Abstract
The role of the endothelium in sepsis-associated disseminated intravascular coagulation (DIC) is multifaceted and may contribute substantially to disease severity and outcome. The purpose of this study was to quantify measures of endothelial function, including markers of activation (endocan, Angiopoietin-2 [Ang-2], and von Willebrand Factor), endogenous anticoagulants (tissue factor pathway inhibitor and protein C), and damage-associated factors (High Mobility Group Box 1 [HMGB-1]) in the plasma of patients with sepsis and DIC, and to determine the relationship of these factors with severity of illness and outcome. Plasma samples were collected from 103 adult patients with sepsis within 48 hours of intensive care unit admission. Biomarker levels were measured using commercially available, standardized methods. Disseminated intravascular coagulation was diagnosed according to the International Society of Thrombosis and Hemostasis scoring algorithm. Twenty-eight-day mortality was used as the primary end point. In this study, endothelial damage and dysfunction were associated with the severity of coagulopathy and mortality in DIC patients. Loss of the endogenous anticoagulant protein C and elevation in the vascular regulator Ang-2 were associated with the development of overt DIC. In addition to Ang-2 and protein C, endocan, a biomarker of endothelial activation, and HMGB-1, a mediator of endothelial damage and activation, were significantly associated with mortality. This underscores the contribution of the endothelium to the pathogenesis of sepsis-associated DIC.
Keywords: sepsis, disseminated intravascular coagulation, DIC, endothelial dysfunction
Introduction
Vascular endothelial cells are in constant contact with the blood and are critical contributors to the pathogenesis of all thromboembolic diseases. In sepsis, both the underlying infection and the overwhelming host response to this infection result in activation, damage, or functional changes to the endothelium. This may contribute to the development of disseminated intravascular coagulation (DIC).
Under physiological conditions, the endothelium prevents inappropriate coagulation. Endothelial cells express or secrete an assortment of endogenous anticoagulants, including tissue factor pathway inhibitor (TFPI), protein C, thrombomodulin, and antithrombin (AT). These molecules act at specific sites along the coagulation cascade to inhibit coagulation. However, the endogenous anticoagulant system is dramatically disrupted in DIC and is a current focus of research for both biomarkers and therapeutic targets. This disruption may occur due to consumption, vascular leakage, or downregulation due to elevated levels of inflammatory cytokines or other factors, such as histones. Increased levels of TFPI may be protective in DIC,1 although administration of endogenous TFPI has not been shown to increase survival.2 Protein C is an endogenous anticoagulant that is of interest in DIC both as a biomarker and as a therapeutic target. Protein C is reduced in DIC, and this reduction is associated with poor outcome.
During sepsis, endothelial cells may also develop procoagulant properties in addition to the loss of anticoagulant function. This is typically described in terms of increased expression of tissue factor (TF) in response to high levels of inflammatory mediators. Activation of the endothelium due to high levels of inflammatory cytokines such as tumor necrosis factor α (TNFα), interleukin 1β (IL-1β), and interferon γ (IFNγ) may be detectable through elevated levels of other biomarkers such as endocan.
In addition to the inflammatory cytokines, more novel mediators may also contribute to endothelial damage or activation in sepsis or DIC. Factors typically restricted to the cell nucleus, including the chromatin-associated protein High Mobility Group Box 1 (HMGB-1) circulate at elevated levels in the blood of patients with sepsis. The HMGB-1 in sepsis may be released from endothelial cells3 as well as immune cells. The HMGB-1, as well as other nuclear components such as histones,4–8 induce significant endothelial dysfunction or an endothelial procoagulant state. Therefore, measurement of these factors may provide insight into endothelial function in this disease state and may provide a link between infection and the development of coagulation dysfunction.
In addition to its role in the development of DIC, the endothelium may also be important in sepsis due to its contributions to vascular leakage. Vascular leakage can be induced by inflammatory factors such as IL-2, vascular endothelial growth factor (VEGF), and monocyte chemoattractant protein 1 (MCP-1).9 In addition to contributing to hypotension and shock in patients with sepsis, increased vascular leakage may also contribute to the development of coagulopathy by permitting the loss of endogenous anticoagulants, particularly AT. Angiopoietin 2 (Ang-2) may contribute to the vascular leakage observed in sepsis by antagonizing Ang-1 at the Tie2 receptor and promoting intercellular gap formation.10,11
The purpose of this study was to measure biomarkers of endothelial function, including the endogenous anticoagulants TFPI and protein C as well as endocan, Ang-2, von Willebrand factor (vWF), and the endothelial damaging protein HMGB-1 and ascertain their relationship to coagulopathy and outcome in a cohort of patients with sepsis and well-defined coagulation dysfunction.
Materials and Methods
Patient Samples
Plasma samples from 103 adult patients with sepsis and suspected DIC were collected under an IRB-approved protocol as described previously.12–14 Samples were collected from adult patients in the intensive care unit (ICU) within 48 hours of ICU admission, and all patients enrolled in the study (or a legally authorized representative) provided informed consent.
