Abstract
The primary end point for sepsis trial is 28-day mortality. However, additional methods for determining the efficacy may have benefits. The purpose of this study was to search a useful indicator of anticoagulant therapy in patients with sepsis with disseminated intravascular coagulation (DIC). Data from 323 patients with sepsis with coagulopathy treated with antithrombin supplementation were analyzed. The changes in the Sequential Organ Failure Assessment (Δ SOFA) score, the overt-DIC (Δ overt-DIC) score, and the Japanese Society for Acute Medicine DIC (Δ JAAM DIC) score from baseline to day 7 were retrospectively analyzed in relation to the 28-day mortality. Significant correlations were found between the 28-day mortality and Δ SOFA, Δ overt-DIC score, and Δ JAAM DIC score. The accuracy of the prediction was higher for Δ SOFA (80.5%) than for Δ overt-DIC (66.7%, P < .001). The areas under the curve for mortality calculated using a receiver operating characteristic curve analysis were 0.812 for Δ SOFA, 0.655 for Δ overt-DIC, and 0.693 for Δ JAAM DIC. The mortality rate was significantly lower among cases with an improved SOFA score compared to those without an improvement. The Δ SOFA had the strongest association with the 28-day mortality in patients with sepsis and DIC.
Keywords: sepsis, sequential organ failure assessment score, disseminated intravascular coagulation, antithrombin, clinical practice
Introduction
Circulatory shock and disseminated intravascular coagulation (DIC) in sepsis decrease organ perfusion1,2 and cause multiorgan dysfunction that is directly correlated with a fatal outcome.3,4 As a result, the mortality rate nearly doubles, compared to that for patients who do not develop shock or DIC.5 Although anticoagulant therapies for sepsis-induced coagulopathy have been reported since the early 2000s,6 their therapeutic benefit for treating sepsis continues to be debated, and anticoagulant therapy has not been recommended for the treatment of sepsis in the current global treatment guidelines.7 However, subanalyses of large-scale randomized controlled trials (RCTs) examining the effect of antithrombin and activated protein C have shown a trend toward a favorable effect in patients with sepsis with DIC.8,9 Also, recent studies consistently report improved survival after anticoagulant therapy in patients with sepsis-associated DIC, but not in patients with non-DIC.10,11 With respect to antithrombin supplementation therapy, data analyses using the Japanese Diagnosis Procedure Combination database have repeatedly shown beneficial effects on survival.12,13 As a result, the Japanese Clinical Practice Guidelines for the Management of Sepsis and Septic Shock 2016 recommend the use of antithrombin for sepsis-associated DIC.14
Although the 28-day mortality rate continues to be the gold-standard end point for evaluating therapeutic approaches in sepsis studies, the development of additional methods to evaluate outcomes may be useful for better understanding potential anticoagulant strategies for treating DIC and for furthering the development of strategies to overcome this critical condition.15 In an RCT, Gando et al16 reported a significant improvement in the DIC score after antithrombin supplementation in patients with sepsis-associated DIC, but with no apparent survival benefit, probably because the number of patients was insufficient to demonstrate an improvement in survival. Of note is the need to include morbidity in evaluations of efficacy, as suggested by Vincent et al.17 Thus, the primary objective of the present study was to search the additional indicators for evaluating the effectiveness of antithrombin therapy in patients with DIC.
