Abstract
Tumor thrombus is a unique characteristic of renal cell carcinoma (RCC). However, only a few studies have reported its clinical influence on the occurrence of venous thromboembolism (VTE). This study aimed to clarify the influence of tumor thrombus and other risk factors for VTE and to elucidate the impact of tumor thrombus on survival outcomes. We retrospectively reviewed data from patients with RCC who underwent radical or partial nephrectomy from September 1999 to August 2015 at Seoul National University Hospital. A total of 2762 patients were enrolled. The 1- and 5-year cumulative incidences of VTE were 0.5% ± 0.1% and 1.5% ± 0.3%, respectively. During a median follow-up of 39.0 months (95% confidence interval [CI], 37.1-41.0 months), deep vein thrombosis occurred in 13 patients and pulmonary embolism in 15 patients. Patients with tumor thrombus (diagnosed by surgical pathology findings) had a significantly higher incidence of VTE than those without thrombus (odds radio 8.160, 95% CI, 1.480-45.004). Older age (≥60 years) and higher preoperative C-reactive protein (>0.5 mg/dL) were also significant risk factors for VTE. Additionally, tumor thrombus was independently associated with worse progression-free survival (PFS) but not with overall survival (OS) in multivariable analysis (hazard ratio [HR] 1.916, 95% CI, 1.295-2.834 for PFS; HR 1.164, 95% CI, 0.755-1.793 for OS). In conclusion, the incidence of VTE was relatively low in patients who underwent surgery for RCC. Nevertheless, patients with tumor thrombus had an increased risk of VTE and should be closely monitored for VTE.
Keywords: renal cell carcinoma, surgery, venous thromboembolism, survival, risk factors
Introduction
Venous thromboembolism (VTE) is one of the major complications of many malignancies. Patients with a malignancy have an approximately 4- to 6.7-fold increased risk of VTE compared to the general population.1–3 Because VTE increases in-hospital morbidity, cost of hospitalization, and possibly mortality, it is an important problem in patients with malignancies.4–6
The incidence of VTE in malignancy is influenced by cancer characteristics, including the type and stage of tumor, as well as the length of time from diagnosis.3,7,8 Previous large population studies showed that the incidence of VTE was highest in the first few months after cancer diagnosis, then decreased over time.3,8 In addition, several large-scale studies reported that patients diagnosed with metastatic gastrointestinal, bladder, uterine, and kidney cancer had a 6 to 25 times increased incidence of VTE, compared to those with other cancer types or localized disease.3,9,10 As the risk of VTE varies according to cancer features, appropriate prophylaxis and monitoring for VTE may depend on a patient’s disease status.
Renal cell carcinoma (RCC) is associated with an increased risk of VTE, with an incidence rate of approximately 1.3% to 3.8% during 2-year follow-up.9 In addition, migration of tumor into the renal vein and inferior vena cava, resulting in tumor thrombus formation, is a unique characteristic of RCC, found in approximately 4% to 10% of patients with this tumor.11 Furthermore, similar to lymph node involvement or metastasis, thrombus has been found to have an important predictive role in patient prognosis.12,13
Venous tumor thrombus can activate the vascular endothelium and disturb venous blood flow, which may increase the risk of VTE.14,15 However, the clinical effects of tumor thrombus on VTE have not been well established, and few studies have reported significantly increased risks of VTE in patients with tumor thrombus.16,17
The aims of this study were to elucidate the influence of tumor thrombus on VTE development and survival in patients who underwent surgical management for RCC. Additionally, we sought to identify other risk factors for VTE, such as patient age, hemogram results, and levels of inflammatory markers.
Materials and Methods
Patient Characteristics and Data Collection
We enrolled patients with pathologically confirmed RCC who underwent surgery at Seoul National University Hospital between September 1999 and August 2015. All patients underwent either radical or partial nephrectomy; for those suspected of having tumor thrombus based on preoperative image studies (eg, computed tomography [CT]), thrombectomy was also performed.
Clinical information regarding patients was retrospectively collected through a medical records review. The data included patient baseline characteristics, tumor histology and characteristics, tumor stage at the time of surgery, the presence or absence of tumor thrombus, and results of preoperative laboratory tests, including C-reactive protein (CRP), hemoglobin, and platelet, neutrophil, and lymphocyte counts. Tumor thrombus was defined as the presence of tumor thrombus confirmed by pathology after surgery. The neutrophil-to-lymphocyte ratio (NLR) was calculated by dividing the neutrophil count by the lymphocyte count. Postoperative VTE events were defined as pulmonary embolism (PE) or deep vein thrombosis (DVT). They were diagnosed using angiography, CT, and Doppler sonography. The value of laboratory tests was obtained before surgery.
Study End Points
The primary objective of this study was to evaluate the incidence and risk factors for VTE in patients with RCC who underwent surgery. We specifically evaluated the association between tumor thrombus and the occurrence of VTE. The secondary objective was to evaluate survival outcomes and to determine prognostic factors for survival.
Statistical Analysis
The cumulative incidence of VTE was calculated from the date of surgery to the detection of thromboembolism by diagnostic imaging. Progression-free survival (PFS) was defined as time from the date of surgery to the date at which disease progression was confirmed by imaging. Overall survival (OS) was defined as the time from the date of surgery to the date of either death or last follow-up. The incidence of VTE was analyzed using Pearson χ2 or Fisher exact test, as appropriate. Kaplan-Meier curves were used to analyze the cumulative incidences of VTE, PFS, and OS. Cutoff values of the continuous variables were either normal values (hemoglobin, platelet count, and CRP) or median values (age and NLR). Variables with P values <.05 in univariable analysis were considered for the multivariable analysis, which was performed using the logistic regression model and Cox proportional hazard model. All statistical tests were 2 sided; significance was defined as P <.05. All analyses were performed using IBM SPSS version 23.0 (IBM, Armonk, New York).
