Abstract
Osteoarthritis, a degenerative disease of the joints, is the most common form of arthritis in the knee. Total joint arthoplasty is a commonly used treatment for joint degeneration and osteoarthritis, and due to these factors, TJA for hip and knee joints is projected to grow by 137% and 601% between 2005 and 2030. Matrix metalloproteases are enzymes found in the extracellular matrix that cleave matrix components. Normally MMPs are downregulated in tissues by Tissue Inhibitors of Metalloproteases, or TIMPs. The relative concentration of TIMPs also may denote some of the activity of the MMPs found in serum. Lubricin (proteoglycan 4) is a molecule found in the synovial fluid that protects joints by dissipating strain energy during locomotion. Lubricin synovial fluid concentration is also diminished in many patients with osteoarthritis, but not all. Given the importance of these three sets of molecules, our lab investigated the correlation between circulating lubricin, MMP levels and TIMPs levels. Blood plasma samples were obtained from de-identified subjects undergoing total joint arthroplasty at Loyola University Medical Center and the University of Utah. Normal blood plasma from pooled healthy individuals served as a control. We analyzed biomarker levels in plasma using ELISA. Our data show that MMP-1 and 9 were increased in TJA patients compared to normal controls, while MMP-2 and 13 were decreased. We also found decreased lubricin and tissue factor in surgical patients relative to controls. These data support the idea that lubricin is vital in protecting the synovial joint and that MMPs play a complex role in the destruction of the joint.
Keywords: osteoarthritis, lubricin, arthroplasty, matrix metalloproteinases, MMP-1, MMP-2, MMP-9, MMP-13, tissue inhibitors of matrix metalloproteinases, vEGF
Introduction
Osteoarthritis (OA), a degenerative joint disease, is the most common form of arthritis in the knee.1 Osteoarthritis is related to both aging and obesity, and so its prevalence is expected to rise in the coming years given America’s demographics.2 Once regarded as a purely mechanical condition involving physical destruction of the articular cartilage, recent research reveals that OA is a complex inflammatory condition with multiple important factors and degradative processes co-occurring.3
At the moment, total joint arthroplasty (TJA) is the most commonly used treatment for joint degeneration and OA. The use of TJA for hip and knee joints is projected to grow by 137% and 601% between 2005 and 2030.4 Currently, there are no Food and Drug Administration approved medical treatments capable of slowing disease progression of OA or ultimately delaying the need for surgery.5 Thus, demand for an effective treatment of OA will only increase as the population ages, and there is room for medical or other therapy to enter the market.
Matrix metalloproteinases (MMPs) are enzymes found in the extracellular matrix that cleave their target proteins at internal peptide bonds.6 Matrix metalloproteinases have a variety of targets and can degrade collagen and extracellular matrix components. Levels of MMPs 1, 2, 9, 13, and others have been shown to be elevated in both plasma and synovial fluid of patients with OA, and likely have significant consequences on the progression of the disease.7,8 Some of these MMPs have been shown to be upregulated by inflammatory cytokines or mononuclear cells.9,10 It has also been shown that synovitis upregulates MMP expression and therefore perpetuates the degradation characteristic of progressive OA.11
Normally MMPs are regulated in tissues by tissue inhibitors of metalloproteinases (TIMPs). Humans have 4 different types of TIMPs (1-4), and TIMPs appear to inhibit all MMPs that have been studied to date.12 The relative concentration of TIMPs will regulate the activity of the MMPs found in plasma. Of the inhibitors, TIMP-1 appears to have the highest affinity for MMPs 1, 3, 9, and 13.13 Research has also suggested that a higher MMP/TIMPs ratio may be indicative of septic arthritis and poorer prognoses.14 Tissue inhibitors of matrix metalloproteinases have been shown to be elevated in patients with OA, but it is not clear if they are elevated as a compensatory response to the increased MMPs or if they are in some way contributing to the progression of disease.15
Lubricin (proteoglycan 4) is a molecule found in the synovial fluid that protects joints by dissipating strain energy during locomotion, protecting cartilage, and decreasing wear on the cartilaginous surfaces.16 It also works to prevent synoviocyte overgrowth and inhibit adhesion-dependent cell growth, thereby protecting the specialized acellular layer of the cartilage within the joint. In the absence of lubricin inhibition, synoviocytes will invade and destroy adjacent cartilage surfaces.17 Lubricin has a similar inhibitory effect on the adhesion of human fibroblasts, furthering its importance within the joint.18 Research has shown that lubricin can prevent chondrocyte apoptosis via inhibition of caspase-3.19,20 This proteoglycan also decreases chondrocyte hypertrophy and terminal differentiation, preserving functional chondrocytes that maintain the joint space.21 Since this important barrier molecule is normally secreted by synoviocytes,22 meniscal cells,23 and superficial chondrocytes,24 damage to these cells in OA can exacerbate the progression of the disease.22–24 Importantly, lubricin is anchored to the joint surface via cartilage oligomeric matrix protein (COMP), fibronectin, and collagen II. The MMP-9 has been shown to digest COMP and liberate lubricin from the joint surface, disrupting its functional capacity.25 Because lubricin itself is not degraded by MMP-9, there is promise for intra-articular injection of lubricin as a therapeutic approach. Lubricin synovial fluid concentration is diminished in many patients with OA but not all.26 However, it has demonstrated potential as an agent to reduce symptoms of OA when injected directly into the joint space in animal models.21,27
In addition to its role as a barrier protein, lubricin also has other anti-inflammatory properties in the context of the joint and may function to diminish MMP action and expression.21,28 Lubricin has been shown to reduce gene expression of some cytokines such as interleukin (IL)-6 and IL-8, while increasing TIMP-2 and cyclooxygenase-2 expression. The same research also showed that lubricin decreased IL-1β-mediated induction of MMP-1, MMP-3, MMP-9, MMP-13, IL-6, IL-8, and COX2 expression.28
Another clinical hallmark of OA is significant inflammation of the joint. Inflammation has been shown to increase the activity of MMPs and reduce the creation of new collagen in vitro.29 Vascular endothelial growth factor (vEGF) is another biomarker that is increased in patients with OA and has some promise as a drug target. In chronic OA, persistent inflammation and vEGF signaling leads to increased invasion of the synovial membrane, greater pain, and increased clinical severity of the disease.30 Animal models have suggested that inhibition of vEGF (via bevacizumab) may inhibit post-traumatic OA,31 and a variety of other studies are being done to assess the viability of vEGF inhibition in humans to treat OA.30
Given the importance of these biochemical parameters, this study investigates the correlation between circulating lubricin, vEGF, MMP levels, and TIMP levels in order to identify the importance of lubricin, MMPs, and TIMPs in joint stability and surgical recovery.