In order to qualify for enrollment in this study, patients were required to meet the criteria for systemic inflammatory response syndrome (SIRS) and have an identified or suspected focus of infection. The SIRS was defined as the presence of 2 or more of the following: (1) temperature <36ºC or >38ºC, (2) heart rate >90 beats/min, (3) respiratory rate >20 beats/min or PaCO2< 32 mm Hg, (4) white blood cell count ≥ 12 000 or ≤ 4000 cells/mm3 or > 10% bands. Patients were excluded from the study if they had received a blood transfusion within the past 4 months, platelet transfusion within the past 14 days, or platelet count of less than 20 K/μl. Patients were also excluded from this study if they had a pre-existing disorder affecting platelet number or function, including idiopathic thrombocytopenic purpura, thrombotic thrombocytopenic purpura, hemolytic uremic syndrome, end-stage liver disease, myeloproliferative disorders, multiple myeloma, Waldenstrom’s macroglobulinemia, end-stage renal disease requiring hemodialysis, or inherited platelet disorders, such as Bernard-Soulier syndrome, gray platelet syndrome, May-Hegglin anomaly, Wiskott-Aldrich syndrome, Glanzmann thrombasthenia, Chediak-Higashi syndrome, Hermansky-Pudlak syndrome, or thrombocytopenia-absent radius syndrome.
Blood was collected into 3.2% sodium citrate and centrifuged to prepare platelet poor plasma. Plasma was collected, aliquoted, and stored at −80ºC prior to analysis.
Plasma from 50 apparently healthy volunteers was purchased from George King Biomedical (Overland, KS). These samples were collected from 25 male and 25 female volunteers, ages 19 to 54 years, with a mean age of 32 years. All volunteers were non-smokers, non-medicated, and of geographically diverse origins.
Clotting Assays: PT and Fibrinogen
Prothrombin time/Inetrnational Normalized Ratio (PT/INR) and fibrinogen, required for computation of the DIC score, were measured using standard operating procedures on an ACL-ELITE automated coagulation analyzer (Instrumentation Laboratory, Bedford, Massachusetts). This instrument uses an optical method to detect clot formation in a plasma sample. Recombiplastin (Instrumentation Laboratory) was used as the PT reagent.
Biomarker Levels
Commercially available enzyme-linked immunosorbent assays (ELISAs) were performed according to the manufacturer’s instructions. The biomarkers measured and assays used were as follows: D-Dimer and vWF (Hyphen BioMed, Neuville-Sur-Oise, France), PAI-1 and TFPI (Stago Asserachrom, Asnieres-Sur-Seine, France), Ang-2 (R&D Systems, Minneapolis, Minnesota), Endocan (Lunginnov (Lille, France), and HMGB-1 (LifeSpan BioSciences, Seattle, Washington).
Functional levels of protein C were measured using a clot-based assay performed using an ST4 coagulation analyzer (STACLOT; Diagnostica Stago, Parsippany, New Jersey). Patient and control plasmas were diluted 1:10 in Owren Koller Buffer. 50 μl of diluted sample, 50 μl of protein C deficient plasma (Diagnostica Stago), and 50 μl of protein C activator (Diagnostica Stago) were incubated in a sample cuvette with a metal mixing ball for 180 seconds at 37ºC. Then 50μl of 0.2 M CaCl2 was added to each sample, initiating the clotting reaction. Time to clot formation was recorded as the time at which the metal ball stopped moving.
Protein C level, measured as percent of normal value, was calculated from the time to clot for each sample based on a standard curve. The standard curve consisted of dilutions of normal human pooled plasma at 100%, 75%, 50%, 25%, 12.5%, and 0%, diluted 1:10 in Owren Koller buffer. Clotting time had an inverse relationship with Protein C activity level.
Disseminated Intravascular Coagulation Score
The DIC score was computed for all patients using the International Society of Thrombosis and Hemostasis (ISTH) scoring algorithm, which assigns points on the basis of platelet count, INR, D-dimer, and fibrinogen and assigns a DIC score to each patient. Patients with a score of 0 to 2 were classified as no DIC, patients with a score of 3 to 4 were classified as non-overt DIC, and patients with a score of ≥5 were classified as overt DIC. The cutoff values for each parameter are presented in Table 1.
Table 1.
ISTH Scoring System for DIC.
| Variables | Values | Points |
|---|---|---|
| Platelets (K/μl) | >100 | 0 |
| 50-100 | 1 | |
| <50 | 2 | |
| INR | <1.3 | 0 |
| 1.3-1.7 | 1 | |
| >1.7 | 2 | |
| D-dimer (ng/ml) | <400 | 0 |
| 400-4000 | 2 | |
| >4000 | 3 | |
| Fibrinogen (mg/dl) | >100 | 0 |
| <100 | 1 |
Abbreviations: DIC, disseminated intravascular coagulation; ISTH, International Society of Thrombosis and Hemostasis.