Ethical Considerations
This study was approved by the Seoul National University Hospital institutional review board (H-1710-095-895). It was conducted in accordance with the Declaration of Helsinki guidelines for biomedical research.
Results
Patient Baseline Characteristics
From September 1999 to August 2015, a total of 2762 patients underwent surgery. Radical nephrectomy was performed in 1436 (52.0%) patients; of these, 921 (33.3%) underwent open surgery, 367 (13.3%) underwent transperitoneal laparoscopic surgery, 124 (4.5%) underwent hand-assisted laparoscopic surgery, 15 (0.5%) underwent retroperitoneal laparoscopic surgery, and 9 (0.3%) underwent robot-assisted surgery. Partial nephrectomy was performed in 1326 (48.0%) patients; of these, 1038 (37.6%) underwent open surgery, 201 (7.3%) underwent robot-assisted surgery, 70 (2.5%) underwent transperitoneal laparoscopic surgery, 15 (0.5%) underwent retroperitoneal laparoscopic surgery, and 2 (0.1%) underwent hand-assisted laparoscopic surgery. Baseline characteristics of the patients are summarized in Table 1.
Table 1.
Baseline Characteristics of Patients.a
| Variables | Values |
|---|---|
| Age, years, median (range) | 56.0 (10.0-87.0) |
| Gender, n (%) | |
| Male | 1927 (69.8) |
| Female | 835 (30.2) |
| Smoking, n (%) | |
| Yes | 429 (15.5) |
| No | 2319 (84.0) |
| Not evaluated | 14 (0.5) |
| ECOG, n (%) | |
| 0 | 1934 (70.0) |
| 1 | 693 (25.1) |
| 2 | 116 (4.2) |
| Not evaluated | 19 (0.7) |
| Histology, n (%) | |
| Clear cell type | 2163 (78.3) |
| Papillary type | 289 (10.5) |
| Chromophobe type | 215 (7.8) |
| Others | 95 (3.4) |
| Pathologic T stage | |
| 1 | 2090 (75.7) |
| 2 | 255 (9.2) |
| 3 | 316 (11.4) |
| 4 | 17 (0.6) |
| Not evaluated | 84 (3.0) |
| Pathologic N stage | |
| Negative | 310 (11.2) |
| Positive | 2431 (88.0) |
| Not evaluated | 21 (0.8) |
| Clinical M stage | |
| Negative | 2577 (93.3) |
| Positive | 154 (5.6) |
| Not evaluated | 31 (1.1) |
| Tumor thrombosis, n (%) | |
| No thrombosis | 2589 (93.7) |
| Renal vein thrombosis | 119 (4.3) |
| Thrombosis involved IVC below the diaphragm | 25 (0.9) |
| Thrombosis involved IVC above the diaphragm | 9 (0.3) |
| Not evaluated | 20 (0.7) |
| Tumor extrusion, n (%) | |
| Exophytic | 1253 (45.4) |
| Mesophytic | 747 (27.0) |
| Endophytic | 707 (25.6) |
| Not evaluated | 55 (2.0) |
| Preoperative hemoglobin, g/dL, median (range) | 13.9 (4.5-21.6) |
| Preoperative platelet, /μL, median (range) | 229 × 103 (17.3 × 103-747 × 103) |
| Preoperative NLR, median (range) | 1.87 (0.02-80.6) |
| Preoperative CRP, mg/dL, median (range) | 0.12 (0.0-25.5) |
Abbreviations: CRP, C-reactive protein; ECOG, Eastern Cooperative Oncology Group; IVC, inferior vena cava; NLR, neutrophil-to-lymphocyte ratio.
a N = 2762.
Incidence of VTE
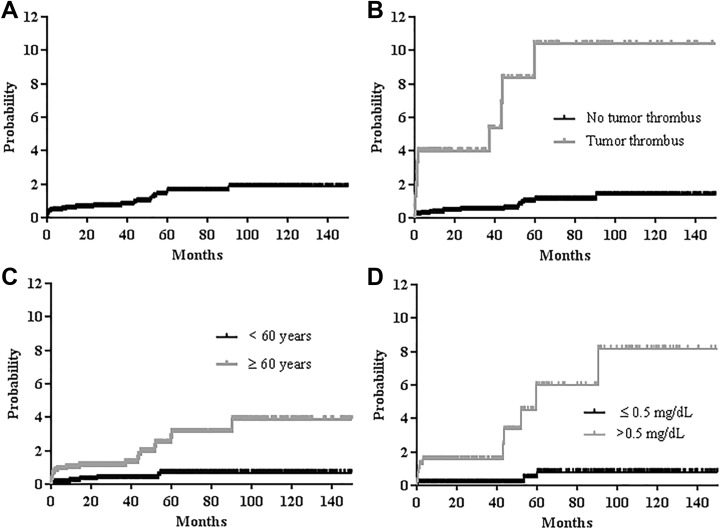
The median follow-up duration of all 2762 patients was 39.0 months (95% confidence interval [CI], 37.1-41.0 months). The 1- and 5-year cumulative incidences of VTE in all patients were 0.5% ± 0.1% and 1.5% ± 0.3%, respectively (Figure 1A). During follow-up, 28 VTE events occurred in 28 patients (1.0%); DVT occurred in 13 patients and PE occurred in 15 patients. The median time to occurrence of VTE was 9.4 months (95% CI, 0.0-23.9 months) after surgery.
