Abstract
Platelet aggregation can be measured using optical aggregation (light transmission aggregometry, LTA) as well as by impedance (Multiplate analyzer). The LTA (the gold standard method) can be influenced by many preanalytical variables. Several guidelines differ in recommendations for the duration patients should refrain from smoking, coffee, fatty meals, and physical exercise prior to blood collection for performing platelet function tests. In this pilot study, the influence of smoking, coffee, high-fat meal, or physical exercise on platelet aggregation was investigated to improve patient friendliness and laboratory logistics in platelet function diagnostics. Standardized blood collection was performed when participants were fasting and after each parameter (n=5 per group). As a control for diurnal fluctuations, participants (n=6) were fasting during both blood collections. Platelet aggregation was executed using standardized methods for LTA and Multiplate analyzer. Statistical analysis of the results using Wilcoxon signed-rank test did not show any significant differences in platelet aggregation in healthy participants under different preanalytical variables. Therefore, these variables are not expected to adversely affect testing, which can avoid canceling tests for those patients who inevitably did.
Keywords: clinical laboratory techniques, electric impedance, light, platelet aggregation, platelet function tests
Introduction
Platelet aggregation can be measured in vitro by light transmission aggregometry (LTA) or by multiple electrode impedance aggregometry (MEIA; Multiplate analyzer, Roche Diagnostics, Almere, the Netherlands). Since 1962, LTA has been considered the gold standard for platelet function testing and is carried out in citrated platelet-rich plasma (PRP).1 Besides LTA, MEIA is also performed in platelet function testing and monitoring of antiplatelet drugs. In vitro platelet aggregation with the Multiplate analyzer is carried out in whole blood, which reflects the in vivo interactions between blood cells.2 MEIA is not as labor intensive as LTA, which leads to less analytical variables in performing aggregation with Multiplate, while LTA can be influenced by many preanalytical variables. Several guidelines differ in recommendations for executing platelet function tests in the preanalytical phase.3–5 In Table 1, the guidelines from the International Society on Thrombosis and Haemostasis, Clinical and Laboratory Standards Institute, and the British Committee for Standards in Haematology are compared (Table 1). Their recommendations differ in the duration patients should abstain smoking, caffeine, fatty meals, and physical exercise prior to a blood collection for platelet function testing. These variables could possibly stimulate or inhibit platelet aggregation and therefore can lead to less reliable results by LTA or MEIA. When performing platelet function tests, patients should be fasting for 10 to 12 hours prior to blood collection; therefore, platelet function tests can only be carried out before noon. In this study, the influence of smoking, drinking coffee, consuming a high-fat meal, or performing physical exercise were investigated to possibly improve patient friendliness and logistics in platelet function diagnostics.
Table 1.
Comparison Between the ISTH,3 CLSI,4 and BCSH5 Guidelines for Preanalytical Variables in Platelet Function Tests.
| ISTH | CLSI | BCSH |
|---|---|---|
| Blood samples for LTA should be collected after a short rest period for the participant, to attenuate the effect of exercise-induced adrenaline release on platelet aggregation. | – | Blood samples should only be collected from resting participants. |
| Blood samples for LTA should be collected from participants who refrain from smoking for at least 30 minutes, to attenuate the effect of exercise-induced adrenaline release on platelet aggregation. | – | Blood samples should only be collected from participants who have refrained from smoking on the day of testing. |
| Blood samples for LTA should be collected from participants who abstain from caffeine for at least 2 hours. | – | Blood samples should only be collected from participants who have refrained from caffeine ingestion on the day of testing. |
| It is uncertain whether blood samples for LTA should be collected from fasting participants. Although variations in glycemia and lipemia might slightly affect the results of LTA studies, it is uncertain whether these effects interfere significantly with the diagnosis of platelet function disorders. | If medically possible, patients should be fasting and should also avoid fatty meals prior to collection. | Blood samples should only be collected from fasting participants. |
Abbreviations: BCSH, British Society for Hematology; CLSI, Clinical and Laboratory Standards Institute; ISTH, International Society on Thrombosis and Hemostasis; LTA, light transmission aggregometry.
