Abstract
This perspective article is focused on the presentation of the latest advances in NMR methods and applications that are behind the exciting achievements in the understanding of glycan receptors in molecular recognition events. Different NMR-based methodologies are discussed along with their applications to scrutinize the conformation and dynamics of glycans as well as their interactions with protein receptors.
Introduction
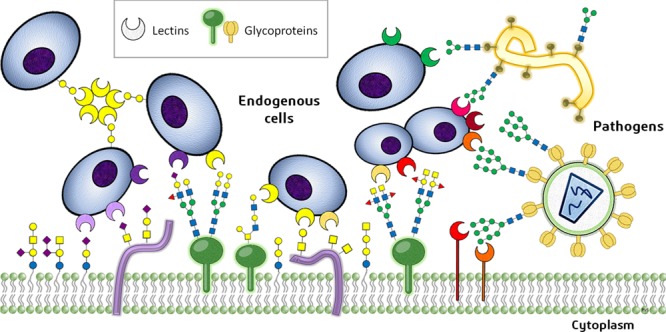
The interactions between carbohydrate molecules and protein receptors are essential for diverse processes of biological and biomedical importance.1 Undeniably, the sugar molecules that decorate lipids or proteins, forming glycolipid and glycoproteins, play key roles as contact points for a multitude of molecular recognition events that steer specific biological phenomena (Figure 1).2 In this context, the malfunction of either partner, the glycan (ligand) or the protein (receptor), may lead to detrimental outcomes for health. For instance, it is rather frequent that tumor cells display altered glycosylation patterns in the initial periods of tumor growth.3 In fact, several glycans have been directly associated with various cancer types. From the receptor’s perspective, the partners, glycan-binding proteins (lectins), may also be modified under different disease conditions with respect to their “correct” structures or cellular concentrations.4
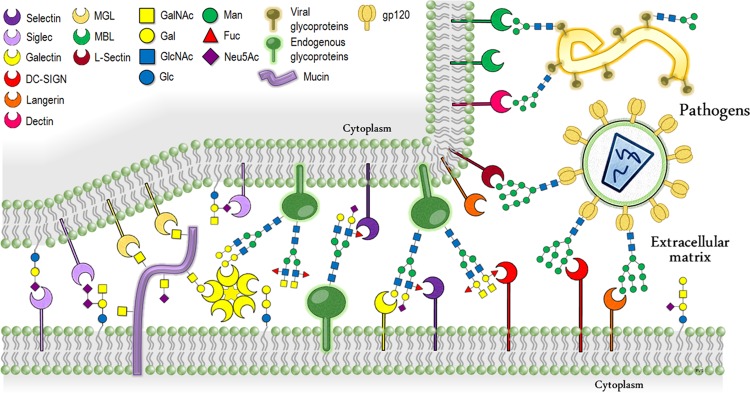
Figure 1.
Schematic summary of the typical protein–carbohydrate recognition events that mediate the cell–cell and host–pathogen interactions and relationship.
Therefore, revealing the fine details of glycan–protein interactions (Figure 2) should permit advancing in the scientific understanding, at the highest possible resolution, of different biological events linked to health and disease. It is nowadays accepted that many factors influence glycan recognition events, including multivalency5 and presentation of epitopes.6 Obviously, kinetics effects are also important, as well as the interplay between enthalpy (van der Waals, CH−π,7 electrostatic, water–ligand, and water–receptor interactions) and entropy factors (rigidification, desolvation–solvation, and hydrophobic effects).8
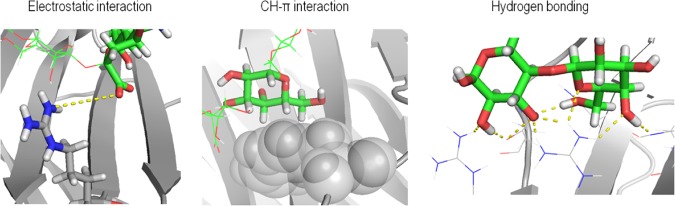
Figure 2.
Typical intermolecular interactions involved in protein–ligand recognition events.
There are different techniques that can be employed to unravel the structural features of protein–carbohydrate interactions (Figure 3). Very frequently, the experimental data acquired by structural techniques (cryo-electron microscopy,9 X-ray crystallography,10 and/or NMR spectroscopy)11 are complemented with additional information generated by complementary biophysical techniques (SPR,12 ITC,13 BLI,14 etc.). The assistance of molecular modeling procedures is also fairly frequent to provide a full picture of the structure and dynamics of the glycan/lectin complex under investigation. Although cryo-electron microscopy methods have started to be employed to characterize glycan-binding events,15 the high level of structural details afforded by employing X-ray crystallography makes this technique the reference for studying these complexes,16 despite the drawbacks that have been identified by experts regarding the refinement protocols of the electron density of the glycans to get their correct structures.17
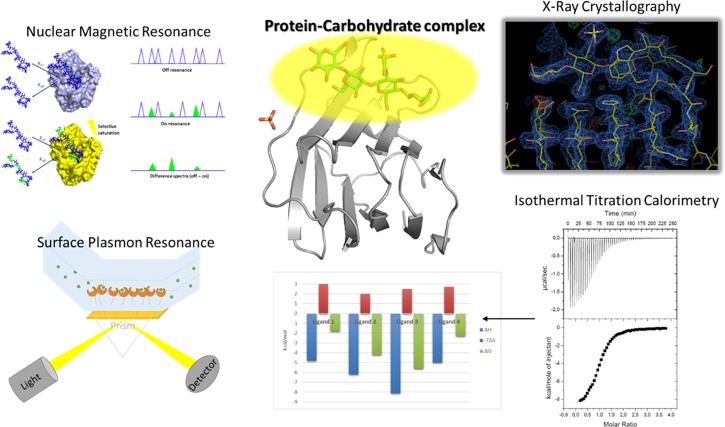
Figure 3.
Different approaches to provide insights into the interaction between carbohydrates and receptors.
NMR has also been extensively used to study the conformation of glycans for several decades.18 However, novel methodologies and protocols have recently permitted discovering new concepts associated with structural features that may permit extraordinary advances in the understanding of how the three-dimensional (3D) structure of glycans is regulated and how the presentation of the key epitope is achieved.
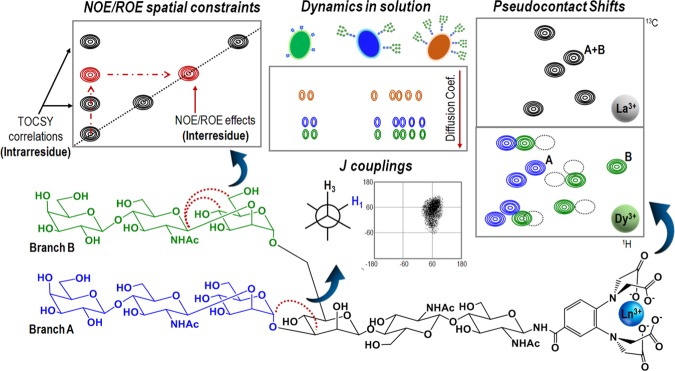
Generally speaking, NMR-based strategies usually include nuclear Overhauser effect (NOE)- and rotating-frame Overhauser effect (ROE)-derived distance constraints19 and/or measurements in the presence of paramagnetic tags (pseudo-contact shifts, PCS, and paramagnetic enhanced relaxation, PRE), as will be described in a specific section, to provide structural details about the sugar arrangement and/or flexibility of the glycan-containing biomolecules. Besides, standard relaxation parameters, diffusion ordered spectroscopy (DOSY)20 and/or J couplings can offer useful information about local flexibility around the glycosidic linkages21 and the ring conformer distribution22 (Figure 4).
Figure 4.
Common NMR methodologies used for studying dynamics and conformation of complex oligosaccharides.
A typical limitation in oligosaccharide conformational studies is the poor NOE enhancement factor for small- and medium-sized oligosaccharides, which generates close-to-zero NOEs. However, such a drawback can be overcome by different alternatives: decreasing the temperature below 10 °C, using very large magnetic fields or conducting ROE-based experiments. An alternative strategy is to covalently attach the glycan to a protein, thus drastically reducing the molecular tumbling of the saccharide and favoring the detection of strongly negative NOE effects. This approach has been masterly employed to assess the conformation of LeX-type antigens.23
Alternatively, in this perspective article, we will focus on the latest advances in NMR spectroscopy that permit spectacular advances in the analysis of glycan–receptor interactions. The enormous variety of NMR methods and protocols make this spectroscopy highly suitable to explore the conformation, dynamics, and interactions of glycans. Since a recent survey on the status of the field was presented in 2018 to the scientific community,24 we will specially focus herein on our perspective of the latest advances and how we feel that they will provide a revolution in the field.
Glycoproteins and Protein–Sugar Interactions
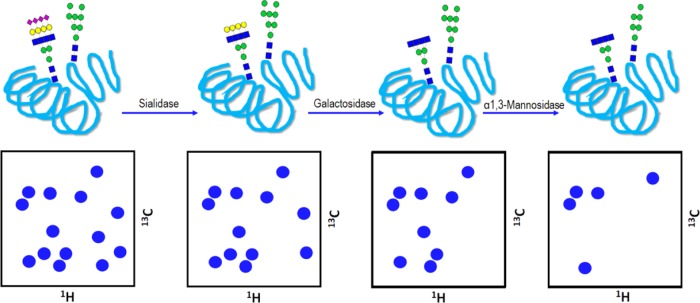
One of the frontiers of NMR in this field is in the study of intact glycoproteins.25 These molecules are intrinsically heterogeneous, and the glycan chains provide them with an additional level of chemical complexity, which may, in turn, be also linked to its biological function.26 Nevertheless, in the past few years, NMR has emerged as a very powerful tool to work with complex glycosylated proteins, providing detailed information on the structure and dynamics of glycans attached to proteins, and enabling the study of recognition events involving such glycoproteins.27 The observation of these events from the protein point of view normally requires uniform isotopic labeling to carry out a consistent NMR-based analysis. However, the major drawback associated with this NMR-based study arises from the slow protein tumbling motion, which precludes, in many cases, that the glycosylation pattern provides an extra layer of solvation, and the effective size of the molecule becomes tremendously high.28 Thus, the acquisition of 3D experiments with multiple coherence transfer steps turns out to be complicated due to faster relaxation processes. A promising solution to decrease relaxation is complete protein deuteration by growing cells in D2O, usually employing insect cells.29 Glycan truncation could be another option to reduce fast relaxation in solution (Figures 5 and 6), as long as the suppressed branches or sugar units do not significantly affect the protein stability or biological properties.30 A more radical option would be directly mutating the asparagine residues attached to the glycans, although these chemical changes will surely affect the structural stability, interaction ability, and the biological function.31
Figure 5.
Enzymatic trimming of a glycoprotein using different glycosidases and its effect on the 1H/13C-heteronuclear single quantum coherence (HSQC) resonances observed in the anomeric region (4.5–5.5 ppm) showing the sugars attached to the protein. Each removed sugar unit will entail the disappearance of one peak, corresponding to its C1 position.
Figure 6.