Statistical Analysis
Data are presented as mean (standard deviation [SD]) or mean ± standard error of the mean (SEM) as specified. P < .05 was used as the cutoff for statistical significance, and computed P values are present throughout this document. Results were tabulated and stored using Microsoft Excel (Microsoft Corporation, Redmond, WA). Statistical analysis was performed and graphs were generated using GraphPad Prism (GraphPad Inc., La Jolla, California).
Biomarker levels in patient populations are presented as mean ± SEM. Nonparametric statistical tests were used throughout as these tests are more appropriate for analysis of data sets with high variability than traditional parametric tests. Differences in biomarker levels between the 2 patient groups (ie, survivors and nonsurvivors) were analyzed using the Mann-Whitney test. Predictive values were analyzed using receiver operating characteristic (ROC) curve analysis, with the main output for this being the area under the curve (AUC).
Results
Patient Cohort Baseline Characteristics
Plasma samples were collected from 103 adult ICU patients with sepsis as described previously in the Materials and Methods section.12–14 Patient cohort baseline characteristics, including disease severity and outcome information, are presented in Table 2. The demographics of this cohort are within the range typical for patients with sepsis in the literature. This includes the age distribution (57 [18.5] years) and the gender balance (46.6% male). The healthy control group was 50% male and had a mean age of 32 years.
Table 2.
Patient Cohort Baseline Characteristics.
| Characteristics | All Patients, (n = 103), Mean (SD) | Survivors, (n = 88), Mean (SD) | Nonsurvivors, (n = 15), Mean (SD) |
|---|---|---|---|
| Age (years) | 57.1 (18.6) | 55.6 (18.1) | 65.4 (19.2) |
| BMI | 31.2 (0.89) | 30.9 (9.6) | 31.2 (7.6) |
| Gender | N (%) | ||
| Male | 48 (46.6%) | 41 (46.6%) | 7 (46.7%) |
| Female | 55 (53.4%) | 47 (56.4%) | 8 (53.3%) |
| Outcome | N (%) | ||
| 28-day mortality | 15 (14.6%) | 0 (0%) | 15 (100%) |
| Vasopressor use | 46 (44.7%) | 34 (38.9%) | 11 (73.3%) |
| Ventilator use | 48 (46.6%) | 43 (48.9%) | 5 (33.3%) |
| Clinical Disease Severity Score | Mean (SD) | ||
| SOFA Score (day 0) | 5.9 (3.7) | 5.4 (3.6) | 8.5 (3.3) |
| APACHE II Score | 17.4 (7.3) | 16.4 (6.9) | 23.1 (6.7) |
| ISTH DIC Score | 3.6 (1.3) | 3.5 (1.3) | 4.1 (1.4) |
Abbreviations: BMI, body mass index; DIC, disseminated intravascular coagulation; ISTH, International Society of Thrombosis and Hemostasis.
The primary outcome measure in this patient cohort was 28-day mortality. This cohort was comprised of 88 survivors and 15 nonsurvivors, resulting in an overall 28-day mortality rate of 14.6%. Severity of illness was further described by the requirement for vasopressors as well as the Sepsis-related Organ Failure Assessment (SOFA) and Acute Physiology, Age, Chronic Health Evaluation II (APACHE-II) scores, all computed at the time of acquisition of the first sample. The severity of disease, quantified by mortality as well as through clinical scoring systems such as SOFA and APACHE II are highly variable based on factors such as study inclusion criteria, standard of care, and variability between institutions and services. The overall 28-day mortality of patients included in this cohort, 14.6%, is relatively low, as mortality in sepsis is often estimated at greater than 20%. However, many studies reporting high mortality are designed to enroll only patients with severe sepsis or septic shock, both of which are associated with increased mortality. Numerous studies enrolling patients with sepsis have reported mortality of <20%. Similarly, the SOFA and APACHE II scores were at the low end of the range typically reported for cohorts of patients with sepsis. Many studies enrolling patients with sepsis report mean SOFA scores between 6 and 9 and mean APACHE II scores between 18 and 25. In this cohort, the SOFA score was 5.9 (3.7) and the APACHE II score was 17.4 (7.3).
As presented in Table 3, markers of endothelial function showed minimal associations with organ failure or disease severity. Statistically significant but weak correlation was observed between SOFA score and protein C (P = .024, R = −0.22). APACHE II score showed no significant correlation with any endothelial biomarker.
Table 3.
Association of Endothelial Biomarkers with Severity of Illness.a
| Spearman Correlation Coefficients | Mann-Whitney Test P Value | |||
|---|---|---|---|---|
| APACHE II Score | SOFA Score | Ventilator | Vasopressor | |
| TFPI | 0.10 | 0.00 | .45 | .55 |
| Protein C | −0.15 | −0.22 | .11 | .25 |
| HMGB-1 | −0.08 | −0.07 | .05 | .29 |
| Endocan | 0.19 | −0.02 | .63 | .35 |
| Ang-2 | 0.05 | 0.14 | .22 | <.0001 |
| vWF | −0.14 | −0.12 | .0006 | .22 |
Abbreviations: Ang-2, Angiopoietin-2; HMGB-1, High Mobility Group Box 1; TFPI, tissue factor pathway inhibitor; vWF, von Willebrand factor.
aFor APACHE II and SOFA scores, Spearman correlation coefficients are shown. Significant correlations (P < .05) are in bold. For ventilator and vasopressor use, Mann-Whitney Test P value is shown for comparison of biomarker levels between patients receiving or not receiving ventilator or vasopressor support.