Figure 1.
Cumulative incidence of venous thromboembolism (VTE) in patients with renal cell carcinoma: (A) in all study patients, (B) according to the presence or absence of tumor thrombus (5-year cumulative incidence: 1.0% vs 10.4%, P < .001), (C) according to age (5-year cumulative incidence: 2.8% vs 0.1%, P < .001), and (D) according to C-reactive protein (5-year cumulative incidence: 6.0% vs 0.1%, P < .001).
In univariable analysis, older age (≥60 years), advanced T stage, the presence of tumor thrombus, and an increased preoperative platelet count (>400 × 103/μL) and CRP (>0.5 mg/dL) were associated with a significantly increased incidence of VTE when all patients were considered (P < .05 for all factors; Table 2; cumulative incidence of VTE: Figure 1B for tumor thrombus, Figure 1C for age, and Figure 1D for CRP). In subgroup analysis of patients who were not diagnosed with stage IV at the initial diagnosis, older age (≥60 years), the presence of tumor thrombus, and an increased preoperative platelet count (>400 × 103/μL) and CRP (>0.5 mg/dL) were associated with an increased incidence of VTE in univariable analysis (P < .05 for all factors; Table 3).
Table 2.
Odds Ratios (ORs) and P Values of Thromboembolic Events in Univariable and Multivariable Analyses (Total Patients).
| Characteristics | No Thrombosis (%) | Thrombosis (%) | P Value (Univariable Analysis) | OR | 95% CI | P Value (Multivariable Analysis) |
|---|---|---|---|---|---|---|
| Age, years | ||||||
| ≥60 | 1118/2734 (40.9) | 21/28 (75.0) | <.001 | 8.608 | 1.845- | .006 |
| <60 | 1616/2734 (59.1) | 7/28 (25.0) | 1 | 40.152 | ||
| Gender | ||||||
| Male | 1907/2734 (69.8) | 20/28 (71.4) | 1.000 | |||
| Female | 827/2734 (30.2) | 8/28 (28.6) | ||||
| Smoking | ||||||
| Yes | 425/2720 (15.6) | 4/28 (14.3) | .913 | |||
| No | 2295/2720 (84.4) | 24/28 (85.7) | ||||
| ECOG | ||||||
| 0 | 1918/2717 (70.6) | 16/26 (61.5) | .162 | |||
| 1 | 686/2717 (25.2) | 7/26 (26.9) | ||||
| 2 | 113/2717 (4.2) | 3/26 (11.5) | ||||
| Histology | ||||||
| Clear cell type | 2144/2734 (78.4) | 19/28 (67.9) | .300 | |||
| Papillary type | 283/2734 (10.4) | 6/28 (21.4) | ||||
| Chromophobe | 213/2734 (7.8) | 2/28 (7.1) | ||||
| Others | 94/2734 (3.4) | 1/28 (3.6) | ||||
| T stage | ||||||
| 1 | 2078/2651 (78.4) | 12/27 (44.4) | <.001 | 1 | .923 | |
| 2 | 249/2651 (9.4) | 6/27 (22.2) | 1.265 | 0.211-7.588 | .797 | |
| 3 | 309/2651 (11.7) | 7/27 (25.9) | 0.666 | 0.102-4.364 | .671 | |
| 4 | 15/2651 (0.6) | 2/27 (7.4) | 0.930 | 0.047-18.398 | .962 | |
| N or M stage | ||||||
| N0M0 | 278/2734 (10.2) | 4/28 (14.3) | .523 | |||
| N+ or M+ | 2456/2734 (89.8) | 24/28 (85.7) | ||||
| Tumor thrombus | ||||||
| No thrombus | 2571/2714 (94.7) | 18/28 (64.3) | <.001 | 1 | 1.480-45.004 | .016 |
| Thrombus | 143/2714 (5.3) | 10/28 (35.7) | 8.160 | |||
| Tumor extrusion | ||||||
| Exophytic | 1242/2681 (46.3) | 11/26 (42.3) | .722 | |||
| Mesophytic | 738/2681 (27.5) | 9/26 (34.6) | ||||
| Endophytic | 701/2681 (26.1) | 6/26 (23.1) | ||||
| Preoperative Hb, g/dL | ||||||
| >16 | 219/2733 (8.0) | 2/28 (7.1) | 1.000 | |||
| ≤16 | 2514/2733 (92.0) | 26/28 (92.9) | ||||
| Preoperative platelet, /μL | ||||||
| >400 × 103 | 88/2732 (3.2) | 4/28 (14.3) | .013 | 2.232 | 0.503-9.907 | .291 |
| ≤400 × 103 | 2644/2732 (96.8) | 24/28 (85.7) | 1 | |||
| Preoperative NLR | ||||||
| ≥1.87 | 1358/2729 (49.8) | 19/28 (67.9) | .060 | |||
| <1.87 | 1371/2729 (50.2) | 9/28 (32.1) | ||||
| Preoperative CRP, mg/dL | ||||||
| >0.5 | 391/1680 (23.3) | 11/15 (73.3) | <.001 | 4.144 | 1.107-15.516 | .035 |
| ≤0.5 | 1289/1680 (76.7) | 4/15 (26.7) | 1 | |||
Abbreviations: CI, confidence interval; CRP, C-reactive protein; ECOG, Eastern Cooperative Oncology Group; Hb, hemoglobin; NLR, neutrophil-to-lymphocyte ratio.
