Abstract
We aimed to evaluate the efficacy and safety of antithrombin (AT) supplementation and concomitant anticoagulation therapy in 65 children who met the Japanese Ministry of Health and Welfare (JMHW) disseminated intravascular coagulation (DIC) criteria and had received AT concentrate and/or other concomitant anticoagulants. The primary efficacy end point was to determine standardized mortality ratio (SMR). The secondary efficacy end points were DIC resolution rate and pediatric sequential organ failure assessment (pSOFA) score on day 3. The 28-day mortality rate was 6.8%; SMR was 0.55. Disseminated intravascular coagulation resolution rate on day 3 was 54.5%. The JMHW DIC scores at day 0 (P = .005) and pSOFA scores at day 3 (P = .018) were significantly lower in patients with resolution of DIC than in those without resolution of DIC. The target cutoff value for JMHW DIC score on day 0 was 6. No bleeding-related adverse events were associated with AT administration. In children with DIC, AT supplementation and concomitant anticoagulation therapy can be safely used as initial treatment when JMHW DIC score is 6; it may improve DIC resolution, organ failure, and mortality rates.
Keywords: antithrombin, children, disseminated intravascular coagulation, JAAM DIC criteria, JMHW DIC criteria
Background
Disseminated intravascular coagulation (DIC) is characterized by systemic activation of blood coagulation, which results in an inadequate supply of blood to various organs,1,2 which may in turn lead to multiple organ dysfunction syndrome (MODS), resulting in poor outcomes.3 A previous report suggested that rapid diagnosis and early treatment of DIC may improve outcomes for these patients.4 The Japanese Association for Acute Medicine (JAAM) DIC criteria were established to facilitate early diagnosis of DIC,5 but in developing the criteria, data were collected from only adults. It remains to be determined whether the JAAM DIC criteria may safely be applied to children. In contrast, the Japanese Ministry of Health and Welfare (JMHW) DIC criteria6 have previously been used for children. However, investigations in the fields of emergency medicine and surgery have shown that diagnoses based on JMHW criteria are often too late.5
Antithrombin (AT) is a key inhibitor of the coagulation system and is estimated to account for 80% of inhibitory activity against thrombin.7 It was recently revealed that AT has a protective effect against the endothelium and capillary leakage.8 Therefore, AT is potentially effective for DIC resolution; conversely, decreased levels of AT are associated with an increase in mortality.9 Several studies conducted in Japan have demonstrated the effectiveness of AT supplementation, which is not associated with an increase in risk of bleeding among patients with DIC with sepsis.10–12
A recent meta-analysis reported beneficial effects of anticoagulant therapy in patients with sepsis-induced DIC.13 Antithrombin concentrate and recombinant human thrombomodulin (rhTM) are recommended agents that are commonly used as anticoagulants in the treatment of sepsis-induced DIC14,15; however, concomitant therapy provided no additional benefit in terms of survival.16
To date, almost all studies performed to investigate anticoagulant therapy have been performed in adult populations, and few studies have examined the issue in pediatric populations.17–20 The clinical benefits of AT and concomitant anticoagulant therapy, as well as the pharmacokinetics of AT, remain to be investigated in a pediatric population.
The objectives of this study were to evaluate the efficacy and safety of AT supplementation and/or concomitant anticoagulant therapy and to establish the target AT activity value after administration of AT concentrate in children.
Methods
This study was conducted in accordance with the Ministry of Health, Labor and Welfare guidelines established for postmarketing surveillance. Multicenter and postmarketing survey data collected by the Japanese Blood Products Organization during the period from 2013 to 2016 were used for analysis. All patients were treated according to the discretion of the attending physician, and there was no limit to the combination of drugs. Personal data were rendered anonymous at the time of data collection. Therefore, although it was not necessary for this surveillance to be approved by the ethics committee or for informed consent to be obtained, the consent of the patient’s family was secured when required by the ethics committee of a particular facility.
Antithrombin concentrate (Nauert; Japan Blood Products Organization, Tokyo, Japan) was administered when patients met JMHW DIC criteria (DIC score ≥ 6) and AT activity was ≤70%. Exclusion criteria were hypersensitivity to AT, history of leukemia, malignant tumor, liver cirrhosis, and history of resuscitation after cardiopulmonary arrest. Antithrombin concentrate was administered by intravenous infusion once a day. Although AT concentrate was always injected after diagnosis of DIC, because the initiation of AT treatment was not standardized, each physician was able to freely determine the timing of AT injections. There was no limitation in terms of the duration of AT administration, and each physician used clinical judgment to determine when to discontinue treatment with AT. The dose of AT concentrate was individualized for each patient, with the goal of achieving AT activity levels between 80% and 120%21 (AT supplementation). There was also no limitation with regard to the use of heparin derivatives (unfractionated heparin [UFH] and low-molecular-weight heparin [LMWH]), protease inhibitors (nafamostat mesylate [NM]), rhTM, or other anticoagulants (including blood products such as fresh frozen plasma and platelet concentrate [PC]).
Disseminated intravascular coagulation was evaluated using the JMHW DIC score (DIC score ≥ 6). According to the JMHW DIC criteria, the cutoff value for DIC scores for the diagnosis of DIC is 7 points, and a score of 6 suggests suspected DIC. In this study, we set the cutoff value at 6 to facilitate early detection of DIC. Organ dysfunction was assessed using the pediatric sequential organ failure assessment (pSOFA) score.22 The survival rate was calculated based on the number of patients who survived 28 days after the first administration of AT. At the onset of AT administration, the following information was collected: age, gender, presence or absence of infection, bleeding, platelet count (PLT), AT activity, prothrombin time–international normalized ratio (PT-INR), fibrinogen plasma levels (FBG), and fibrin/fibrinogen degradation products (FDP).