Patients and Methods
Data Collection
Data from multi-institutional, postmarketing surveys performed between June 2014 and May 2016 by Nihon Pharmaceutical were utilized for the analysis.18 A total of 498 patients with sepsis with coagulopathy who were treated with antithrombin concentrate (Nihon Pharmaceutical Co Ltd, Tokyo, Japan) were registered in the survey. Among them, the data sets from 175 patients did not have the sequential organ failure assessment (SOFA) score of either day 1 or day 7 and those data sets were eliminated from the study. Finally, 323 data sets were utilized for the analysis. Each patient received 30 to 60 IU/kg/d of antithrombin concentrate for 3 consecutive days unless the patient died or treatment was stopped for any justifiable reason. All but 6 of the patients fulfilled the Japanese Association for Acute Medicine (JAAM) DIC criteria,19 while 280 (86.7%) fulfilled the International Society on Thrombosis and Haemostasis overt-DIC criteria at baseline. The SOFA score was calculated on days 1 (before treatment), 2, 4, and 7.20,21 The overt-DIC score22 and the JAAM DIC were recorded on days 1, 2, 4, and 7, and the changes in each indicator between day 1 and day 7 were defined as the Δ SOFA score, the Δ overt-DIC score, and the Δ JAAM DIC score, respectively. The patients’ outcomes on day 28 were also recorded. If the patient died before day 7, the Δ SOFA score, Δ overt-DIC score, and Δ JAAM DIC score were calculated based on the data obtained on the last day of observation.
Ethics, Patient Consent, Study Permissions, and Consent to Publish
The survey was conducted in accordance with the Declaration of Helsinki and Good Vigilance Practice and Good Post-marketing Study Practice. Although the Japanese Ministry of Health, Labour and Welfare judged that the patients’ agreement was not necessary for this survey, the patients’ agreement and consent were obtained when required by the ethics committee of each hospital. The complete anonymization of personal data was performed upon data collection, and the identification of individual patients was impossible; thus, the Institutional Ethics Committee of Juntendo University judged that consent to publish was not required.18
Statistical Analysis
The numerical values in the text and tables represent the median and interquartile range. Differences in patient characteristics between survivors and nonsurvivors were examined using the Fisher exact test or an unpaired Wilcoxon signed-rank test. The association of the Δ SOFA score, the Δ overt-DIC score, and the Δ JAAM DIC score was measured using the coefficient for the slope between each indicator and the 28-day mortality. The overall mortality effect explained by each indicator was quantified using the regression coefficient of determination (R 2). The results were reported as the slope of the logistic regression, the odds ratios (ORs), the 95% confidence intervals (CI), the P values, and R 2. The area under the receiver operating characteristic (ROC) curve (AUC) was used to discriminate between factors with and those without an association with the 28-day mortality rate. The Youden index was calculated for each score as the cutoff offering the best sensitivity and specificity to predict mortality. The mortality differences were examined between patients who showed an improvement in their Δ SOFA score and those who did not show any improvement. The relationships between the improvement of scores and the mortality were also examined with regard to the DIC scores. For all the analyses, a P value <.05 was considered to denote statistical significance. The abovementioned analyses were performed using SPSS 13.0 for Windows (SPSS Inc, Chicago, Illinois).
Results
Among the 323 patients, 235 (72.8%) patients survived, while 88 (27.2%) patients died. Table 1 shows the baseline characteristics of the patients. The median age of the survivors was 73 years, while that of the nonsurvivors was 79 years (P < .001). The gender distribution was similar between the survivors and the nonsurvivors. The most common suspected infection focus was the respiratory tract, and the prevalence of this focus was higher among nonsurvivors (25.1% among survivors vs 47.7% among nonsurvivors, P < .001). Regarding the coagulation and organ failure profiles, the overt-DIC score, JAAM DIC score, and SOFA score were higher among the nonsurvivors (P < .001, < .040, and < .001, respectively).
Table 1.