We hypothesize that there will be positive correlations between MMPs and other pro-inflammatory biomarkers and that these markers will be elevated in TJA patients compared to controls. We also hypothesize that TIMPs will be elevated and that lubricin will be drastically decreased in patients with OA.
Materials and Methods
Methods
Under institutional review board’s ethical approval from Loyola University Chicago Health Sciences Division, de-identified samples of plasma (n = 80) were obtained from patients undergoing total hip arthroplasty and total knee arthroplasty at Loyola University Medical Center and the University of Utah Orthopedics Clinic. Patient mean age was 66 ± 11 with a range of 36 to 86 years. Samples were collected into tubes containing 3.2% sodium citrate during routine preoperative consultations 1 day prior to surgery and 1 day postoperatively, and the plasma was subsequently isolated. Blood samples were centrifuged to isolate plasma which was then aliquoted and stored at −80°C. Patients undergoing revisionary procedures were excluded from our sample set. Patient demographic data were also obtained at the time of surgery and included patient age, gender, BMI, presence of diabetes mellitus, hypertension, and use of statin therapy. Patients were assigned random numerical identifiers in order to match demographic data with plasma marker levels.
We analyzed blood plasma samples with enzyme-linked immunosorbent assay (ELISA) kits, according to manufacturer’s instructions. The ELISAs were run to assay MMPs 1, 2, 9, and 13 levels, as well as TIMP-1, and vEGF levels (obtained from R&D Systems, Minneapolis, Minnesota). Plasma lubricin levels were measured using a PRG4 ELISA kit (obtained from MyBioSource.com).
Controls
A pool of normal human plasma (NHP; n = 31) composed of non-age-matched pooled plasma served as a control. Pathological pooled plasma (PATH) was obtained from Loyola University Medical Center from patients with inflammatory disorders and was aggregated together and used as a pool to compare OA to other generalized pathological and inflammatory conditions.
Statistical Analysis
Statistical analysis was performed using Microsoft Excel and Graphpad Prism Software version 7. Pre- and postoperative levels of MMPs, TIMPs, lubricin, MP-TF, vEGF, tissue factor, and Procollagen type I N-terminal Propeptide were compared to levels in the NHP and PATH samples.
Results were expressed as means ± standard error of the mean, and as percent changes from normal. Analysis of variance identified significant differences between control and experimental groups. We used a standard P value of less than .05 to determine significance. Additionally, we created correlation tables using Spearman R coefficients to identify correlated markers within our cohort groups, while screening for significant values.
Results
Matrix Metalloproteinase Levels in TJA Patients Compared to Pathological and Normal Patients
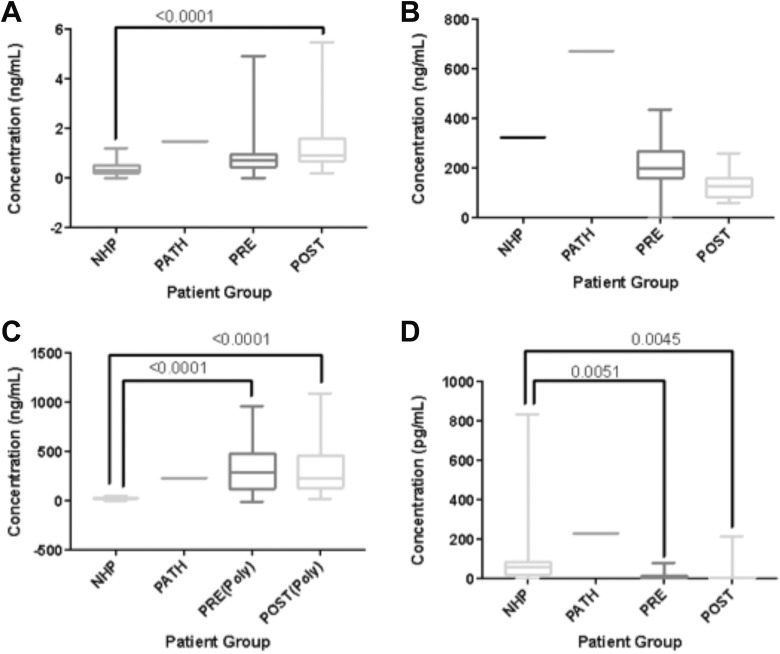
Matrix metalloproteinase-1 was significantly elevated in post-TJA patients compared to normals (P < .0001) but not significantly different from pathological samples. Results are displayed in Figure 1. The MMP-2 levels were higher in the pathological group than our normal human plasma (P = .0047), and post-operative MMP-2 was decreased compared to preoperative MMP-2 (P < .0001). However, there was not a demonstrable statistically significant difference between our patient group and our control group. Results are displayed in Figure 1. The MMP-9 was significantly increased both pre- and postoperatively compared to normals (P < .0001, < .0001). We found no significant difference between our pathological control group and our surgical group, nor between the normal control and the pathological control. Results are displayed in Figure 1. The MMP-13 was significantly decreased both pre- and postoperatively compared to normals (P = .0051, .0045). Pathological pooled plasma samples appeared to have elevated levels of MMP-13 when compared to both normals and our TJA patients, but there was not a statistically significant difference. Results are displayed in Figure 1.
Figure 1.
Boxplots comparing biomarker levels in control groups versus total joint arthroplasty (TJA) patients. (A) Matrix metalloproteinase (MMP)-1. (B) MMP-2 (C) MMP-9 (D) MMP-13. Significant P values (< .05) are indicated.