Methods
Participants and Study Design
Participants (n=26) were included in 6 groups regarding the different parameters. All participants gave their written consent to participate in this study, and the study was approved by our local medical ethics committee. The included participants were healthy and had no bleeding history. All participants did not take any medication that could have an effect on platelet function for at least 3 days in the case of non-steroidal anti-inflammatory drugs and 7 days for medication containing acetylsalicylic acid and platelet aggregation inhibitors. All participants were 10 to 12 hours fasting, nonsmoking, and nonexercising prior to the first blood collection. Participants in the smoking group were included in our study if they were smoking for at least 5 years prior to participating in our study. Blood was drawn between 8 and 9 o’clock in the morning. A second blood collection was performed 2 hours after the first collection. In one group, the participants were allowed to smoke 1 cigarette half an hour prior to the second blood collection (based on stereotypical behavior). Another group of participants drank 1 cup of coffee after the first blood collection, and another group consumed a high-fat meal. The high-fat meal contained on average 55.8 grams of fat (12.4% fat) of which 8.1% of the meal consisted of saturated fats. The meal consisted of croissants with cheese or high-fat meat, a dessert, and milk products. Two hours (based on average digestion period) after drinking the coffee or consuming the high-fat meal, a second blood collection was performed. In a separate group, participants were included who performed physical exercise directly before the second blood collection (to represent patients who are in a hurry). These participants rapidly walked up and down the stairs (5 levels). As a control on diurnal fluctuations, a group of participants who were fasting, in physical rest, and nonsmoking during both blood collections were included. Complete blood counts (CBCs) were measured from each blood collection. To control the fasting state of the participants and to monitor the effect of the high-fat meal consumption, triglyceride concentrations were measured using Cobas 8000 modular analyzer series (Roche Diagnostics, Almere, the Netherlands).
Blood Collection
One EDTA tube (BD Vacutainer 7.2 mg K2EDTA, 4.0 mL, Becton Dickinson & Company, Plymouth, UK), 2 citrated tubes ( 9NC Coagulation Sodium Citrate 3.2%, 9.0 mL, Greiner Bio-One GmbH, Frickenhausen, Germany), 1 hirudin tube (Hirudin tube, 3.0 mL, Roche Diagnostics, Almere, the Netherlands), and a serum tube (Z Serum Sep Clot Activator, 9.0 mL, Greiner Bio-One GbmH, Frickenhausen, Germany,) were filled using a BD Vacutainer Eclipse (Becton Dickinson & Company, Plymouth, UK) needle of 21 Gauge with preattached holder. Blood collection was performed following a standardized protocol with minimal use of a tourniquet.
Light Transmission Aggregometry
The collected blood was analyzed within 4 hours. Citrated PRP was obtained by centrifuging for 10 minutes at 170 × g. Platelet-poor plasma (PPP) was obtained by first centrifuging the remaining PRP at 2500 × g during 5 minutes. Second, the tubes were centrifuged at 10.000 × g during 10 minutes (at 18°C). Complete blood counts were performed of the PRP (Sysmex XN-9000; Sysmex Corporation, Kobe, Japan). Hereafter, the PRP was standardized and diluted with autologous PPP to 250 × 109 platelets/L. The standardized PRP was allowed to rest for 30 minutes to stabilize prior to testing. To induce platelet aggregation, agonists were added to the cuvette (250 µL PRP) with a final concentration of 1 mM arachidonic acid (NAAA, LS101297; Bio/Data Corporation, Horsham, Pennsylvania, USA), 5 µM adenine diphosphate (ADP, CH384, Chrono-Par Chrono-log Corporation, Havertown, Pennsylvania, USA), 2 µg/mL collagen (CH385, Chrono-Par, Chrono-log Corporation, Havertown, Pennsylvania, USA), and 5 µM epinephrine (CH393, Chrono-Par, Chrono-log Corporation, Havertown, Pennsylvania, USA; final concentrations) and measured with the aggregometer (Chronolog model 700, Chrono-log Corporation, Havertown, Pennsylvania, USA). After 15-minute measurements, AGGRO/LINK8 software (Chrono-log Corporation, Havertown, Pennsylvania, USA) was used to determine the amplitudes.
Multiple Electrode Impedance Aggregometry
Hirudin whole blood was tested within 30 to 180 minutes after blood collection. Of this, 20 µL of the agonists were added to the test tube with final concentrations of 6.4 µM ADP (06675794190, Roche Diagnostics, Almere, the Netherlands), 0.5 mM arachidonic acid (ASPI, 06675816190, Roche Diagnostics, Almere, the Netherlands), 3.2 µg/mL collagen (CH385, Chrono-Par, Chrono-log Corporation, Havertown, Pennsylvania, USA), and 32µM thrombin receptor-activating peptide (TRAP, 06675883190, Roche Diagnostics, Almere, the Netherlands). Of this, 300 µL hirudin whole blood was 1:1 diluted with 0.9% NaCl in each test cell of the Multiplate analyzer (Roche Diagnostics, Almere, the Netherlands). After a 3-minute incubation step at 37°C, the agonist was added to each test cell. After 6minutes, the area under the curve (AUC) was determined using the Multiplate software. Remaining blood in the tube was centrifuged for 5 minutes at 2000 × g and checked for hemolysis. None of the samples showed a hemolytic aspect; therefore, all samples could be included in this study.