Minimal associations were observed between the endothelial markers and the presence of shock or ventilator use. Angiopoietin-2 was significantly elevated in patients requiring vasopressor support. High Mobility Group Box 1 and vWF were significantly elevated in patients requiring mechanical ventilation. This low degree of association between endothelial markers and disease severity is somewhat surprising, as previous studies have demonstrated associations between endothelial damage and organ failure.
Disseminated Intravascular Coagulation Score Distribution and Association with Endothelial Dysfunction
Disseminated intravascular coagulation was diagnosed using the ISTH scoring algorithm for overt DIC.15 This algorithm assigns points based on reduced platelet count, elevated INR, elevated D-dimer, and reduced fibrinogen. Using this scoring system in patients with a predisposing condition such as sepsis, a score of 0 to 2 indicates no DIC, a score of 3 to 4 indicates non-overt DIC, and a score of ≥5 indicates overt DIC. Of the 103 patients, 20 had sepsis without DIC, 59 had sepsis and non-overt DIC, and 24 had sepsis and overt DIC. Overt DIC describes a scenario of severe, decompensated coagulopathy with marked perturbations to multiple aspects of the hemostatic system. Non-overt DIC represents a heterogeneous phenotype, with a variable degree and manifestation of coagulopathy. Patients in the no DIC category were still severely ill with sepsis; however, these patients did not have significant coagulation dysfunction. Differences in biomarker levels between the 3 groups and from the healthy control cohort were assessed using the Kruskal-Wallis analysis of variance with Dunn multiple comparison test and P < .05 as the cutoff for significance. Markers were measured in 50 healthy individuals as well as in samples from 20 patients with no DIC, 59 patients with non-overt DIC, and 24 patients with overt DIC.
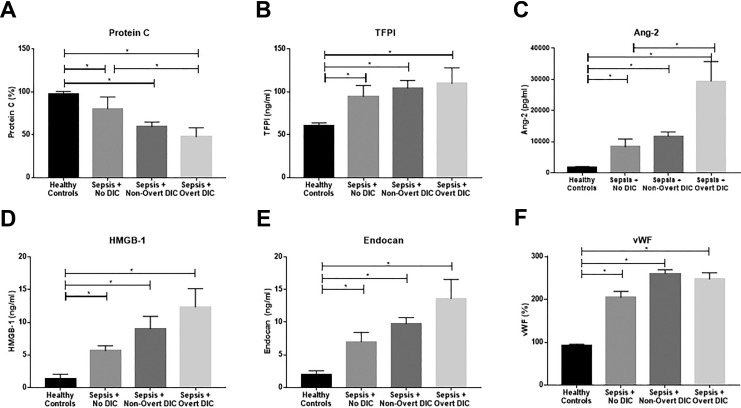
Significant variation of levels of endothelial biomarkers based on DIC score was observed, as shown in Figure 1 and Table 4.
Figure 1.
Baseline endothelial biomarker levels stratified by DIC score. Significance calculated between groups using the Kruskal-Wallis ANOVA with Dunn’s multiple comparison test and P < .05 as the cutoff for significance (indicated by *). Data are shown as mean ± SEM. DIC, disseminated intravascular coagulation.
Table 4.
Baseline Endothelial Biomarker Levels Stratified by DIC Score.