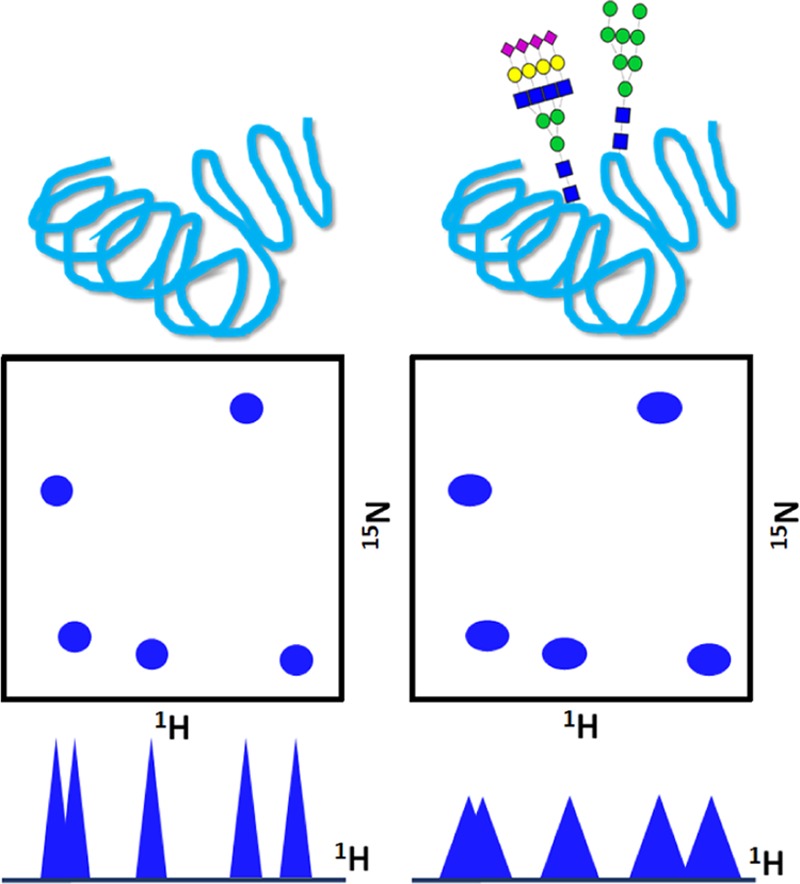
Effect of glycosylation in the 1H–15N backbone resonances of proteins observed by two-dimensional (2D) heteronuclear NMR.
Nevertheless, some of these strategies have been employed to study glycoproteins by NMR, suppressing the entire glycan ramifications that are not or barely important for structural integrity and activity.32 Alternative studies on large glycan-containing proteins have made use of specific amino acid labeling strategies to simplify the 2D-HSQC spectra and to cope with line-broadening issues. These methodologies include [13C]-labeling of methyl groups of methionines and/or the aliphatic amino acids (Ile, Leu, and Val), as well as labeling of the carbonyl groups.33
Alternatively, sugar labeling has also been used to exclusively extract information from the external N-glycans. This method has been efficiently used to observe the first sugar residue directly attached to the asparagine side chain, which displays a particular carbon resonance around δ 80 ppm, derived from its linkage to a nitrogen atom.34 Another approach would be the so-called postpurification labeling, consisting in ordinarily expressing the unlabeled glycoprotein and then using [13C]-labeled sugar nucleotides with specific glycosyltransferases to attach them to the terminal positions of glycan branches.35
Chemical shifts provide key information on perturbations arising from particular chemical environments, and this parameter is used as a tool to detect flexibility changes in glycoproteins. This approach has been employed to study the interaction of Skp1 from the ameba Dictyostelium (DdSkp1) with its F-box counterpart protein,36 which are involved in protein polyubiquitination processes. This protein is unusually hydroxylated at Pro143 and subsequently glycosylated with a pentameric glycan chain. Isotopic enrichment of the Skp1 fragments with 2H, 15N, and 13C enabled the collection of the corresponding 1HN, 15N, 13Cα, and 13CO backbone chemical shifts for further conformational analysis. As expected, the mono- and polyglycosylated forms of Skp1 (Gn-DdSkp1 and GGFGGn-DdSkp1, respectively) displayed noticeable chemical shift perturbations (CSP) around residues 139–153 upon glycosylation. The analysis of the chemical shift index (CSI) for the unmodified DdSkp1 strongly predicted a random coil conformation in the C-termini. However, the same CSI values were consistent with the presence of a short α-helix region for the glycosylated Gn-DdSkp1, thus demonstrating that this conformational change actually takes place and specifically affects the C-terminal segment of DdSkp1. 15N-edited nuclear Overhauser effect spectroscopy (NOESY) spectra confirmed this finding by providing the typical sequential 1HN–1HN NOE cross-peaks for an α-helix, whereas the random coil index values from 15N{1H} NOE data supported a reduction in the degree of structural disorder upon glycosylation, although the global mobility of this segment is still high, as assessed by molecular dynamics (MD) simulations. From these conclusions, this glycan-mediated structural rearrangement has been proposed to enhance the association with the F-box counterpart by defining a distribution of preferential conformational states.
Apart from the effect of the attached sugars on the glycoprotein conformations, the influence of the natural, external glycan substrates upon binding can also lead to notorious changes in the 3D structure of the protein counterpart, as exemplified by the human blood group A and B glycosyltransferases (GTA and GTB).38 Both enzymes experience transitions between “open”, “semi-closed”, and “closed” conformations when interacting with the corresponding substrates, the histo-blood group H (HBG-H) and the sugar donor (UDP-Gal/GalNAc). For this particular system, “hot-spot labeling” enabled conformational studies through the observation of the HN side chain from 15N-Trp and the ε-methyl of 13C-Met. Monitoring of CSP through 1H–15N-HSQC–TROSY and 1H–13C-HSQC–TROSY provided key information on GTA and GTB dynamics during binding events. HSQC-based titrations revealed different exchange kinetics depending on the presence of one substrate or both at the same time. The progressive addition of UDP led to continuous CSP on the HN side chain signals of Trp residues, indicative of a fast exchange binding process. Fittingly, some methionine methyl groups showed fast and intermediate exchange. These near-to-coalescence trajectories allowed calculating exchange rates, with kex values of up to 70 Hz (fast–intermediate regime). Interestingly, the subsequent addition of HBG-H to the UDP-saturated enzyme revealed a slow exchange process in the NMR chemical shift time scale, as supported by the estimated exchange rates and the presence of two different peaks for some methionine residues. Consistently, these results highlight the allosteric regulation of HBG-H binding mediated by the prior interaction with the sugar donor, and how this allosteric control affects enzyme dynamics. In fact, a mutual allosteric regulation is observed since first titrating both enzymes with HBG-H and then with UDP actually guide to the same results. The large CSP detected for some remote methionines provided further evidence on local conformational rearrangements in regions far away from the binding site, as a potential consequence of transitions from the open to the “close” states. How these local movements contribute to switch between conformational states remains unclear yet, and can be exemplified, for instance, by the differences in protein motions reported for some mutants, which suggest the existence of more intermediate conformers.
Amazingly, Schubert et al. have shown how recognition of glycosylation patterns can be achieved by simple NMR experiments.37 By means of 2D correlation NMR spectra acquired at natural isotopic abundance, they have been able to distinguish among differentiated patterns of glycosylation present in glycoproteins from different organisms (bacteria, fungi, plants, and animals). This analysis relies on the fingerprint patterns characteristic of each monosaccharide and linkage type: in the [1H, 13C]-HSQC 2D NMR experiment, the N-linked glycans can be easily differentiated from the O-linked ones. The remaining components of the saccharidic skeleton can be deduced from a thorough examination of the visible spin systems through the set of 2D experiments. Then, the monosaccharide fragments can be reasonably linked step by step, inferring the entire glycan structure. In this procedure, no labeled protein samples are needed and the experiments are independent of the macromolecule size. However, high concentrations of the protein are required. With lower protein concentrations, assignment of oligosaccharides could be still accomplished but would necessarily require [13C]-labeling as well as sequential assignments via 3D NMR spectra.
As mentioned above, the enormous compositional heterogeneity of sugars in glycoproteins is a notorious disadvantage for NMR assignment, which becomes an arduous work when the structure of the oligosaccharide is unknown. To identify saccharides making use of the available NMR data, several databases and computer algorithms have been developed over time. GlycoNMRSearch is a new promising bioinformatics tool39 that could boost the applicability of NMR for complex glycan identification and assignment. This search algorithm novelty combines experimental chemical shift values with a new library of assigned spin systems, not contained in other databases. It is designed to efficiently find correlations between any chemical shift in a given spin system and all described spin systems deposited in the NMR database.
The increasing amount of deposited NMR data regarding oligosaccharides has also eased the observation of novel spatial arrangements that glycans with certain scaffolds tend to adopt, highlighting the importance of developing tools for studying complex, unaltered structures instead of individual fragments. A particular case has recently gained increasing interest. The LeX-type branched oligosaccharides display an intramolecular C–H···O hydrogen bond, associated with the stacking of two nonvicinal sugar residues.40 The detection of this nonconventional hydrogen bond has raised the question on its actual importance for the conformational stabilization of the particular 3D geometry and whether this particular C–H···O interaction could have any relevant biological role, not only in glycans but also in proteins and nucleic acids.41 A detailed inspection of the oligosaccharides that show this feature has revealed that all of them share a common set of structural features: the interacting fucose (Fuc) residue and its stacked residue are both bound to a third central one through α(1–3) and β(1–4) glycosidic linkages, respectively, or through the α(1–4) and β(1–3) alternatives (Figure 7). The central residue is always Glc or GlcNAc, whereas the residue involved in the interaction with the Fuc unit may be Gal, GalNAc, Glc, or GlcNAc. Consequently, this unusual hydrogen bond does not appear in other histo-blood groups (HBG) such as HBG-A or HBG-B, for which the Fuc unit is α(1–2)-linked to the core residue.42
Figure 7.
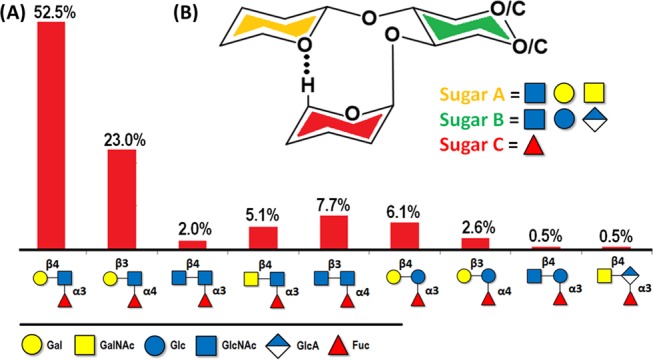
(A) Nine categories of glycan motifs that share the same kind of stabilizing hydrogen bond C–H···O between the H5-Fuc and the stacking residue. The percentage corresponds to the amount of glycan structures in the Glycosciences.de database which contain each trisaccharide. (B) The generic structure of the fucose-containing trisaccharide that possesses the mentioned inter-residue hydrogen bond and the possible substitutions that have been reported in the surrounding positions.23,40
Use of Paramagnetic NMR
This methodology, which is transferred from the protein field, has provoked a revolution in the glycan conformational analysis in the past few years.43 Indeed, the use of oligosaccharides decorated with lanthanide-binding tags has arisen as new NMR protocols to unravel the 3D shape of complex glycans and to afford key elements for unraveling fine details of their interactions.44 From the physics perspective, the origin of this methodology is perfectly established. The occurrence of a paramagnetic nucleus in a molecule generates strong chemical shift variations, pseudo-contact shifts45 (PCS), in the NMR resonance signals of its adjacent nuclei. These PCS depend on the distance with the inverse of the third power of the distance; therefore, they decrease more slowly with the distance than the NOEs and paramagnetic relaxation enhancements (PRE). Thus, these PCS can be employed to deduce distance constraints in a quantitative manner (Figure 4).