Statistics
The maximal variation coefficient of the LTA was estimated at 12.5%. With the Multiplate, variation coefficients of 9.9%, 9.1%, 8.8%, and 8.4% were determined for, respectively, 6.4 µM ADP, 0.5mM ASPI, 3.2 µg/mL collagen, and 32µM TRAP in an internal validation report. Sample size calculations showed that 3 participants per group for the Multiplate were sufficient enough to detect a clinically relevant difference, based on a standard deviation of 10 U. For the LTA, 5 participants per group are sufficient enough, based on the standard deviation estimated around 12.5%. Medians of the LTA and Multiplate per group were analyzed with a paired Wilcoxon signed-rank test using GraphPad Prism software (version 5.5; La Jolla, California). Results were considered significant at P < .05.
Results
Complete Blood Counts and Triglyceride Measurements
The results of CBCs and triglyceride measurements from every blood collection are shown in Table 2 for each group. No statistical differences in CBCs were observed between before and after measurements in each group. Platelet counts and mean platelet volume were excluded for 3 patients due to the presence of platelet clumps in the EDTA tubes. We did observe an increase, although not significant, in triglyceride levels in participants who consumed the high-fat meal.
Table 2.
Complete Blood Count and Triglyceride Results for Each Observed Parameter.
| Participant's Characteristics | Parameter | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Smoking (n = 5) | Drinking Coffee (n = 5) | Consuming High-Fat Meal (n = 5) | Performing Physical Exercise (n = 5) | Control (n = 6) | ||||||
| Male:female ratio | 0:5 | 0:5 | 2:3 | 1:4 | 1:5 | |||||
| Age (years) | 55 (40-59) | 22 (21-50) | 26 (23-42) | 31 (22-45) | 32 (23-41) | |||||
| CBC and Triglyceride | Before | After | Before | After | Before | After | Before | After | Before | After |
| WBC (×109/L) | 6.87 (5.56-9.91) | 8.31 (6.91-10.15) | 6.80 (5.83-7.22) | 7.06 (6.22-7.44) | 6.18 (5.63-6.69) | 6.62 (5.97-7.95) | 6.07 (4.90-7.23) | 8.70 (7.19-9.96) | 7.06 (5.44-7.49) | 7.56 (5.45-8.15) |
| RBC (×1012/L) | 5.00 (4.36-5.07) | 4.76 (3.02-4.88) | 4.49 (4.31-4.70) | 4.56 (4.40-4.74) | 4.90 (4.51-5.13) | 4.76 (4.41-4.95) | 4.66 (4.25-5.06) | 4.71 (4.32-5.17) | 4.83 (4.48-4.98) | 4.85 (4.51-5.03) |
| HGB (mmol/L) | 8.8 (8.4-9.0) | 8.5 (8.3-9.0) | 8.1 (6.9-8.5) | 8.0 (6.9-8.5) | 8.4 (8.3-9.7) | 8.5 (8.2-9.4) | 9.0 (7.9-9.9) | 8.8 (8.0-9.9) | 8.7 (8.3-9.1) | 8.8 (8.1-9.2) |
| HCT (L/L) | 0.440 (0.404-0.444) | 0.420 (0.415-0.432) | 0.399 (0.357-0.408) | 0.408 (0.361-0.409) | 0.421 (0.411-0.464) | 0.408 (0.402-0.449) | 0.422 (0.387-0.454) | 0.426 (0.398-0.467) | 0.425 (0.397-0.432) | 0.426 (0.396-0.441) |
| PLT (×109/L) | 287 (269-332)a | 285 (279-351)a | 361 (316-437) | 356 (310-458) | 357 (275-416)a | 326 (265-409)a | 242 (210-343) | 270 (214-355) | 249 (212-334)a | 261 (218-327)a |
| MPV (fL) | 10.2 (9.0-11.5)a | 10.5 (9.1-14.0)a | 9.8 (9.5-10.4) | 9.6 (9.5-10.6) | 9.4 (9.0-9.8)a | 9.2 (8.7-9.8)a | 10.4 (9.9-10.7) | 10.3 (9.9-10.5) | 9.5 (9.2-10.5)a | 9.6 (9.1-10.5)a |
| Triglyceride (mmol/L) | 1.36 (0.90-2.18) | 1.06 (0.86-1.99) | 0.68 (0.56-1.00) | 0.74 (0.50-0.92) | 1.23 (0.68-1.46) | 1.40 (0.96-2.16) | 0.76 (0.59-1.04) | 0.71 (0.56-1.04) | 0.80 (0.62-1.39) | 0.83 (0.62-1.12) |
Abbreviations: CBC, complete blood count; HCT, hematocrit; HGB, hemoglobin; MPV, mean platelet volume; PLT, platelet count; RBC, red blood cell count; WBC, white blood cell count.
aPLT medians and interquartile ranges were calculated based on 4 measurements in smoking and high-fat meal group and 5 measurements in control group, values were excluded due to the presence of platelet clumps in EDTA tube. Median and interquartile ranges of before and after values in each group were used to calculate P values with Wilcoxon signed rank test. No statistical differences were observed for each variable measured by CBC and triglyceride measurements.