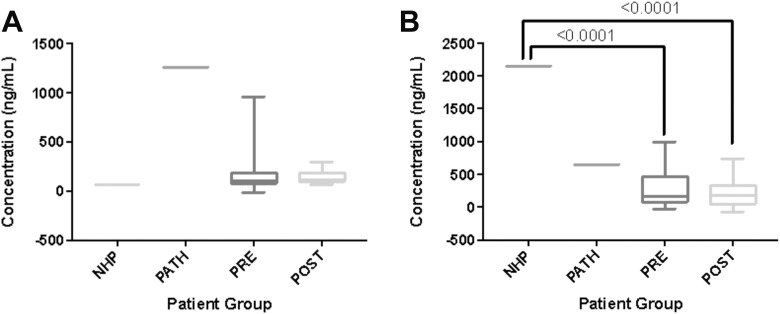
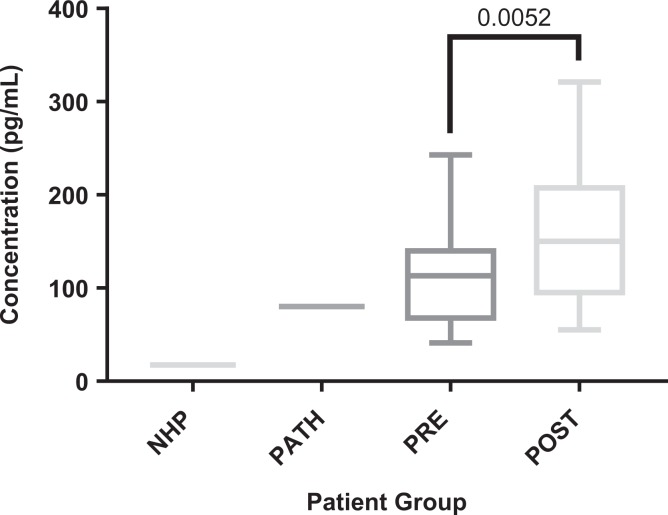
The TIMP-1 showed increases in our surgical group compared to normal samples, but there were not enough normal controls for the increase to be statistically significant. Pathological pooled plasma appeared to be elevated compared to both normal controls as well as surgical patients, but the difference was not statistically significant. Results are displayed in Figure 2. Lubricin showed significant decreases both pre- and postoperatively when compared to normals (P < .0001, < .0001). There was no statistically significant difference compared to pathological controls. Results are displayed in Figure 2. Vascular endothelial growth factor showed a significant increase postoperatively (P = .0052). There were no significant differences between the surgical patients and the normals or the pathological controls. Results are displayed in Figure 3.
Figure 2.
Boxplots comparing biomarker levels in control groups versus total joint arthroplasty (TJA) patients. (A) Tissue inhibitors of matrix metalloproteinase (TIMP-1) (B) Lubricin. Significant P values (< .05) are indicated.
Figure 3.
Boxplots comparing vascular endothelial growth factor (vEGF) levels in control groups versus total joint arthroplasty (TJA) patients. Significant P values (< .05) are indicated.
Percent Differences Between Patient Population and Controls
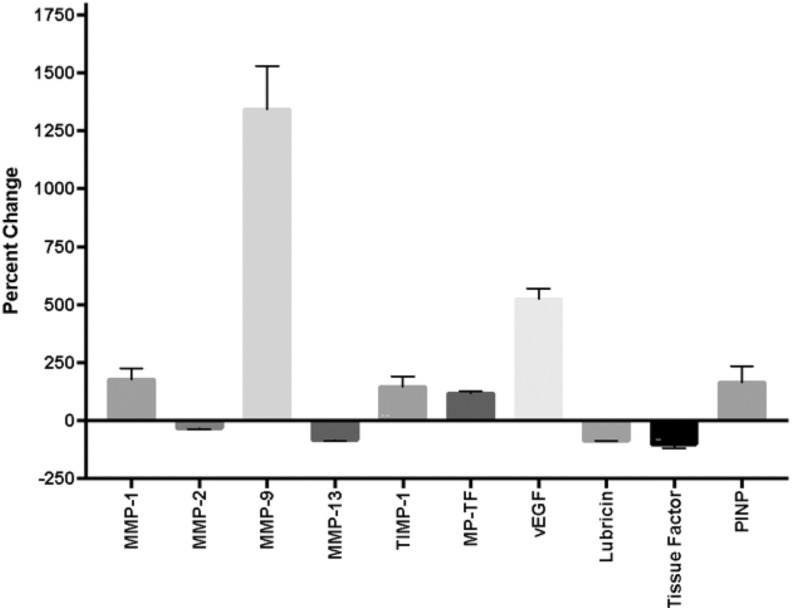
Data were also analyzed as percent changes between different groups. These are summarized in Figure 4.
Figure 4.
Percent changes of matrix metalloproteinases (MMPs) and biomarkers in presurgical total joint arthroplasty (TJA) patients with osteoarthritis (OA) compared to normal controls (normal human plasma).