| Biomarker | Patient Group | Mean | Median | SD | SEM | Range |
|---|---|---|---|---|---|---|
| TFPI, (ng/ml) | Healthy controls (n = 50) | 61 | 59 | 19 | 2.7 | 24-106 |
| No DIC (n = 20) | 95 | 81 | 58 | 13 | 32-285 | |
| Non-overt DIC (n = 59) | 104 | 94 | 69 | 8.9 | 4.8-423 | |
| Overt DIC (n = 24) | 110 | 89 | 90 | 18 | 11-407 | |
| Protein C, (%) | Healthy controls (n = 50) | 98 | 94 | 18 | 2.5 | 71-142 |
| No DIC (n = 20) | 80 | 71 | 62 | 14 | 0-309 | |
| Non-overt DIC (n = 59) | 59 | 51 | 40 | 5.3 | 2.5-309 | |
| Overt DIC (n = 24) | 48 | 41 | 52 | 11 | 2.7-277 | |
| HMGB-1, (ng/ml) | Healthy controls (n = 50) | 1.4 | 0.13 | 4.9 | 0.69 | 0.04-23 |
| No DIC (n = 20) | 5.6 | 4.8 | 3.5 | 0.77 | 2.5-18 | |
| Non-overt DIC (n = 59) | 9 | 5.1 | 15 | 1.9 | 0.18-87 | |
| Overt DIC (n = 24) | 12 | 7.5 | 14 | 2.8 | 3.3-66 | |
| Endocan, (ng/ml) | Healthy controls (n = 50) | 1.9 | 0.85 | 4.5 | 0.63 | 0.17-25 |
| No DIC (n = 20) | 7 | 5.1 | 6.4 | 1.4 | 1.4-24 | |
| Non-overt DIC (n = 59) | 9.8 | 7.4 | 7.3 | 0.95 | 2-34 | |
| Overt DIC (n = 24) | 14 | 6.5 | 14 | 3 | 1.9-60 | |
| Ang-2, (pg/ml) | Healthy controls (n = 50) | 1869 | 1566 | 1070 | 151 | 503-5538 |
| No DIC (n = 20) | 8343 | 5754 | 11 187 | 2501 | 961-53 612 | |
| Non-overt DIC (n = 59) | 11 736 | 8274 | 10 563 | 1387 | 650-44 167 | |
| Overt DIC (n = 24) | 29 440 | 17 618 | 30 732 | 6273 | 1816-136 317 | |
| vWF, (%) | Healthy controls (n = 50) | 93 | 93 | 19 | 2.7 | 59-131 |
| No DIC (n = 20) | 205 | 183 | 63 | 14 | 107-349 | |
| Non-overt DIC (n = 59) | 260 | 271 | 69 | 9 | 111-370 | |
| Overt DIC (n = 24) | 247 | 251 | 73 | 15 | 122-379 |
Abbreviations: Ang-2, Angiopoietin-2; DIC, disseminated intravascular coagulation; HMGB-1, High Mobility Group Box 1; TFPI, tissue factor pathway inhibitor; vWF, von Willebrand factor.
Protein C is known to be implicated in the pathogenesis of sepsis-associated DIC, and decreased levels are generally associated with poor outcome. In this cohort, protein C was decreased in patients with sepsis compared to healthy controls regardless of coagulation status (Figure 1A). Additionally, protein C showed a significant decrease in patients with overt DIC compared to patients with sepsis and no DIC. This corroborates prior research regarding Protein C in sepsis-associated coagulopathy. Depletion of this endogenous anticoagulant contributes to the development of coagulopathy in patients with sepsis, and this pathway is a major therapeutic target. In contrast, another endogenous anticoagulant, TFPI, showed no significant variation based on DIC status, although it was elevated in patients with sepsis compared to healthy controls regardless of DIC score (Figure 1B). The TFPI release is induced by heparin therapy. All patients enrolled in this study received prophylactic doses of unfractionated heparin (UFH); no additional UFH or low molecular weight heparin (LMWH) use was reported; therefore, this treatment does not represent a confounding factor for TFPI levels in this cohort.
Angiopoietin-2 also varied significantly based on DIC status, with significant elevation in patients with overt DIC compared to those with sepsis and no DIC as well as significant elevations in all patient groups compared to healthy controls (Figure 1C). High Mobility Group Box 1 (Figure 1D), endocan (Figure 1E), and vWF (Figure 1F) were elevated in patients with sepsis compared to controls regardless of DIC status; however, no variation was seen in patients with sepsis based on DIC status.
Association of Endothelial Dysfunction with Mortality
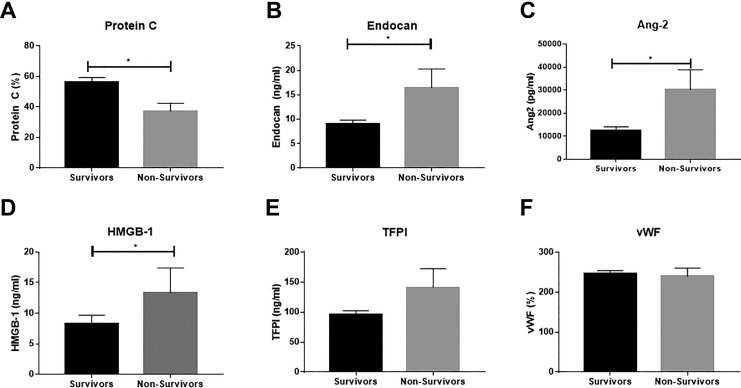
The 28-day mortality in this patient cohort was 14.6% (88 survivors and 15 nonsurvivors). Information on time to mortality was not available. Differences in baseline biomarker levels between survivors and nonsurvivors were evaluated using the Mann-Whitney t test with P < .05 as the cutoff for significance. The predictive power of each biomarker for mortality was evaluated using ROC curve analysis; the AUC is reported as the quantification of this analysis.
As shown in Figure 2, significant association was seen between markers of endothelial function and mortality. In contrast to almost all other evaluated markers, protein C showed a significant reduction in nonsurvivors compared to survivors (Figure 2A; P = .0093, AUC = 0.71). Both endocan (Figure 2B; P = .025, AUC = 0.58) and Ang-2 (Figure 2C; P = .001, AUC = 0.76) were significantly elevated in nonsurvivors compared to survivors. High Mobility Group Box 1 was also significantly elevated in nonsurvivors compared to survivors (Figure 2D; P = .031, AUC = 0.67). High Mobility Group Box 1 may be a direct mechanistic link between infection response and the physiological dysfunction that ultimately results in death. The TFPI (Figure 2E; AUC = 0.55) and vWF (Figure 2F; AUC = 0.58) showed no significant variation based on survival.