Light Transmission Aggregometry
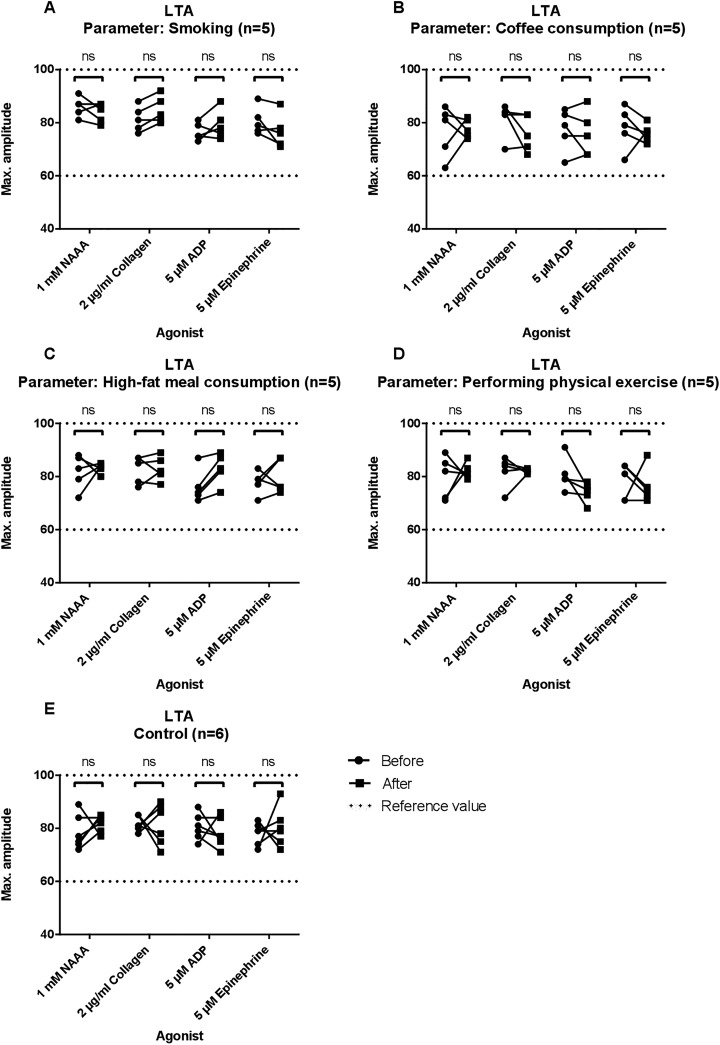
Aggregation using the LTA was measured with NAAA, collagen, ADP, and epinephrine as agonists. Results are shown in Figure 1 and Table 3. Median amplitudes before and after every parameter, including the control group, did not show significant differences. After consuming the high-fat meal with 55.8 g (±2.9) fat, the PPP aspect became very lipemic for 1 participant and slightly turbid for the other 4 participants.
Figure 1.
Results of light transmission aggregometry (LTA) for each group. Maximum amplitudes in percentages are depicted for 1 mM arachidonic acid (NAAA), 2 μg/mL collagen, 5 μM adenosine diphosphate (ADP), and 5μM epinephrine. Measurements before and after smoking/drinking coffee/consuming a high-fat meal or physical exercise are shown and linked by a continuous line. Reference values are shown as dotted lines. In panel A, amplitudes before and after smoking are observed. In panel B, amplitudes before and after coffee consumption are shown. In panel C, amplitudes before and after consuming a high-fat meal are observed. In panel D, amplitudes before and after physical exercise are shown. Control group is shown in panel E. NS indicates not significant.
Table 3.