The hemostatic system in children differs significantly from that of adults. Normal adult levels of AT are achieved at about 6 months of age.23 Physiologically, AT activity is 63% of normal adult levels by first day after birth, 78% at 1 month of age, 97% at 3 months of age, 104% at 6 months of age, and 105% in adults.23 The influence of the age could be eliminated by expressing measured AT value as a percentage of normal AT value, and the percentage AT activity compared to normal (corrected AT [c-AT] activity) was evaluated. Laboratory and coagulation tests (the JMHW DIC score, the JAAM DIC score, pSOFA score, and AT activity) were performed at 2 time points: before administration of AT (day 0) and 3 days after AT was first administered (day 3). Antithrombin activity was measured daily until 3 days after AT administration.
Discrimination Capacity of JMHW DIC Scores for the JAAM DIC Criteria
The JAAM DIC algorithm may be used to diagnose DIC early at a high diagnostic rate. In this study, DIC was diagnosed with the JMHW DIC scoring system rather than the JAAM DIC scoring system. The patients were divided into 2 groups: patients who met the JAAM DIC criteria (JAAM DIC score >4) and those who did not meet the criteria both on day 0 and day 3. Receiver operating characteristic curve analysis was performed to evaluate the cutoff level of the JMHW DIC score to predict patients meeting the JAAM DIC criteria.
Efficacy End Point
The primary efficacy end point was to calculate standardized mortality ratio (SMR) as the ratio of observed deaths to expected deaths. Estimations of the expected number of deaths were based on pSOFA score22; death occurred in about 70% of patients with scores of 21 to 24, 50% of those with scores of 17 to 20, 30% of those with scores of 13 to 16, 8% of those with scores of 9 to 12, 2% of those with scores of 5 to 8, and 0% of those with scores of 0 to 4.
The secondary efficacy end point was rate of DIC resolution and pSOFA score at day 3. Disseminated intravascular coagulation resolution was defined as a JMHW DIC score <6. The effectiveness of administration of AT alone (AT group) was compared to that of AT plus rhTM (AT + rhTM group) and AT plus UFH/LMWH (AT + H group). Subgroup analysis was performed to evaluate changes in clinical and laboratory findings.
Safety Assessment
Safety data were coded using preferred terms from version 14.1 of the Japanese version of the Medical Dictionary for Regulatory Activities.24 Adverse events (AEs) and adverse drug reactions (ADRs) were classified according to International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) guidelines.25 The safety assessment included questions pertaining to bleeding AEs and all ADRs observed up to 28 days after administration of AT.
Statistical Analysis
In the descriptive analysis of baseline characteristics, numerical data are expressed as median (Q1-Q3; interquartile ranges). Nominal variables were compared between groups with Fisher exact test. Continuous variables were compared with the Mann-Whitney U test. The Kruskal-Wallis test was used for comparison of 3 continuous variables. Multiple comparisons were evaluated using the Steel-Dwass post hoc test. The correlation was examined with Spearman correlation coefficient test. Receiver operating curve analysis, including the area under the curve (AUC), was used to compare cutoff values of the JMHW/JAAM DIC score and c-AT activity. The results of the analysis were considered significant when P < .05. Statistical tests were performed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient Demographics and Characteristics
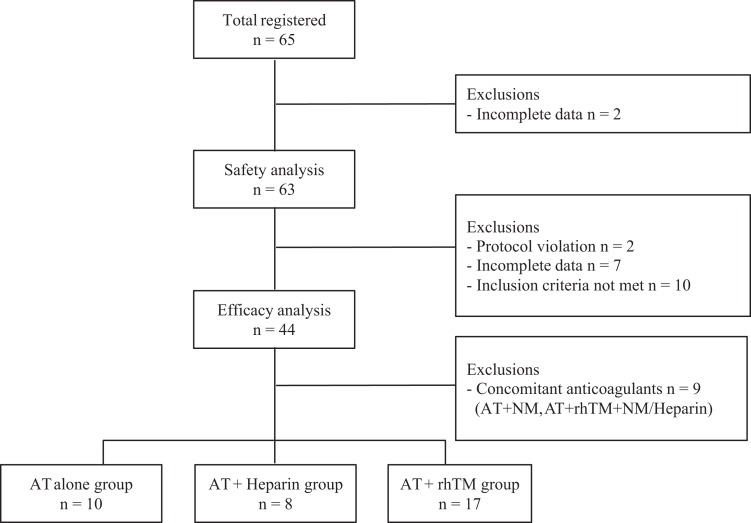
A total of 65 patients were included in the study. Two patients were excluded from the safety analysis due to lack of safety data. Furthermore, 19 patients were excluded from the efficacy analysis: 2 due to protocol violation, 7 due to incomplete data, and 10 due to failure to meet inclusion criteria. Finally, 63 patients were included in the safety analysis and 44 in the efficacy analysis (Figure 1). Of the 44 patients, 16 (36.4%) were female; median age for the entire study population was 1.0 year (0.2-4.0 years). Infections were found in 24 (54.5%) patients, and the focus of infection was commonly pulmonary (n = 10, 41.7%). Other anticoagulants, rhTM, UFH, LMWH, and NM, were given in 24 (54.5%), 8 (18.2%), 4 (9.1%), and 5 (11.4%) patients, respectively. Fresh frozen plasma and PC were administered in 28 (63.6%) and 23 (52.3%), respectively. The number of survivors at day 28 was 41 (93.2%).
