Abstract
Both vitamin K antagonists (VKAs) and novel oral anticoagulants (NOACs) are effective for stroke prevention in nonvalvular atrial fibrillation (NVAF) patients. This study evaluated the utilization of VKA and NOACs in NVAF patients before and after catheter ablation in China. Prescription data were retrospectively collected between January 1, 2016, and December 31, 2016, including indication of use, dose, renal function, and risk assessment (CHA2DS2-VASc score and HAS-BLED score) in Zhongshan Hospital of Fudan University. Trends and factors associated with anticoagulants use before and after ablation were evaluated. A total of 475 patients with NVAF who received ablation were included in the analysis. Of all, 53.26% of them received antithrombotic therapy preablation. Warfarin was prescribed in 35.26%, with NOACs in 11.37%. Four hundred seventy-three patients received antithrombotic therapy (99.58%) postablation, 236 patients with NOACs (49.68%). CHA2DS2-VASc score, HAS-BLED score, hypertension, diabetes mellitus, and alcohol were independently associated with anticoagulant utilization before catheter ablation. The higher CHA2DS2-VASc score was associated with less frequent prescription of NOACs postablation. The preablation anticoagulation use was still inadequate in China, and CHA2DS2-VASc score was a significant factor influencing the preablation anticoagulant utilization. The utilization rate of NOACs increased significantly postablation, especially for dabigatran, which implied that more physicians prefer to prescribe NOACs for NVAF patients after ablation in our country and may be attributed to the aspects such as ease of NOAC use but also possibly the greater safety and efficacy. Furthermore, the physicians may reluctant to use NOACs for high stroke risk atrial fibrillation patients after catheter ablation.
Keywords: anticoagulant utilization, atrial fibrillation, novel oral anticoagulant, warfarin
Introduction
Atrial fibrillation (AF) is the most common sustained cardiac rhythm disorder in clinical practice.1 Thromboembolic events associated with AF are leading cause of morbidity and mortality worldwide.2,3 Both vitamin K antagonist (VKA) and novel oral anticoagulants (NOACs) are effective for the prevention of stroke in nonvalvular atrial fibrillation (NVAF) patients and can prolong life.4 Because of the greater efficacy, safety, and no international normalized ratio (INR) monitoring of NOACs compared to the VKA, most guidelines now recommend that NOACs should be considered instead of VKA.4,5 Furthermore, NOACs are considered to be preferentially indicated in Asians, owning to their significantly lower relative risk than VKA for bleeding and intracranial hemorrhage (ICH) in Asians, while maintaining their efficacy profile.6,7
During the past decade, transcatheter ablation has developed from a specialized, experimental procedure to a common treatment to prevent this prevalent cardiac rhythm disorder and recurrent AF at most medical centers worldwide.8,9 In line with the 2016 guidelines of European Society of Cardiology (ESC), oral anticoagulants (OAC) after catheter ablation should be maintained for at least 8 weeks for stroke prevention.10–14
Compared to Europe and the United States, few studies of anticoagulants utilization after catheter ablation have been conducted in Asian patients with AF, especially in the Chinese population. In this study, the prescription pattern and predictors of the anticoagulants use in hospitalized patients before and after catheter ablation at Shanghai of China were studied. Furthermore, the application of NOACs and VKA was analyzed to assess the utilization of anticoagulants compared to guideline recommendations, as well as secular trends in prescription of OAC, and NOACs especially, in patients with NVAF.
Method
Data Source
This study was performed using data from the Hospital Information System (HIS) of Zhongshan Hospital of Fudan University. The HIS is a comprehensive, integrated information system designed to manage all the aspects of a hospital’s operation, containing medical, pharmaceutical, and financial information of patients.
Study Population
A retrospective study was performed to investigate anticoagulants use for the prevention of stoke in NVAF patients after successful radiofrequency catheter ablation in Zhongshan Hospital of Fudan University. Patients studied in this analysis were enrolled in the HIS between January 1, 2016, and December 31, 2016. All patients diagnosed with NVAF as their primary (ie, AF was the presenting complaint) or secondary condition (ie, AF was listed as a current illness in the medical history or discharge summary) and received successful radiofrequency catheter ablation in our hospital were included. This study was achieved by excluding everyone with a history of artificial heart valve replacement or pacemaker installment or other heart surgery.
