Abstract
A variety of viral infections are associated with hypercoagulable states and may be linked to the development of deep venous thrombosis and pulmonary embolism. The Zika and Chikungunya viral infections spread through the South and Central American continents, moving to North America in 2016, with severe cases of polyarthralgia, fever, and Guillain-Barré syndrome leading eventually to death. A decreased trend for both infections was reported in the first quarter of 2017. In this article, we report the possible association of venous thromboembolic events associated with Zika infection. After 2 cases of deep venous thrombosis in patients with acute Zika infections, D-dimer levels were measured in 172 consecutive patients who presented to the emergency department of a university hospital in an endemic region of Brazil with either Zika or Chikungunya infections confirmed by polymerase chain reaction tests. D-dimer levels were increased in 19.4% of 31 patients with Zika and in 63.8% of 141 patients with Chikungunya infections. The mechanisms behind this association are yet to be elucidated as well as the potential for venous thromboembolism prevention strategies for in-hospital patients affected by Zika and Chikungunya infections.
Keywords: Zika virus, Chikungunya virus, deep venous thrombosis
Introduction
Zika and Chikungunya virus infections are transmitted by various species of aedes mosquitoes, both resulting in an outbreak of cases (Zika in 2014 and Chikungunya in 2015), with peaks in 2016 and a decrease of new cases in 2017. Fever, headache, arthralgia, myalgia, and maculopapular rash are symptoms common for both diseases and other viruses, such as dengue.1 Although for Zika-infected patients the disease is self-limiting, cases of neurologic manifestations and the Guillain-Barré syndrome were described in Brazil during Zika virus epidemics.2 An association between Zika infection in pregnant women and newborn microcephaly have also been reported.3–5 As for Chikungunya, in association with the rash and febrile syndrome, muscular and joint pain as well as intense asthenia with chronic leg pain have also been reported.6
During the outbreak of Zika infection, a fair number of young patients suspicious of the virus presented to the emergency department with unusual edema of upper or lower limbs. Initially, this was imputed to the systemic inflammation associated with such viruses. However, in some patients, the extremity edema took a longer time to recover. Reports of high D-dimer levels (greater than 15.00 µg/mL fibrinogen-equivalent units [FEU]) in a patient with Guillain-Barré syndrome in the intense care unit brought to our attention the possibility of linking this infection to the development of venous thromboembolism (VTE). This finding supports Parra and colleagues that also reported a significant number of patients (66) with Zika symptoms before the onset of Guillain-Barré syndrome in 2017.7
In early January 2016, a 46-year-old Brazilian non-Caucasian female previously healthy arrived at the emergency department of the University Hospital of Sergipe with a history of 3 days with high fever (>39°C), joint pain, arthralgia, and a red cutaneous rash. She was treated with hydration and discharged from the hospital with a paracetamol prescription. She returned to the emergency department 4 days later with right upper extremity painful edema, with no prior peripherally inserted central catheter or intravenous infusions in the arm that had brachial thrombosis (Figure 1). An upper limb venous duplex scan revealed right brachial vein thrombosis. She was treated with unfractionated heparin followed by vitamin K antagonists (VKAs). Polymerase chain reaction (PCR) test confirmed Zika infection.
Figure 1.
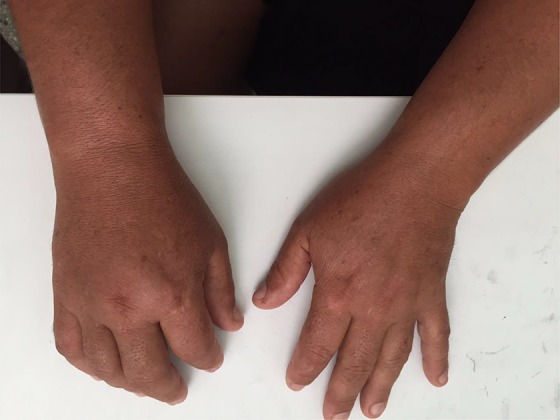
A 46-year-old female with Zika infection and upper limb deep venous thrombosis.