Figure 1.
Flowchart of patients. AT indicates antithrombin; NM, nafamostat mesylate; rhTM, recombinant human thrombomodulin.
At the time when AT was started (on day 0), median PLT was 79 000/μL (40 000-117 000), median PT-INR was 1.81 (1.41-2.29), median FBG was 180 mg/dL (118-298), and median FDP was 26.5 μg/mL (11.0-91.6); furthermore the JMHW DIC score was 7.5 (6.0-9.0) and the JAAM DIC score was 5.0 (4.0-6.0). The pSOFA score was 10 (8.0-13.0). The number of expected deaths was 5.5; the number of observed deaths was 3. The SMR was 0.55, which was less than 1.0, but not significant (95% confidence interval [CI]: −0.06 to 1.17). Rate of DIC resolution at day 3 was 54.5%. The median total dose of AT concentrate was 85.3 U/kg (53.7-120 U/kg). The median single dose of AT concentrate was 30 U/kg (30-50 U/kg). The median duration of AT administration was 3.0 days (1.0-4.0 days). With regard to the timing of AT administration, 84.1% (n = 37) of patients were treated with AT on the same day that they were diagnosed according to JMHW DIC score; 18.2% (n = 8) were started 1 day after being diagnosed with DIC.
Discrimination Capacity of JMHW DIC Scores for the JAAM DIC Criteria
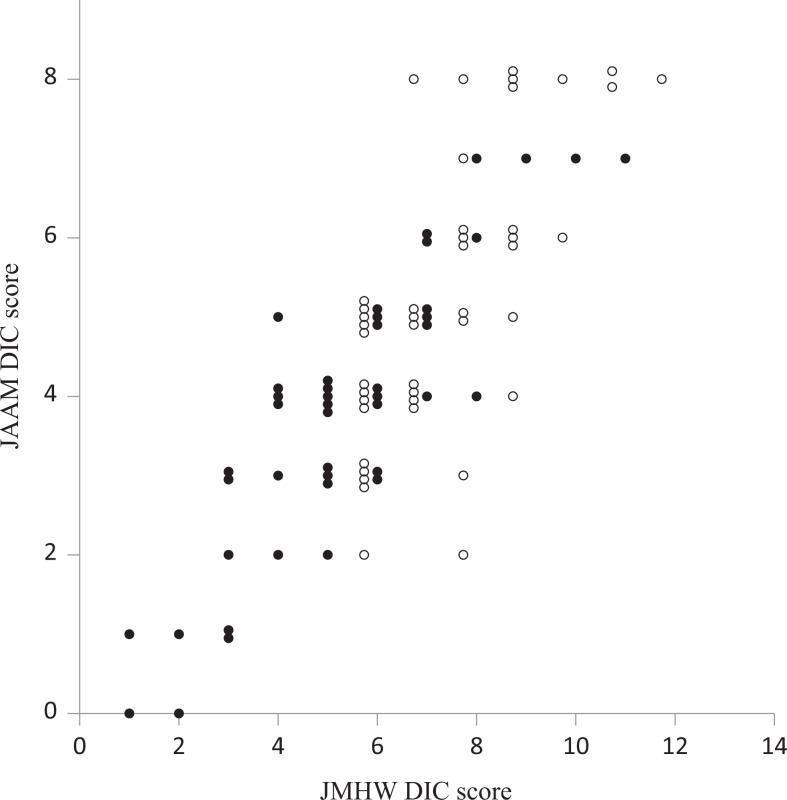
The correlation between JMHW DIC scores and JAAM DIC scores is shown in Figure 2. Both JAAM score and JMHW score showed a downward trend on day 3 (closed circle) compared to day 0 (open circle). There was a generally linear relationship between JMHW DIC scores and JAAM DIC scores; however, the same JAAM DIC score had several JMHW DIC scores (ie, for JAAM score 4, six different JMHW scores were obtained ranging from 4 to 9). Receiver operating characteristic curve analysis showed that the cutoff level of JMHW DIC score for discrimination of the JAAM DIC was 6 (sensitivity 0.725, specificity 0.757, P < .001), with an AUC of 0.822 (95% CI: 0.739-0.906).
Figure 2.
Correlation between Japanese Ministry of Health and Welfare (JMHW) disseminated intravascular coagulation (DIC) score and Japanese Association for Acute Medicine (JAAM) DIC score. Both JMHW DIC and JAAM DIC scores on day 0 (open circle) and day 3 (closed circle) were plotted for 44 patients.