Results LTA and Multiplate.a
| Platelet Function Tests | Smoking (n = 5) | Coffee (n = 5) | High-Fat Meal (n = 5) | Physical Exercise (n = 5) | Control (n = 6) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | Before | After | Before | After | |
| LTA | ||||||||||
| 1 mM NAAA | 87.0 (84.0-87.0) | 85.0 (81.0-87.0) | 81.0 (71.0-83.0) | 77.0 (75.0-81.0) | 83.0 (79.0-87.0) | 84.0 (83.0-84.0) | 82.0 (72.0-85.0) | 82.0 (81.0-83.0) | 80.5 (75.5-86.3) | 83.0 (79.8-84.0) |
| 2 µg/mL collagen | 81.0 (78.0-84.0) | 83.0 (81.0-88.0) | 84.0 (83.0-84.0) | 75.0 (71.0-83.0) | 85.0 (78.0-87.0) | 82.0 (810-86.0) | 84.0 (81.0-85.0) | 82.0 (81.0-83.0) | 81.0 (80.3-84.0) | 82.0 (75.8-87.5) |
| 5 µM ADP | 75.0 (75.0-79.0) | 78.0 (76.0-81.0) | 79.0 (75.0-83.0) | 75.0 (68.0-80.0) | 74.0 (73.0-76.0) | 83.0 (82.0-87.0) | 79.0 (79.0-81.0) | 75.0 (73.0-76.0) | 80.0 (77.5-83.3) | 77.0 (75.5-82.3) |
| 5 µM epinephrine | 79.0 (77.0-82.0) | 76.0 (72.0-78.0) | 79.0 (76.0-83.0) | 76.0 (74.0-77.0) | 79.0 (77.0-79.0) | 76.0 (76.0-87.0) | 81.0 (71.0-84.0) | 75.0 (73.0-76.0) | 79.0 (75.3-80.5) | 79.5 (76.0-82.3) |
| Multiplate | ||||||||||
| 6.4 µM ADP | 85.0 (84.0-102.0) | 87.0 (87.0-97.0) | 96.0 (96.0-110.0) | 95.0 (86.0-118.0) | 88.0 (82.0-89.0) | 90.0 (79.0-94.0) | 96.0 (95.0-100.0) | 105.0 (84.0-113.0) | 89.0 (85.3-91.5) | 84.0 (82.5-86.5) |
| 0.5 µM ASPI | 119.0 (110.0-120.0) | 126.0 (105.0-127.0) | 110.0 (99.0-118.0) | 107.0 (106.0-127.0) | 100.0 (76.0-110.0) | 102.0 (86.0-108.0) | 108.0 (86.0-108.0) | 105.0 (104.0-114.0) | 106.0 (98.8-110.3) | 99.0 (95.3-108.8) |
| 3.2 µg/mL collagen | 114.0 (105.0-116.0) | 112.0 (97.0-114.0) | 108.0 (105.0-118.0) | 127.0 (107.0-128.0) | 92.0 (73.0-94.0) | 95.0 (89.0-101.0) | 104.0 (97.0-120.0) | 119.0 (102.0-126.0) | 101.5 (95.3-107.0) | 95.5 (91.3-113.3) |
| 32 µM TRAP | 137.0 (137.0-147.0) | 140.0 (134.0-141.0) | 124.0 (123.0-125.0) | 125.0 (1230-130.0) | 114.0 (98.0-137.0) | 133.0 (122.0-137.0) | 118.0 (106.0-129.0) | 130.0 (122.0-147.0) | 118.5 (116.2-126.8) | 137.5 (116.5-148) |
Abbreviations: ADP, adenosine diphosphate; LTA, light transmission aggregometry; TRAP, thrombin receptor-activating peptide.
aFor LTA, the median amplitudes of the first and second blood collection with interquartile ranges are shown. For Multiplate, the median area under the curve (AUC) with interquartile ranges is shown before and after each parameter. No statistical differences were observed before and after each parameter.
Multiple Electrode Impedance Aggregometry (Multiplate Analyzer)
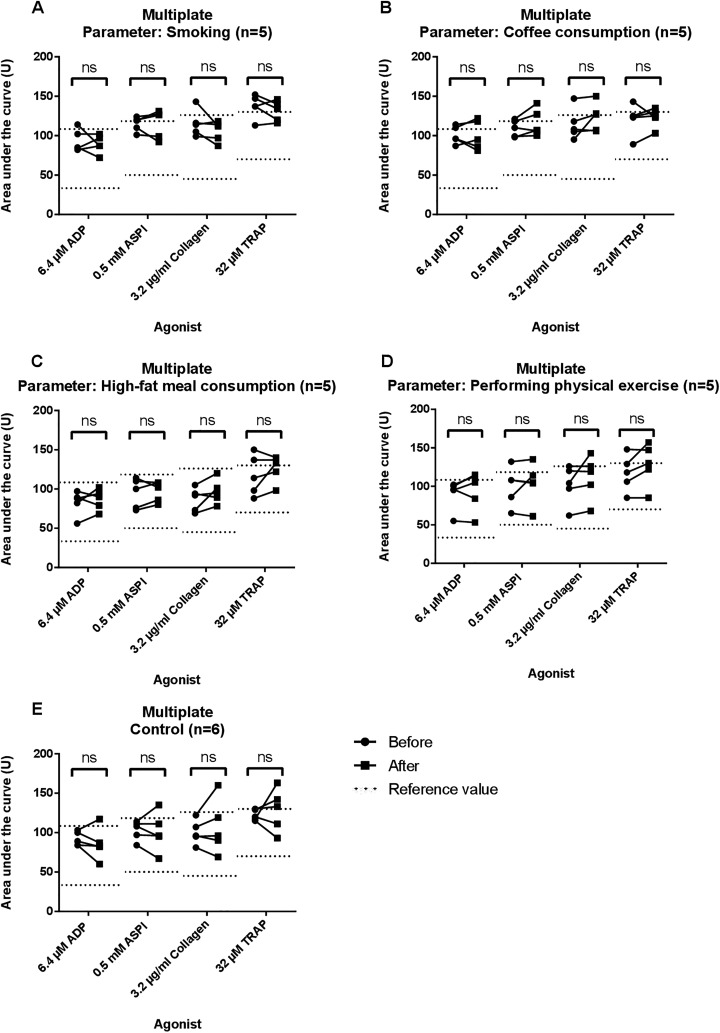
Platelet aggregation using the Multiplate analyzer was performed with ADP, ASPI, collagen, and TRAP as agonists. Results are shown in Figure 2 and Table 3. Median AUC before and after every parameter as well as in the control group did not show significant differences.