Data Collection
Data regarding patient demographics, anticoagulants on admission, and history of comorbid conditions were collected. The CHA2DS2-VASc (congestive heart failure, hypertension, age ≥75 [doubled], Diabetes, Stroke [doubled], Vascular disease, Age 65 to 74, and Sex [female]) score was evaluated for assessing stoke risk and the HAS-BLED (Hypertension, abnormal renal function, abnormal liver function, bleeding predisposition [doubled], age >65 years, the use of drugs predisposing patients to bleeding [nonsteroidal anti-inflammatory drugs], alcohol use [>8 drinks per week], previous stroke and labile INRs [doubled]) score for assessing bleeding risk. In general, patients without clinical stroke risk factors do not need antithrombotic therapy, while patients with stroke risk factors (CHA2DS2-VASc score ≥1 for men, and ≥2 for women) are likely to benefit from OAC.4 We regarded the CHA22DS2-VASc score ≥1 for men and ≥2 for women as the high risk, while CHA2DS2-VASc score <1 for men and <2 for women as low risk.
The labile INRs was defined as unstable/high INRs as recorded in the medical record of our patients. Anticoagulants using pattern before catheter ablation was collected by us based on the in-hospital records of the patient’s medical records. All antithrombotic prescription patterns in our population were identified. Vitamin K antagonist included warfarin, and NOAC included dabigatran and rivaroxaban. The NOAC apixaban and edoxaban were not licensed for the prevention of stoke in NVAF patients in China during the study period.
Statistical Analysis
Data were analyzed using SPSS version 21.0 (Prentice Hall, New Jersey). Continuous variables are expressed as mean (standard deviation) and were compared with Student t test or Mann-Whitney U test as appropriate. Categorical variables were compared by χ2 analysis or Fisher’s exact test, as appropriate. A P < .05 indicated statistical significance. Logistic regression modeling was used to identify the independent association between patient characteristics and antithrombotic therapy prescription in eligible patients.
Results
Demographics
A total of 475 patients with NVAF who received successful radiofrequency catheter ablation were included in the analysis. The baseline characteristics of the 475 patients are displayed in Table 1. The mean age was 59.88 years with a range from 21 to 82. Notably, more than half of the patients were male, and only 6.32% (n = 30) of them were aged >75 years. Paroxysmal AF was present in 82.53% (n = 392) and persistent in 17.47% (n = 83) of the patients. Congestive heart failure was present in 6.53% (n = 31), hypertension in 24.70% (n = 184), diabetes mellitus in 13.26% (n = 63), previous stoke, transient ischemic attacks, or thromboembolism in 13.26% (n = 28), and coronary atherosclerosis in 9.47% (n = 45) of the patients. Of all, 45.89% (n = 218) received Medicare, while 54.11% (n = 257) were self-paying.
Table 1.
Patient Characteristics.
| Total | N = 475 |
|---|---|
| Ages (average) | 59.88 (10.89) |
| Gender | |
| Male | 312 (65.68%) |
| Female | 163 (34.32%) |
| Duration of AF (years) | 2.95 (4.17) |
| Patterns of atrial fibrillation | |
| Paroxysmal AF | 392 (82.53%) |
| Persistent AF | 83 (17.47%) |
| CHA2DS2-VASc score | |
| Low (score 0) | 124 (26.11%) |
| Intermediate (score 1) | 132 (27.79%) |
| High (score ≥2) | 219 (46.11%) |
| HAS-BLED score | |
| Low (score 0) | 248 (52.21%) |
| Intermediate (score 1or 2) | 222 (46.74%) |
| High (score ≥3) | 5 (1.05%) |
| Insurance | |
| Self-paying | 218 (45.89%) |
| Medicare | 257 (54.11%) |
| Complication | |
| Congestive heart failure | 32 (6.74%) |
| Hypertension | 204 (42.95%) |
| Diabetes mellitus | 70 (14.74) |
| Previous stroke, TIA, or TE | 33 (6.95%) |
| Coronary heart disease | 47 (9.89%) |
| Abnormal renal/liver function | 9 (1.89%) |
| Alcohol | 31 (6.53%) |
Abbreviations: AF, atrial fibrillation; TIA, transient ischemic attacks; TE, thromboembolism.
The average CHA2DS2-VASc score in the population was 1.60 (1.43), nearly half scored more than 1 points. The average HAS-BLED score was 0.59 (0.71), among them, only 5 persons scored higher than 2. Only 6 of the all patients had renal insufficiency and no one required renal adjustments.