Efficacy End Point
There was no significant difference in the demographics and laboratory findings between patients with and without infection (data not shown). The median JMHW DIC and pSOFA scores at day 0 among patients with infection were 7.5 (6.0-8.0) and 10 (8-12), respectively, and among patients without infection were 7.0 (6.8-9.0) and 11 (10-13), respectively. Patients with infection had a mortality rate of 4.2% (1/24) and tended to have high JMHW DIC and pSOFA scores at day 3 (P = .398 and 0.176, respectively). The SMRs of both groups were not significantly less than 1.0 (with infection group 0.35 vs without infection group 0.76). Table 1 shows the demographics and clinical characteristics of patients <6 months versus >6 months of age. The JMHW DIC score at day 3 was significantly lower in the <6 months of age-group, compared with the >6 months of age-group (P = .030). Antithrombin activity at day 0 was significantly lower in the <6 months of age-group (P = .005), but there was no significant difference in c-AT activity at day 0 (P = .980). Antithrombin activity at day 3 was lower in the <6 months of age-group, compared to the >6 months of age-group; however, c-AT activity at day 3 was higher in the <6 months of age-group, compared to the >6 months of age-group (P = .067, 0.108, respectively). Total dose of AT concentrate was significantly higher in the <6 months group (P = .039). Table 2 shows the patient demographics and laboratory findings compared to the AT group, AT + H group, and AT + rhTM group. There were no significant differences in baseline demographics or laboratory findings among groups. Table 3 shows clinical and demographic characteristics of the patients with or without resolution of DIC at day 3. The patients with resolution of DIC had significantly lower JMHW DIC scores at day 0 (P = .005) and day 3 (P < .001) and pSOFA (P = .018) at day 3, as well as lower SMR than those without resolution of DIC. Although there was no significant difference in total dose of AT concentrate, patients with resolution of DIC tended to have higher levels of c-AT activity at day 3 (P = .076), compared to those without resolution of DIC. Receiver operating curve analysis showed that the target cutoff level of JMHW DIC score on day 0 was 7 (sensitivity 0.708, specificity 0.750, P = .005), with AUC of 0.743 (95% CI: 0.595-0.890). Receiver operating characteristic curve analysis showed that the target cutoff level for JAAM DIC score on day 0 was 4 (sensitivity: 0.583, specificity: 0.900, P = .005), with AUC of 0.747 (95% CI: 0.594-0.900). The target cutoff level of c-AT activity at day 3 was 90.0% (sensitivity 57.1%, specificity 77.8%), with AUC of 0.667 (95% CI: 0.487-0.846, P = .078).
Table 1.
Patient Characteristics and Variables Between Age Groups.a
| Variables | <6 Months of Age (n = 14) | ≥6 Months of Age (n = 30) | P Value |
|---|---|---|---|
| Patient characteristics | |||
| Age, years | 0.1 (0.1-0.2) | 2.5 (1.0-6.8) | <.001 |
| Gender, male/female | 8/6 | 20/10 | .738 |
| Infection | 6 (42.9%) | 18 (60.0%) | .342 |
| Blood products using | |||
| FFP | 9 (64.3%) | 19 (63.3%) | 1.000 |
| PC | 8 (57.1%) | 15 (50.0%) | .752 |
| Bleeding AEs | 0 (0.00%) | 2 (6.7%) | 1.000 |
| Survivors at day 28 | 13 (92.9%) | 28 (93.3%) | 1.000 |
| Standardized mortality ratio | 0.39 (−0.38 to 1.17) | 0.69 (−0.26 to 1.63) | – |
| Severity | |||
| JMHW DIC score at day 0 | 8.0 (7.0 to 8.0) | 7.0 (6.0 to 9.0) | .560 |
| JMHW DIC score at day 3 | 4.0 (3.3 to 5.8) | 6.0 (5.0 to 7.0) | .030 |
| DIC resolution at day 3 | 10 (71.4%) | 14 (46.7%) | .195 |
| pSOFA score at day 0 | 10.0 (10.0 to 14.0) | 10.0 (6.3 to 12.0) | .093 |
| pSOFA score at day 3 | 10.0 (8.0 to 13.0) | 9.0 (7.0 to 12.0) | .393 |
| AT administration | |||
| AT activity at day 0, % | 35.5 (28.3 to 40.6) | 45.5 (38.3 to 58.8) | .005 |
| AT activity at day 3, % | 69.0 (61.0 to 78.0) | 83.0 (71.3 to 96.8) | .067 |
| c-AT activity day 0, % | 46.8 (35.0 to 59.5) | 43.3 (36.5 to 56.0) | .980 |
| c-AT activity day 3, % | 92.3 (78.2 to 110) | 79.1 (67.9 to 92.2) | .108 |
| Total dose, U/kg | 90.0 (60.0 to 124) | 60.0 (32.0 to 86.5) | .039 |
| Duration, days | 2.0 (1.3 to 3.0) | 2.0 (1.0 to 2.0) | .302 |
Abbreviations: AE, adverse event; AT, antithrombin; DIC, disseminated intravascular coagulation; FFP, fresh frozen plasma; JMHW, Japanese Ministry of Health and Welfare; PC, platelet concentrate; pSOFA, pediatric sequential organ failure assessment.
aData for continuous variables are expressed as medians and interquartile ranges and analyzed using the Mann-Whitney U test. Data for categorical variables are expressed as count or percentage and analyzed using Fisher exact test.
Table 2.