Figure 2.
Association of baseline endothelial biomarker levels with survival. Significance calculated between groups using the Mann-Whitney test with P < .05 as the cutoff for significance (indicated by *). Data are shown as mean ± SEM.
Discussion
This study examined the relationship of biomarkers of endothelial function with severity of illness, mortality, and the severity of DIC in a cohort of patients with sepsis and well-defined coagulopathy.
The patient cohort used in this study is composed of patients with sepsis and DIC defined according to well-established criteria. While the overall severity of illness, as defined by 28-day mortality, SOFA score and APACHE-II score was relatively mild, patients were well distributed in terms of severity of DIC. Using the ISTH criteria, 19.4% of patients were diagnosed with no DIC, 57.3% with non-overt DIC, and 23.3% with overt DIC. This distribution of DIC scores enables analysis of the association of biomarker levels with the severity of coagulopathy.
Disseminated intravascular coagulation was strictly defined and patients were subdivided into 3 groups based on DIC score. Many studies categorize patients as either overt DIC (ISTH score ≥5) or no DIC (ISTH score <5). In these studies, non-overt DIC (ISTH score 3-4) is not treated as an independent category. This results in a highly heterogeneous patient population in the no DIC category, resulting in reduced ability to identify factors associated with the development of severe coagulation dysfunction. Separation of patients with non-overt DIC from those who do not demonstrate coagulation dysfunction (ISTH score ≤2), as was performed in this study, is required for improved understanding of factors involved in the development of coagulation dysfunction. Furthermore, post hoc analysis of clinical trials has demonstrated that patients with overt DIC may respond differently to treatments than patients with less-severe manifestations of coagulopathy.16,17 In this study, 57.3% of patients had non-overt DIC at baseline.
The demographics of this cohort are within the range typical for patients with sepsis in the literature. This includes the age distribution (57 [18.5] years) and the gender balance (46.6% male). The racial and ethnic composition of this cohort is reasonable for the region in which the samples were collected. The highest prevalence comorbidities in this cohort included hypertension (45.6%), diabetes mellitus (25.2%), and cardiovascular disease (21.4%), all of which are common medical conditions. History of recent surgery was also highly prevalent in this cohort (22.3%). This is reasonable, as sepsis often develops as a complication of surgery.
The severity of disease, quantified by mortality as well as through clinical scoring systems such as SOFA and APACHE II are highly variable based on factors such as study inclusion criteria, standard of care, and variability between institutions and services. The overall 28-day mortality of patients included in this cohort, 14.6%, is relatively low, as mortality in sepsis is often estimated at greater than 20%. However, many studies reporting high mortality are designed to enroll only patients with severe sepsis or septic shock, both of which are associated with increased mortality. Numerous studies enrolling patients with sepsis have reported mortality of <20%. Similarly, the SOFA and APACHE II scores were at the low end of the range typically reported for cohorts of patients with sepsis. Many studies enrolling patients with sepsis report mean SOFA scores between 6 and 9 and mean APACHE II scores between 18 and 25. In this cohort, the SOFA score was 5.9 (3.7) and the APACHE II score was 17.4 (7.3). In this study, the associations of endothelial biomarkers with organ failure were relatively weak, and association with ventilator or vasopressor use was minimal.
Endothelial dysfunction demonstrated significant association with the development of DIC and with mortality. This is logical from a pathophysiological perspective, as endothelial damage is cited in Virchow’s Triad as one of the main requirements for thrombosis. The variation of protein C based on DIC score is expected as the role of this endogenous anticoagulant in the pathophysiology of DIC is well accepted. Protein C was notable in this study as the only biomarker to maintain an association with DIC status throughout the course of hospitalization.
Angiopoietin-2 also demonstrated a significant association with DIC, with elevation in overt DIC patients compared to sepsis and no DIC. Angiopoietin-2 has not previously been strongly associated with coagulopathy in patients with sepsis, although an association of Ang-2 with coagulopathy in trauma patients has been noted.18 Angiopoietin-2 has been more strongly tied to regulation of endothelial barrier function10,11 and the development of respiratory dysfunction in critically ill patients.10,11,19,20 Angiopoietin-2 acts as an antagonist to Ang-1 at the Tie2 receptor on the endothelial cell surface. While Ang-1 promotes vascular stability and preserves cell–cell contacts, Ang-2 acts in opposition to these effects. In addition to Ang-2, Ang-1 and the Ang-Tie system may represent a new avenue of study in sepsis-associated DIC. Surprisingly, Ang-2 demonstrated the highest predictive value for mortality of the measured endothelial markers, superior to protein C (AUC = 0.71). Angiopoietin-2 is predominately involved in the maintenance of endothelial cell barrier function. Increased Ang-2 is associated with increased intracellular gap formation. Patients with sepsis already suffer from hypotension, shock, and impaired perfusion; increased loss of fluid into the intravascular space further impairs perfusion and increases mortality. Elevated Ang-2 has been implicated in the development of respiratory dysfunction, another contributor to mortality. The mechanisms by which Ang-2 may contribute to hemostatic dysfunction remain unclear. This suggests Ang-2 and the Ang-Tie system is a new avenue for investigation in the molecular pathophysiology of sepsis and DIC. High Mobility Group Box 1 was significantly elevated in nonsurvivors compared to survivors. High Mobility Group Box 1 may contribute to both thrombosis and inflammation21,22 and is a potential therapeutic target in DIC.23,24 Endocan, was also elevated in nonsurvivors compared to survivors, underscoring the importance of endothelial function to this disease.
