Ogura H, Gando S, Saitoh D, et al. Japanese Association for Acute Medicine Sepsis Registry (JAAMSR) Study Group . Epidemiology of severe sepsis in Japanese intensive care units: a prospective multicenter study. J Infect Chemother. 2014;20(3):157–162. [DOI] [PubMed] [Google Scholar]
- 5. Ranieri VM, Thompson BT, Barie PS, et al. PROWESS-SHOCK Study Group . Drotrecogin alfa (activated) in adults with septic shock. N Engl J Med. 2012;366:2055–2064. [DOI] [PubMed] [Google Scholar]
- 6. Warren BL, Eid A, Singer P, et al. KyberSept Trial Study Group . Caring for the critically ill patient. High-dose antithrombin III in severe sepsis: a randomized controlled trial. JAMA. 2001;286(15):1869–1878. [DOI] [PubMed] [Google Scholar]
- 7. Abraham E, Reinhart K, Opal S, et al. Efficacy and safety of tifacogin (recombinant tissue factor pathway inhibitor) in severe sepsis: a randomized controlled trial. JAMA. 2003;290(2):238–247. [DOI] [PubMed] [Google Scholar]
- 8. Dhainaut JF, Yan SB, Joyce DE, et al. Treatment effects of Drotrecogin alfa (activated) in patients with severe sepsis with or without overt disseminated intravascular coagulation. J Thromb Haemost. 2004;2(11):1924–1933. [DOI] [PubMed] [Google Scholar]
- 9. Kienast J, Juers M, Wiedermann CJ, et al. Treatment effects of high-dose antithrombin without concomitant heparin in patients with severe sepsis with or without disseminated intravascular coagulation. J Thromb Haemost. 2006;4(1):90–97. [DOI] [PubMed] [Google Scholar]
- 10. Dhainaut JF, Laterre PF, Janes JM, et al. Recombinant Human Activated Protein C Worldwide Evaluation in Severe Sepsis (PROWESS) Study Group . Drotrecogin alfa (activated) in the treatment of severe sepsis patients with multiple-organ dysfunction: data from the PROWESS trial. Intensive Care Med. 2003;29(6):894–903. [DOI] [PubMed] [Google Scholar]
- 11. Wiedermann CJ, Hoffmann JN, Juers M, et al. KyberSept Investigators . High-dose antithrombin III in the treatment of severe sepsis in patients with a high risk of death: efficacy and safety. Crit Care Med. 2006;34(2):285–292. [DOI] [PubMed] [Google Scholar]
- 12. Umemura Y, Yamakawa K. Efficacy and safety of anticoagulant therapy in three specific populations with sepsis: a meta-analysis of randomized controlled trials: reply. J Thromb Haemost. 2016;14(3):518–530. [DOI] [PubMed] [Google Scholar]
- 13. Yamakawa K, Umemura Y, Hayakawa M, et al. Japan Septic Disseminated Intravascular Coagulation (J-Septic DIC) Study Group . Benefit profile of anticoagulant therapy in sepsis: a nationwide multicentre registry in Japan. Crit Care. 2016;20:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kobayashi N, Maekawa T, Takada M, Tanaka H, Gonmori H. Criteria for diagnosis of DIC based on the analysis of clinical and laboratory findings in 345 DIC patients collected by the Research Committee on DIC in Japan. Bibl Haematol. 1983;49:265–275. [DOI] [PubMed] [Google Scholar]
- 15. Taylor FB, Jr, Toh CH, Hoots WK, Wada H, Levi M; Scientific Subcommittee on Disseminated Intravascular Coagulation of the International Society on Thrombosis and Haemostasis. Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost. 2001;86(5):1327–1330. [PubMed] [Google Scholar]
- 16. Gando S, Iba T, Eguchi Y, et al. Japanese Association for Acute Medicine Disseminated Intravascular Coagulation (JAAM DIC) Study Group. A multicenter, prospective validation of disseminated intravascular coagulation diagnostic criteria for critically ill patients: comparing current criteria. Crit Care Med. 2006;34(3):625–631. [DOI] [PubMed] [Google Scholar]