Baseline Characteristics of the Patients.
| Characteristics | Survivors, n = 235 | Nonsurvivors, n = 88 | P Value | Missing Value |
|---|---|---|---|---|
| Age, years | 73.0 (64.0-80.0) | 79.0 (70.0-85.0) | <.001 | 0 |
| Sex, male/female | 148/87 | 61/27 | .299 | 0 |
| Overt-DIC score | 4.0 (3.0-5.0) | 5.0 (4.0-6.0) | <.001 | 43 |
| JAAM DIC score | 5.0 (4.8-6.3) | 6.0 (5.0-7.0) | .040 | 6 |
| Antithrombin activity | 47.4 (39.0-57.6) | 44.0 (34.5-51.3) | .005 | 8 |
| Total SOFA score | 11.0 (7.0-13.0) | 13.0 (10.0-16.0) | <.001 | 0 |
| Respiratory score | 2.0 (1.0-3.0) | 3.0 (2.0-3.8) | <.001 | 0 |
| Coagulation score | 2.0 (1.0-3.0) | 2.0 (1.0-3.0) | .357 | 0 |
| Hepatic score | 0.0 (0.0-1.0) | 1.0 (0.0-2.0) | .055 | 0 |
| Cardiovascular score | 3.0 (1.0-4.0) | 3.0 (2.0-4.0) | .146 | 0 |
| Neurological score | 2.0 (1.0-3.0) | 3.0 (2.0-4.0) | <.001 | 0 |
| Renal score | 1.0 (0.0-2.0) | 2.0 (1.0-3.0) | .059 | 0 |
| Suspected source of infection (%) | ||||
| Respiratory tract | 59 (25.1) | 42 (47.7) | <.001 | 0 |
| Digestive tract | 72 (30.6) | 20 (22.7) | .202 | 0 |
| Urinary tract | 28 (11.9) | 10 (11.4) | 1.000 | 0 |
| Biliary tract | 27 (11.5) | 8 (9.1) | .686 | 0 |
Abbreviations: DIC, disseminated intravascular coagulation; JAAM, Japanese Association for Acute Medicine; SOFA, sequential organ failure assessment.
Table 2 summarizes the relationship between each indicator and the mortality end points. Significant correlations were observed between all the indicators and the mortality rate (P < .001, respectively). The slope and OR were similar for the Δ SOFA score, Δ overt-DIC score, and the Δ JAAM DIC score. Overall, the R 2 statistic showed that 31.5% of the mortality effects were explained by the Δ SOFA score. The R 2 values of the Δ overt-DIC score and the Δ JAAM DIC score were 8.2% and 12.4%, respectively. The sensitivity, specificity, positive predictive value, negative predictive value, and accuracy are summarized in Table 3. The specificity and accuracy of the Δ SOFA score for the prediction of 28-day mortality were higher than those of the Δ overt-DIC score (P < .001, respectively).
Table 2.
Comparison of the Predictive Values of Δ SOFA Score, Δ Overt-DIC Score, and Δ JAAM DIC Score.
| Δ SOFA Score | Δ Overt-DIC Score | Δ JAAM DIC Score | |
|---|---|---|---|
| Slope | 0.323 | 0.339 | 0.324 |
| Odds ratio | 1.381 | 1.403 | 1.383 |
| 95% CI | 1.264-1.508 | 1.152-1.709 | 1.203-1.590 |
| P value | <.001 | <.001 | <.001 |
| R 2 | 0.315 | 0.082 | 0.124 |
Abbreviations: CI, confidence interval; DIC, disseminated intravascular coagulation; JAAM, Japanese Society for Acute Medicine; SOFA, sequential organ failure assessment.
Table 3.
Accuracy of Δ mSOFA Score, Δ overt-DIC Score, and Δ JAAM DIC Score to Predict 28-day Mortality in Patients With Sepsis-Associated DIC and Treated With Antithrombin.a
| Δ SOFA Score (%) | Δ Overt-DIC Score (%) | Δ JAAM DIC Score (%) | |
|---|---|---|---|
| Sensitivity | 72.7 | 68.3 (NS) | 70.7 (NS) |
| Specificity | 83.4 | 66.0 (P = .001) | 77.0 (NS) |
| PPV | 62.1 | 45.3 | 54.1 |
| NPV | 89.1 | 83.5 | 87.3 |
| Accuracy | 80.5 | 66.7 (P < .001) | 75.3 (NS) |
Abbreviations: DIC, disseminated intravascular coagulation; JAAM, Japanese Society for Acute Medicine; NPV, negative predictive value; NS, not significant; PPV, positive predictive value; SOFA, sequential organ failure assessment.
aThe statistical difference was calculated between Δ SOFA score and Δ overt-DIC score and between Δ SOFA score and Δ JAAM DIC score.