Patient Characteristics and Anticoagulant Therapy.a
| Variables | AT, n = 10 | AT + H, n = 8 | AT + rhTM, n = 17 | P Value |
|---|---|---|---|---|
| Patient characteristics | ||||
| Age, years | 0.9 (0.2-1.0) | 1.1 (0.2-3.3) | 2.0 (0.6-6.0) | .427 |
| Gender, male/female | 5/5 | 6/2 | 11/6 | .603 |
| Infection | 6 (60.0%) | 4 (50.0%) | 11 (64.7%) | .510 |
| Blood products using | ||||
| FFP | 6 (60.0%) | 7 (87.5%) | 8 (47.1%) | .172 |
| PC | 3 (30.0%) | 5 (62.5%) | 8 (47.1%) | .380 |
| Bleeding AEs | 1 (10.0%) | 1 (12.5%) | 0 (0.0%) | .257 |
| Survivors at day 28 | 10 (100%) | 8 (100%) | 16 (94.1%) | 1.000 |
| Standardized mortality ratio | 0 | 0 | 0.55 (−0.53 to 1.62) | – |
| Severity | ||||
| JMHW DIC score at day 0 | 7.5 (7.0 to 8.8) | 7.0 (6.0 to 8.8) | 8.0 (6.0 to 9.0) | .847 |
| JMHW DIC score at day 3 | 5.0 (3.3 to 6.0) | 5.5 (3.0 to 7.0) | 5.0 (4.0 to 6.0) | .857 |
| DIC resolution at day 3 | 6 (60.0%) | 4 (50.0%) | 9 (52.9%) | 1.000 |
| pSOFA score at day 0 | 10.0 (7.5 to 12.3) | 10.0 (8.0 to 12.0) | 10.0 (7.5 to 12.0) | .982 |
| pSOFA score at day3 | 8.5 (7.0 to 9.0) | 9.0 (5.5 to 11.0) | 10.0 (7.0 to 11.5) | .804 |
| AT administration | ||||
| AT activity at day 0, % | 36.5 (34.3 to 52.0) | 42.8 (33.3 to 54.0) | 41.0 (39.0 to 53.0) | .496 |
| AT activity at day 3, % | 74.0 (57.0 to 92.6) | 75.0 (62.1 to 87.5) | 76.5 (63.0 to 88.5) | .832 |
| c-AT activity day 0, % | 40.1 (25.7 to 63.9) | 44.8 (31.4 to 80.8) | 45.7 (22.9 to 75.6) | .558 |
| c-AT activity day 3, % | 81.2 (54.3 to 132) | 80.1 (45.0 to 176) | 80.5 (58.9 to 149) | .941 |
| Total dose, U/kg | 69.0 (30.0 to 90.0) | 51.0 (30.0 to 159) | 60.0 (16.0 to 150) | .947 |
| Duration, days | 2.0 (1.0 to 3.0) | 1.5 (1.0 to 3.0) | 2.0 (1.0 to 3.0) | .786 |
Abbreviations: AE, adverse event; AT, antithrombin; c-AT, corrected antithrombin; DIC, disseminated intravascular coagulation; FFP, fresh frozen plasma; JMHW, Japanese Ministry of Health and Welfare; PC, platelet concentrate; pSOFA, pediatric sequential organ failure assessment; rhTM, recombinant human thrombomodulin.
aData for continuous variables are expressed as medians and interquartile ranges. Results were analyzed using the Kruskal-Wallis test. Multiple comparisons were performed using the Steel-Dwass post hoc test. Data for categorical variables are expressed as count or percentage. Results were analyzed using Fisher exact test.
Table 3.
Patient Characteristics and Variables in the Resolution of DIC.a
| Variables | DIC Resolution (JMHW DIC Score <6 at Day 3), n = 24 | DIC Nonresolution (JMHW DIC Score ≥6 at Day 3), n = 20 | P Value |
|---|---|---|---|
| Patient characteristics | |||
| Age, years | 0.9 (0.1-4.3) | 1.5 (0.7 to 3.3) | .585 |
| Gender, male/female | 17/7 | 11/9 | .352 |
| Infection | 11 (45.8%) | 13 (65.0%) | .238 |
| Blood products using | |||
| FFP | 14 (58.3%) | 14 (70.0%) | .534 |
| PC | 10 (41.7%) | 13 (65.0%) | .143 |
| Bleeding AEs | 0 (0.00%) | 2 (10.0%) | .201 |
| Survivors at day 28 | 23 (95.8%) | 18 (90.0%) | .583 |
| Standardized mortality ratio | 0.35 (−0.34 to 1.04) | 0.76 (−0.29 to 1.82) | – |
| Severity | |||
| JMHW DIC score at day 0 | 7.0 (6.0 to 8.0) | 8.0 (7.8 to 9.0) | .005 |
| JMHW DIC score at day 3 | 4.0 (3.0 to 5.0) | 7.0 (6.0 to 8.0) | <.001 |
| pSOFA score at day 0 | 10.0 (6.5 to 12.0) | 12.0 (9.0 to 13.0) | .385 |
| pSOFA score at day 3 | 7.5 (5.3 to 10.8) | 11.0 (9.0 to 12.0) | .018 |
| AT administration | |||
| AT activity at day 0, % | 40.3 (35.8 to 51.5) | 42.8 (36.0 to 59.8) | .443 |
| AT activity at day 3, % | 78.0 (69.0 to 97.0) | 74.0 (63.1 to 91.0) | .382 |
| c-AT activity day 0, % | 46.3 (36.9 to 56.3) | 43.3 (35.0 to 59.5) | .897 |
| c-AT activity day 3, % | 92.0 (74.3 to 110) | 74.8 (65.1 to 89.7) | .076 |
| Total dose, U/kg | 60.0 (39.0 to 89.0) | 71.0 (51.0 to 104) | .400 |
| Per dose, U/kg | 30.0 (30.0 to 47.0) | 32.0 (30.0 to 50.0) | .563 |
| Duration, days | 2.0 (1.0 to 2.0) | 2.0 (1.0 to 3.0) | .224 |
Abbreviations: AE, adverse event; AT, antithrombin; c-AT, corrected antithrombin; DIC, disseminated intravascular coagulation; FFP, fresh frozen plasma; JMHW, Japanese Ministry of Health and Welfare; PC, platelet concentrate; pSOFA, pediatric sequential organ failure assessment.
aData for continuous variables are expressed as medians and interquartile ranges. Results were analyzed using the Mann-Whitney U test. Data for categorical variables are expressed as count or percentage. Results were analyzed using Fisher exact test.