Table 3.
Odds Ratios (ORs) and P Values of Thromboembolic Events in Univariable and Multivariable Analyses.a
| Characteristics | No Thrombosis (%) | Thrombosis (%) | P Value (Univariable Analysis) | OR | 95% CI | P Value (Multivariable Analysis) |
|---|---|---|---|---|---|---|
| Age, years | ||||||
| ≥60 | 662/1609 (41.1) | 10/13 (76.9) | .011 | 14.935 | 1.671-133.529 | .016 |
| <60 | 947/1609 (58.9) | 3/13 (23.1) | 1 | |||
| Gender | ||||||
| Male | 1101/1609 (68.4) | 8/13 (61.5) | .595 | |||
| Female | 508/1609 (31.6) | 5/13 (38.5) | ||||
| Smoking | ||||||
| Yes | 242/1605 (15.1) | 2/13 (15.4) | .921 | |||
| No | 1363/1605 (84.9) | 11/13 (84.6) | ||||
| ECOG | ||||||
| 0 | 1180/1594 (74.0) | 10/12 (83.3) | .695 | |||
| 1 | 361/1594 (22.6) | 2/12 (16.7) | ||||
| 2 | 53/1594 (3.3) | 0 | ||||
| Histology | ||||||
| Clear cell type | 1224/1609 (76.1) | 7/13 (53.8) | .064 | |||
| Papillary type | 176/1609 (10.9) | 4/13 (30.8) | ||||
| Chromophobe | 118/1609 (7.3) | 2/13 (15.4) | ||||
| Others | 91/1609 (5.7) | 0 | ||||
| T stage | ||||||
| 1 | 1235/1528 (80.8) | 8/13 (61.5) | .073 | |||
| 2 | 127/1528 (8.3) | 1/13 (7.7) | ||||
| 3 | 166/1528 (10.9) | 4/13 (30.8) | ||||
| 4 | 0 | 0 | ||||
| N or M stage | ||||||
| N0M0 | 275/1609 (17.1) | 3/13 (23.1) | .476 | |||
| N+ or M+ | 1334/1609 (82.9) | 10/13 (76.9) | ||||
| Tumor thrombus | ||||||
| No thrombus | 1540/1595 (96.6) | 9/13 (69.2) | .001 | 1 | .010 | |
| Thrombus | 55/1595 (3.4) | 4/13 (30.8) | 8.222 | 1.660-40.720 | ||
| Tumor extrusion | ||||||
| Exophytic | 747/1572 (47.5) | 5/12 (41.7) | .804 | |||
| Mesophytic | 394/1572 (25.1) | 4/12 (33.3) | ||||
| Endophytic | 431/1572 (27.4) | 3/12 (25.0) | ||||
| Preoperative Hb, g/dL | ||||||
| >16 | 107/1609 (6.7) | 1/13 (7.7) | .593 | |||
| ≤16 | 1502/1609 (93.3) | 12/13 (92.3) | ||||
| Preoperative platelet, /μL | ||||||
| >400 × 103 | 37/1609 (2.3) | 2/13 (15.4) | .037 | 7.745 | 1.146-52.368 | .036 |
| ≤400 × 103 | 1572/1609 (97.7) | 11/13 (84.6) | 1 | |||
| Preoperative NLR | ||||||
| ≥1.87 | 757/1608 (47.1) | 8/13 (61.5) | .298 | |||
| <1.87 | 851/1608 (52.9) | 5/13 (38.5) | ||||
| Preoperative CRP, mg/dL | ||||||
| >0.5 | 248/1225 (20.2) | 6/9 (66.7) | .003 | 3.613 | 0.753-17.343 | .109 |
| ≤0.5 | 977/1225 (79.8) | 3/9 (33.3) | 1 | |||
Abbreviations: ECOG, Eastern Cooperative Oncology Group; Hb, hemoglobin; NLR, neutrophil-to-lymphocyte ratio; CRP, C-reactive protein; CI, confidence interval.
a Patients who were not diagnosed with stage IV at the initial diagnosis, n = 1622.
In multivariable analysis, older age (≥60 years), the presence of tumor thrombus, and increased preoperative CRP (>0.5 mg/dL) were risk factors for an increased incidence of VTE when all patients were considered (odds ratio [OR] 8.608, P = .006 for age; OR 8.160, P = .016 for tumor thrombus; OR 4.144, P = .035 for preoperative CRP; Table 2). In subgroup analysis of patients who were not diagnosed with stage IV at the initial diagnosis, older age (≥60 years), the presence of tumor thrombus, and increased preoperative platelet count (>400 × 103/μL) were associated with a significantly increased incidence of VTE in multivariable analysis (OR 14.935, P = .016 for age; OR 8.222, P = .010 for tumor thrombus; OR 7.745, P = .036 for preoperative platelets; Table 3).