In the subsequent month, a 53-year-old Brazilian Caucasian male presented to the same emergency department with fever, asthenia, headache, and arthralgia in the elbows, knees, and shoulders, with onset 5 days previously. The arthralgia had increased in intensity and was accompanied by pain in the left calf and bilateral edema of the ankles, more intense on the left (Figure 2). No additional patient risk factors for thrombosis that may have predisposed them was recorded. Color Doppler ultrasonography examination confirmed thrombosis of the left popliteal vein. The patient was treated with low-molecular weight heparin and VKA therapy and had a full recovery. He also tested positive for Zika virus.
Figure 2.
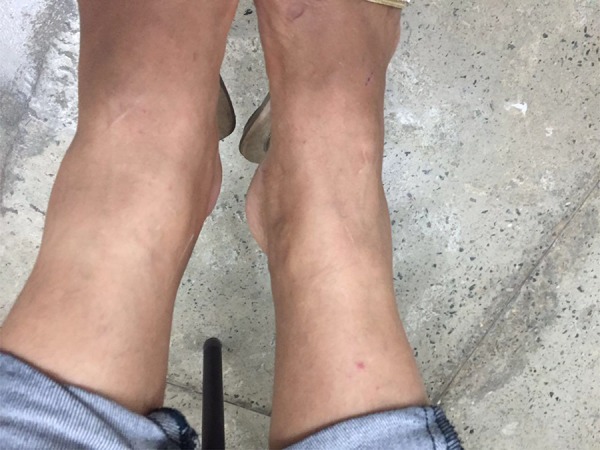
A 53-year-old Caucasian male with Zika infection and left lower limb deep venous thrombosis.
In January 2017, a case was reported of a patient who developed thrombosis of the right popliteal vein after being admitted for treatment of severe polyarthralgia and fever caused by Chikungunya infection.8 This clinical cases claimed our attention for the possible association between this viral infections and VTE.
As of now, there is no strong data supporting the relationship between Zika and Chikungunya and VTE. We, therefore, measured D-dimer levels in 172 consecutive patients who presented to the emergency department of a university hospital in an endemic region of Brazil (Sergipe) with either Zika or Chikungunya infections confirmed by PCR tests. The nature of the study was exploratory.
Methods
Patients
This study was approved by local institutional review board and followed the federal recommendations of the resolutions of the National Health Council that refer to the clinical research involving human beings of CONEP (196/1996 and 251/1997, 347/2005 and CAAE 54835916.2.0000.5546). All patients signed an informed consent.
Patients with symptoms of Zika and Chikungunya infections were treated at the University Hospital of the Federal University of Sergipe in the northeast area of Brazil. After the confirmation of the reported cases of DVT, blood samples were prospectively collected for D-dimer analysis for all patients with confirmed viral (Zika and Chikungunya) infections. There were 172 infected patients studied, 31 with Zika and 141 with Chikungunya confirmed by PCR tests.
Sample Collection
Five milliliter of whole blood was collected in a tube without an anticoagulant, and 10 mL whole blood was collected in a tube containing EDTA. Tubes were centrifuged at 3000 rpm for 10 minutes. Plasmas and serum were stored at −80°C until evaluated.
Quantitative Real-Time (QRT)-Polymerase Chain Reaction
For virus detection, viral RNAs were extracted from 140 μL of the serum sample using the QIAamp Viral RNA Mini kit (QIAGEN, Hilden, Germany) according to the manufacturer’s instructions.
Reverse transcription (RT) followed by real-time polymerase chain reaction was performed using Invitrogen’s SuperScript III Platinum kit (One-Step qRT-PCR System; Thermo Fisher, Norcross, Georgia): 2× reaction mix (13.5 μL), H2O free (0.5 μL), primer reverse (0.5 μL), 10 μM probe (0.5 μL), SuperScript III RT/Platinum Taq Mix (0.5 μL), with addition of 5 μL of extracted RNA for each reaction. The thermal cycling conditions for reverse RT were 1 cycle at 50°C for 15 minutes, for inactivation of RT and activation of Taq Polymerase 1 cycle at 95°C for 2 minutes, denaturation occurred in 40 cycles at 95°C for 15 seconds, and 40 cycles at 60°C for 30 seconds for annealing/extension. Amplification was performed using the ABI 7500 apparatus (Applied Biosystems, Foster City, California). Samples with a threshold number (cycle threshold [CT]) ≤38 were considered positive for infection caused by the Zika and Chikungunya viruses.