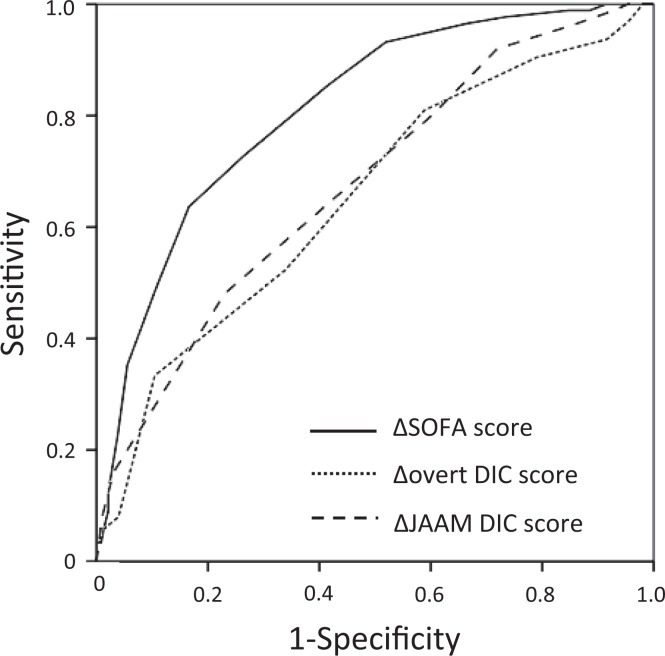
Figure 1 shows the ROC curves of the Δ SOFA score, Δ overt-DIC score, and Δ JAAM DIC score for mortality. The AUCs for the 3 indicators were 0.812 (95% CI, 0.762-0.863, P < .001), 0.653 (95% CI, 0.572-0.735, P < .001), and 0.677 (95% CI, 0.606-0.748, P < .001), respectively.
Figure 1.
Comparison of the receiver operating characteristic (ROC) curves for the Δ SOFA score, the Δ overt-DIC score, and the Δ JAAM DIC score for mortality. The ROC curves for 28-day mortality of the changes from baseline to day 7 in the modified sequential organ failure assessment (SOFA) score (solid line), the overt-DIC score (dotted line), and the Japanese Association for Acute Medicine (JAAM) DIC score (dashed line) are shown. The areas under the ROC curves (AUCs) for the 3 indicators were 0.812, 0.655, and 0.693, respectively.
Discussion
The improvement in the 28-day mortality rate has long been used as the primary end point for sepsis trials. However, patient outcome is affected by many factors other than treatment or infection-related events. Patients with severe sepsis who develop shock and DIC and survive in the hospital for only a few days are included in conventional 28-day mortality assessments, and evaluating the 28-day mortality rate may miss some of the potentially beneficial effects of initial interventions.23 Therefore, the evaluation of therapeutic agents based on differences in 28-day mortality might not be adequate under specific conditions.15 Pocock et al24 proposed changing the outcome measure as an option when the primary outcome of the study fails, and one candidate for a surrogate end point is the SOFA score. The usefulness of serial evaluations of the SOFA score to predict outcome among critically ill patients has been previously reported.25 The idea of using the delta (Δ) SOFA score (ie, the trajectory from the baseline score) as an end point for studies examining treatment effects in critically ill patients was first proposed by de Grooth et al.26 They analyzed 87 RCTs and recommended using Δ SOFA, rather than a fixed-day SOFA. This approach is interesting because Δ SOFA seems to be a more direct and faster measure in relation to the effects of antisepsis therapies.