Survival Outcomes
During follow-up, 5-year PFS and OS were 85.2% ± 0.9% and 87.7% ± 0.8%, respectively. In univariable analysis, older age, poor Eastern Cooperative Oncology Group (ECOG) status, papillary type, advanced T stage, the presence of lymph node or distant metastases, tumor thrombus, exophytic type, and elevated preoperative platelet count, NLR, and CRP were prognostic factors for poor PFS (Table 4). In addition, age, ECOG status, histology, T stage, N or M stage, tumor thrombus, tumor extrusion, and preoperative hemoglobin, platelet count, NLR, and CRP were prognostic factors for OS in univariable analysis (Table 5).
Table 4.
Hazard Ratios (HRs) and P Values of Progression-Free Survival (PFS) Using Cox Regression Analysis in Univariable and Multivariable Analyses.
| Univariable Analysis | Multivariable Analysis | ||||
|---|---|---|---|---|---|
| Characteristics | 5-Year PFS (95% CI) (%) | P Value | HR | 95% CI | P Value |
| Age, years | |||||
| ≥60 | 82.2 (80.7-83.7) | <.001 | 1.153 | 0.857-1.551 | .347 |
| <60 | 87.2 (86.1-88.3) | 1 | |||
| Gender | |||||
| Male | 84.8 (83.8-85.8) | .181 | |||
| Female | 87.0 (85.6-88.4) | ||||
| Smoking | |||||
| Yes | 87.8 (85.8-90.8) | .387 | |||
| No | 85.2 (84.3-86.1) | ||||
| ECOG | |||||
| 0 | 88.1 (87.2-89) | <.001 | 1 | .015 | |
| 1 | 78.1 (76.1-80.1) | 1.509 | 1.106-2.059 | .009 | |
| 2 | 76.1 (71.1-81.1) | 1.695 | 0.997-2.882 | .051 | |
| Histology | |||||
| Clear cell type | 84.4 (83.4-85.4) | .004 | 1 | .222 | |
| Papillary type | 83.6 (80.8-86.4) | 0.996 | 0.642-1.544 | .985 | |
| Chromophobe | 92.1 (89.9-94.3) | 0.482 | 0.243-0.956 | .037 | |
| Others | 98.6 (97.2-100) | 0 | .956 | ||
| T stage | |||||
| 1 | 91.5 (90.7-92.3) | <.001 | 1 | <.001 | |
| 2 | 71.4 (68.1-74.7) | 4.115 | 2.701-6.268 | <.001 | |
| 3 | 55.0 (51.3-58.7) | 4.745 | 3.133-7.185 | <.001 | |
| 4 | 0 | 8.750 | 3.919-19.534 | <.001 | |
| N or M stage | |||||
| N0M0 | 86.1 (85.2-87.0) | .003 | 1 | .212 | |
| N+ or M+ | 77.4 (74.3-80.5) | 1.286 | 0.866-1.910 | ||
| Tumor thrombus | |||||
| No thrombosis | 88.0 (87.2-88.8) | <.001 | 1 | .001 | |
| Thrombosis | 37.4 (32.6-42.2) | 1.916 | 1.295-2.834 | ||
| Tumor extrusion | |||||
| Exophytic | 82.7 (81.4-84.1) | .001 | 1 | .751 | |
| Mesophytic | 84.4 (82.6-86.2) | 0.904 | 0.639-1.279 | .569 | |
| Endophytic | 90.4 (89.1-91.7) | 0.884 | 0.609-1.285 | .519 | |
| Preoperative Hb, g/dL | |||||
| >16 | 88.4 (85.8-91.0) | .223 | |||
| ≤16 | 84.8 (83.9-85.7) | ||||
| Preoperative platelet, /μL | |||||
| >400 × 103 | 51.1 (44.2-58.0) | <.001 | 1.253 | 0.780-2.011 | .351 |
| ≤400 × 103 | 86.4 (85.5-87.3) | 1 | |||
| Preoperative NLR | |||||
| ≥1.87 | 78.6 (77.2-80.0) | <.001 | 1.710 | 1.201-2.435 | .003 |
| <1.87 | 91.8 (90.9-92.7) | 1 | |||
| Preoperative CRP, mg/dL | |||||
| >0.5 | 69.4 (66.4-72.4) | <.001 | 1.395 | 0.998-1.950 | .051 |
| ≤0.5 | 88.8 (87.6-90.0) | 1 | |||
Abbreviations: CI, confidence interval; CRP, C-reactive protein; ECOG, Eastern Cooperative Oncology Group; Hb, hemoglobin; NLR, neutrophil-to-lymphocyte ratio.
Table 5.
Hazard Ratios (HRs) and P Values of Overall Survival (OS) Using Cox Regression Analysis in Univariable and Multivariable Analyses.