Anticoagulation Use in Relation to CHA2DS2-VASc Score
The prevalence of anticoagulation use preablation is described in Table 2 according to female and male, respectively. Out of 163 female patients, 48 (29.45%) of them had a CHA2DS2-VASc score ≤1 with low embolism risk and 115 (70.55%) had a CHA2DS2-VASc score ≥2 with high embolism risk. Fifty-five of the high embolism risk female patients were on no OAC preablation. Among the male patients, 124 (39.74%) had a CHA2DS2-VASc score = 0 with low embolism risk, while 188 (60.26%) had a CHA2DS2-VASc score ≥1 with high embolism risk. Seventy-nine of the high embolism risk male patients were on no OAC preablation.
Table 2.
The Anticoagulants Use in Relation to CHA2DS2-VASc Score Before Ablation.
| In Female | ||||
|---|---|---|---|---|
| No Therapy | NOACs | Warfarin | Antiplateleta | |
| CHA2DS2-VASc score ≤1 (n = 48) | 24 | 4 | 15 | 5 |
| CHA2DS2-VASc score ≥ 2 (n = 115) | 55 | 16 | 38 | 6 |
| Total (163) | 79 | 20 | 53 | 11 |
| In Male | ||||
| No Therapy | NOACs | Warfarin | Antiplateleta | |
| CHA2DS2-VASc score = 0 (n = 124) | 64 | 9 | 37 | 14 |
| CHA2DS2-VASc score ≥ 1 (n = 188) | 79 | 25 | 68 | 16 |
| Total (312) | 143 | 34 | 105 | 30 |
Abbreviation: NOACs, novel oral anticoagulants.
a Lone antiplatelet therapy, aspirin 0.1 mg once daily.
Anticoagulation use in relation to CHA2DS2-VASc score postablation is reported in Table 3. In total, 124 (26.11%) had a CHA2DS2-VASc score of 0, 132 (27.79%) had a CHA2DS2-VASc score of 1, whereas the remaining 219 (46.10%) patients had a CHA2DS2-VASc score ≥2. Patients with CHA2DS2-VASc score of 0 were preferred to receive NOACs (56.45%), whereas patients with CHA2DS2-VASc score ≥2 were likely to receive warfarin (50.68%). Table 3 indicates that the higher CHA2DS2-VASc score is associated with less frequent prescription of NOACs.
Table 3.
The Anticoagulants Use Relation to CHA2DS2-VASc Score After Ablation.
| No Therapy | NOACs | Warfarin | Antiplateleta | |
|---|---|---|---|---|
| CHA2DS2-VASc score = 0 (n = 124) | 1 (0.81%) | 70 (56.45%) | 50 (40.32%) | 3 (2.42%) |
| CHA2DS2-VASc score = 1 (n = 132) | 0 | 61 (46.21%) | 69 (52.27%) | 2 (1.52%) |
| CHA2DS2-VASc score ≥ 2 (n = 219) | 1 (0.46%) | 105 (47.95%) | 111 (50.68%) | 2 (0.91%) |
| Total (163) | 2 | 236 | 230 | 7 |
Abbreviation: NOACs, novel oral anticoagulants.
aLone antiplatelet therapy, aspirin 0.1 mg once daily.
Preablation and Postablation Anticoagulation
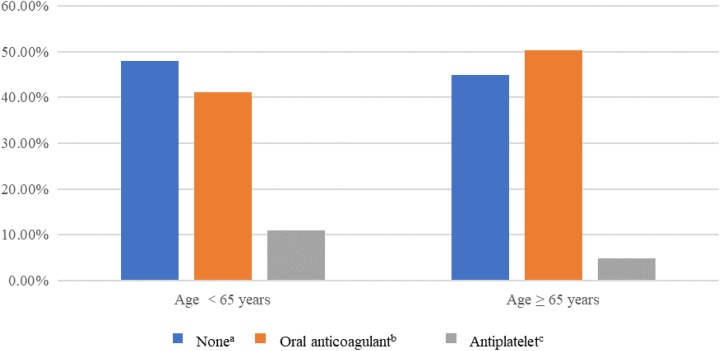
Table 4 shows the pre- and postablation anticoagulation regimens. Before ablation, there were 222 (46.74%) patients on no OAC. The preablation OAC was antiplatelet in 41 (8.63%), warfarin in 158 (33.26%) patients, dabigatran in 43 (9.05%), and rivaroxaban in 11 (2.32%). The antithrombotic prescription patterns before ablation according to age are presented in Figure 1. The patients over 65 years old received more anticoagulation prescriptions than those less than 65.
Table 4.