Correlations Between Biomarkers
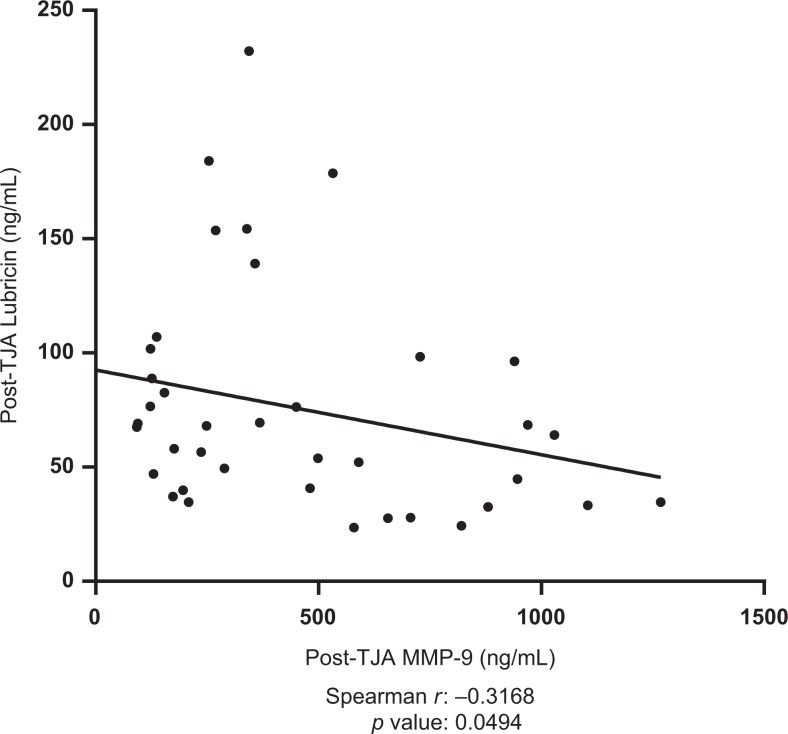
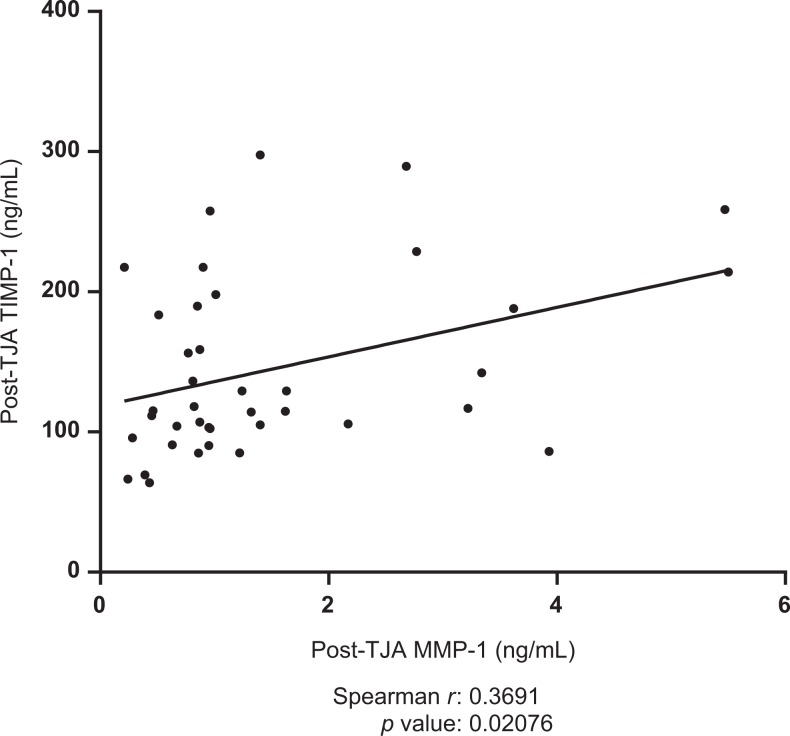
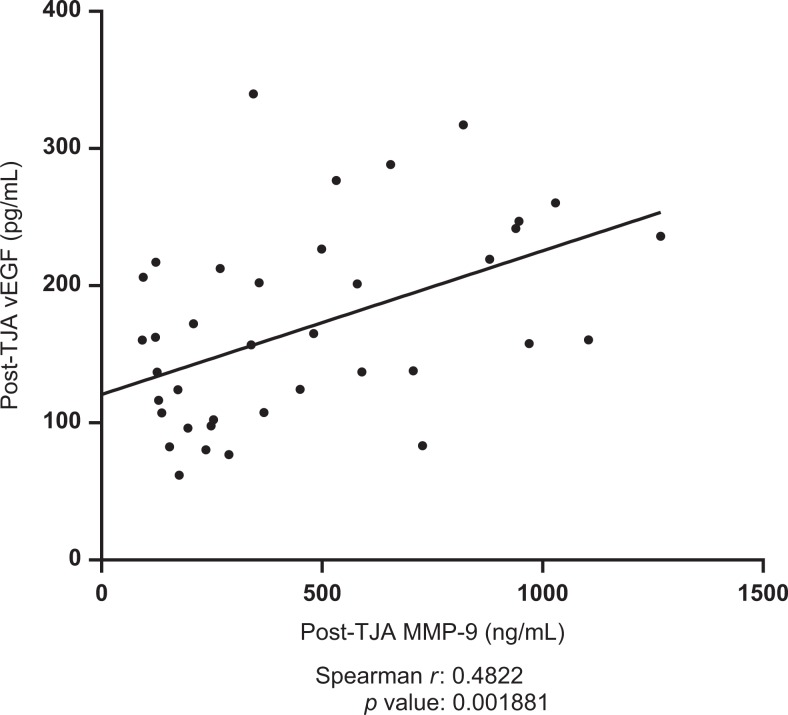
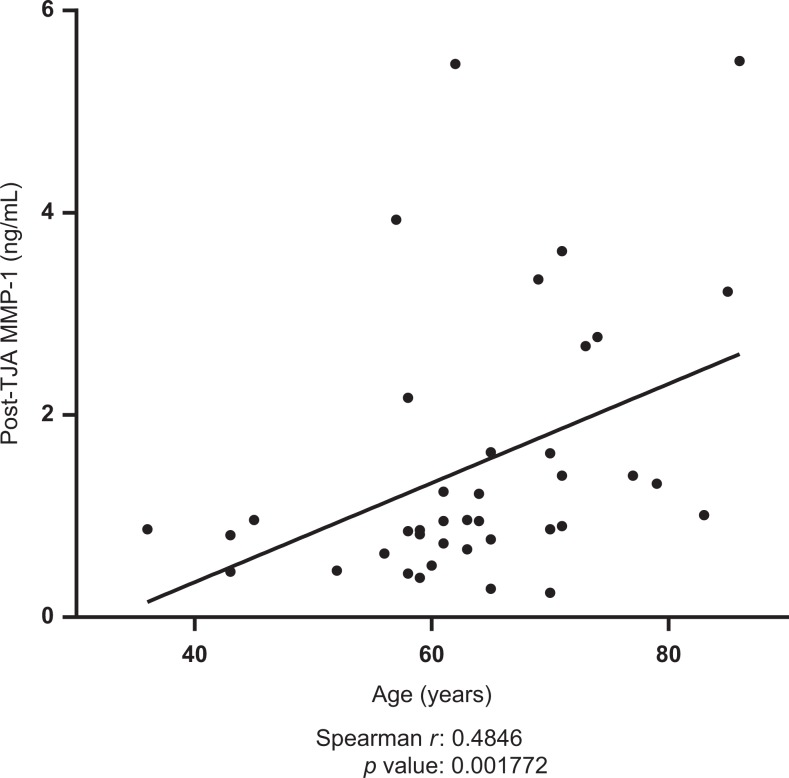
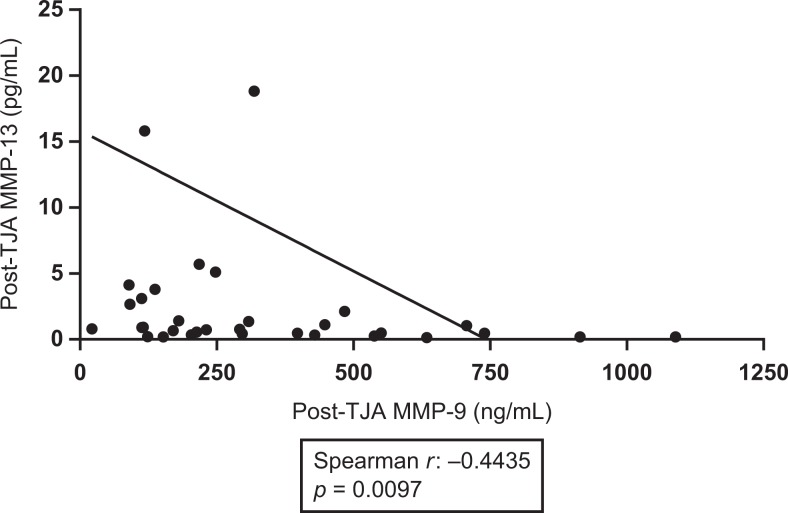
Postoperative plasma levels of MMP-9 were shown to be negatively correlated with post-operative lubricin levels (spearman r = −0.3168, P = .0494). Results are displayed in Figure 5. Postoperative TIMP-1 was shown to be positively correlated with postoperative MMP-1 (spearman r = 0.3691, P =.02076). Results are displayed in Figure 6. Preoperative TIMP-1 was shown to be positively correlated with preoperative MMP-1 (spearman r = 0.3945, P = .01903). Postoperative MMP-9 was shown to be positively correlated with postoperative vEGF (spearman r = 0.4822, P = .001891). Results are displayed in Figure 7. The MMP-1 was shown to be positively correlated with age (spearman r = 0.4846, P = .001772). Results are displayed in Figure 8. The MMP-9 was shown to be negatively correlated with MMP-13 (spearman r = −0.4435, P = .0097). Results are displayed in Figure 9.
Figure 5.
Correlation between postoperative Lubricin levels and postoperative matrix metalloproteinase-9 (MMP-9) levels in total joint arthroplasty (TJA) patients. Best-fit line shown; Spearman r and P values indicated.
Figure 6.
Correlation between postoperative tissue inhibitors of matrix metalloproteinase-1 (TIMP-1) and postoperative matrix metalloproteinase-1 (MMP-1) in total joint arthroplasty (TJA) patients. Best-fit line shown; Spearman r and P values indicated.
Figure 7.
Correlation between postoperative vascular endothelial growth factor (vEGF) and postoperative matrix metalloproteinase-9 (MMP-9). Best-fit line shown; Spearman r and P values indicated.
Figure 8.
Correlations between postoperative matrix metalloproteinase-1 (MMP-1) and patient age. Best-fit line shown; Spearman r and P values indicated.
Figure 9.
Correlations between postoperative matrix metalloproteinase-13 (MMP-13) and postoperative MMP-9. Best fit line shown.
Discussion