| Univariable Analysis | Multivariable Analysis | ||||
|---|---|---|---|---|---|
| Characteristics | 5-Year OS (95% CI) (%) | P Value | HR | 95% CI | P Value |
| Age, years | |||||
| ≥60 | 82.2 (80.7-83.7) | <.001 | 1.585 | 1.167-2.152 | .003 |
| <60 | 91.2 (90.3-92.1) | 1 | |||
| Gender | |||||
| Male | 87.0 (86.0- 88.0) | .350 | |||
| Female | 89.3 (87.9-90.7) | ||||
| Smoking | |||||
| Yes | 85.9 (83.7-88.1) | .687 | |||
| No | 88.0 (72.2-88.8) | ||||
| ECOG | |||||
| 0 | 90.5 (89.7-91.3) | <.001 | 1 | .001 | |
| 1 | 80.9 (82.9-78.9) | 1.478 | 1.062-2.057 | .020 | |
| 2 | 74.2 (69.1-79.3) | 2.401 | 1.457-3.959 | .001 | |
| Histology | |||||
| Clear cell type | 87.3 (86.4-88.2) | <.001 | 1 | .026 | |
| Papillary type | 82.1 (79.3-84.9) | 1.612 | 1.043-2.492 | .032 | |
| Chromophobe | 93.3 (86.9-99.7) | 0.461 | 0.213-0.999 | .050 | |
| Others | 97.1 (95.8-98.4) | 0 | .966 | ||
| T stage | |||||
| 1 | 93.7 (93.0-94.4) | <.001 | 1 | <.001 | |
| 2 | 76.8 (73.7-79.9) | 3.078 | 1.950-4.857 | <.001 | |
| 3 | 64.3 (61.1-67.5) | 4.221 | 2.704-6.591 | <.001 | |
| 4 | 0 | 8.485 | 3.842-18.741 | <.001 | |
| N or M stage | |||||
| N0M0 | 88.1 (87.3-88.9) | .017 | 1 | .046 | |
| N+ or M+ | 84.9 (82.2-87.6) | 1.550 | 1.009-2.381 | ||
| Tumor thrombus | |||||
| No thrombosis | 90.0 (89.2-90.8) | <.001 | 1 | .492 | |
| Thrombosis | 52.3 (47.8-56.8) | 1.164 | 0.755-1.793 | ||
| Tumor extrusion | |||||
| Exophytic | 84.2 (82.9-85.5) | <.001 | 1 | .894 | |
| Mesophytic | 88.7 (87.3-90.1) | 1.058 | 0.738-1.519 | .758 | |
| Endophytic | 92.4 (91.2-93.6) | 0.951 | 0.637-1.418 | .804 | |
| Preoperative Hb, g/dL | |||||
| >16 | 93.6 (91.8-95.4) | .007 | 1 | .942 | |
| ≤16 | 87.1 (86.3-87.9) | 1.026 | 0.510-2.065 | ||
| Preoperative platelet, /μL | |||||
| >400 × 103 | 55.1 (48.8-61.4) | <.001 | 1.144 | 0.713-1.836 | .577 |
| ≤400 × 103 | 88.9 (88.1-89.7) | 1 | |||
| Preoperative NLR | |||||
| ≥1.87 | 80.7 (79.4-82.0) | <.001 | 1.863 | 1.273-2.727 | .001 |
| <1.87 | 94.8 (94.0-95.6) | 1 | |||
| Preoperative CRP, mg/dL | |||||
| >0.5 | 88.8 (87.6-90.0) | <.001 | 3.101 | 2.217-4.336 | <.001 |
| ≤0.5 | 93.8 (92.8-94.8) | 1 | |||
Abbreviations: CI, confidence interval; CRP, C-reactive protein; ECOG, Eastern Cooperative Oncology Group; Hb, hemoglobin; NLR, neutrophil-to-lymphocyte ratio.
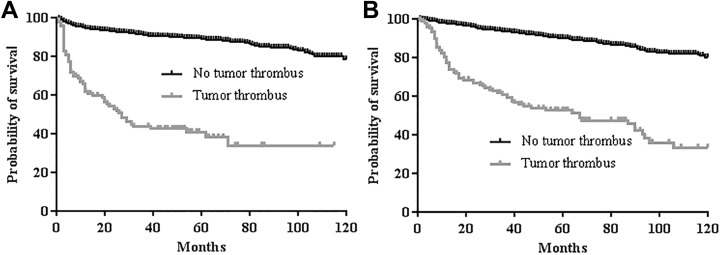
Tumor thrombus was an independent factor for poor PFS but not for OS in the final multivariable analysis (hazard ratio [HR] 1.916, 95% CI, 1.295-2.834, P = .001 for PFS; HR 1.164, 95% CI, 0.755-1.793, P = .492 for OS; Tables 4 and 5, Figure 2A and B). Poor ECOG status, advanced T stage, and increased preoperative NLR (≥1.87) were also associated with poor PFS in multivariable analysis (HR 1.509, P = .009 for ECOG 1; HR 4.115, P < .001 for T2; HR 4.745, P < .001 for T3; HR 8.750, P < .001 for T4; HR 1.710, P = .003 for preoperative NLR; Table 4). Additionally, older age, worse ECOG status, papillary type, advanced T stage, advanced N or M stage, and increased preoperative NLR (≥1.87) and CRP (>0.5 mg/dL) were risk factors for worse OS in multivariable analysis (HR 1.585, P = .003 for age; HR 1.478, P = .020 for ECOG 1; HR 2.401, P = .001 for ECOG 2; HR 1.612, P = .032 for papillary type; HR 3.078, P < .001 for T2; HR 4.221, P < .001 for T3; HR 8.485, P < .001 for T4; HR 1.550, P = .046 for N+ or M+ stage; HR 1.863, P = .001 for preoperative NLR; HR 3.101, P < .001 for preoperative CRP; Table 5).
Figure 2.
Survival outcomes according to the presence or absence of tumor thrombus: (A) progression-free survival (5-year survival: 88.0% vs 37.4%, P < .001) and (B) overall survival (5-year survival: 90.0% vs 52.3%, P < .001).