Using this methodology, insights into the conformational details of simple oligosaccharides46 and, in particular, bi-antennary47 and tetra-antennary complex type48 and high mannose N-glycans have been deduced.49 The different NMR-active nuclei in these molecules show isochronous chemical shifts for the units located at the end of the arms, and therefore, their complete NMR analysis is highly difficult or almost impossible.50 Nevertheless, the initial employment of this approach has also allowed the direct evaluation of the interaction of saccharides (lactose-type), decorated with the lanthanide-binding tag, with a lectin (human galectin-3).51 More recently, the experimental conformational analysis of extremely complex bi- and tetra-antennary oligosaccharides has also been achieved, since the use of the paramagnetic approach permits distinguishing all specific NMR signals of these molecules, thus removing the intrinsic 2-fold or 4-fold pseudosymmetry of the glycans. In this manner, their interactions with different lectins have also been studied. This approach represents a key breakthrough in the field, beyond current expertise.52 Obviously, using regular NMR experiments, the 1H and 13C nuclei belonging to the four terminal Gal residues at the tetra-antennary N-glycan display chemical shift degeneracy and are indistinguishable. As a major recent innovation in the field, the analysis of the conformation, dynamics, and molecular recognition properties of a long sialylated tetradecasaccharide bi-antennary N-glycan with two LacNAc repetitions at each arm has been effectively achieved. In fact, this complex N-glycan is the receptor of the hemagglutinin of several pathogenic influenza viruses.53 Using the paramagnetic approach, the 1H and 13C signals of each sugar unit in the glycan were identified and the combined use of PCS and STD data allowed deducing the binding epitope of the N-glycan toward Hong-Kong HK/68 hemagglutinin.54
For molecular recognition studies, there is one alternative strategy, which involves the use of either a spin label or a paramagnetic ion, but now placed at the receptor. In this case, a metal locus for attaching the paramagnetic ion to the protein should be designed, which involves a precise design. As examples, short lanthanide-binding peptides have been synthesized using a fusion construct for protein expression.55 This approach may also permit obtaining complementary NMR parameters, such as residual dipolar couplings (RDCs) without employing external alignment media. RDC and PCS data have been employed to build the three-dimensional model of a lectin/lactose complex using this methodology. The use of a similar strategy, inserting a lanthanide-binding peptide into the loop between two helixes of a known IgG binding protein, has allowed the characterization of the dynamic properties of Fc glycans.56
Moreover, paramagnetic relaxation enhancements (PRE) constitute another source of detailed structural information, extensively used in NMR to gain insights into conformational features of highly complex oligosaccharides and for analyzing protein–carbohydrate interactions as well. They are particularly useful as they provide long-range structural information. In this regard, the radical marker TEMPO is one of the frequent choices to undertake these kinds of studies. A recent work by Moure et al. exemplifies the rational use of TEMPO to unveil the structural details underlying the interaction between the human Roundabout 1 (Robo1) protein and heparan sulfate.57 In particular, for the introduction of TEMPO into the protein, the authors employed enzyme-catalyzed glycosylation reactions. The protein was expressed in mammalian cells that lack N-acetylglucosaminyltransferase, preventing extension of the glycans and leaving only the core that was cleaved to a single GlcNAc residue. Thus, a monosaccharide with an azide group was coupled to this GlcNAc moiety and afterward, the paramagnetic tag was attached by a Cu-mediated “click” reaction. The presence of TEMPO caused PRE effects, giving rise to a complete loss of the NMR cross-peak intensities in the 1H–15N-HSQC for those nuclei closer than 10 Å and a decrease for those at distances up to 24 Å, depending on their proximity to the TEMPO ring. The combination of these experimental data with MD simulations allowed describing protein dynamics and examining the binding of a ligand. In this case, the use of diffusion ordered spectroscopy58 (DOSY) permitted exclusively obtaining the NMR spectra of the bound ligand, whose analysis allowed inferring the exact position and orientation of the ligand in the binding site.
Use of Stable Isotopes: 13C- and 19F-Labeled Oligosaccharides
Advances in synthesis and molecular biology methods now permit accessing extremely complex oligosaccharides, which were almost impossible to access a few years ago for detailed structural, conformational, and interaction studies.59 The use of these methodologies permits the generation of extremely valuable saccharide samples that contain one or several residues labeled with stable NMR-active isotopes (13C, 15N).60 Clearly, 13C-labeling offers significant advantages for oligosaccharide NMR analyses: The large spectral dispersion of this nucleus and the option of combining this information with that of 1H through 2D or 3D NMR experiments allow solving the usual signal overlapping in glycan NMR spectroscopy61 (Figure 8). However, an important concern when using 13C-labeled carbohydrates is the suppression of the large 13C–13C couplings. Over the past few years, the application of constant-time periods (CT) in the indirect dimension or the use of virtual decoupling schemes have been proved to be very useful to avoid the 13C–13C coupling problem.62
Figure 8.
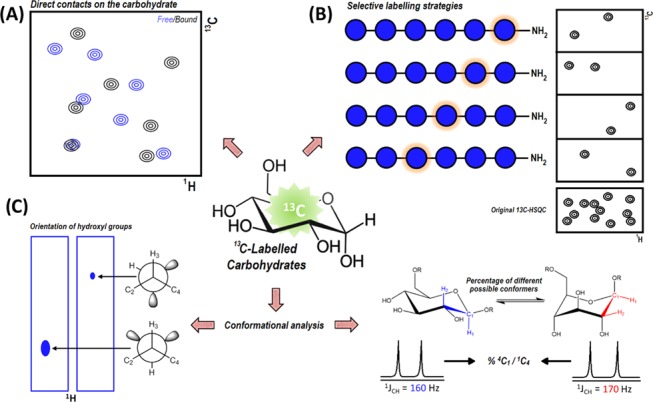
Scheme of the various possible advantages provided by 13C-labeled carbohydrates for NMR studies. (A) 2D-1H–13C-HSQC experiments to study interactions and recognition events involving carbohydrates. (B) Selective labeling strategies to reduce overlapping and signal crowding of NMR spectra in long and complex oligosaccharides. (C) 13C-labeling applied to study conformations through the analysis of 1H–13C coupling constants.
The presence of a labeled molecule opens the possibility of using 13C- and/or 15N-filtered NOESY experiments, which are ideally suited to extract intermolecular interactions.63 NMR approaches that use 13C/15N-labeled proteins in conjunction with 13C-labeled carbohydrates have been efficiently applied by Nestor et al. to describe the interaction between the 13C-labeled Manα(1–2)Manα(1–2)ManαOMe (Man3) trisaccharide core and cyanovirin-N (CV-N) P51G.64 This protein displays binding sites, A and B, which preferentially bind to the Man3 trisaccharide and Man2 disaccharide, respectively.65 Due to the known slow-exchange regime in which this interaction takes place, STD-NMR experiments66 cannot be used for directly monitoring the ligand signals. Instead, the use of 1H–13C-CT–HSQC spectra, complemented with 1H–13C-HSQC–NOESY data, allowed assessing the CV-N bound conformation of Man3, which was very similar to that in the free state. Noteworthily, the authors efficiently exploited the 1H–13C and 15N–13C heteronuclear correlations to accurately describe the protein–ligand close contacts in the complex of 13C-Man3 with the 15N-labeled protein. Fittingly, all of these results led to a description of the binding pose that appropriately correlated with the X-ray structure (PDB: 3GXZ).67
In a parallel work, the 13C-labeled Man3 trisaccharide has been shown to display well-resolved NMR signals for its polar hydroxyl protons even at room temperature.68 The presence of these signals encouraged the characterization of additional recognition features not feasible with regular unlabeled carbohydrates. The assignment of the hydroxyl groups was performed with simple 1H,13C-HSQC–TOCSY experiments that also allowed estimating the different 3JCHOH values, and thus deducing the major H–C–O–H rotamer for each hydroxyl group. Nevertheless, the most remarkable output of this work is the very detailed description of the hydrogen bond network present in the complex, an unprecedented result if considering that these protons are usually inaccessible by NMR due to the typical proton-water exchange that affects the polar 1H nuclei.69
Although 13C-labeled saccharides have been proved to be highly advantageous for NMR studies on carbohydrates, their synthesis is still troublesome.70 Hence, there are multiple lines of work that keep focusing on improving the synthetic routes to afford these biomolecules. An interesting synthetic strategy is the automated glycan assembly scheme,71 which has been recently used to readily synthesize several well-defined oligosaccharides, containing selectively 13C-labeled residues.72 This selective labeling enables breaking the chemical shift degeneracy, making possible the precise NMR analysis of each residue. One advantage of the chemical shift degeneracy is that the 1JCH values for each residue are accessible. Since it is widely known that the 1JCH values provide information on the preferred axial or equatorial orientation of the anomeric hydrogen,73 the observed 1JCH values for the different β-pyranose rings served to deduce the percentage of the 4C1 chair and the alternative 1C4 chair conformations in each case. For some of the synthesized structures, differences in the 4C1–1C4 ratio were found between internal and external sugar residues, which nicely concurred with the results obtained from MD simulations.
Besides 13C-labeling, carbohydrates are often labeled with fluorine nuclei. The advantages of using this probe in 19F NMR studies are enormous: the active isotope is highly abundant in nature and its intrinsic sensitivity is almost as good as that of the 1H nucleus. Moreover, the spectral dispersion of 19F NMR signals is remarkably larger than that of the protons. Usually, only one or a few 19F atoms are chemically introduced in the studied molecule, thus increasing the simplicity of the recorded spectra and facilitating their interpretation.74
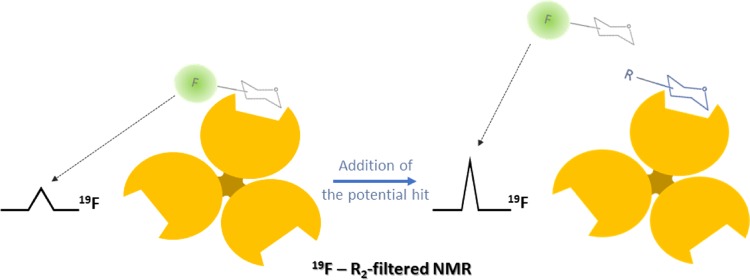
Broadening effects in ligand signals upon binding may be successfully used to describe protein–ligand interactions.75 For this purpose, T2-filtered NMR relaxation experiments constitute a robust method to characterize such recognition events, and fluorinated probes provided the simplest way to observe linewidth changes regarding the aforementioned advantages.7619F-NMR-based experiments have been employed for screening a library of analogues of 2-deoxy-2-trifluoroacetamido-α-mannoside to the C-type lectin, Langerin, to target the additional pockets on the Langerin surface, close to the calcium-binding site.77 These analogues are decorated with different molecular fragments on the acetyl branch, substituting the −CF3 group of the reference compound. Indeed, the −CF3 group of the reporter molecule showed substantial line broadening, evidencing binding to the protein, and the interaction was quantified by competition experiments. In particular, the observed relaxation rates R2,obs were measured for the probe in the presence of increasing amounts of glycomimetics (Figure 9). The recovery of the trifluoroacetamide peak afforded the corresponding dissociation constants for the library.