While TJA remains the only definitive treatment for OA, research continues to identify substances or pharmaceuticals that may help slow the progression of OA or work in combination with current therapies to reduce symptomatology. At this point, there are several targets (ie, lubricin) that have shown promise in animal models, yet there remains no clinically approved drug.30,32 The objective of this research is to identify novel targets, such as MMPs, for OA treatment, and to add to the growing literature on biomarkers related to the disease process.
Our measurements of plasma biomarkers proved to be varied, which is consistent with the variety of results seen in contemporary research. While MMPs 1, 2, 9, and 13 have been shown to be elevated in patients with OA in some reports,7,8 we did not see quite as consistent results. There is also other research that has not found elevated MMPs in OA or has found no correlation.33
While it appeared to be the trend that patients with OA had increased plasma levels of MMP-1, only our postsurgical group had a statistically significant value. The MMP-1 is a collagenase that has been shown to play a role in cancer disease course and has been implicated in OA, but some studies suggest that its elevation may be different in different ethnic groups and that Caucasians may not show elevated MMP-1 levels in OA.7 One study has found that levels of MMP-1 may even decrease with progression of OA due to deterioration of the superficial cartilage.34 Our finding of elevated MMP-1 postoperatively may simply be consistent with postsurgical inflammation and tissue remodeling at the joint, or it could be indicative of a true elevation seen in OA.
The body of research for MMP-2 is similar to that of MMP-1; some studies have found elevations of MMP-2 in certain populations but not others.7,35 While MMP-2 also functions as a gelatinase and is capable of degrading collagens I, II, and IV, its role in OA is not entirely clear, and it may be more prominently involved in angiogenesis of the affected joint. Some research suggests that other MMPs have a more central role in the disease process of OA.36 Our data show a decrease in MMP-2 in our diseased group versus the control and also show a decrease after surgery in our TJA patients. It appears that MMP-2 may have a secondary role in OA, but it does not appear to be the driving force behind the degradation of the joint cartilage. Since functional levels differ from the levels we measured via ELISA, it may be useful to therefore perform activity assays in future studies.