D-Dimer
Frozen plasma was shipped to Albert Einstein Israeli Hospital in São Paulo, Brazil for D-dimer analysis. The D-dimer levels were quantified using an enzyme-linked immunosorbent assay (ELISA) method (Hyphen BioMed, Andresy, France). Absorbance measurements were obtained using an ELISA plate reader SPECTRAmax PLUS (Molecular Devices, Sunnyvale, California). The reference concentration of D-dimer was less than 0.5 µg/mL FEU.
Results
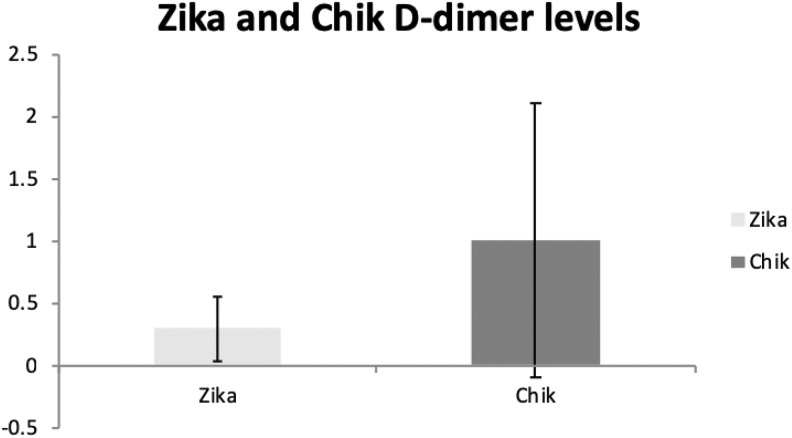
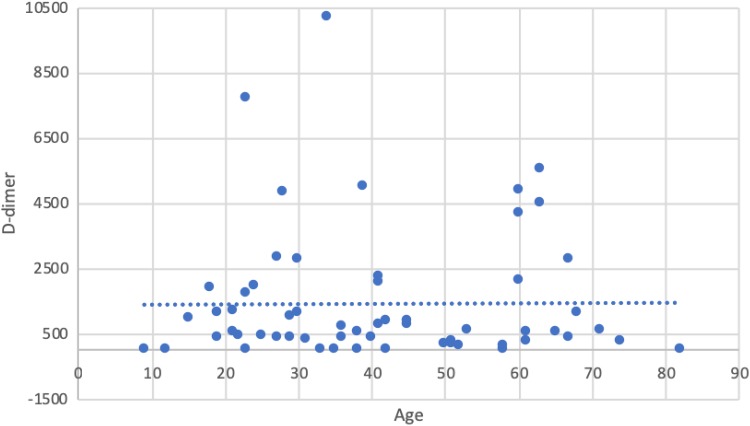
Demographics of included patients are depicted in Table 1. No screening for patients. All positives for both Zika and Chikungunya were tested. D-dimer levels were elevated above the reference range in 19.4% of 31 patients with Zika and in 63.8% of 141 patients with Chikungunya viral infections (Table 2 and Figure 3). A sensitivity analysis regarding a gender- and age-matched comparison for the D-dimer evaluation was performed. Neither the age (P = .659—Zika; P = .405—Chik) nor sex (P = .735—Zika; P = .826—Chik) was a significant covariate in the analysis—Figure 4 and Table 3. None of the patients had developed symptomatic VTE at the time of the study.
Table 1.