Protein C was significantly reduced in nonsurvivors compared to survivors. Protein C is the most studied endothelial factor in sepsis-associated DIC, and reductions in protein C levels have previously been associated with poor outcome in patients with sepsis and DIC,25–30 and this pathway has been pursued as a therapeutic target. Protein C functions as an endogenous anticoagulant as well as performing other anti-inflammatory functions, including the destruction of extracellular histones. Protein C depletion leads to the loss of these antithrombotic and cytoprotective functions, resulting in increased severity of coagulopathy and increased mortality. Protein C is activated by the thrombin–thrombomodulin complex. Thrombomodulin is expressed on endothelial cells in both bound and plasmatic forms. It binds to thrombin and the thrombin–thrombomodulin complex accelerates the activation of protein C. Increased thrombomodulin levels have also been shown to be associated with endothelial cell injury. Although TFPI did not show significant variation based on mortality, the increased level of TFPI in patients with sepsis and DIC compared to healthy controls further emphasizes the role of endogenous anticoagulants in this disease process. Measurement of functional TFPI levels in addition to protein levels may provide further insight into the role of TFPI in sepsis-associated DIC.
In conclusion, this study underscores the importance of endothelial function to the pathophysiology of sepsis-associated DIC and to outcome in patients with sepsis. Several aspects of endothelial function were significantly associated with DIC severity or outcome, including endogenous anticoagulants, represented by protein C; mediators of endothelial damage, represented by HMGB-1; and regulators of vascular function and permeability, represented by Ang-2. Therefore, endothelial function should be evaluated in future studies regarding both the diagnosis and treatment of sepsis and DIC.
Acknowledgments
The authors would like to acknowledge the skillful assistance of the staff of the Hemostasis Research Laboratories of the Department of Pathology and the Loyola University Medical Center. The authors are thankful to Dr Eva Wojick, Chair of the Department of Pathology, for her support in facilitating this study. This work was supported by the NHLBI and NIA (HL112311 and HL126547 to M.T.R. and A.S.W and AG048022 to M.T.R.). This material is the result of work supported with resources and use of facilities at the George E. Wahlen VA Medical Center, Salt Lake City, Utah. The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
Authors’ Note: Written informed consent was obtained from the patients or a legally authorized representative for anonymized patient information to be published in this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Amanda Walborn  https://orcid.org/0000-0001-8235-3624
https://orcid.org/0000-0001-8235-3624
Jawed Fareed  https://orcid.org/0000-0003-3465-2499
https://orcid.org/0000-0003-3465-2499
References
- 1. Gando S, Levi M, Toh CH. Disseminated intravascular coagulation. Nature Rev. 2016;2:1–16. [DOI] [PubMed] [Google Scholar]
- 2. Abraham E, Reinhart K, Opal S, et al. Efficacy and safety of tifacogin (recombinant tissue factor pathway inhibitor) in severe sepsis. JAMA. 2003;290(2):238–247. [DOI] [PubMed] [Google Scholar]
- 3. Bae J-S, Rezaie AR. Activated protein C inhibits high mobility group box 1 signaling in endothelial cells. Blood. 2011;118(14):3952–3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Abrams ST, Zhang N, Manson J, et al. Circulating histones are mediators of trauma-associated lung injury. Am J Respir Crit Care Med. 2013;187(2):160–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Daigo K, Nakakido M, Ohashi R, et al. Protective effect of the long pentraxin PTX3 against histone-mediated endothelial cell cytotoxicity in sepsis. Sci Signal. 2014;7(343):ra88. [DOI] [PubMed] [Google Scholar]
- 6. Gould T, Lysov Z, Liaw P. Extracellular DNA and histones: double-edged swords in immunothrombosis. J Thromb Haemost. 2015;13(suppl1):S82–S91. [DOI] [PubMed] [Google Scholar]
- 7. Kim JE, Yoo HJ, Gu JY, Kim HK. Histones induce the procoagulant phenotype of endothelial cells through tissue factor up-regulation and thrombomodulin down-regulation. Plos One. 2016;11(6):e0156763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yang X, Li L, Liu J, Lv B, Chen F. Extracellular histones induce tissue factor expression in vascular endothelial cells via TLR and activation of NF-kB and AP-1. Thromb Res. 2016;137:211–218. [DOI] [PubMed] [Google Scholar]