The MMP-9 may be involved in the activation of pro-MMP-13, and along with MMP-13, may have a more central role in the pathogenesis of OA.36,37 We found an increased concentration of MMP-9 in our patients with OA when compared to normal patient populations, and no impact of the surgical interval. This elevation of MMP-9 is consistent with other previously mentioned research into the role of MMP-9 in OA.7,8
Increased TIMP-1 was associated with attenuation of OA changes in an animal model,38 but the literature in humans is not necessarily in agreement. While it may seem that TIMP-1 presence would retard the progression of OA, the picture is not clear, and some research has found increased expression of TIMP-1 to be associated with progress of the disease.39 The TIMPs, much like the MMPs they regulate, affect many cellular processes, and may serve either a constructive or destructive role in the setting of OA, depending on the local context.39 While TIMP-1 appears to be elevated in our patients with OA, the change was not significant, and the concentration of TIMP-1 in PATH, in fact, was much higher than it was in the patients with OA, supporting TIMP-1’s pleiotropic role in the body. It may also be that, while TIMP-1 is increased in some patients with OA, it is overridden by the increases in MMPs such that the net effect is still degradative.
The MMP-13 has been shown to be especially important in the pathogenesis of OA. Because it is one of the rate-limiting components of the collagen degradation process,40 it has a wide range of possible targets of degradation,41 and it is very active against the type II collagen of which cartilage is predominantly made. It has also been shown to be activated by other MMPs37 and is the most common inappropriately activated MMP in the process of OA.42 Our data shows that in pre- and postoperative TJA patients, plasma MMP-13 concentrations were significantly lowered from our normal controls, and they were also low relative to our pathological controls. However, while MMP-13 is broadly increased in OA, the temporality of the OA may affect the amount of MMP-13 present in the tissue, as a higher expression of MMP-13 has been seen in intact OA cartilage than in damaged cartilage.43 Given that our patient cohort included primarily surgical candidates with advanced age, it is possible that the reduced MMP-13 concentration we observed was a product of later stage degradation of the joint and decreased cartilage.
Our data showed an increase in vEGF in patients postoperatively, consistent with an inflammatory process secondary to surgery. Vascular endothelial growth factor also appeared to show an increase in preoperative patients, but the difference was not significant. This is broadly consistent with previously published research that shows vEGF and angiogenesis as part of the disease process of OA.
The concentrations of lubricin in our patients with OA showed similar reductions as that of the published literature. There was no significant difference between the pre- and postoperative patients. This fits with our understanding of lubricin as a chondroprotectant that is diminished within the joint space in OA progression. Unfortunately, this does not mesh with previously published data from this lab.44 Our research sought to measure plasma levels of lubricin as an index of synovial fluid lubricin concentrations, and it is possible that the 2 environments do not correlate exactly during the course of OA. In the future, it would be of value to measure synovial fluid directly, as well as to examine histological segments of synovium for lubricin levels.
Interestingly, lubricin also has other anti-inflammatory properties in the context of the joint and may function to diminish MMP action and expression.21,28 This may help to explain the relationship we found between lubricin and MMPs wherein increasing MMP levels are correlated with lower lubricin. Lubricin also generally inhibits inflammation through inhibition of nuclear factor kappa B (NFκB) via CD44 receptor binding45 and an interaction with toll-like receptors 2 and 4.46 A recent study shows that it also may serve an autocrine role by reducing total cellular NFκB.28
Our findings broadly agree with the current literature and help contribute to the published data about MMP levels and lubricin in the role of OA. Our patient base is interesting because they are all patients whose OA was severe enough to warrant TJA, and therefore, our data may paint a picture of more severe, more chronic OA.
Conclusion
Our data showed many significant increases in both MMPs and other inflammatory biomarkers, as well as a significant decrease in lubricin in patients with OA. Surprisingly, our data showed decreases in MMP-2 and MMP-13, though only the MMP-13 decrease was significant. These results differ from some of the published literature, though the literature also shows that MMP-2 and MMP-13 were less elevated than MMPs 1, 8, and 10. It is possible that different patient groups and different joint locations of OA have different MMP profiles. Our data also showed a nonsignificant increase in TIMP-1, though the increase in TIMP-1 was not as large as the increase in other MMPs. We postulate that while there is increased TIMP-1 in OA, it is not sufficient to compete with the increased MMP presence.
We also observed a strong negative correlation between MMP-9 and lubricin, which may add to the idea that increased MMP activity is just one part of a large cascade of inflammation and degradation of the synovium. We also found a positive correlation between MMP-9 and vEGF, reflecting published data. The positive correlation between MMP-1 and TIMP-1 may indicate an increase in TIMP-1 in patients with OA that is insufficient to moderate the MMP activity.
Our correlations were performed with TJA patients, and it would be interesting to see whether there are similar correlations in milder forms of OA or in healthy individuals. Additionally, for some of our biomarkers, we did not have enough normal controls for the data to be significant.
While the synovium becomes permeable during OA, synovial fluid and plasma are compositionally different. It would be interesting to compare plasma levels to synovial fluid levels in both healthy patients and those with OA.