Figure 2.
Results of multiple electrode impedance aggregometry (MEIA) for each group using Multiplate. Areas under the curves (AUC) in U are shown for 6.4 μM adenine diphosphate (ADP), 0.5 mM arachidonic acid (ASPI), 3.2 μg/mL collagen, and 32 μM thrombin receptor-activating peptide (TRAP). Measurements before and after smoking/drinking coffee/consuming a high-fat meal or physical exercise are shown and linked by a continuous line. Reference values are shown as dotted lines. In panel A, AUC before and after smoking are observed. In panel B, AUC before and after coffee consumption are shown. In panel C, AUC before and after consuming a high-fat meal are observed. In panel D, AUC before and after physical exercise are shown. Control group is shown in panel E. NS indicates not significant.
Discussion
The results of this study indicate that it might not be necessary for patients to refrain from coffee, cigarette smoking, or remain completely fasting prior to blood collection for platelet function testing. Limitations of our study however are the use of healthy individuals instead of patients who are typically assessed for platelet function with a presumed hemostatic dysfunction, and the use of a single intervention in each case (1 cigarette, 1 cup of coffee, 1 meal, short pretest exercise), where we do not know if our findings also hold true for all types of fatty meals/cigarettes, larger volumes of (stronger) coffee/meals, and long-term consumption of fatty meals and coffee. For the smoking of cigarettes, we, however, handed the criteria that participants had a history of smoking for at least 5 years, and our results, therefore, give a long-term indication of the influence of smoking on platelet aggregation in healthy individuals. Also, if there was a clinically relevant difference (platelet aggregation lower than the reference values used), this would be observed in our study, despite the small number of participants used.
Casey et al6 observed a significant difference in platelet aggregation between young male smokers and a control group using 2µM ADP. However, Casey et al did not carry out measurements that point out the direct effect of smoking before a blood collection, since they only compared long-term smokers with nonsmokers. Also, platelet aggregation was measured using a different aggregometer (PAP-4 model), different preparation of the PPP, and only 2 µM ADP was used as agonist. Platelet-poor plasma from the samples in our study was obtained with a 2-step procedure to eliminate platelet debris and microparticles from plasma, while Casey et al used a single-step method. The method that is used in our study is a standardized procedure that is currently being used in our laboratory and is preferred, since the generated plasma could also be used for other purposes such as thrombin generation, where a second centrifugation step is preferred for plasma.7 In our experience and based on a study of Hayward et al,8 the use of lower concentration ADP leads to higher variation even in healthy volunteers. Therefore, we chose to use a higher concentration of 5µM ADP.
Natella et al9 showed that coffee consumption 30 and 60 minutes prior to a blood collection leads to a decrease in platelet aggregation with 0.5 µM NAAA and 3 µg/mL collagen, while caffeine intake (capsules) did not affect platelet aggregation induced by 0.5 µM NAAA, 3 µg/mL collagen, and 2µM ADP. However, platelet function in their study was determined using a different methodology for performing LTA (different concentration of agonists, preparation of PRP, and other time points for blood collection).