Discussion
Venous thromboembolism (VTE) is a risk factor for poor prognosis in patients with cancer; previous studies have reported that patients with VTE have a higher mortality rate than those without VTE.6
In the present study, we found that patients with RCC tumor thrombus had an approximately 8-fold increased risk of VTE during follow-up. With the unique ability of RCC to produce tumor thrombus, the risk of VTE might increase as tumor thrombus enters the pulmonary circulation after surgery.18 Furthermore, tumor thrombus can stimulate procoagulant factors through activation of vascular endothelial cells, which might contribute to an increased risk of VTE.14 In a previous study, Ihaddadene et al reported that the incidence of VTE was higher in patients with residual tumor thrombus after surgery than in those with complete tumor thrombus resection or no tumor thrombus (HR 8.7, 95% CI, 1.7-43.4 for complete resection; HR 6.5, 95% CI, 1.7-24.7 for no thrombosis).17 Our current findings are consistent with these results, as our patients with tumor thrombus had a higher incidence of thromboembolic events than those without thrombus. However, we did not classify patients according to whether they had residual tumor thrombus postoperatively. Nevertheless, we examined the effects of variables that could be related to VTE, such as smoking, tumor extent, inflammatory markers, and hemogram results.19–22 In addition, we performed subgroup analysis in patients who were not diagnosed with stage IV at the initial diagnosis because advanced stage is associated with increased biological activity of the tumor, and previous studies have reported that the biological aggressiveness of tumors could be positively correlated with thromboembolic events.7,21 Our results show that tumor thrombus was one of the statistically significant risk factors for VTE in the total study population, as well as in the subgroup of patients who were not diagnosed with an advanced stage initially.
Although hemogram results, such as the platelet count and hemoglobin, were not associated with an increased incidence of VTE, older age and increased CRP were identified as risk factors for VTE in the present study. The CRP is a representative inflammatory marker; an inflammatory state could contribute to VTE through activation of vascular endothelial cells and adhesion molecules, which could activate the coagulation cascade.15,23,24 Older age is an established risk factor for VTE, which may be associated with underlying illnesses or reduced blood flow and stasis that increase the potential for coagulation.25
Renal cell carcinoma (RCC) is a type of malignancy frequently associated with the development of VTE, with a 1.3- to 5.3-fold higher risk of VTE than cancers with a low thromboembolic risk.9,26 In the current study, the incidence of VTE was low (1%) compared to that found in previous studies.9,21,27 This difference could be explained by the characteristics of our patients. We evaluated a homogeneous group of patients who had undergone surgery, most of whom (93.3%) did not have metastatic lesions. In malignant neoplasms, tumor cells can activate the hemostatic system and coagulation cascade through procoagulant factors, including tissue factor, cancer procoagulants, and inflammatory cytokines. Additionally, interaction of tumor cell surface adhesion molecules with platelets, leukocytes, and vascular endothelial cells can promote clotting activation and thrombus formation. Through these processes, tumor cells can promote the development of VTE. Additionally, procoagulant proteins can support the metastasis of tumors by promoting neoangiogenesis.26 This synergistic interaction may explain the positive correlation between cancer biological activity and VTE formation.7,21 Therefore, our patients likely had a relatively low incidence of VTE because of fewer biologically aggressive tumor features.
In the present study, we found that tumor thrombus predicted poor PFS but not OS. The effect of tumor thrombus on survival has been controversial in previous studies.13,17,28,29 Ihaddadene et al reported that residual tumor thrombus was not associated with worse survival compared with those without a tumor thrombus or completely resected tumor thrombus, and their result is similar to the result in this study 17. However, Haferkamp et al found that tumor thrombus extension was an independent prognostic factor for survival.13 Therefore, further large-scale studies might be necessary to verify this.
Our study has some limitations, including its retrospective and single-center design. Therefore, our results should be interpreted with caution and will require validation in future prospective studies from other centers. Despite these limitations, this study clearly demonstrates that tumor thrombus is associated with an increased thromboembolic risk in patients who underwent surgery for RCC and that it has a negative impact on PFS.
In conclusion, the incidence of VTE was relatively low in patients with RCC who underwent surgery. Nevertheless, patients with tumor thrombus, older age, and increased preoperative CRP had a 4 to 8 times increased risk of VTE and should be monitored closely for the development of VTE.