Acknowledgments
The authors gratefully acknowledge the expert assistance of the staff of the Hemostasis and Thrombosis research laboratories. Special thanks to the staff of the clinical laboratories for the collection of de-identified samples. The authors also thank Dr Banos for his input and expertise. Additional thanks to Dr Elissa Davis, Dr Daniel Schmitt and Trung Phan. The research reported in this poster represents part of the investigations carried out as a component of the Student Training in Approaches to Research 2016 program at the Loyola Stritch School of Medicine. The authors are thankful to Dr Gail Hecht and Dean Brubaker for their oversight and support. Special thanks to Dr Ed Truitt of Lubris Pharma for his expert advice during this study. The authors are also grateful to Mr Jonas Kingo of Anira Diagnostics for their generous gifts of reagents and kits.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Riis A, Rathleff MS, Jensen MB, Simonsen O. Low grading of the severity of knee osteoarthritis pre-operatively is associated with a lower functional level after total knee replacement. Bone Joint J. 2014;96-B(11):1498–1502. [DOI] [PubMed] [Google Scholar]
- 2. Christensen R, Henriksen M, Leeds AR, et al. Effect of weight maintenance on symptoms of knee osteoarthritis in obese patients: a twelve-month randomized controlled trial. Arthritis Care Res. 2015;67(5):640–650. PMC. Web. 8 Feb. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Szychlinska MA, Leonardi R, Al-Qahtani M, Mobasheri A, Musumeci G. Altered joint tribology in osteoarthritis: reduced lubricin synthesis due to the inflammatory process. New horizons for therapeutic approaches. Ann Phys Rehabil Med. 2016;59(3):149–156. [DOI] [PubMed] [Google Scholar]
- 4. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780–785. [DOI] [PubMed] [Google Scholar]
- 5. Losina E, Daigle ME, Suter LG, et al. Disease-modifying drugs for knee osteoarthritis: can they be cost-effective? Osteoarthritis Cartilage. 2013;21(5):655–667. doi: 10.1016/j.joca.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bourboulia D, Stetler-Stevenson WG. Matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs): positive and negative regulators in tumor cell adhesion. Semin Cancer Biol. 2010;20(3):161–168. PMC. Web. 9 Feb. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zeng GQ, Chen AB, Li W, Song JH, Gao CY. High MMP-1, MMP-2, and MMP-9 protein levels in osteoarthritis. Genet Mol Res. 2015;14(4):14811–14822. [DOI] [PubMed] [Google Scholar]
- 8. Son YO, Park S, Kwak JS, et al. Estrogen-related receptor γ causes osteoarthritis by upregulating extracellular matrix-degrading enzymes. Nat Commun. 2017;8(1):2133 doi: 10.1038/s41467-017-01868-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yang Q, Ding W, Cao Y, et al. Interferonregulatoryfactor-8(IRF-8) regulates the expression of matrix metalloproteinase-13 (MMP-13) in chondrocytes. Cell Stress Chaperones. 2017. doi: 10.1007/s12192-017-0849-y [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Luo S, Shi Q, Chen J, Wang H, Wu W, Zha Z. Expression and significance of MMPs in synovial fluid, serum and PBMC culture supernatant stimulated by LPS in osteoarthritis patients with or without diabetes. Exp Clin Endocrinol Diabetes. 2017. doi: 10.1055/s-0043-122223 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 11. Berenbaum F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthr Cartil. 2013;21(1):16–21. [DOI] [PubMed] [Google Scholar]
- 12. Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases. Circ Res. 2003;92(8):827–839. [DOI] [PubMed] [Google Scholar]
- 13. Dean DD, Woessner JF., Jr Extracts of human articular cartilage contain an inhibitor of tissue metalloproteinases. Biochem J. 1984;218(1):277–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fotopoulos VC, Tzinia A, Tzurbakis M, et al. Expression levels of matrix metalloproteinase (MMP)-9 and its specific inhibitor TIMP-1, in septic and aseptic arthritis of the knee. Knee Surg Sports Traumatol Arthrosc. 2012;20:1159 doi: 10.1007/s00167-011-1676-9. [DOI] [PubMed] [Google Scholar]
- 15. Naito K, Takahashi M, Kushida K, et al. Measurement of matrix metalloproteinases (MMPs) and tissue inhibitor of metalloproteinases-1 (TIMP-1) in patients with knee osteoarthritis: comparison with generalized osteoarthritis. Rheumatology. 1999;38(6):510–515. [DOI] [PubMed] [Google Scholar]
- 16. Jay GD, Torres JR, Warman ML, Laderer MC, Breuer KS. The role of lubricin in the mechanical behavior of synovial fluid. Send to Proc Natl Acad Sci U S A. 2007;104(15):6194–6199. doi: 10.1073/pnas.0608558104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rhee DK, Marcelino J, Baker M, et al. The secreted glycoprotein lubricin protects cartilage surfaces and inhibits synovial cell overgrowth. J Clin Invest. 2005;115(3):622–631. PMC. Web. January 31, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Noyori K, Jasin HE. Inhibition of human fibroblast adhesion by cartilage surface proteoglycans. Arthritis Rheum. 1994;37(11):1656–1663. doi: 10.1002/art.1780371115. [DOI] [PubMed] [Google Scholar]
- 19. Musumeci G, Loreto C, Leonardi R, et al. The effects of physical activity on apoptosis and lubricin expression in articular cartilage in rats with glucocorticoid-induced osteoporosis. J Bone Miner Metab. 2013;31:274 doi: 10.1007/s00774-012-0414-9. [DOI] [PubMed] [Google Scholar]
- 20. Waller KA, Zhang LX, Elsaid KA, Fleming BC, Warman ML, Jay GD. Lubricin prevents chondrocyte apoptosis. P Natl A Sci. 2013;110(15):5852–5857. doi: 10.1073/pnas.1219289110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ruan MZC, Erez A, Guse K, et al. Proteoglycan 4 expression protects against the development of osteoarthritis. Sci Transl Med. 2013;5(176):176ra34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schumacher BL, Hughes CE, Kuettner KE, Caterson B, Aydelotte MB. Immunodetection and partial cDNA sequence of the proteoglycan, superficial zone protein, synthesized by cells lining synovial joints. J Orthop Res. 1999;17(1):110–120. [DOI] [PubMed] [Google Scholar]
- 23. Schumacher BL, Schmidt TA, Voegtline MS, Chen AC, Sah RL. Proteoglycan 4 (PRG4) synthesis and immunolocalization in bovine meniscus. J Orthop Res. 2005;23(3):562–568. [DOI] [PubMed] [Google Scholar]