In the current study, no differences in platelet aggregation were observed after consuming a high-fat meal. Earlier research revealed conflicting results whether consuming a high-fat meal has an effect on platelet aggregation.10–14 This might be due to the use of different methodology: different meals with higher/lower fat concentrations, differences in executing LTA, and the use of other agonist concentrations. Also, one of the factors that may play a role in affecting platelet aggregation is the increased cloudiness of a lipemic sample. This interferes with the ability of optical assays, such as LTA, to properly measure platelet aggregation. According to Sanders,14 an intake of 40 to 50 g of fat in a meal results in significant lipemia in healthy adults and limiting the fat intake to 30 g on each eating occasion would minimize postprandial lipemia. In our study, only an increase, although not significant, in triglyceride levels after consuming the high-fat meal was observed. This insignificance could be due to the broad range of triglyceride levels among our participants. The interference of a lipemic sample with LTA assays could also differ with the type of aggregometer that is used. In our study, we used the Chronolog model 700, although the PAP models are also often used to carry out platelet function tests. The PAP models could be more sensitive for changes in testing with a lipemic sample since a different wavelength is used in these models. We have not performed experiments with the PAP or other models than the Chronolog 700; therefore, we can only state that we observed no difference in platelet aggregation with the Chronolog 700. Dobesh et al11 showed no significant change in platelet aggregation between fasting and nonfasting states for 5 and 20 µM ADP when measured with the Chronolog 700. However, a small, significant change was observed in the AUC with 5µM from fasting to nonfasting time points. Dobesh et al state that a meaningful increase in platelet aggregation is not demonstrated without extreme fat intake.11 However, platelet function can also be affected by the type of fat that is ingested. Bachmair et al15 state that saturated fatty acids and trans-fatty acids have been shown to increase platelet aggregation, while in other studies cis-, mono-, and polyunsaturated fatty acids have been shown to decrease platelet aggregation.16–18 Also, in a review of McEwen,19 it is stated that polyunsaturated fatty acids, present in fish, can decrease platelet aggregation when this was induced with ADP and collagen. Nevertheless, in a study of O’Brien,20 it was described that by replacing a normal diet with 120 g of polyunsaturated fats in the form of linoleic acid, no significant results were found when aggregation was tested with ADP and collagen. This was also observed in a study from Cohen et al,21 where platelet activation with omega-3 polyunsaturated fatty acids did not lead to significant changes in platelet aggregation when measured with NAAA and ADP. However, variation in study populations, time points, and lack of comparability in methods that assess platelet aggregation make it hard to draw a firm conclusion from preexisting studies. Moreover, we believe that the influence of specific fat types on platelet function is more likely related to long-term use than by testing as a single meal.
Several studies have examined the influence of physical exercise on platelet aggregation. However, this effect seems to differ with the type, intensity, and duration of the exercise, where especially strenuous exercise could lead to hypercoagulation.22,23 Therefore, we believe that the effect of short physical exercise, such as rapidly walking up the stairs, would not have a significant impact on platelet aggregation.
Although the influence of antiplatelet therapy, hematocrit levels, or pressure or temperature changes in platelet function assessed with multiple electrode aggregometry is described in other studies, other preanalytical variables remain unstudied.
Recommendations
Based on our findings, it might not be necessary for patients to refrain from coffee, cigarette smoking, or remain completely fasting prior to blood collection for platelet function testing. Therefore, we believe that platelet function testing can proceed if patients have had a cigarette, a cup of coffee, a meal, or have had to race up the stairs to come to their appointment. The real-life events that we have investigated during our study are therefore not expected to adversely affect testing, which can avoid canceling tests for those patients. However, definitive studies need to be undertaken to ensure that this would also be the case in patients with hemostatic dysfunction and in continuous consumption of a fatty diet, more/stronger coffee consumption, different types of cigarettes, and longer physical activity.
Acknowledgments
The authors thank the Hemostasis Working Group of the Dutch Society for Haematological Laboratories for critically reviewing this research project.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Joyce P.M. Krekels, BSc  http://orcid.org/0000-0001-8445-8411
http://orcid.org/0000-0001-8445-8411