With regard to the usefulness of the dynamic change of DIC score, Park et al27 reported that the change in DIC score was significantly associated with the hospital mortality. In the present study, we compared the predictive performance of the Δ SOFA score, Δ overt-DIC score, and Δ JAAM DIC score relative to the 28-day mortality rate and found that the performance of the Δ SOFA score was superior to those of the DIC scores. We previously reported the usefulness of the JAAM DIC score for evaluating the treatment effect of anticoagulant therapies.28 In the present study, the AUC of the Δ SOFA score was higher than that of the Δ JAAM DIC score, and the predictive performances of the Δ SOFA score (specificity, negative predictive value, and accuracy) all exceeded 80%. Though the present study should be repeated in another series of patients, we think that the performance of this measure is sufficient. Of note, de Grooth et al also reported that the Δ SOFA score was strongly associated with mortality, explaining 32% of the treatment effects on mortality, and this finding was consistent with the results of our present study. Though we reported the usefulness of measuring Δ SOFA score, we have to keep in mind that these kinds of disease-oriented end points do not always correspond with the most important patient-oriented outcomes.29
The timing of SOFA evaluations is an important issue. Dhainaut et al30 reported that the continuation or worsening of coagulopathy during the first day of sepsis was associated with increases in the development of new organ failure and the 28-day mortality rate. In the present study, we did not compare the performances of the Δ SOFA score at an earlier time. However, we previously reported that the mortality rate of patients whose JAAM-DIC score recovered before day 4 was 7.9%, while that of patients whose score had not recovered by day 7 was 58.2%.31 Furthermore, we previously examined the changes in the JAAM DIC score after 3 days of antithrombin substitution and reported that the AUC for the Δ day 4 JAAM DIC score for 28-day mortality was 0.69.28 Since the AUCs of the Δ day 4 JAAM DIC score in the previous study and the Δ day 7 JAAM DIC score in the present study were almost identical, the predictive value of the Δ SOFA score might also be comparable to that at an earlier timing, and this possibility should be examined in additional studies.
This study has some limitations. First, it should be reminded that the current study was an observational study and therefore could only show a statistical association to the mortality. Second, this study could not establish whether antithrombin influenced the SOFA score or DIC scores. The efficacy of antithrombin for improving overall mortality in sepsis has not been confirmed in the sufficiently powered RCT. Furthermore, since antithrombin is not widely used in the rest of the world, it may not be possible to generalize the results. Although the effectiveness of supplementation therapy has been repeatedly reported12,13 and antithrombin use is recommended for sepsis-associated DIC in the Japanese treatment guidelines,14 its efficacy should be confirmed in adequately powered RCT.32 After all, the usefulness of Δ SOFA score and Δ DIC scores should also be examined in the patients with sepsis-DIC who are not treated with antithrombin.
Conclusions
The Δ SOFA score can be used to predict the outcomes of patients with sepsis-induced DIC who are treated with antithrombin. Since the calculation of the Δ SOFA score is relatively easy and is a component of routine ICU care, its use is applicable in studies examining sepsis. The use of this score may provide an additional means of evaluating the effectiveness of treatments for sepsis-associated DIC at an earlier stage. These results should be examined in the future prospective study performed in septic DIC cohort and not treated with antithrombin.
Acknowledgments
The authors thank all the institutes that cooperated with this study.
Authors’ Note: Our institution does not require ethical approval for reporting individual cases or case series. Informed consent for patient information to be published in this article was not obtained because the complete anonymization of personal data were performed upon data collection and the identification of individual patients was impossible.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: This work was performed using data from a postmarketing surveillance conducted by Nihon Pharmaceutical Co Ltd. M.A. is an employee of Nihon Pharmaceutical Co Ltd.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Ministry of Education, Culture, Sports, Science and Technology-Supported Program for the Strategic Research Foundation at Private Universities 2018.