Figure 9.
Schematic conception of the competition experiments to screen the affinities of a library of compounds using a monofluorinated probe. The signal recovery after displacing the probe from the binding site can be correlated with differences in binding constants.
Toward in-Cell NMR: Recognizing Carbohydrates in Crowded Environments
The last frontier in glycan recognition by NMR is to explore these interactions in cells. As a general rule, most of the NMR studies oriented to get insights into molecular recognition processes are performed in vitro. Those conditions usually include the sole presence of the macromolecule of interest under diluted conditions and a few ligands, as well as other buffer components, commonly salts and/or small organic compounds. In contrast, the biological milieu is far from these experimental conditions: a complex and crowded environment in which several additional factors can simultaneously affect the nature of protein–ligand interactions in different ways as they are detected under in vitro conditions.78 Thus, the challenge is to employ NMR methods in cells, but still there are many examples of these applications, which have been specially focused on the study of the interaction of the histo-blood group antigens with virus-like particles, as a previous step to monitor their recognition by specific capsid viral proteins.79
Molecular crowding primarily affects the viscosity, which consequently disturbs the diffusion-related processes and causes an important reduction in the available volume, enhancing steric repulsion phenomena.80 Whereas repulsions tend to favor protein stability by self- and non-self-assembly, the existence of nonspecific protein–protein soft interactions, both hydrophobic and hydrophilic, contributes to misfolding and aggregation. In cellular environments, protein interactions at this level of complexity have already been shown to be crucial for establishing signaling cascades and organizing multi-enzyme complexes through the electrostatic interaction of specific residues at protein surfaces.81 Therefore, an expanded understanding of recognition processes in crowded media requires considering these so-called “quinary interactions”.81 Part of the most recent studies related to this issue have been primarily focused on antibodies,82 since such therapeutic biomolecules function in the blood stream, a very heterogeneous environment.83 In this context, NMR may be used as a tool to elucidate the structural details that play a key role in molecular recognition events in solution involving glycans. Obviously, all of these studies imply using isotopic labeling as a strategy to distinguish the system of interest in the heterogeneous media.
Along this line, the receptor–ligand interactions between human galectin-3 and β-galactoside-containing glycans have been studied in a crowded milieu.84 This work exemplifies the effects of the viscosity and steric repulsion combined with additional specific interactions ascribed to other glycoproteins present in the heterogeneous environment. Line broadening was observed in the 1H–15N-HSQC spectrum when 15N-Gal-3 was titrated with increasing amounts of four different crowding agents: bovine serum albumin (BSA-I), human serum albumin (HSA-I), Ficoll, and PEG 3350. The decrease in signal intensities is strongly correlated with the increment in solution viscosity. However, the addition of both polymers, Ficoll and PEG 3350, increased the viscosity much more than in the case of both albumins. The intense signal broadening observed in the presence of HSA and BSA could be, therefore, ascribed to the formation of large-sized Gal-3/crowder complexes through additional specific and nonspecific interactions. Instead, Ficoll and PEG 3350 seemed to be more inert although still evoked line-broadening effects in the Gal-3 1H–15N-HSQC. Since HSA and BSA samples contained other serum glycoproteins and Gal-3 recognizes the β-Gal epitope, it would be reasonable to think about an actual specific recognition process between both proteins. Indeed, the subsequent addition of saturating amounts of lactose to the BSA-I/Gal-3 and HSA-I/Gal-3 samples allowed disrupting the suggested interactions, which led to a partial recovery of Gal-3 signal intensities. The lactose did not significantly affect the viscosity of the samples, and the same experiments with Ficoll and PEG 3350 showed no changes in the HSQC spectra, thus confirming the specificity of this recognition event. Similarly, the 1H–15N-HSQC in the presence of the non-glycosylated serum albumin HSA-II displayed negligible line-broadening effects, exclusively due to viscosity issues. The addition of lactose also gave rise to the corresponding chemical shift perturbations that were identical to those measured on diluted Gal-3/lactose samples.85 This observation underlines the ability of the lactose to find the proper binding site even in physiological crowded media, even though the Kd would reasonably be lower due to slower diffusion processes (reduced kon) and competition with other substrates for the Gal-3 binding site. Finally, the interaction of Gal-3 with serum glycoproteins from both HSA and BSA samples was demonstrated to occur via recognition of the nonreducing 2,3-sialyllactose motif present in those glycoproteins, as evidenced by the identical set of CSP obtained for the Gal-3 HSQC spectrum when titrated with the pure 2,3-sialyllactose trisaccharide.
Glycan Interactions Monitored by Solid-State NMR
Solid-State NMR, ssNMR, has emerged in the last decades as a formidable, realistic, and versatile tool in structural biology.86 Indeed, the ssNMR approach may also be very informative to observe the behavior of different oligosaccharides in their intact chemical form and when bound to prokaryotic or eukaryotic receptors.
As one of the first examples in the area, Simorre et al.87 studied the structure and dynamics of Escherichia coli peptidoglycan, an essential component of the bacterial cell wall, which was obtained in its double labeled form using 13C, 15N-isotopically labeled E. coli BL21 cells as medium. The important flexibility of the PG was inferred from the observed reduced dipolar coupling values. Moreover, its interaction with YajG, a E. coli protein, with a yet unknown role in PG metabolism was later evaluated through the changes in the relaxation behavior of the partners.88
In a related context, Baldus and co-workers have investigated the structure and dynamics of the Gram-negative bacterial cell.89 This is characterized by a molecular complex that consists of two lipid bilayers, inner and outer membranes, separated by the peptidoglycan layer. The outer membrane is asymmetrical and consists of phospholipids, lipopolysaccharides (LPS), lipoproteins, and membrane proteins.90 In this particular case, the PG and lipopolysaccharide (LPS) components could be assigned through 1H–13C and 13C–13C correlation experiments.91
As landmarks in the molecular recognition field, the interaction between Colicin N, an antibacterial protein toxin secreted by E. coli, with the lipopolysaccharide close to the membrane surface has also been investigated,92 while the interaction of the intact PG from Bacillus subtilis with the L,D-transpeptidase LdtBS has also been scrutinized using U-[13C, 15N]-labeled LdtBS and PG molecules.93 A detailed NMR analysis of the behavior of PG in its free and bound forms was carried out through relaxation and RDC measurements to deduce the effect of protein binding on PG conformations and dynamics. Interestingly, PG dynamics was clearly affected, although no drastic chemical shift perturbations in the PG signals were observed in the presence of the protein. More recently,94 two different and archetypal LPS have been studied in a membrane-like environment. In particular, the structures of LPS from different bacterial strains, a rough-type LPS from E. coliK-12 and a smooth-type LPS from Pseudomonas aeruginosa PAO1 strains, were used as systems. P. aeruginosa PAO1 displays variable LPS O-antigens, which are composed of the same lipid-A-core oligosaccharide but decorated with two different O-antigens.95 The A-band O-antigen is based on a D-rhamnose repeating unit, whereas the B-band shows a complex trisaccharide with two N-acylated mannuronic acids and one FucNAc moiety. Initial studies permitted assigning the last four carbons of the lipid chains only a few resonances of the inner core oligosaccharides.95 However, the analysis of the ssNMR data permitted identifying the signals of the major B-band O-antigen trisaccharide, allowing the study of the chemical shift perturbations and the relaxation behavior in the presence of gentamicin, allowing confirming that a specific oligosaccharide is indeed involved in the recognition of the antibiotic.96
Obviously, the use of ssNMR for the direct observation of LPS endotoxins in their natural environments constitutes an important step in the field of molecular microbiology with special emphasis on the investigation of novel antibiotic molecules.
Perspectives
Glycosciences are enjoying from exciting developments in the field. Novel NMR methodologies, technologies, and applications are emerging continuously, advancing our knowledge on glycans, glycan interactions, and their key roles in health and disease. Indeed, the application of novel NMR methodologies (paramagnetic, RDC, and RCSA constraints)97 will allow further advances and breakthroughs in the structural and dynamic analysis of many complex glycans as well as to disentangle key features of their interactions with receptors. These methodologies together with the impressive synthetic efforts98 carried out by expert chemists around the world will allow breaking the limits in accessing information for highly complex glycan structures and providing a detailed understanding of the molecular mechanisms behind the concomitant recognition events. In fact, the application of NMR methodologies is of enormous importance to uncover the glycan epitope presentation to the receptors, a key information for vaccine development, drug discovery programs, and for the invention of novel therapeutics for combating different pathologies, including infection and immune diseases and cancer.99 The new NMR methodologies described herein together with the continuous advances in sensitivity provided by the manufacturers (new probes, hardware, and technologies) will also permit directly studying intact glycoproteins100 and their interactions so that, together with chemoenzymatic remodeling tools, they will have applications for designing novel glycosylated therapeutic antibodies. Concomitantly, novel glycan biomarkers will be characterized. In this context, we also expect a tremendous development of the application of high-field NMR methods in cells, in the true biological environment. Thus, we envisage a large contribution of NMR methods and applications to the exciting glycoscience field.
Acknowledgments
We thank Agencia Estatal de Investigación (Spain) for grants CTQ2015-64597-C2-1-P and RTI2018-094751-B-C21, for a FPU fellowship to P.V. (FPU 14/06147), and for the Severo Ochoa Excellence Accreditation (SEV-2016-0644). J.J.-B. also thanks the European Research Council (RECGLYCANMR, Advanced Grant no. 788143).
The authors declare no competing financial interest.