References
- 1. Cattaneo M, Cerletti C, Harrison P, et al. Recommendations for the standardization of light transmission aggregometry: a consensus of the working party from the platelet physiology subcommittee of SSC/ISTH. J Thromb Haemost. 2013;11:1183–1189. [DOI] [PubMed] [Google Scholar]
- 2. Seyfert UT, Haubelt H, Vogt A, Hellstern P. Variables influencing Multiplate(TM) whole blood impedance platelet aggregometry and turbidimetric platelet aggregation in healthy individuals. Platelets. 2007;18(3):199–206. [DOI] [PubMed] [Google Scholar]
- 3. Cattaneo M, Hayward CP, Moffat KA, Pugliano MT, Liu Y, Michelson AD. Results of a worldwide survey on the assessment of platelet function by light transmission aggregometry: a report from the platelet physiology subcommittee of the SSC of the ISTH. J Thromb Haemost. 2009;7(6):1029. [DOI] [PubMed] [Google Scholar]
- 4. Clinical and Laboratory STandards Institute (CLSI). Platelet Function Testing by Aggregometry; Approved Guideline. CLSI document H58-A (ISBN 1-56238683-2). Clinical and Laboratory Standards Institute, 950 West Valley Road, Suite 2500, Wayne, Pennsylvania 19087 USA; 2008. [Google Scholar]
- 5. Harrison P, Mackie I, Mumford A, et al. Guidelines for the laboratory investigation of heritable disorders of platelet function. Br J Haematol. 2011;155(1):30–44. [DOI] [PubMed] [Google Scholar]
- 6. Casey RG, Joyce M, Roche-Nagle G, Cox D, Bouchier-Hayes DJ. Young male smokers have altered platelets and endothelium that precedes atherosclerosis. J Surg Res. 2004;116(2):227–233. [DOI] [PubMed] [Google Scholar]
- 7. Loeffen R, Kleinegris MC, Loubele ST, et al. Preanalytic variables of thrombin generation: towards a standard procedure and validation of the method. J Thromb Haemost. 2012;10(12):2544–2554. [DOI] [PubMed] [Google Scholar]
- 8. Hayward CP, Moffat KA, Pai M, et al. An evaluation of methods for determining reference intervals for light transmission platelet aggregation tests on samples with normal or reduced platelet counts. Thromb Haemost. 2008;100(1):134–145. [PubMed] [Google Scholar]
- 9. Natella F, Nardini M, Belelli F, et al. Effect of coffee drinking on platelets: inhibition of aggregation and phenols incorporation. Br J Nutr. 2008;100(6):1276–1282. [DOI] [PubMed] [Google Scholar]
- 10. Ahuja KD, Thomas GA, Adams MJ, Ball MJ. Postprandial platelet aggregation: effects of different meals and glycemic index. Eur J Clin Nutr. 2012;66(6):722–726. [DOI] [PubMed] [Google Scholar]
- 11. Dobesh PP, Urban JF, Shurmur SW, Oestreich JH. Impact of a high-fat meal on assessment of clopidogrel-induced platelet inhibition in healthy subjects. Thromb J. 2015;13(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Freese R, Mutanen M. Postprandial changes in platelet function and coagulation factors after high-fat meals with different fatty acid compositions. Eur J Clin Nutr. 1995;49(9):658–664. [PubMed] [Google Scholar]
- 13. Jakubowski JA, Ardlie NG, Chesterman CN, McGready JF, Morgan FJ. Acute postprandial lipaemia does not influence the in vivo activity of human platelets. Thromb Res. 1985;39(6):725–732. [DOI] [PubMed] [Google Scholar]
- 14. Sanders TA. Dietary fat and postprandial lipids. Curr Atheroscler Rep. 2003;5(6):445–451. [DOI] [PubMed] [Google Scholar]
- 15. Bachmair E, Ostertag L, Zhang X, de Roos B. Dietary manipulation of platelet function. Pharmacol Ther. 2014;144(2):97–113. [DOI] [PubMed] [Google Scholar]
- 16. Al-Madaney MM, Kramer JK, Deng Z, Vanderhoek JY. Effects of lipid-esterified conjugated linoleic acid isomers on platelet function: evidence for stimulation of platelet phospholipase activity. Biochim Biophys Acta. 2003;1635(2-3):75–82. [DOI] [PubMed] [Google Scholar]
- 17. Sofi F, Buccioni A, Cesari F, et al. Effects of a dairy product (pecorino cheese) naturally rich in cis-9, trans-11 conjugated linoleic acid on lipid, inflammatory and haemorheological variables: a dietary intervention study. Nutr Metab Cardiovasc Dis. 2010;20(2):117–124. [DOI] [PubMed] [Google Scholar]
- 18. Benito P, Nelson GJ, Kelley DS, Bartolini G, Schmidt PC, Simon V. The effect of conjugated linoleic acid on platelet function, platelet fatty acid composition, and blood coagulation in humans. Lipids. 2001;36(3):221–227. [DOI] [PubMed] [Google Scholar]
- 19. McEwen BJ. The influence of diet and nutrients on platelet function. Semin Thromb Hemost. 2014;40(2):214–226. [DOI] [PubMed] [Google Scholar]
- 20. O’Brien J. Effect of a diet of polyunsaturated fats on some platelet-function tests. Lancet. 1976;308(7993):995–997. [DOI] [PubMed] [Google Scholar]
- 21. Cohen MG, Rossi JS, Garbarino J, et al. Insights into the inhibition of platelet activation by omega-3 polyunsaturated fatty acids: beyond aspirin and clopidogrel. Thromb Res. 2011;128(4):335–340. [DOI] [PubMed] [Google Scholar]
- 22. Lippi G, Maffulli N. Biological influence of physical exercise on hemostasis. Semin Thromb Hemost. 2009;35(3):269–276. [DOI] [PubMed] [Google Scholar]
- 23. Posthuma JJ, Loeffen R, van Oerle R, et al. Long-term strenuous exercise induces a hypercoagulable state through contact activation. Thromb Haemost. 2014;111(6):1197–1199. [DOI] [PubMed] [Google Scholar]