ORCID iD: Toshiaki Iba, MD  https://orcid.org/0000-0002-0255-4088
https://orcid.org/0000-0002-0255-4088
Hideo Wada, MD  https://orcid.org/0000-0001-9021-8633
https://orcid.org/0000-0001-9021-8633
References
- 1. Levi M, Schultz M, van der Poll T. Sepsis and thrombosis. Semin Thromb Hemost. 2013;39(5):559–566. [DOI] [PubMed] [Google Scholar]
- 2. Semeraro N, Ammollo CT, Semeraro F, et al. Sepsis, thrombosis and organ dysfunction. Thromb Res. 2012;129(3):290–295. [DOI] [PubMed] [Google Scholar]
- 3. Kidokoro A, Iba T, Fukunaga M, et al. Alterations in coagulation and fibrinolysis during sepsis. Shock. 1996;5(3):223–228. [DOI] [PubMed] [Google Scholar]
- 4. Iba T, Levy JH. Inflammation and thrombosis: roles of neutrophils, platelets and endothelial cells and their interactions in thrombus formation during sepsis. J Thromb Haemost. 2017;16(2):231–241. [DOI] [PubMed] [Google Scholar]
- 5. Ogura H, Gando S, Saitoh G, et al. Epidemiology of severe sepsis in Japanese intensive care units: a prospective multicenter study. J Infect Chemother. 2014;20(3):157–162. [DOI] [PubMed] [Google Scholar]
- 6. Iba T, Gando S, Thachil J. Anticoagulant therapy for sepsis-associated disseminated intravascular coagulation: the view from Japan. J Thromb Haemost. 2014;12(7):1010–1019. [DOI] [PubMed] [Google Scholar]
- 7. Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis AND Septic Shock: 2016. Crit Care Med. 2017;45(3):486–552. [DOI] [PubMed] [Google Scholar]
- 8. Kienast J, Juers M, Wiedermann CJ, et al. Treatment effects of high-dose antithrombin without concomitant heparin in patients with severe sepsis with or without disseminated intravascular coagulation. J Thromb Haemost. 2006;4(1):90–97. [DOI] [PubMed] [Google Scholar]
- 9. Dhainaut JF, Yan SB, Joyce DE, et al. Treatment effects of drotrecogin alfa (activated) in patients with severe sepsis with or without overt disseminated intravascular coagulation. J Thromb Haemost. 2004;2(11):1924–1933. [DOI] [PubMed] [Google Scholar]
- 10. Yamakawa K, Umemura Y, Hayakawa M, et al. Benefit profile of anticoagulant therapy in sepsis: a nationwide multicentre registry in Japan. Crit Care. 2016;20(1):229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Umemura Y, Yamakawa K, Ogura H, et al. Efficacy and safety of anticoagulant therapy in three specific populations with sepsis: a meta-analysis of randomized controlled trials. J Thromb Haemost. 2016;14(3):518–530. [DOI] [PubMed] [Google Scholar]
- 12. Tagami T, Matsui H, Horiguchi H, et al. Antithrombin and mortality in severe pneumonia patients with sepsis-associated disseminated intravascular coagulation: an observational nationwide study. J Thromb Haemost. 2014;12(9):1470–1479. [DOI] [PubMed] [Google Scholar]
- 13. Tagami T, Matsui H, Fushimi K, et al. Supplemental dose of antithrombin use in disseminated intravascular coagulation patients after abdominal sepsis. Thromb Haemost. 2015;114(3):537–545. [DOI] [PubMed] [Google Scholar]
- 14. Nishida O, Ogura H, Egi M, et al. The Japanese Clinical Practice Guidelines for Management of Sepsis and Septic Shock 2016 (J-SSCG 2016). Acute Med Surg. 2018;5(1):3–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Opal SM, Dellinger RP, Vincent JL, et al. The next generation of sepsis clinical trial designs: what is next after the demise of recombinant human activated protein C. Crit Care Med. 2014;42(7):1714–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gando S, Saitoh D, Ishikura H, et al. A randomized, controlled, multicenter trial of the effects of antithrombin on disseminated intravascular coagulation in patients with sepsis. Crit Care. 2013;17(6):R297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vincent JL, Angus D, Annane D, et al. Clinical expert round table discussion (session 5) at the Margaux Conference on Critical Illness: outcomes of clinical trials in sepsis: lessons learned. Crit Care Med. 2001;29(suppl 7):S136–S137. [DOI] [PubMed] [Google Scholar]