References
- a Zhao Y. Y.; Takahashi M.; Gu J. G.; Miyoshi E.; Matsumoto A.; Kitazume S.; Taniguchi N. Functional roles of N-glycans in cell signaling and cell adhesion in cancer. Cancer Sci. 2008, 99, 1304–1310. 10.1111/j.1349-7006.2008.00839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Marth J. D.; Grewal P. K. Mammalian glycosylation in immunity. Nat. Rev. Immunol. 2008, 8, 874–887. 10.1038/nri2417. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Sun L.; Middleton D. R.; Wantuch P. L.; Ozdilek A.; Avci F. Y. Carbohydrates as T-cell antigens with implications in health and disease. Glycobiology 2016, 26, 1029–1040. 10.1093/glycob/cww062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Varki A. Biological roles of glycans. Glycobiology 2017, 27, 3–49. 10.1093/glycob/cww086. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Taylor M. E.; Drickamer K. Paradigms for glycan-binding receptors in cell adhesion. Curr. Opin. Cell Biol. 2007, 19, 572–577. 10.1016/j.ceb.2007.09.004. [DOI] [PubMed] [Google Scholar]
- a Hakomori S. Tumor-associated carbohydrate antigens. Ann. Rev. Immunol. 1984, 2, 103–126. 10.1146/annurev.iy.02.040184.000535. [DOI] [PubMed] [Google Scholar]; b Hakomori S. Aberrant Glycosylation In Tumors And Tumor-Associated Carbohydrate Antigens. Adv. Cancer Res. 1989, 52, 257–331. 10.1016/S0065-230X(08)60215-8. [DOI] [PubMed] [Google Scholar]
- a Figueiredo G. G.; Cezar R. D.; Freire N. M.; Teixeira V. G.; Baptista P.; Cordeiro M.; Carmo R. F.; Vasconcelos L. R. S.; Moura P. Mannose-binding lectin gene (MBL2) polymorphisms related to the mannose-binding lectin low levels are associated to dengue disease severity. Hum. Immunol. 2016, 77, 571–575. 10.1016/j.humimm.2016.05.006. [DOI] [PubMed] [Google Scholar]; b Piao W.; Liu C.; Kao A. H.; Manzi S.; Vogt M. T.; Ruffing M. J.; Ahearn J. M. Mannose-binding lectin is a disease-modifying factor in North American patients with systemic lupus erythematosus. J. Rheumatol. 2007, 34, 1506–1513. [PubMed] [Google Scholar]
- a Müller C.; Despras G.; Lindhorst T. K. Organizing multivalency in carbohydrate recognition. Chem. Soc. Rev. 2016, 45, 3275–3302. 10.1039/C6CS00165C. [DOI] [PubMed] [Google Scholar]; b Bernardi A.; et al. Multivalent glycoconjugates as anti-pathogenic agents. Chem. Soc. Rev. 2013, 42, 4709–4727. 10.1039/C2CS35408J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Gimeno A.; Delgado S.; Valverde P.; Bertuzzi S.; Berbís M. A.; Echavarren J.; Lacetera A.; Martín-Santamaría S.; Surolia A.; Cañada F. J.; Jiménez-Barbero J.; Arda A. Minimizing the Entropy Penalty for Ligand Binding: Lessons from the Molecular Recognition of the Histo Blood-Group Antigens by Human Galectin-3. Angew. Chem., Int. Ed. 2019, 58, 7268–7272. 10.1002/anie.201900723. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Valverde P.; Delgado S.; Martínez J. D.; Vendeville J. B.; Malassis J.; Linclau B.; Reichardt N. C.; Cañada F. J.; Jiménez-Barbero J.; Ardá A. Molecular Insights into DC-SIGN Binding to Self-Antigens: The Interaction with the Blood Group A/B Antigens. ACS Chem. Biol. 2019, 14, 1660–1671. 10.1021/acschembio.9b00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asensio L.; Arda A.; Canada F. J.; Jimenez-Barbero J. Carbohydrate–Aromatic Interactions. Acc. Chem. Res. 2013, 46, 946–954. 10.1021/ar300024d. [DOI] [PubMed] [Google Scholar]
- a Nagae M.; Kanagawa M.; Morita-Matsumoto K.; Hanashima S.; Kizuka Y.; Taniguchi N.; Yamaguchi Y. Atomic visualization of a flipped-back conformation of bisected glycans bound to specific lectins. Sci. Rep. 2016, 6, 22973 10.1038/srep22973. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Calabrese C.; Écija P.; Compañón I.; Vallejo-López M.; Cimas Á.; Parra M.; Basterretxea F. J.; Santos J. I.; Jiménez-Barbero J.; Lesarri A.; Corzana F.; Cocinero E. J. Conformational Behavior of d-Lyxose in Gas and Solution Phases by Rotational and NMR Spectroscopies. J. Phys. Chem. Lett. 2019, 10, 3339–3345. 10.1021/acs.jpclett.9b00978. [DOI] [PubMed] [Google Scholar]; c Bermejo I. A.; Usabiaga I.; Compañón I.; Castro-López J.; Insausti A.; Fernández J. A.; Avenoza A.; Busto J. H.; Jiménez-Barbero J.; Asensio J. L.; Peregrina J. M.; Jiménez-Osés G.; Hurtado-Guerrero R.; Cocinero E. J.; Corzana F. Water Sculpts the Distinctive Shapes and Dynamics of the Tumor-Associated Carbohydrate Tn Antigens: Implications for Their Molecular Recognition. J. Am. Chem. Soc. 2018, 140, 9952–9960. 10.1021/jacs.8b04801. [DOI] [PubMed] [Google Scholar]
- Sirohi D.; Chen Z.; Sun L.; Klose T.; Pierson T. C.; Rossmannand M. G.; Kuhn R. J. The 3.8 Å resolution cryo-EM structure of Zika virus. Science 2016, 352, 467–470. 10.1126/science.aaf5316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agirre J. Strategies for carbohydrate model building, refinement and validation. Acta Crystallogr., Sect. D: Struct. Biol. 2017, 73, 171–186. 10.1107/S2059798316016910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Blaum B. S.; Neu U.; Peters T.; Stehlea T. Spin ballet for sweet encounters: saturation-transfer difference NMR and X-ray crystallography complement each other in the elucidation of protein–glycan interactions. Acta Crystallogr., Sect. F: Struct. Biol. Commun. 2018, 74, 451–462. 10.1107/S2053230X18006581. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Roldós V.; Canada F. J.; Jimenez-Barbero J. Carbohydrate-protein interactions: a 3D view by NMR. ChemBioChem 2011, 12, 990–1005. 10.1002/cbic.201000705. [DOI] [PubMed] [Google Scholar]
- a Duverger E.; Lamerant-Fayel N.; Frison N.; Monsigny M.. Surface Plasmon Resonance: Methods and Protocols, (Methods in Molecular Biology); De Mol N. J., Fischer M. J. E., Eds.; Springer: Utrecht, 2010; Vol. 627, pp 157–178. [DOI] [PubMed] [Google Scholar]; b Penezic A.; Deokar G.; Vignaud D.; Pichonat E.; Happy H.; Subramanian P.; Gasparovic B.; Boukherroub R.; Szunerits S. Carbohydrate-Lectin Interaction on Graphene-Coated Surface Plasmon Resonance (SPR) Interfaces. Plasmonics 2014, 9, 677–683. 10.1007/s11468-014-9686-3. [DOI] [Google Scholar]
- a Dam T. K.; Talaga M. L.; Fan N.; Brewer C. F. Measuring Multivalent Binding Interactions by Isothermal Titration Calorimetry. Methods Enzymol. 2016, 567, 71–95. 10.1016/bs.mie.2015.08.013. [DOI] [PubMed] [Google Scholar]; b Murthy B. N.; Sinha S.; Surolia A.; Indi S. S.; Jayaraman N. SPR and ITC determination of the kinetics and the thermodynamics of bivalent versus monovalent sugar ligand-lectin interactions. Glycoconjugate J. 2008, 25, 313–321. 10.1007/s10719-007-9076-6. [DOI] [PubMed] [Google Scholar]; c Wang Z.; Chen G.; Lu J.; Hong L.; Ngai T. Investigation of the factors affecting the carbohydrate–lectin interaction by ITC and QCM-D. Colloid Polym. Sci. 2014, 292, 391–398. 10.1007/s00396-013-3080-0. [DOI] [Google Scholar]
- Ji Y.; Woods R. J. Quantifying Weak Glycan-Protein Interactions Using a Biolayer Interferometry Competition Assay: Applications to ECL Lectin and X-31 Influenza Hemagglutinin. Adv. Exp. Med. Biol. 2018, 1104, 259–273. 10.1007/978-981-13-2158-0_13. [DOI] [PubMed] [Google Scholar]
- Wild R.; Kowal J.; Eyring J.; Ngwa E. M.; Aebi M.; Locher K. P. Structure of the yeast oligosaccharyltransferase complex gives insight into eukaryotic N-glycosylation. Science 2018, 359, 545–550. 10.1126/science.aar5140. [DOI] [PubMed] [Google Scholar]
- a Chaptal V.; Kwon S.; Sawaya M. R.; Guan L.; Kaback H. R.; Abramson J. Crystal structure of lactose permease in complex with an affinity inactivator yields unique insight into sugar recognition. Proc. Natl. Acad. Sci. U.S.A. 2011, 108, 9361–9366. 10.1073/pnas.1105687108. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Ideo H.; Matsuzaka T.; Nonaka T.; Seko A.; Yamashita K. Galectin-8-N-domain recognition mechanism for sialylated and sulfated glycans. J. Biol. Chem. 2011, 286, 11346–11355. 10.1074/jbc.M110.195925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Agirre J.; Davies G. J.; Wilson K. S.; Cowtan K. D. Carbohydrate anomalies in the PDB. Nat. Chem. Biol. 2015, 11, 303 10.1038/nchembio.1798. [DOI] [PubMed] [Google Scholar]; b Agirre J.; Davies G. J.; Wilson K. S.; Cowtan K. D. Carbohydrate structure: the rocky road to automation. Curr. Opin. Struct. Biol. 2017, 44, 39–47. 10.1016/j.sbi.2016.11.011. [DOI] [PubMed] [Google Scholar]
- Marchetti R.; Perez S.; Arda A.; Imberty A.; Jimenez-Barbero J.; Silipo A.; Molinaro A. “Rules of Engagement” of Protein–Glycoconjugate Interactions: A Molecular View Achievable by using NMR Spectroscopy and Molecular Modeling. ChemistryOpen 2016, 5, 274–296. 10.1002/open.201600024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters T.; Scheffler K.; Ernst B.; Katopodis A.; Magnani J. L.; Wang W. T.; Weisemann R. Determination of the Bioactive Conformation of the Carbohydrate Ligand in the E-Selectin/Sialyl LewisX Complex. Angew. Chem., Int. Ed. Engl. 1995, 34, 1841–1844. 10.1002/anie.199518411. [DOI] [Google Scholar]
- a Jütten L.; Ramírez-Gualito K.; Weilhard A.; Albrecht B.; Cuevas G.; Fernández-Alonso M. C.; Jiménez-Barbero J.; Schlörer N. E.; Diaz D. Exploring the Role of Solvent on Carbohydrate–Aryl Interactions by Diffusion NMR-Based Studies. ACS Omega 2018, 3, 536–543. 10.1021/acsomega.7b01630. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Groves P.; Rasmussen M. O.; Molero M. D.; Samain E.; Cañada F. J.; Driguez H.; Jiménez-Barbero J. Diffusion ordered spectroscopy as a complement to size exclusion chromatography in oligosaccharide analysis. Glycobiology 2004, 14, 451–456. 10.1093/glycob/cwh037. [DOI] [PubMed] [Google Scholar]