Safety Assessment
Adverse events were identified in 11 (17.5%) patients; no ADRs were observed. Severe AEs observed during the course of the study included cardiac failure, respiratory distress, pneumothorax, brain edema, sepsis, and acute liver failure. None of these serious AEs was considered related to treatment. Bleeding AEs occurred in 2 patients. These AEs were pulmonary and intracranial. These bleeding AEs were considered not to be associated with treatment.
Discussion
The purpose of the present study was to evaluate the efficacy and safety of AT supplementation and concomitant anticoagulant therapy and to evaluate target AT activity after administration of AT concentrate in children. The results showed that AT supplementation and concomitant anticoagulation therapy are safe for use as early treatment for DIC in the pediatric population (when JMHW DIC score is 6 or JAAM DIC score is 4). Antithrombin supplementation and concomitant anticoagulation therapy may accelerate the resolution of DIC and MODS, leading to improved 28-day survival. Increasing AT activity to 90.0% of normal 3 days after administration of AT concentrate may improve recovery from DIC in pediatric patients.
In the present study, DIC was diagnosed with the JMHW DIC scoring system rather than the JAAM DIC scoring system, because age <15 years was one of the exclusion criteria in data collection for development of the JAAM DIC criteria and it remains to be determined whether the JAAM DIC criteria can be safely applied to children. Recently, it has been revealed that rapid diagnosis using the JAAM criteria (JAAM DIC score ≥4) and early treatment of DIC improve outcomes4 and diagnoses based on JMHW criteria (JMHW DIC score ≥7) are often too late.5 In our study, as patients with DIC diagnosed by the JAAM DIC criteria could be detected using a JMHW DIC score of 6 as a cutoff value, it was considered that the JAAM DIC score of 4 corresponded to the JMHW DIC score of 6.
Infection is associated with coagulation disorders, resulting in DIC. Disseminated intravascular coagulation contributes to MODS and is associated with high mortality.26 Matics and Sanchez-Pinto showed that pSOFA scores have strong discrimination for predicting in-hospital mortality in a pediatric intensive care unit, as well as in the subgroup of patients with infection.22 The median pSOFA score for patients with sepsis was 8 (5-12); this group had a mortality rate of 12.1%. In the present study, the median pSOFA score among patients with infection was 10 (8-12); the mortality rate was 4.2%. Although these patients had higher pSOFA scores than those reported for patients with sepsis by Matics and Sanchez-Pinto, the mortality rate was lower. Because Matics and Sanchez-Pinto’s report did not mention DIC treatment, anticoagulation therapy may reduce mortality.
The hemostatic system in children is significantly different from that in adults. Antithrombin activity in children is lower than that in adults.23 A low AT level in septic children is a good prognostic marker for MODS and is associated with higher mortality.27 In the present study, median AT activity at day 0 was significantly lower for patients <6 months of age, compared to patients >6 months of age (35.5% and 45.5%, P = .005). However, median c-AT activity corrected by an age-equivalent AT activity of <6 months of age was almost equal to that of patients >6 months of age (46.8% and 43.3%, P = .980). Because there were no significant differences in pSOFA and JMHW DIC scores between groups at day 0, there seemed to be no significant difference in patient severity between groups. Therefore, c-AT activity could be a universal marker, independent of age, for assessing the severity DIC in pediatric patients.
The basis of DIC treatment is the vigorous treatment of underlying disease. So far, there is no universal consensus on the treatment of DIC. Anticoagulation therapy is widely used for sepsis-induced DIC in Japan. Antithrombin concentrate and rhTM are the agents used most frequently, and concomitant use of these 2 anticoagulants was associated with reduced mortality among patients with sepsis-induced DIC.28 Only a few analyses performed in pediatric populations have been reported. Kreuz et al reported that administration of AT concentrate resulted in normalization of hemostasis parameters within 48 hours.18 Shirahata et al investigated the effectiveness of rhTM in 210 children: 58.5% (48/82) of patients recovered from DIC and 71.6% (149/208) had survived on day 28.20 The present study showed that 54.5% (24/44) recovered from DIC on day 3 and 93.2% (41/44) were alive on day 28. It was revealed that AT supplementation therapy might be as useful as rhTM administration therapy, for the treatment of pediatric DIC; concomitant therapy did not enhance the beneficial effect. However, Umemura and Yamakawa reported the benefit from rhTM only in patients with severe disease.29 Therefore, it cannot be definitively concluded from the current study data that concomitant anticoagulation therapy is not useful in pediatric patients with DIC. Future studies will need to investigate these issues among patients who present with severe disease.
In the present study, rate of DIC resolution at day 3 was 54.5%. Compared to patients without resolution of DIC, patients with resolution of DIC had significantly lower JMHW DIC scores and a trend toward lower pSOFA scores at day 0. Patients with resolution of DIC also had significantly lower JMHW DIC and pSOFA scores at day 3. These results suggest that early treatment of DIC (eg, administration of AT concentrate) may improve DIC and MODS. Starting therapy when the JMHW score is 7 or the JAAM score is 4 may lead to improved prognosis. If the cutoff value of the JMHW DIC score is 6, cutoff sensitivity is 0.460 and specificity is 0.850. Because the JMHW DIC score on day 0 for all patients in this study was ≥6, the sensitivity of a JMHW DIC score of 6 was low. When treatment is started when the JMHW score is 6, the rate of DIC resolution should be even more rapid.