Baseline Characteristics of the Participants.
| Baseline Characteristics | ||
|---|---|---|
| Characteristic | Zika (n = 31) | Chik (n = 141) |
| Age, years | 37.5 ± 13.35 | 41.8 ± 17.3 |
| Male (%) | 64 | 13.2 |
| Fever (%) | 84.6 | 85.9 |
| Arthralgia (%) | 65.4 | 85.9 |
| Exanthema (%) | 53.8 | 19 |
| Conjunctivitis (%) | 7.7 | 4.9 |
| Myalgia (%) | 42.3 | 15.7 |
| Retro-orbital pain (%) | 26.9 | 47.1 |
| Lymphadenopathy (%) | 19.2 | 4.1 |
Table 2.
D-dimer Levels Are Given in µg/mL on 31 Plasma Samples From Patients Positive for Zika Virus and 141 Plasma Samples From Patients Positive for Chikungunya Virus.a
| Variables | Zika | Chik |
|---|---|---|
| Mean (µg/mL) | 0.29 | 1.01 |
| SD (µg/mL) | 0.26 | 1.102 |
| N | 31 | 141 |
| D-dimer samples above upper limit | 19.35% | 63.83% |
Abbreviation: SD, standard deviation.
a The reference concentration of D-dimer is less than 0.5 µg/mL fibrinogen-equivalent units (FEU).
Figure 3.
Comparative Zika and Chikungunya D-dimer levels.
Figure 4.
Raw data regarding D-dimer levels from both groups (Zika and Chik) and age distribution.
Table 3.
Sensitivity Analysis—Chik/Zika Gender and D-dimer Levels.a,b
| Groups | Calculated P Value for Age | Calculated P Value for Sex |
|---|---|---|
| Zika | .659 | .735 |
| Chik | .405 | .826 |
a All analyses were carried out in G*Power 3.1.9.2 (Kiel Universität, Germany).
b Neither the age (P = .659—Zika; P = .405—Chik) nor sex (P = .735—Zika; P = .826—Chik) was a significant covariate in the analysis.
Discussion
A relationship between systemic viruses (such as H1N1, HIV, and hepatitis) and inflammation/hypercoagulable states has already been demonstrated.9 All aspects of the coagulation cascade, including coagulation, platelet function, and fibrinolysis can be affected.8 The mechanism behind this interplay is not yet well elucidated. Increased procoagulant factors, direct endothelial injury with increased expression of tissue factor might be involved. Viral infection can induce production and release of microparticles that are procoagulant, as well as increased platelet adhesion could also be involved.10,11
As yet, there are no reliable data supporting the relationship between Zika and Chikungunya and VTE, but the progressive increase in its global incidence, the fact that this infection very often causes severe locomotion restrictions due to polyarthralgia, and the possibility of direct endothelial injury suggest that cases of VTE may begin to be described.8 Moreover, severe patients who develop Guillain-Barré syndrome could be at increased risk of VTE given their restricted mobility and acute medically ill status. Many of them are in the intensive care unit. In addition to specifically identifying thrombosis, the use of D-dimer can help determine in general the severity of the disease.
In the 2 case reports presented, we describe an association between venous thrombosis and Zika infection. Chikungunya association to VTE has already started to be described.
D-Dimer and Infection
The prospective D-dimer data described herein suggests an increase of this biomarker in Zika patients but clearly, a higher elevation in Chikungunya-infected participants. A drawback of this study was that we were unable to correlate levels of D-dimer with thrombotic events, particularly in the Chikungunya virus population. However, the elevated D-dimer levels, particularly in the Chikungunya-infected patients, support the risk of thrombosis associated with these infections and the enhanced severity of disease with Chikungunya infection. It is important to stress out that the value of D-dimer (a nonspecific marker of inflammation that is often elevated in infections of varying types) is best validated in the literature when the result is negative in conjunction with a low or intermediate pretest probability of VTE (as opposed to being suggestive of thrombosis).