- 9. Hawiger J, Veach R, Zienkiewicz J. New paradigms in sepsis: from prevention to protection of failing microcirculation. J Thromb Haemost. 2015;13(10):1743–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Parikh SM, Mammoto T, Schultz A, et al. Excess circulating angiopoietin-2 may contribute to pulmonary vascular leak in sepsis in humans. Plos Med.2006;3(3):e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gallagher DC, Parikh SM, Balonov K, et al. Circulating angiopoietin 2 correlates with mortality in a surgical population with acute lung injury/adult respiratory distress syndrome. Shock. 2008;29(6):656–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rondina M, Schwertz H, Harris E, et al. The septic milieu triggers expression of spliced tissue factor mRNA in human platelets. J Thromb Haemost. 2011;9(4):748–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rondina MT, Brewster B, Grissom CK, et al. In vivo platelet activation in critically ill patients with primary 2009 influenza a(H1N1). Chest. 2012;141(6):1490–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rondina MT, Carlisle M, Fraughton T, et al. Platelet-monocyte aggregate formation and mortality risk in older patients with severe sepsis and septic shock. J Gerontol A Biol Sci Med Sci.2015;70(2):224–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Taylor FB, Toh C-H, Hoots WK, et al. Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost. 2001;86(5):1327–1330. [PubMed] [Google Scholar]
- 16. Dhainaut J, Yan S, Joyce D, et al. Treatment effects of drotrecogin alfa (activated) in patients with severe sepsis with or without overt disseminated intravascular coagulation. J Thromb Haemost. 2004;2(11):1924–1933. [DOI] [PubMed] [Google Scholar]
- 17. Shakoory B, Carcillo J, Chatham W, et al. Interleukin-1 receptor blockade is associated with reduced mortality in sepsis patients with features of the macrophage activation syndrome: Re-analysis of a prior Phase III trial. Crit Care Med.2016;44(2):275–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ganter MT, Cohen MJ, Brohi K, et al. Angiopoietin-2, marker and mediator of endothelial activation with prognostic significance early after trauma? Ann Surg.2008;247(2):320–326. [DOI] [PubMed] [Google Scholar]
- 19. Kumpers P, Lukasz A, David S, et al. Excess circulating angiopoietin-2 is a strong predictor of mortality in critically ill medical patients. Crit Care. 2008;12(6):R147 doi:10.1186/cc7130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lin SM, Chung FT, Kuo CH, et al. Circulating angiopoietin-1 correlates with the clinical course of multiple organ dysfunction syndrome and mortality in patients with severe sepsis. Medicine. 2015;94(20):e878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stark K, Philippi V, Stockhausen S, et al. Disulfide HMGB1 derived from platelets coordinates venous thromboembolism in mice. Blood. 2016;128(20):2435–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ito T, Kawahara K, Nakamura T, et al. High-mobility group box 1 protein promotes development of microvascular thrombosis in rats. J Thromb Haemost.2006;5:109–116. [DOI] [PubMed] [Google Scholar]
- 23. Suda K, Kitagawa Y, Ozawa S, et al. Anti-high-mobility group box chromosomal protein 1 antibodies improve survival of rats with sepsis. World J Surg. 2006;30(9):1755–1762. [DOI] [PubMed] [Google Scholar]
- 24. Yang H, Ochani M, Li J, et al. Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proc Nat Acad Sci. 2004;101(1):296–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shorr AF, Nelson DR, Wyncoll DL, et al. Protein C: a potential biomarker in severe sepsis and a possible tool for monitoring treatment with drotrecogin alfa (activated). Crit Care. 2008;12(2):R45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Macias WL, Nelson DR. Severe protein C deficiency predicts early death in severe sepsis. Crit Care Med. 2004;32(5):S223–S228. [DOI] [PubMed] [Google Scholar]
- 27. Bouchard J, Malhotra R, Shah S, et al. Levels of protein c and soluble thrombomodulin in critically ill patients with acute kidney injury: a multicenter prospective observational study. PLoS ONE. 2015;10(3):e0120770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shorr AF, Janes JM, Artigas A, et al. Randomized trial evaluating serial protein C levels in severe sepsis patients treated with variable doses of drotrecogin alfa (activated). Crit Care. 2010;14(6):R229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Collins PW, Macchiavello LI, Lewis SJ, et al. Global tests of haemostasis in critically ill patients with severe sepsis syndrome compared to controls. Brit J Haematol. 2006;135(2):220–227. [DOI] [PubMed] [Google Scholar]
- 30. LaRosa SP, Opal SM, Utterback B, et al. Decreased protein C, protein S, and antithrombin levels are predictive of poor outcome in Gram-negative sepsis caused by Burkholderia pseudomallei. Int J Infect Dis. 2006;10(1):25–31. [DOI] [PubMed] [Google Scholar]