Among patients with resolution of DIC, median c-AT activity on day 3 was higher than that of patients with resolution of DIC regardless of AT concentrate dose. Gando et al evaluated the changes in AT activity after administration of a fixed dose of AT (30 IU/kg/d). Patients who achieved an AT activity level of more than 60% had better outcomes.30 In our study, it was suggested that after administration AT (30 IU/kg/d for 2 days), the pediatric patients who achieved a c-AT activity level of >90.0% on day 3 had improved recovery from DIC. Therefore, at 3 days after administration of AT concentrate, target AT activity was 90.0% of normal.
Among patients who received AT concentrate alone or AT with concomitant anticoagulants (UFH, NM and rhTM), bleeding AEs affected 4.5% of patients; ADRs affected 0%. Antithrombin supplementation and concomitant anticoagulation therapy have been shown to be safe in children.
Limitations
There were several limitations to this study. First, this study was an examination of a single arm with no comparison arms. Second, there were small sample sizes in the subgroup analysis. Data were collected from patients who were not subjected to any inclusion criteria, as patients with DIC were consecutively registered at the initiation of AT administration. As a result, 21 patients were excluded. Although their clinical characteristics, such as severity scores or mortality, were similar to those of included patients, they could be a bias to the results. Third, this study was performed under daily clinical practice conditions (patients undergoing concomitant use of other anticoagulants or other treatments of DIC were not excluded from this study). Fourth, there was no uniform protocol for therapeutic intervention among patients with DIC, which could be a confounding factor in the outcome. Despite these limitations, this study offers new information about the effectiveness and safety of AT for children with DIC. Further study is needed to confirm these findings.
Conclusion
Among pediatric patients, AT supplementation and concomitant anticoagulation therapy may be used safely as early treatment for DIC, when DIC score is 6 or JAAM DIC score is 4 and target AT activity is 90.0% of normal at 3 days after administration of AT concentrate. This approach may improve the DIC resolution rate, decrease risk of MODS, and decrease mortality rate.
Acknowledgments
The authors would like to thank all participating physicians, registered patients, and families who took part in this postmarketing surveillance study at the following centers: Obihiro Kosei General Hospital, Kindai University Nara Hospital, Saitama Medical Center, Juntendo University Urayasu Hospital, Showa University Northern Yokohama Hospital, Nihon University Itabashi Hospital, Nara Medical University Hospital, National Center for Global Health and Medicine, Ehime University Hospital, Okayama University Hospital, Kanazawa University Hospital, Kyushu University Hospital, Gunma University Hospital, Mie University Hospital, Kagoshima City Hospital, Kanagawa Children’s Medical Center, Nagano Children’s Hospital, Shonai Hospital, and Japanese Red Cross Society Himeji Hospital. The authors would like to thank Enago (www.enago.jp) for the English language review.
Authors’ Note: This work was performed as a postmarketing surveillance by the Japanese Blood Products Organization, which participated in study design as well as collection of the data. H.N. wrote the initial draft of the manuscript. Y.E. and T.I. contributed to analysis and interpretation of data and assisted in preparation of the manuscript. All authors revised and approved the final version of the manuscript.
Declaration of Conflicting Interests: The author(s) declare no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Levi M, Ten Cate H. Disseminated intravascular coagulation. N Engl J Med. 1999;341(8):586–592. [DOI] [PubMed] [Google Scholar]
- 2. Levi M. Disseminated intravascular coagulation. Crit Care Med. 2007;35(9):2191–2195. [DOI] [PubMed] [Google Scholar]
- 3. Gando S, Saitoh D, Ogura H, et al. Natural history of disseminated intravascular coagulation diagnosed based on the newly established diagnostic criteria for critically ill patients: results of a multicenter, prospective survey. Crit Care Med. 2008;36(1):145–150. [DOI] [PubMed] [Google Scholar]
- 4. Wada H, Wakita Y, Nakase T, et al. Outcome prediction of disseminated intravascular coagulation in relation to the score when treatment was begun. Thromb Haemost. 1995;74(3):848–852. [PubMed] [Google Scholar]
- 5. Gando S, Iba T, Eguchi Y, et al. A multicenter, prospective validation of disseminated intravascular coagulation diagnostic criteria for critically ill patients: comparing current criteria. Crit Care Med. 2006;34(3):625–631. [DOI] [PubMed] [Google Scholar]
- 6. Kobayashi N, Maegawa K, Takada M, Tanaka H, Gonmori H. Criteria for diagnosis of DIC based on the analysis of clinical and laboratory findings in 345 DIC patients collected by the Research Committee on DIC in Japan. Bibl Haematol. 1983;(49):265–275. [DOI] [PubMed] [Google Scholar]
- 7. Liumbruno GM, Franchini M, Lanzoni M, et al. Clinical use and the Italian demand for antithrombin. Blood Transfus. 2013;11(suppl 4):s86–s93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Iba T, Saitoh D. Efficacy of antithrombin in preclinical and clinical applications for sepsis-associated disseminated intravascular coagulation. J Intensive Care. 2014;2(1):66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Levi M, van der Poll T. The role of natural anticoagulants in the pathogenesis and management of systemic activation of coagulation and inflammation in critically ill patients. Semin Thromb Hemost. 2008;34(5):459–468. [DOI] [PubMed] [Google Scholar]