- Xu B.; Unione L.; Sardinha J.; Wu S.; Etheve-Quelquejeu M.; Rauter A. P.; Bleriot Y.; Zhang Y.; Martín-Santamaría S.; Díaz D.; Jiménez-Barbero J.; Sollogoub M. gem-Difluorocarbadisaccharides: restoring the exo-anomeric effect. Angew. Chem., Int. Ed. 2014, 53, 9597–9602. 10.1002/anie.201405008. [DOI] [PubMed] [Google Scholar]
- Unione L.; Xu B.; Díaz D.; Martín-Santamaría S.; Poveda A.; Sardinha J.; Rauter A. P.; Bleriot Y.; Zhang Y.; Cañada F. J.; Sollogoub M.; Jiménez-Barbero J. Conformational Plasticity in Glycomimetics: Fluorocarbamethyl-L-idopyranosides Mimic the Intrinsic Dynamic Behaviour of Natural Idose Rings. Chem. - Eur. J. 2015, 21, 10513–10521. 10.1002/chem.201501249. [DOI] [PubMed] [Google Scholar]
- Zierke M.; Smiesko M.; Rabbani S.; Aeschbacher T.; Cutting B.; Allain F. H.; Schubert M.; Ernst B. Stabilization of Branched Oligosaccharides: Lewisx Benefits from a Nonconventional C–H···O Hydrogen Bond. J. Am. Chem. Soc. 2013, 135, 13464–13472. 10.1021/ja4054702. [DOI] [PubMed] [Google Scholar]
- Ardá A.; Jimenez-Barbero J. The recognition of glycans by protein receptors. Insights from NMR spectroscopy. Chem. Commun. 2018, 54, 4761–4769. 10.1039/C8CC01444B. [DOI] [PubMed] [Google Scholar]
- Skrisovska L.; Schubert M.; Allain F. H. T. Recent advances in segmental isotope labeling of proteins: NMR applications to large proteins and glycoproteins. J. Biomol. NMR 2010, 46, 51–58. 10.1007/s10858-009-9362-7. [DOI] [PubMed] [Google Scholar]
- Higel F.; Seidl A.; Sörgel F.; Friess W. N-glycosylation heterogeneity and the influence on structure, function and pharmacokinetics of monoclonal antibodies and Fc fusion proteins. Eur. J. Pharm. Biopharm. 2016, 100, 94–100. 10.1016/j.ejpb.2016.01.005. [DOI] [PubMed] [Google Scholar]
- a Yamaguchi Y.; Kato K. Dynamics and Interactions of Glycoconjugates Probed by Stable-Isotope-Assisted NMR Spectroscopy. Methods Enzymol. 2010, 478, 305–322. 10.1016/S0076-6879(10)78015-0. [DOI] [PubMed] [Google Scholar]; b Barb A. W.; Freedberg D. I.; Battistel M. D.; Prestegard J. H. NMR Detection and Characterization of Sialylated Glycoproteins and Cell Surface Polysaccharides. J. Biomol. NMR 2011, 51, 163–171. 10.1007/s10858-011-9550-0. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Barb A. W.; Glushka J. N.; Prestegard J. H. Kinetics of Neuraminidase Action on Glycoproteins by 1D and 2D NMR. J. Chem. Educ. 2011, 88, 95–97. 10.1021/ed900054b. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Barb A. W.; Borgert A. J.; Liu M.; Barany G.; Live D. Intramolecular glycan-protein interactions in glycoproteins. Methods Enzymol. 2010, 478, 365–388. 10.1016/S0076-6879(10)78018-6. [DOI] [PubMed] [Google Scholar]
- a Nagae M.; Yamaguchi Y. Function and 3D Structure of the N-Glycans on Glycoproteins. Int. J. Mol. Sci. 2012, 13, 8398–8429. 10.3390/ijms13078398. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Lu D.; Yang C.; Liu Z. How Hydrophobicity and the Glycosylation Site of Glycans Affect Protein Folding and Stability: A Molecular Dynamics Simulation. J. Phys. Chem. B 2012, 116, 390–400. 10.1021/jp203926r. [DOI] [PubMed] [Google Scholar]
- Opitz C.; Isogai S.; Grzesiek S. An economic approach to efficient isotope labeling in insect cells using homemade 15N-, 13C- and 2H-labeled yeast extracts. J. Biomol. NMR 2015, 62, 373–385. 10.1007/s10858-015-9954-3. [DOI] [PubMed] [Google Scholar]
- a Li W.; Zhu Z.; Chen W.; Feng Y.; Dimitrov D. S. Crystallizable Fragment Glycoengineering for Therapeutic Antibodies Development. Front. Immunol. 2017, 8, 1554 10.3389/fimmu.2017.01554. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Venetz D.; Hess C.; Lin C.; Aebi M. Glycosylation profiles determine extravasation and disease-targeting properties of armed antibodies. Proc. Nat. Ac. Sci. U.S.A 2015, 112, 2000–2005. 10.1073/pnas.1416694112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ereño-Orbea J.; Sicard T.; Cui H.; Mazhab-Jafari M. T.; Benlekbir S.; Guarné A.; Rubinstein J. L.; Julien J. Molecular basis of human CD22 function and therapeutic targeting. Nat. Commun. 2017, 8, 764 10.1038/s41467-017-00836-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barb A. W.; Falconer D. J.; Subedi G. P. The Preparation and Solution NMR Spectroscopy of Human Glycoproteins Is Accessible and Rewarding. Methods Enzymol. 2019, 614, 239–261. 10.1016/bs.mie.2018.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyanoiri Y.; Ishida Y.; Takeda M.; Terauchi T.; Inouye M.; Kainosho M. Highly efficient residue-selective labeling with isotope-labeled Ile, Leu, and Val using a new auxotrophic E. coli strain. J. Biomol. NMR 2016, 65, 109–119. 10.1007/s10858-016-0042-0. [DOI] [PubMed] [Google Scholar]
- Schwarz F.; Fan Y. Y.; Schubert M.; Aebi M. Cytoplasmic N-Glycosyltransferase of Actinobacillus pleuropneumoniae Is an Inverting Enzyme and Recognizes the NX(S/T) Consensus Sequence. J. Biol. Chem. 2011, 286, 35267–35274. 10.1074/jbc.M111.277160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu T.; Yamaguchi T.; Satoh T.; Kato K. A Hybrid Strategy for the Preparation of 13C-labeled High-mannose-type Oligosaccharides with Terminal Glucosylation for NMR Study. Chem. Lett. 2015, 44, 1744–1746. 10.1246/cl.150898. [DOI] [Google Scholar]
- Xu X.; Eletsky A.; Sheikh M. O.; Prestegard J. H.; West C. M. Glycosylation Promotes the Random Coil to Helix Transition in a Region of a Protist Skp1 Associated with F-Box Binding. Biochemistry 2018, 57, 511–515. 10.1021/acs.biochem.7b01033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissbach S.; Flegge F.; Peters T. Substrate Binding Drives Active-Site Closing of Human Blood Group B Galactosyltransferase as Revealed by Hot-Spot Labeling and NMR Spectroscopy Experiments. ChemBioChem 2018, 19, 970–978. 10.1002/cbic.201800019. [DOI] [PubMed] [Google Scholar]
- Schubert M.; Walczak M. J.; Aebi M.; Wider G. Posttranslational modifications of intact proteins detected by NMR spectroscopy: application to glycosylation. Angew. Chem., Int. Ed. 2015, 54, 7096–7100. 10.1002/anie.201502093. [DOI] [PubMed] [Google Scholar]
- Klukowski P.; Schubert M. Chemical shift-based identification of monosaccharide spin-systems with NMR spectroscopy to complement untargeted glycomics. Bioinformatics 2019, 35, 293–300. 10.1093/bioinformatics/bty465. [DOI] [PubMed] [Google Scholar]
- a Aeschbacher T.; Zierke M.; Smieško M.; Collot M.; Mallet J. M.; Ernst B.; Allain F.; Schubert M. A Secondary Structural Element in a Wide Range of Fucosylated Glycoepitopes. Chem. - Eur. J. 2017, 23, 11598–11610. 10.1002/chem.201701866. [DOI] [PubMed] [Google Scholar]; b Battistel M. D.; Azurmendi H. F.; Frank M.; Freedberg D. I. Uncovering Nonconventional and Conventional Hydrogen Bonds in Oligosaccharides through NMR Experiments and Molecular Modeling: Application to Sialyl Lewis-X. J. Am. Chem. Soc. 2015, 137, 13444–13447. 10.1021/jacs.5b03824. [DOI] [PubMed] [Google Scholar]
- Horowitz S.; Trievel R. C. Carbon-Oxygen Hydrogen Bonding in Biological Structure and Function. J. Biol. Chem. 2012, 287, 41576–41582. 10.1074/jbc.R112.418574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimeno A.; Delgado S.; Valverde P.; Bertuzzi S.; Berbís M. A.; Echavarren J.; Lacetera A.; Martín-Santamaría S.; Surolia A.; Cañada F. J.; Jiménez-Barbero J.; Ardá A. Minimizing the Entropy Penalty for Ligand Binding: Lessons from the Molecular Recognition of the Histo Blood-Group Antigens by Human Galectin-3. Angew. Chem., Int. Ed. 2019, 58, 7268–7272. 10.1002/anie.201900723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Cornish V. W.; Benson D. R.; Altenbach C. A.; Hideg K.; Hubbell W. L.; Schultz P. G. Site-specific incorporation of biophysical probes into proteins. Proc. Natl. Acad. Sci. U.S.A. 1994, 91, 2910–2914. 10.1073/pnas.91.8.2910. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Battiste J. L.; Wagner G. Utilization of Site-Directed Spin Labeling and High-Resolution Heteronuclear Nuclear Magnetic Resonance for Global Fold Determination of Large Proteins with Limited Nuclear Overhauser Effect Data. Biochemistry 2000, 39, 5355–5365. 10.1021/bi000060h. [DOI] [PubMed] [Google Scholar]; c Yamaguchi T.; Kamiya Y.; Choo Y.; Yamamoto S.; Kato K. Terminal Spin Labeling of a High-mannose-type Oligosaccharide for Quantitative NMR Analysis of Its Dynamic Conformation. Chem. Lett. 2013, 42, 544–546. 10.1246/cl.130040. [DOI] [Google Scholar]
- a Brath U.; Swamy S. I.; Veiga A. X.; Tung C.; Van Petegem F.; Erdélyi M. Paramagnetic Ligand Tagging To Identify Protein Binding Sites. J. Am. Chem. Soc. 2015, 137, 11391–11398. 10.1021/jacs.5b06220. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Zong C. L.; Huang R. R.; Condac E.; Chiu Y. L.; Xiao W. Y.; Li X. R.; Lu W. G.; Ishihara M.; Wang S.; Ramiah A.; Stickney M.; Azadi P.; Amster I. J.; Moremen K. W.; Wang L. C.; Sharp J. S.; Boons G. J. Integrated Approach to Identify Heparan Sulfate Ligand Requirements of Robo1. J. Am. Chem. Soc. 2016, 138, 13059–13067. 10.1021/jacs.6b08161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otting G. Prospects for lanthanides in structural biology by NMR. J. Biomol. NMR 2008, 42, 1–9. 10.1007/s10858-008-9256-0. [DOI] [PubMed] [Google Scholar]