- 24. Schumacher BL, Block JA, Schmid TM, Aydelotte MB, Kuettner KE. A novel proteoglycan synthesized and secreted by chondrocytes of the superficial zone of articular cartilage. Arch Biochem Biophys. 1994;311(1):144–152. [DOI] [PubMed] [Google Scholar]
- 25. Flowers SA, Zieba A, Örnros J, et al. Lubricin binds cartilage proteins, cartilage oligomeric matrix protein, fibronectin and collagen II at the cartilage surface. Sci Rep. 2017;7(1):13149 doi: 10.1038/s41598-017-13558-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ludwig TE, McAllister JR, Lun V, Wiley JP, Schmidt TA. Diminished cartilage-lubricating ability of human osteoarthritic synovial fluid deficient in proteoglycan 4: Restoration through proteoglycan 4 supplementation. Arthritis Rheum. 2012;64(12):3963–3971. doi: 10.1002/art.34674. [DOI] [PubMed] [Google Scholar]
- 27. Bao J, Chen W, Wu L. Lubricin: a novel potential biotherapeutic approaches for the treatment of osteoarthritis. Mol Biol Rep. 2011;38(5):2879–2885. doi: 10.1007/s11033-010-9949-9. [DOI] [PubMed] [Google Scholar]
- 28. Alquraini A, Jamal M, Zhang L, Schmidt T, Jay GD, Elsaid KA. The autocrine role of proteoglycan-4 (PRG4) in modulating osteoarthritic synoviocyte proliferation and expression of matrix degrading enzymes. Arthritis Res Ther. 2017;19(1):89 doi: 10.1186/s13075-017-1301-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stone AV, Loeser RF, Vanderman KS, Long DL, Clark SC, Ferguson CM. Pro-inflammatory stimulation of meniscus cells increases production of matrix metalloproteinases and additional catabolic factors involved in osteoarthritis pathogenesis. Osteoarthritis Cartilage. 2014;22(2):264–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hamilton JL, Nagao M, Levine BR, Chen D, Olsen BR, Im HJ. Targeting VEGF and its receptors for the treatment of osteoarthritis and associated pain. J Bone Miner Res. 2016;31(5):911–924. PMC. Web. 9 Feb. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nagai T, Sato M, Kobayashi M, Yokoyama M, Tani Y, Mochida J. Bevacizumab, an anti-vascular endothelial growth factor antibody, inhibits osteoarthritis. Arthritis Res Ther. 2014;16(5):427 PMC. Web. February 1, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cho H, Walker A, Williams J, Hasty KA. Study of osteoarthritis treatment with anti-inflammatory drugs: cyclooxygenase-2 inhibitor and steroids. Biomed Res Int. 2015;2015:595273 PMC. Web. February 1, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Heard BJ, Martin L, Rattner JB, Frank CB, Hart DA, Krawetz R. Matrix metalloproteinase protein expression profiles cannot distinguish between normal and early osteoarthritic synovial fluid. BMC Musculoskelet Disord. 2012;13:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rübenhagen R, Schüttrumpf JP, Stürmer KM, Frosch KH. Interleukin-7 levels in synovial fluid increase with age and MMP-1 levels decrease with progression of osteoarthritis. Acta Orthop 2012;83(1):59–64. PMC. Web. February 1, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Xue M, McKelvey K, Shen K, et al. Endogenous MMP-9 and not MMP-2 promotes rheumatoid synovial fibroblast survival, inflammation and cartilage degradation. Rheumatology. 2014;53(12):2270–2279. [DOI] [PubMed] [Google Scholar]
- 36. Kim KS, Choi HM, Lee YA, et al. Expression levels and association of gelatinases MMP-2 and MMP-9 and collagenases MMP-1 and MMP-13 with VEGF in synovial fluid of patients with arthritis. Rheumatol Int. 2011;31(4):543–547. doi: 10.1007/s00296-010-1592 -1. [DOI] [PubMed] [Google Scholar]
- 37. Kim KS, Lee YA, Choi HM, Yoo MC, Yang HI. Implication of MMP-9 and urokinase plasminogen activator (uPA) in the activation of pro-matrix metalloproteinase (MMP)-13. Rheumatol Int. 2012;32(10):3069.-3075. doi: 10.1007/s00296-011-2095-4. [DOI] [PubMed] [Google Scholar]
- 38. Qu H, Li J, Wu L, Chen W. Trichostatin A increases the TIMP-1/MMP ratio to protect against osteoarthritis in an animal model of the disease. Mol Med Rep. 2016;14(3):2423–2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hsieh JL, Shiau AL, Lee CH, et al. CD8+ T cell-induced expression of tissue inhibitor of metalloproteinses-1 exacerbated osteoarthritis. Int J Mol Sci. 2013;14(10):19951–19970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Burrage PS, Mix KS, Brinckerhoff CE. Matrix metalloproteinases: role in arthritis. Front Biosci. 2006;11:529–543. [DOI] [PubMed] [Google Scholar]
- 41. Knauper V, Cowell S, Smith B, et al. The role of the C-terminal domain of human collagenase-3 (MMP-13) in the activation of procollagenase-3, substrate specificity, and tissue inhibitor of metalloproteinase interaction. J Biol Chem. 1997;272(12):7608–7616. [DOI] [PubMed] [Google Scholar]
- 42. Goldring MB, Otero M, Plumb DA, et al. Roles of inflammatory and anabolic cytokines in cartilage metabolism: signals and multiple effectors converge upon MMP-13 regulation in osteoarthritis. Eur Cell Mater. 2011;21:202–220. Print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sato T, Konomi K, Yamasaki S, et al. Comparative analysis of gene expression profiles in intact and damaged regions of human osteoarthritic cartilage. Arthritis Rheumatism. 2006;54(3):808–817. doi: 10.1002/art.21638. [DOI] [PubMed] [Google Scholar]
- 44. Wanderling C, Liles J, Davis E, et al. Levels of matrix-degrading enzymes and lubricin in patients with degenerative joint disease requiring arthroplasty. Clin Appl Thromb Hemost. 2017:1–6. doi: 10.1177/1076029617724231. [DOI] [PMC free article] [PubMed]
- 45. Al-Sharif A, Jamal M, Zhang LX, et al. Lubricin/proteoglycan 4 binding to CD44 receptor: a mechanism of lubricin’s suppression of pro-inflammatory cytokine induced synoviocyte proliferation. Arthritis Rheumatol (Hoboken, N.J.). 2015;67(6):1503–1513. PMC. Web. February 6, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Alquraini A, Garguilo S, D’Souza G, et al. The interaction of lubricin/proteoglycan 4 (PRG4) with toll-like receptors 2 and 4: an anti-inflammatory role of PRG4 in synovial fluid. Arthritis Res Ther. 2015;17:353 PMC. Web. February 6, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]