- 10. Iba T, Saito D, Wada H, Asakura H. Efficacy and bleeding risk of antithrombin supplementation in septic disseminated intravascular coagulation: a prospective multicenter survey. Thromb Res. 2012;130(3):e129–e133. [DOI] [PubMed] [Google Scholar]
- 11. Gando S, Saitoh D, Ishikura H, et al. A randomized, controlled, multicenter trial of the effects of antithrombin on disseminated intravascular coagulation in patients with sepsis. Crit Care. 2013;17(6):R297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Iba T, Saitoh D, Wada H, Asakura H. Efficacy and bleeding risk of antithrombin supplementation in septic disseminated intravascular coagulation: a secondary survey. Crit Care. 2014;18(5):497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Umemura Y, Yamakawa K, Ogura H, Yuhara H, Fujimi S. Efficacy and safety of anticoagulant therapy in three specific populations with sepsis: a meta-analysis of randomized controlled trials. J Thromb Haemost. 2016;14(3):518–530. [DOI] [PubMed] [Google Scholar]
- 14. Hayakawa M, Yamakawa K, Saito S, et al. Recombinant human soluble thrombomodulin and mortality in sepsis-induced disseminated intravascular coagulation. A multicenter retrospective study. Thromb Haemost. 2016;115(6):1157–1166. [DOI] [PubMed] [Google Scholar]
- 15. Hayakawa M, Kudo D, Saito S, et al. Antithrombin supplementation and mortality in sepsis-induced disseminated intravascular coagulation: a multicenter retrospective observational study. Shock. 2016;46(6):623–631. [DOI] [PubMed] [Google Scholar]
- 16. Umemura Y, Yamakawa K, Hayakawa M, Kudo D, Fujimi S. Concomitant versus individual administration of antithrombin and thrombomodulin for sepsis-induced disseminated intravascular coagulation: a nationwide Japanese registry study. Clin Appl Thromb Hemost. 2018;24(5):734–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Göbel U, von Voss H, Jürgens H, et al. Efficiency of heparin in the treatment of newborn infants with respiratory distress syndrome and disseminated intravascular coagulation. Eur J Pediatr. 1980;133(1):47–49. [DOI] [PubMed] [Google Scholar]
- 18. Kreuz WD, Schneider W, Nowak-Göttl U. Treatment of consumption coagulopathy with antithrombin concentrate in children with acquired antithrombin deficiency—a feasibility pilot study. Eur J Pediatr. 1999;158(suppl 3):S187–S191. [DOI] [PubMed] [Google Scholar]
- 19. Yagasaki H, Kato M, Shimozawa K, et al. Treatment responses for disseminated intravascular coagulation in 25 children treated with recombinant thrombomodulin: a single institution experience. Thromb Res. 2012;130(6):e289–e293. [DOI] [PubMed] [Google Scholar]
- 20. Shirahata A, Mimuro J, Takahashi H, et al. Recombinant soluble human thrombomodulin (thrombomodulin alfa) in the treatment of neonatal disseminated intravascular coagulation. Eur J Pediatr. 2014;173(3):303–311. [DOI] [PubMed] [Google Scholar]
- 21. Simpson E, Liebman M. Common products used to manage bleeding and clotting In: Blanchette VS, Breakey VR, Revel Vilk S, eds. SickKids Handbook of Pediatric Thrombosis and Hemostasis. 1st ed Basel, Switzerland: Karger; 2013:235–241. [Google Scholar]
- 22. Matics TJ, Sanchez-Pinto LN. Adaptation and validation of a pediatric sequential organ failure assessment score and evaluation of the sepsis-3 definitions in critically ill children. JAMA Pediatr. 2017;171(10):e172352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Andrew M, Paes B, Milner R, et al. Development of the human coagulation system in the full-term infant. Blood. 1987;70(1):165–172. [PubMed] [Google Scholar]
- 24. International Federation of Pharmaceutical Manufacturers and Associations Medical Dictionary for Regulatory Activities MedDRA/J Version. 2011. http://www.meddra.org/sites/default/files/guidance/file/intguide_14_1_english.pdf. Accessed September 11, 2013.
- 25. ICH Steering Committee. ICH Harmonised Tripartite Guideline: Clinical safety data management: definitions and standards for expedited reporting. 2011. http://www.pmda.go.jp/ich/e/e2a_95_3_20e.pdf.
- 26. van Gorp EC, Suharti C, ten Cate H, et al. Review: infectious diseases and coagulation disorders. J Infect Dis. 1999;180(1):176–186. [DOI] [PubMed] [Google Scholar]
- 27. Niederwanger C, Hell T, Hofer S, et al. Antithrombin deficiency is associated with mortality and impaired organ function in septic pediatric patients: a retrospective study. PeerJ. 2018;6:e5538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Iba T, Hagiwara A, Saitoh D, et al. Effects of combination therapy using antithrombin and thrombomodulin for sepsis-associated disseminated intravascular coagulation. Ann Intensive Care. 2017;7(1):110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Umemura Y, Yamakawa K. Optimal patient selection for anticoagulant therapy in sepsis: an evidence-based proposal from Japan. J Thromb Haemost. 2018;16(3):462–464. [DOI] [PubMed] [Google Scholar]
- 30. Gando S, Sawamura A, Hayakawa M, et al. First day dynamic changes in antithrombin III activity after supplementation have a predictive value in critically ill patients. Am J Hematol. 2006;81(12):907–914. [DOI] [PubMed] [Google Scholar]