- a Yamamoto S.; Zhang Y.; Yamaguchi T.; Kameda T.; Kato K. Lanthanide-assisted NMR evaluation of a dynamic ensemble of oligosaccharide conformations. Chem. Commun. 2012, 48, 4752–4754. 10.1039/c2cc30353a. [DOI] [PubMed] [Google Scholar]; b Zhang Y.; Yamamoto S.; Yamaguchi T.; Kato K. Application of paramagnetic NMR-validated molecular dynamics simulation to the analysis of a conformational ensemble of a branched oligosaccharide. Molecules 2012, 17, 6658–6671. 10.3390/molecules17066658. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Yamamoto S.; Yamaguchi T.; Erdelyi M.; Griesinger C.; Kato K. Paramagnetic lanthanide tagging for NMR conformational analyses of N-linked oligosaccharides. Chem. - Eur. J. 2011, 17, 9280–9282. 10.1002/chem.201100856. [DOI] [PubMed] [Google Scholar]
- Canales A.; Mallagaray A.; Perez-Castells J.; Boos I.; Unverzagt C.; André S.; Gabius H. J.; Cañada F. J.; Jiménez-Barbero J. Breaking pseudo-symmetry in multiantennary complex N-glycans using lanthanide-binding tags and NMR pseudo-contact shifts. Angew. Chem., Int. Ed. 2013, 52, 13789–13793. 10.1002/anie.201307845. [DOI] [PubMed] [Google Scholar]
- Canales A.; Boos I.; Perkams L.; Karst L.; Luber T.; Karagiannis T.; Domínguez G.; Cañada F. J.; Pérez-Castells J.; Häussinger D.; Unverzagt C.; Jiménez-Barbero J. Breaking the Limits in Analyzing Carbohydrate Recognition by NMR Spectroscopy: Resolving Branch-Selective Interaction of a Tetra-Antennary N-Glycan with Lectins. Angew. Chem., Int. Ed. 2017, 56, 14987–14991. 10.1002/anie.201709130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T.; Kajino M.; Yanaka S.; Zhu T.; Yagi H.; Satoh T.; Yamaguchi T.; Kato K. Conformational Analysis of a High-Mannose-Type Oligosaccharide Displaying Glucosyl Determinant Recognised by Molecular Chaperones Using NMR-Validated Molecular Dynamics Simulation. ChemBioChem 2017, 18, 396–401. 10.1002/cbic.201600595. [DOI] [PubMed] [Google Scholar]
- Ardá A.; Blasco P.; Silva D. V.; Schubert V.; André S.; Bruix M.; Cañada F. J.; Gabius H.-J.; Unverzagt C.; Jiménez-Barbero J. Molecular recognition of complex-type biantennary N-glycans by protein receptors: a three-dimensional view on epitope selection by NMR. J. Am. Chem. Soc. 2013, 135, 2667–2675. 10.1021/ja3104928. [DOI] [PubMed] [Google Scholar]
- Canales Á.; Mallagaray Á.; Berbís M. Á.; Navarro-Vázquez A.; Domínguez G.; Cañada F. J.; André S.; Gabius H.-J.; Pérez-Castells J.; Jiménez-Barbero J. Lanthanide-chelating carbohydrate conjugates are useful tools to characterize carbohydrate conformation in solution and sensitive sensors to detect carbohydrate-protein interactions. J. Am. Chem. Soc. 2014, 136, 8011–8017. 10.1021/ja502406x. [DOI] [PubMed] [Google Scholar]
- Gimeno A.; Reichardt N. C.; Cañada F. J.; Perkams L.; Unverzagt C.; Jiménez-Barbero J.; Ardá A. NMR and Molecular Recognition of N-Glycans: Remote Modifications of the Saccharide Chain Modulate Binding Features. ACS Chem. Biol. 2017, 12, 1104–1112. 10.1021/acschembio.6b01116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng W.; de Vries R. P.; Grant O. C.; Thompson A. J.; McBride R.; Tsogtbaatar B.; Lee P. S.; Razi N.; Wilson I. A.; Woods R. J.; Paulson J. C. Recent H3N2 Viruses Have Evolved Specificity for Extended, Branched Human-type Receptors, Conferring Potential for Increased Avidity. Cell Host Microbe 2017, 21, 23–34. 10.1016/j.chom.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández de Toro B.; Peng W.; Thompson A. J.; Domínguez G.; Cañada F. J.; Pérez-Castells J.; Paulson J. C.; Jiménez-Barbero J.; Canales A. Avenues to Characterize the Interactions of Extended N-Glycans with Proteins by NMR Spectroscopy: The Influenza Hemagglutinin Case. Angew. Chem., Int. Ed. 2018, 57, 15051–15055. 10.1002/anie.201807162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saio T.; Ogura K.; Yokochi M.; Kobashigawa Y.; Inagaki F. Two-point anchoring of a lanthanide-binding peptide to a target protein enhances the paramagnetic anisotropic effect. J. Biomol. NMR 2009, 44, 157–166. 10.1007/s10858-009-9325-z. [DOI] [PubMed] [Google Scholar]
- Barb A. W.; Ho T. G.; Flanagan-Steet H.; Prestegard J. H. Lanthanide binding and IgG affinity construct: potential applications in solution NMR, MRI, and luminescence microscopy. Protein Sci. 2012, 21, 1456–1466. 10.1002/pro.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moure M. J.; Eletsky A.; Gao Q.; Morris L. C.; Yang J.; Chapla D.; Zhao Y.; Zong C.; Amster I. J.; Moremen K. W.; Boons G. J.; Prestegard J. H. Paramagnetic Tag for Glycosylation Sites in Glycoproteins: Structural Constraints on Heparan Sulfate Binding to Robo1. ACS Chem. Biol. 2018, 13, 2560–2567. 10.1021/acschembio.8b00511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D. H.; Chen A. D.; Johnson C. S. An Improved Diffusion-Ordered Spectroscopy Experiment Incorporating Bipolar-Gradient Pulses. J. Magn. Reson., Ser. A 1995, 115, 260–264. 10.1006/jmra.1995.1176. [DOI] [Google Scholar]
- Yanaka S.; Yagi H.; Yogo R.; Yagi-Utsumi M.; Kato K. Stable isotope labeling approaches for NMR characterization of glycoproteins using eukaryotic expression systems. J. Biomol. NMR 2018, 71, 193–202. 10.1007/s10858-018-0169-2. [DOI] [PubMed] [Google Scholar]
- Martín-Pastor M.; Canales A.; Corzana F.; Asensio J. L.; Jiménez-Barbero J. Limited flexibility of lactose detected from residual dipolar couplings using molecular dynamics simulations and steric alignment methods. J. Am. Chem. Soc. 2005, 127, 3589–3595. 10.1021/ja043445m. [DOI] [PubMed] [Google Scholar]
- Kamiya Y.; Yanagi K.; Kitajima T.; Yamaguchi T.; Chiba Y.; Kato K. Application of Metabolic 13C Labeling in Conjunction with High-Field Nuclear Magnetic Resonance Spectroscopy for Comparative Conformational Analysis of High Mannose-Type Oligosaccharides. Biomolecules 2013, 3, 108–123. 10.3390/biom3010108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana C.; Kovacs H.; Widmalm G. NMR structure analysis of uniformly 13C-labeled carbohydrates. J. Biomol. NMR 2014, 59, 95–110. 10.1007/s10858-014-9830-6. [DOI] [PubMed] [Google Scholar]
- Canales A.; Angulo J.; Ojeda R.; Bruix M.; Fayos R.; Lozano R.; Giménez-Gallego G.; Martín-Lomas M.; Nieto P. M.; Jiménez-Barbero J. Conformational Flexibility of a Synthetic Glycosylaminoglycan Bound to a Fibroblast Growth Factor. FGF-1 Recognizes Both the 1C4 and 2SO Conformations of a Bioactive Heparin-like Hexasaccharide. J. Am. Chem. Soc. 2005, 127, 5778–5779. 10.1021/ja043363y. [DOI] [PubMed] [Google Scholar]
- Nestor G.; Anderson T.; Oscarson S.; Gronenborn A. M. Exploiting Uniformly 13C-Labeled Carbohydrates for Probing Carbohydrate-Protein Interactions by NMR Spectroscopy. J. Am. Chem. Soc. 2017, 139, 6210–6216. 10.1021/jacs.7b01929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Bewley C. A.; Kiyonaka S.; Hamachi I. Site-specific discrimination by cyanovirin-N for alpha-linked trisaccharides comprising the three arms of Man(8) and Man(9). J. Mol. Biol. 2002, 322, 881–889. 10.1016/S0022-2836(02)00842-2. [DOI] [PubMed] [Google Scholar]; b Shenoy S. R.; Barrientos L. G.; Ratner D. M.; O’Keefe B. R.; Seeberger P. H.; Gronenborn A. M.; Boyd M. R. Multisite and Multivalent Binding between Cyanovirin-N and Branched Oligomannosides: Calorimetric and NMR Characterization. Chem. Biol. 2002, 9, 1109–1118. 10.1016/S1074-5521(02)00237-5. [DOI] [PubMed] [Google Scholar]
- Angulo J.; Nieto P. M. STD-NMR: application to transient interactions between biomolecules-a quantitative approach. Eur. Biophys. J. 2011, 40, 1357–1369. 10.1007/s00249-011-0749-5. [DOI] [PubMed] [Google Scholar]
- Botos I.; O’Keefe B. R.; Shenoy S. R.; Cartner L. K.; Ratner D. M.; Seeberger P. H.; Boyd M. R.; Wlodawer A. Structures of the complexes of a potent anti-HIV protein cyanovirin-N and high mannose oligosaccharides. J. Biol. Chem. 2002, 277, 34336–34342. 10.1074/jbc.M205909200. [DOI] [PubMed] [Google Scholar]
- Nestor G.; Anderson T.; Oscarson S.; Gronenborn A. M. Direct Observation of Carbohydrate Hydroxyl Protons in Hydrogen Bonds with a Protein. J. Am. Chem. Soc. 2018, 140, 339–345. 10.1021/jacs.7b10595. [DOI] [PubMed] [Google Scholar]
- a Siebert H. C.; Andre S.; Vliegenthart J. F. G.; Gabius H. -J.; Minch M. J. Suitability of binary mixtures of water with aprotic solvents to turn hydroxyl protons of carbohydrate ligands into conformational sensors in NOE and transferred NOE experiments. J. Biomol. NMR 2003, 25, 197–215. 10.1023/A:1022898428465. [DOI] [PubMed] [Google Scholar]; b Battistel M. D.; Pendrill R.; Widmalm G.; Freedberg D. I. Direct evidence for hydrogen bonding in glycans: a combined NMR and molecular dynamics study. J. Phys. Chem. B 2013, 117, 4860–4869. 10.1021/jp400402b. [DOI] [PubMed] [Google Scholar]
- Islam M.; Shinde G. P.; Hotha S. Expedient synthesis of the heneicosasaccharyl mannose capped arabinomannan of the Mycobacterium tuberculosis cellular envelope by glycosyl carbonate donors. Chem. Sci. 2017, 8, 2033–2038. 10.1039/C6SC04866H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeberger P. H. The logic of automated glycan assembly. Acc. Chem. Res. 2015, 48, 1450–1463. 10.1021/ar5004362. [DOI] [PubMed] [Google Scholar]
- Delbianco M.; Kononov A.; Poveda A.; Yu Y.; Diercks T.; Jiménez-Barbero J.; Seeberger P. H. Well-Defined Oligo- and Polysaccharides as Ideal Probes for Structural Studies. J. Am. Chem. Soc. 2018, 140, 5421–5426. 10.1021/jacs.8b00254. [DOI] [PubMed] [Google Scholar]
- Zhang X.; Pagadala V.; Jester H. M.; Lim A. M.; Pham T. Q.; Goulas A. M. P.; Liu J.; Linhardt R. J. Chemoenzymatic synthesis of heparan sulfate and heparin oligosaccharides and NMR analysis: paving the way to a diverse library for glycobiologists. Chem. Sci. 2017, 8, 7932–7940. 10.1039/C7SC03541A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diercks T.; Infantino A. S.; Unione L.; Jiménez-Barbero J.; Oscarson S.; Gabius H.-J. Fluorinated Carbohydrates as Lectin Ligands: Synthesis of OH/F-Substituted N-Glycan Core Trimannoside and Epitope Mapping by 2D STD-TOCSYreF NMR spectroscopy. Chem. - Eur. J. 2018, 24, 15761–15765. 10.1002/chem.201803217. [DOI] [PubMed] [Google Scholar]