- 17. Hayakawa M, Saito S, Uchino S, et al. Characteristics, treatments, and outcomes of severe sepsis of 3195 ICU-treated adult patients throughout Japan during 2011-2013. J Intensive Care. 2016;4:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101(6):1644–1655. [DOI] [PubMed] [Google Scholar]
- 19. Knaus WA, Draper EA, Wanger DP, Zimmerman JE. APACHE II: a severity classification system. Crit Care Med. 1985;13(10):818–829. [PubMed] [Google Scholar]
- 20. Ferreira FL, Bota DP, Bross A, Mélot C, Vincent JL. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA. 2001;286(14):1754–1758. [DOI] [PubMed] [Google Scholar]
- 21. Gando S, Saitoh D, Ogura H, et al. Japanese Association for Acute Medicine Disseminated Intravascular Coagulation (JAAM DIC) Study Group . Natural history of disseminated intravascular coagulation diagnosed based on the newly established diagnostic criteria for critically ill patients: results of a multicenter, prospective survey. Crit Care Med. 2008;36(1):145–150. [DOI] [PubMed] [Google Scholar]
- 22. Vincent JL. The coming era of precision medicine for intensive care. Crit Care. 2017;21(suppl 3):314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Giamarellos-Bourboulis EJ. The failure of biologics in sepsis: where do we stand? Int J Antimicrob Agents. 2013;42(suppl):S45–S47. [DOI] [PubMed] [Google Scholar]
- 24. Iba T, Gando S, Thachil J. Anticoagulant therapy for sepsis-associated disseminated intravascular coagulation: the view from Japan. J Thromb Haemost. 2014;12(7):1010–1019. [DOI] [PubMed] [Google Scholar]
- 25. Umemura Y, Yamakawa K. Optimal patient selection for anticoagulant therapy in sepsis: an evidence-based proposal from Japan. J Thromb Haemost 2018;16(3):462–464. [DOI] [PubMed] [Google Scholar]
- 26. Umemura Y, Yamakawa K, Hayakawa M, Hamasaki T, Fujimi S; Japan Septic Disseminated Intravascular Coagulation (J-Septic DIC) Study Group . Screening itself for disseminated intravascular coagulation may reduce mortality in sepsis: a nationwide multicenter registry in Japan. Thromb Res. 2018;161:60–66. [DOI] [PubMed] [Google Scholar]
- 27. Wada H, Wakita Y, Nakase T, et al. Outcome of disseminated intravascular coagulation in relation to the score when treatment was begun. Mie DIC Study Group. Thromb Haemost. 1995;74(3):848–852. [PubMed] [Google Scholar]
- 28. Martí-Carvajal AJ, Solà I, Gluud C, Lathyris D, Cardona AF. Human recombinant protein C for severe sepsis and septic shock in adult and paediatric patients. Cochrane Database Syst Rev. 2012;(12):CD004388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Allingstrup M, Wetterslev J, Ravn FB, Møller AM, Afshari A. Antithrombin III for critically ill patients. Cochrane Database Syst Rev. 2016;(2):CD005370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Umemura Y, Yamakawa K, Hayakawa M, Kudo D, Fujimi S. Concomitant versus individual administration of antithrombin and thrombomodulin for sepsis-induced disseminated intravascular coagulation: a nationwide Japanese Registry Study. Clin Appl Thromb Hemost. 2018;5(5):734–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplmental_Tables for Optimal Timing and Early Intervention With Anticoagulant Therapy for Sepsis-Induced Disseminated Intravascular Coagulation by Kazuma Yamakawa, Yutaka Umemura, Shuhei Murao, Mineji Hayakawa, and Satoshi Fujimi in Clinical and Applied Thrombosis/Hemostasis