Limitations
This report is preliminary and exploratory, no sample size calculations. We understand that more samples would be mandatory to achieve a statistical significance, however, due to geographical and logistic issues, our team could not increase sample size and unfortunately, more biomarker analyses could not be performed as well, due to small sample volumes. Possible positives for VTE might have been lost to follow-up given the nature of the region and lack of resources for VTE diagnosis in the north part of Brazil. Nevertheless, our findings are extremely relevant for our community and contribute greatly to an important cause that compromises poor regions of Brazil (northeast) and other Latin-American countries.
Future Directions
A better understanding of the pathophysiology underlying the association of viral infections and coagulation disorders is crucial for developing therapeutic strategies. This is of special importance in the case of severe complications, such as those seen in hemorrhagic viral infections, the incidence of which is increasing worldwide.9
Further understanding of these mechanisms is warranted. Patients at high risk of VTE with viral infections may be good candidates for VTE prophylaxis with current anticoagulants. These strategies could help improve the medical care of patients affected by these pandemic viruses that carry high emotional and economic impact on the society.
Conclusion
Zika and Chikungunya may be associated with an increased risk of clinical VTE, supported by increased D-dimer levels. The mechanisms are yet to be elucidated as well as strategies to prevent VTE in this fragile population.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was self-funded by Hospital e Maternidade Dr. Christóvão da Gama, SP, Brazil and Albert Einstein Israeli Hospital, SP, Brazil. Roque Almeida reports a FINEP grant (Financiadora de Estudos e Projetos) number 0116005600. Juliana Alves reports that this study was financed in part by the “Coordenação de Aperfeiçoamento de Pessoal de Nível Superior”—Brazil (CAPES)—Finance Code 001. Laboratory kits were provided by Loyola University Medical Center, Maywood, Illinois.
ORCID iD: Juliana Cardoso Alves  https://orcid.org/0000-0001-9294-3257
https://orcid.org/0000-0001-9294-3257
References
- 1. Fauci AS, Morens DM. Zika virus in the Americas—yet another arbovirus threat. N Engl J Med. 2016;374(7):601–604. [DOI] [PubMed] [Google Scholar]
- 2. Broutet N, Krauer F, Riesen M, et al. Zika virus as a cause of neurologic disorders. N Engl J Med. 2016;374(16):1506–1509. [DOI] [PubMed] [Google Scholar]
- 3. McCarthy M. Microcephaly risk with Zika infection is 1-13% in first trimester, study shows. BMJ. 2016;353:i3048. [Google Scholar]
- 4. Johansson MA, Mier y Teran Romero L, Reefhuis J, Gilboa SM, Hills SL. Zika and the risk of microcephaly. N Engl J Med. 2016;375(1):1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brasil P, Pereira JP, Jr, et al. Zika virus infection in pregnant women in Rio de Janeiro. N Engl J Med. 2016;375(24):2321–2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Morens DM, Fauci AS. Chikungunya at the door—déjà vu all over again?. N Engl J Med. 2014;371(10):885–887. [DOI] [PubMed] [Google Scholar]
- 7. Parra B, Lizarazo J, Jiménez-Arango JA, et al. Guillain-Barré syndrome associated with Zika virus infection in Colombia. N Engl J Med. 2016;375(16):1513–1523. [DOI] [PubMed] [Google Scholar]
- 8. Marques MA, Adami de Sá FP, Lupi O, Brasil P, von Ristow A. Deep venous thrombosis and chikungunya virus. J Vasc Bras. 2017;16(1):60–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Goeijenbier M, van Wissen M, van de Weg C, et al. Review: viral infections and mechanisms of thrombosis and bleeding. J Med Virol. 2012;84(10):1680–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Avnon LS, Munteanu D, Smoliakov A, Jotkowitz A, Barski L. Thromboembolic events in patients with severe pandemic influenza A/H1N1. Eur J Intern Med. 2015;26(8):596–598. [DOI] [PubMed] [Google Scholar]
- 11. Wang CC, Chang CT, Lin CL, Lin IC, Kao CH. Hepatitis C virus infection associated with an increased risk of deep vein thrombosis: a population-based cohort study. Medicine (Baltimore). 2015;94(38):e1585. [DOI] [PMC free article] [PubMed] [Google Scholar]