- 18. Iba T, Hagiwara A, Saitoh D, et al. Effects of combination therapy using antithrombin and thrombomodulin for sepsis-associated disseminated intravascular coagulation. Ann Intensive Care. 2017;7(1):110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gando S, Iba T, Eguchi Y, et al. A multicenter, prospective validation of disseminated intravascular coagulation diagnostic criteria for critically ill patients: comparing current criteria. Crit Care Med. 2006;34(3):625–631. [DOI] [PubMed] [Google Scholar]
- 20. Moreno R, Vincent JL, Matos R, et al. The use of maximum SOFA score to quantify organ dysfunction/failure in intensive care. Results of a prospective, multicentre study. Working Group on Sepsis Related Problems of the ESICM. Intensive Care Med. 1999;25(7):686–696. [DOI] [PubMed] [Google Scholar]
- 21. Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-Related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22(7):707–710. [DOI] [PubMed] [Google Scholar]
- 22. Taylor FB, Toh CH, Hoots WK, et al. Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost. 2001:86:1327–1330. [PubMed] [Google Scholar]
- 23. Iba T, Arakawa M, Ohchi Y, et al. Prediction of early death in patients with sepsis-associated coagulation disorder treated with antithrombin supplementation. Clin Appl Thromb Hemost. 2018. doi:10.1177/1076029618797474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pocock SJ, Stone GW. The primary outcome fails—what next? N Engl J Med. 2016;375(9):861–870. [DOI] [PubMed] [Google Scholar]
- 25. Ferreira FL, Bota DP, Bross A, Mélot C, Vincent JL. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA. 2001;286(14):1754–1758. [DOI] [PubMed] [Google Scholar]
- 26. de Grooth HJ, Geenen IL, Girbes AR, Vincent JL, Parienti JJ, Oudemans-van Straaten HM. SOFA and mortality endpoints in randomized controlled trials: a systematic review and meta-regression analysis. Crit Care. 2017;21(1):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Park JY, Park S, Park SY, et al. Day 3 versus Day 1 disseminated intravascular coagulation score among sepsis patients: a prospective observational study. Anaesth Intensive Care. 2016;44(1):57–64. [DOI] [PubMed] [Google Scholar]
- 28. Iba T, Saitoh D, Gando S, et al. The usefulness of antithrombin activity monitoring during antithrombin supplementation in patients with sepsis-associated disseminated intravascular coagulation. Thromb Res. 2015;135(5):897–901. [DOI] [PubMed] [Google Scholar]
- 29. de Grooth HJ, Parienti JJ, Oudemans-van Straaten HM. Should we rely on trials with disease rather than patient-oriented endpoints? Intensive Care Med. 2018;44(4):464–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dhainaut JF, Shorr AF, Macias WL, et al. Dynamic evolution of coagulopathy in the first day of severe sepsis: relationship with mortality and organ failure. Crit Care Med. 2005;33(2):341–348. [DOI] [PubMed] [Google Scholar]
- 31. Iba T, Gando S, Saitoh D, Wada H, Di Nisio M, Thachil J. Antithrombin supplementation and risk of bleeding in patients with sepsis-associated disseminated intravascular coagulation. Thromb Res. 2016;145:46–50. [DOI] [PubMed] [Google Scholar]
- 32. Levi M, Scully M. How I treat disseminated intravascular coagulation. Blood. 2017;131(8):845–854. [DOI] [PubMed] [Google Scholar]