- Unione L.; Alcalá M.; Echeverria B.; Serna S.; Ardá A.; Franconetti A.; Cañada F. J.; Diercks T.; Reichardt N. C.; Jiménez-Barbero J. Fluoroacetamide Moieties as NMR Spectroscopy Probes for the Molecular Recognition of GlcNAc-Containing Sugars: Modulation of the CH−π Stacking Interactions by Different Fluorination Patterns. Chem. - Eur. J. 2017, 23, 3957–3965. 10.1002/chem.201605573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Dalvit C. Ligand- and substrate-based 19F NMR screening: Principles and applications to drug discovery. Prog. Nucl. Magn. Reson. Spectrosc. 2007, 51, 243–271. 10.1016/j.pnmrs.2007.07.002. [DOI] [Google Scholar]; b de Las Rivas M.; Coelho H.; Diniz A.; Lira-Navarrete E.; Compañón I.; Jiménez-Barbero J.; Schjoldager K. T.; Bennett E. P.; Vakhrushev S. Y.; Clausen H.; Corzana F.; Marcelo F.; Hurtado-Guerrero R. Structural Analysis of a GalNAc-T2 Mutant Reveals an Induced-Fit Catalytic Mechanism for GalNAc-Ts. Chem. - Eur. J. 2018, 24, 8382–8392. 10.1002/chem.201800701. [DOI] [PubMed] [Google Scholar]; c Martínez J. D.; Valverde P.; Delgado S.; Romanò C.; Linclau B.; Reichardt N. C.; Oscarson S.; Ardá A.; Jiménez-Barbero J.; Cañada F. J. Unraveling Sugar Binding Modes to DC-SIGN by Employing Fluorinated Carbohydrates. Molecules 2019, 24, 2337 10.3390/molecules24122337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wamhoff E.; Hanske J.; Schnirch L.; Aretz J.; Grube M.; Silva D. V.; Rademacher C. 19)F NMR-Guided Design of Glycomimetic Langerin Ligands. ACS Chem. Biol. 2016, 11, 2407–2413. 10.1021/acschembio.6b00561. [DOI] [PubMed] [Google Scholar]
- a Inomata K.; Ohno A.; Tochio H.; Isogai S.; Tenno T.; Nakase I.; Takeuchi T.; Futaki S.; Ito Y.; Hiroaki H.; Shirakawa M. High-resolution multi-dimensional NMR spectroscopy of proteins in human cells. Nature 2009, 458, 106–109. 10.1038/nature07839. [DOI] [PubMed] [Google Scholar]; b Theillet F. X.; Binolfi A.; Bekei B.; Martorana A.; Rose H. M.; Stuiver M.; Verzini S.; Lorenz D.; van Rossum M.; Goldfara D.; Selenko P. Structural disorder of monomeric α-synuclein persists in mammalian cells. Nature 2016, 530, 45–50. 10.1038/nature16531. [DOI] [PubMed] [Google Scholar]
- a Fiege B.; Leuthold M.; Parra F.; Dalton K. P.; Meloncelli P. J.; Lowary T. L.; Peters T. Epitope mapping of histo blood group antigens bound to norovirus VLPs using STD NMR experiments reveals fine details of molecular recognition. Glycoconjugate J. 2017, 34, 679–689. 10.1007/s10719-017-9792-5. [DOI] [PubMed] [Google Scholar]; b Mallagaray A.; Creutznacher R.; Dülfer J.; Mayer P. H. O.; Grimm L. L.; Orduña J. M.; Trabjerg E.; Stehle T.; Rand K. D.; Blaum B. S.; Uetrecht C.; Peters T. A post-translational modification of human Norovirus capsid protein attenuates glycan binding. Nat. Commun. 2019, 10, 1320 10.1038/s41467-019-09251-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Qin S.; Zhou H. X. Protein folding, binding, and droplet formation in cell-like conditions. Curr. Opin. Struct. Biol. 2017, 43, 28–37. 10.1016/j.sbi.2016.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Sarkar M.; Smith A. E.; Pielak G. J. Impact of reconstituted cytosol on protein stability. Proc. Natl. Acad. Sci. U.S.A. 2013, 110, 19342–19347. 10.1073/pnas.1312678110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Smith A. E.; Zhou L. Z.; Gorensek A. H.; Senske M.; Pielak G. J. In-cell thermodynamics and a new role for protein surfaces. Proc. Natl. Acad. Sci. U S A. 2016, 113, 1725–1730. 10.1073/pnas.1518620113. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Monteith W. B.; Cohen R. D.; Smith A. E.; Guzman-Cisneros E.; Pielak G. J. Quinary structure modulates protein stability in cells. Proc. Natl. Acad. Sci. U. S. A. 2015, 112, 1739–1742. 10.1073/pnas.1417415112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanaka S.; Yamazaki T.; Yogo R.; Noda M.; Uchiyama S.; Yagi H.; Kato K. NMR Detection of Semi-Specific Antibody Interactions in Serum Environments. Molecules 2017, 22, 1619–1626. 10.3390/molecules22101619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psychogios N.; Hau D. D.; Peng J.; Guo A. C.; Mandal R.; Bouatra S.; et al. The human serum metabolome. PLoS One 2011, 6, e16957 10.1371/journal.pone.0016957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diniz A.; Dias J. S.; Jiménez-Barbero J.; Marcelo F.; Cabrita E. J. Protein-Glycan Quinary Interactions in Crowding Environment Unveiled by NMR Spectroscopy. Chem. - Eur. J. 2017, 23, 13213–13220. 10.1002/chem.201702800. [DOI] [PubMed] [Google Scholar]
- Rahkila J.; Ekholm F. S.; Ardá A.; Delgado S.; Savolainen J.; Jiménez-Barbero J.; Leino R. Novel Dextran-Supported Biological Probes Decorated with Disaccharide Entities for Investigating the Carbohydrate-Protein Interactions of Gal-3. ChemBioChem 2019, 20, 203–209. 10.1002/cbic.201800423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton D. A.Structural Biology. In NMR Crystallography; Harris R. K., Wasylishen R. E., Duer M. J., Eds.; John Wiley & Sons, 2009; Chapter 27, pp 417–434. [Google Scholar]
- Kern T.; Hediger S.; Müller P.; Cécile G.; Joris B.; Bougault C.; Vollmer W.; Simorre J. P. Toward the characterization of peptidoglycan structure and protein-peptidoglycan interactions by solid state NMR spectroscopy. J. Am. Chem. Soc. 2008, 130, 5618–5619. 10.1021/ja7108135. [DOI] [PubMed] [Google Scholar]
- Boudet J.; Chuuquet A.; Chahboune A.; Giustini C.; Joris B.; Simorre J. P.; Bougault C. 1H, 13C and 15N resonance assignments of YajG, an Escherichia coli protein of unknown structure and function. Biomol. NMR Assignments 2007, 1, 89–91. 10.1007/s12104-007-9025-0. [DOI] [PubMed] [Google Scholar]
- Renault M.; Tommassen-van Boxtel R.; Bos M. P.; Post J. A.; Tommassen J.; Baldus M. Cellular solid state nuclear magnetic resonance spectroscopy. Proc. Natl Acad. Sci. U.S.A. 2012, 109, 4863–4868. 10.1073/pnas.1116478109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raetz C. R.; Reynolds C. M.; Trent M. S.; Bishop R. E. Lipid A modification systems in gram-negative bacteria. Annu. Rev. Biochem. 2007, 76, 295–329. 10.1146/annurev.biochem.76.010307.145803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mares J.; Kumaran S.; Gobbo M.; Zerbe O. Interactions of lipopolysaccharide and polymyxin studied by NMR spectroscopy. J. Biol. Chem. 2009, 284, 11498–11506. 10.1074/jbc.M806587200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson C. L.; Ridley H.; Marchetti R.; Silipo A.; Griffin D. C.; Crawford L.; Bonev B.; Molinaro A.; Lakey J. H. The antibacterial toxin colin N binds to the inner core of lipopolysaccharade and close to its translocator protein. Mol. Microbiol. 2014, 92, 440–452. 10.1111/mmi.12568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schanda P.; Triboulet S.; Lugari C.; Bougault C. M.; Ayala I.; Callon M.; Arthur M.; Simorre J. P. Atomic model of a cell-wall cross-linking enzyme in complex with an intact bacterial peptidoglycan. J. Am. Chem. Soc. 2014, 136, 17852–17860. 10.1021/ja5105987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laguri C.; Silipo A.; Martorana A. M.; Schanda P.; Marchetti R.; Polissi A.; Molinaro A.; Simorra J. P. Solid State NMR studies of intact lipopolysaccharide endotoxin. ACS Chem. Biol. 2018, 13, 2106–2113. 10.1021/acschembio.8b00271. [DOI] [PubMed] [Google Scholar]
- Knirel Y. A.; Bystrova O. V.; Kocharova N. A.; Zähringer U.; Pier G. B. Conserved and variable structural features in the lipopolysaccharide of Pseudomonas aeruginosa. J. Endotoxin Res. 2006, 12, 324–336. 10.1179/096805106X118906. [DOI] [PubMed] [Google Scholar]
- Di Lorenzo F.; Silipo A.; Bianconi I.; Lore’ N. I.; Scamporrino A.; Sturiale L.; Garozzo D.; Lanzetta R.; Parrilli M.; Bragonzi A.; Molinaro A. Persistent cystic fibrosis isolate Pseudomonas aeruginosa strain RP73 exhibits an under-acylated LPS structure responsible of its low inflammatory activity. Mol. Immunol. 2015, 63, 166–175. 10.1016/j.molimm.2014.04.004. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Navarro-Vázquez A.; Gil R. R.; Griesinger C.; Martin G. E.; Williamson R. T. Application of anisotropic NMR parameters to the confirmation of molecular structure. Nat. Protoc. 2019, 14, 217–247. 10.1038/s41596-018-0091-9. [DOI] [PubMed] [Google Scholar]
- Zhang Q.; Gimeno A.; Santana D.; Wang Z.; Valdés-Balbin Y.; Rodríguez-Noda L.; Hansen T.; Kong L.; Shen M.; Overkleeft H. S.; Vérez-Bencomo V.; van der Marel G.; Jiménez-Barbero J.; Chiodo F.; Codée J. D. C. Synthetic, Zwitterionic Sp1 Oligosaccharides Adopt a Helical Structure Crucial for Antibody Interaction. ACS Cent. Sci. 2019, 454 10.1021/acscentsci.9b00454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.; Sun J.; Liu L. N.; Flies D. B.; Nie X.; Toki M.; Zhang J.; Song C.; Zarr M.; Zhou X.; Han X.; Archer K. A.; O’Neill T.; Herbst R. S.; Boto A. N.; Sanmamed M. F.; Langermann S.; Rimm D. L.; Chen L. Siglec-15 as an immune suppressor and potential target for normalization cancer immunotherapy. Nat. Med. 2019, 25, 656–666. 10.1038/s41591-019-0374-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unione L.; Lenza M. P.; Ardá A.; Urquiza P.; Laín A.; Falcón-Pérez J. M.; Jiménez-Barbero J.; Millet O. Glycoprofile Analysis of an Intact Glycoprotein As Inferred by NMR Spectroscopy. ACS Central Sci. 2019, 540 10.1021/acscentsci.9b00540. [DOI] [PMC free article] [PubMed] [Google Scholar]