Footnotes
Authors’ Note: Hyunkyung Park, MD and Chang Wook Jeong, MD, are the first co-authors. Cheol Kwak, MD and Inho Kim, MD, are the corresponding authors. Informed consent for patient information to be published in this article was not obtained because of the retrospective nature of this study.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Lee AY, Levine MN. Venous thromboembolism and cancer: risks and outcomes. Circulation. 2003;107(23 suppl 1):I17–I21. [DOI] [PubMed] [Google Scholar]
- 2. Heit JA, Silverstein MD, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ., III Risk factors for deep vein thrombosis and pulmonary embolism: a population-based case–control study. Arch Intern Med. 2000;160(6):809–815. [DOI] [PubMed] [Google Scholar]
- 3. Blom JW, Doggen CJ, Osanto S, Rosendaal FR. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA. 2005;293(6):715–722. [DOI] [PubMed] [Google Scholar]
- 4. Khorana AA, Francis CW, Culakova E, Fisher RI, Kuderer NM, Lyman GH. Thromboembolism in hospitalized neutropenic cancer patients. J Clin Oncol. 2006;24(3):484–490. [DOI] [PubMed] [Google Scholar]
- 5. Elting LS, Escalante CP, Cooksley C, et al. Outcomes and cost of deep venous thrombosis among patients with cancer. Arch Intern Med. 2004;164(15):1653–1661. [DOI] [PubMed] [Google Scholar]
- 6. Sorensen HT, Mellemkjaer L, Olsen JH, Baron JA. Prognosis of cancers associated with venous thromboembolism. N Engl J Med. 2000;343(25):1846–1850. [DOI] [PubMed] [Google Scholar]
- 7. Wun T, White RH. Venous thromboembolism (VTE) in patients with cancer: epidemiology and risk factors. Cancer Invest. 2009;27(suppl 1):63–74. [DOI] [PubMed] [Google Scholar]
- 8. Chew HK, Wun T, Harvey DJ, Zhou H, White RH. Incidence of venous thromboembolism and the impact on survival in breast cancer patients. J Clin Oncol. 2007;25(1):70–76. [DOI] [PubMed] [Google Scholar]
- 9. Chew HK, Wun T, Harvey D, Zhou H, White RH. Incidence of venous thromboembolism and its effect on survival among patients with common cancers. Arch Intern Med. 2006;166(4):458–464. [DOI] [PubMed] [Google Scholar]
- 10. Levitan N, Dowlati A, Remick SC, et al. Rates of initial and recurrent thromboembolic disease among patients with malignancy versus those without malignancy. Risk analysis using Medicare claims data. Medicine. 1999;78(5):285–291. [DOI] [PubMed] [Google Scholar]
- 11. Nouh MA, Inui M, Kakehi Y. Renal cell carcinoma with IVC thrombi; current concepts and future perspectives. Clin Med Oncol .2008;2:247–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zisman A, Wieder JA, Pantuck AJ, et al. Renal cell carcinoma with tumor thrombus extension: biology, role of nephrectomy and response to immunotherapy. J Urol. 2003;169(3):909–916. [DOI] [PubMed] [Google Scholar]
- 13. Haferkamp A, Bastian PJ, Jakobi H, et al. Renal cell carcinoma with tumor thrombus extension into the vena cava: prospective long-term followup. J Urol. 2007;177(5):1703–1708. [DOI] [PubMed] [Google Scholar]
- 14. Mackman N. New insights into the mechanisms of venous thrombosis. J Clin Invest. 2012;122(7):2331–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lopez JA, Chen J. Pathophysiology of venous thrombosis. Thromb Res. 2009;123(suppl 4):S30–S34. [DOI] [PubMed] [Google Scholar]
- 16. Yokom DW, Ihaddadene R, Moretto P, et al. Increased risk of preoperative venous thromboembolism in patients with renal cell carcinoma and tumor thrombus. J Thromb Haemost. 2014;12(2):169–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ihaddadene R, Yokom DW, Le Gal G, et al. The risk of venous thromboembolism in renal cell carcinoma patients with residual tumor thrombus. J Thromb Haemost. 2014;12(6):855–859. [DOI] [PubMed] [Google Scholar]
- 18. Ayyathurai R, Garcia-Roig M, Gorin MA, et al. Bland thrombus association with tumour thrombus in renal cell carcinoma: analysis of surgical significance and role of inferior vena caval interruption. BJU Int. 2012;110(11 pt B):E449–E455. [DOI] [PubMed] [Google Scholar]
- 19. Ferroni P, Riondino S, Formica V, et al. Venous thromboembolism risk prediction in ambulatory cancer patients: clinical significance of neutrophil/lymphocyte ratio and platelet/lymphocyte ratio. Int J Cancer. 2015;136(5):1234–1240. [DOI] [PubMed] [Google Scholar]
- 20. Pomp ER, Rosendaal FR, Doggen CJ. Smoking increases the risk of venous thrombosis and acts synergistically with oral contraceptive use. Am J Hematol. 2008;83(2):97–102. [DOI] [PubMed] [Google Scholar]
- 21. Wun T, White RH. Epidemiology of cancer-related venous thromboembolism. Best Pract Res Clin Haematol. 2009;22(1):9–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Connolly GC, Phipps RP, Francis CW. Platelets and cancer-associated thrombosis. Semin Oncol. 2014;41(3):302–310. [DOI] [PubMed] [Google Scholar]
- 23. Clyne B, Olshaker JS. The C-reactive protein. J Emerg Med. 1999;17(6):1019–1025. [DOI] [PubMed] [Google Scholar]
- 24. Aksu K, Donmez A, Keser G. Inflammation-induced thrombosis: mechanisms, disease associations and management. Curr Pharm Des. 2012;18(11):1478–1493. [DOI] [PubMed] [Google Scholar]
- 25. Cushman M. Epidemiology and risk factors for venous thrombosis. Semin Hematol. 2007;44(2):62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Falanga A, Russo L, Verzeroli C. Mechanisms of thrombosis in cancer. Thromb Res. 2013;131(suppl 1):S59–S62. [DOI] [PubMed] [Google Scholar]
- 27. Smith AB, Horvath-Puho E, Nielsen ME, Lash TL, Baron JA, Sorensen HT. Effect of comorbidity on risk of venous thromboembolism in patients with renal cell carcinoma. Urol Oncol. 2014;32(4):466–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Quencer KB, Friedman T, Sheth R, Oklu R. Tumor thrombus: incidence, imaging, prognosis and treatment. Cardiovasc Diagn Ther. 2017;7(suppl 3):S165–S177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sidana A, Goyal J, Aggarwal P, Verma P, Rodriguez R. Determinants of outcomes after resection of renal cell carcinoma with venous involvement. Int Urol Nephrol. 2012;44(6):1671–1679. [DOI] [PubMed] [Google Scholar]