Number of Patients on Each Anticoagulant Preablation and Postablation.
| Preablation Anticoagulation | Number Preablation | Postablation Anticoagulation | ||||
|---|---|---|---|---|---|---|
| None | Antiplateleta | Warfarin | Dabigatran | Rivaroxaban | ||
| None | 222 | 1 | 6 | 98 | 100 | 17 |
| Antiplatelet | 41 | 0 | 1 | 14 | 24 | 2 |
| Warfarin | 158 | 1 | 0 | 115 | 35 | 7 |
| Dabigatran | 43 | 0 | 0 | 3 | 38 | 2 |
| Rivaroxaban | 11 | 0 | 0 | 0 | 0 | 11 |
| Total | 475 | 2 | 7 | 230 | 197 | 39 |
aLone antiplatelet therapy, aspirin 0.1 mg once daily.
Figure 1.
Antithrombotic prescriptions before catheter ablation described according to age.aThe patients with no antithrombotic therapy.b The patients received warfarin or novel oral anticoagulants. cThe patients received aspirin, 0.1 mg once daily.
After catheter ablation, almost all the patients received antithrombotic therapy (n = 473, 99.58%), nearly half patients with NOACs (n = 236, 49.68%). Out of 49.68% with NOACs, 41.47% were given dabigatran and 8.21% were given rivaroxaban. Still 2 patients did not receive antithrombotic therapy and 7 patients were discharged only on antiplatelet therapy due to afraid of bleeding. The postablation anticoagulation prescriptions of those patients were not always the same as the preablation anticoagulation (Table 4). Of the 475 patients, 166 (34.95%) were on the same anticoagulation before and after ablation, while 309 (65.05%) were on a different anticoagulant postprocedure. Among the patients of prescription change, 185 changed to NOACs, while 115 turned to warfarin. Of 263 patients on antiplatelet or no preablation anticoagulation, 112 began warfarin, 124 began dabigatran, 19 on rivaroxaban, and 7 remained on antiplatelets. Of 212 patients on warfarin, dabigatran, or rivaroxaban preprocedure, 164 (77.36%) remained on the same anticoagulation and 48 (22.64%) changed to a different anticoagulant postprocedure. For warfarin, 42 of 158 changed to dabigatran, while 1 changed to aspirin. For dabigatran, 3 patients changed to warfarin, 1 for gastrointestinal hemorrhage and the other 2 for mucocutaneous hemorrhage, and 2 patients changed to rivaroxaban for the more convenient administration than dabigatran.
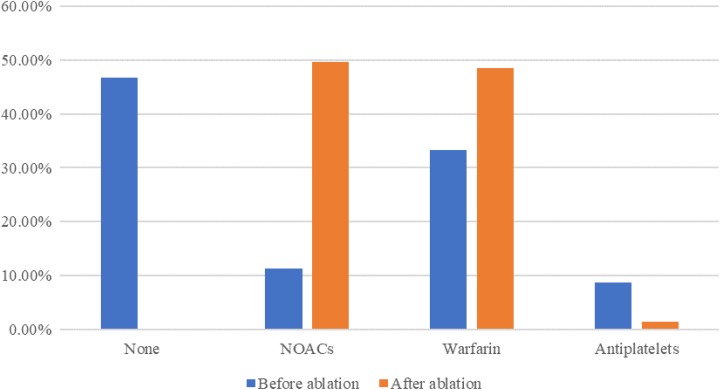
Variation in antithrombotic therapy prescriptions before and after ablation is seen in Figure 2. Among these drugs, NOAC use has seen the greatest increase percentage, reached 337.04%.
Figure 2.
Variation of antithrombotic therapy prescriptions before and after ablation. NOACs indicates novel oral anticoagulants.
Factors Associated With Anticoagulant Prescribing at Discharge
Logistic regression was performed to identify the impact of a number of factors on the antithrombotic therapy prescription in eligible patients before and after ablation (Table 5). Eight variables were included: age, gender, patterns of AF, CHA2DS2-VASc score, HAS-BLED score, the type of insurance, concomitant disease (included congestive heart failure, hypertension, diabetes, previous stroke, coronary heart disease), and alcohol. The model was statistically significant (P < .05). Age between 65 and 75 years (odds ratio [OR]: 7.523, 95% confidence interval [CI]: 1.76-32.163; P = .006), CHA2DS2-VASc score = 1 (OR: 0.001, CI: 0.001-0.004; P < .0001), CHA2DS2-VASc score ≥2 (OR: 0.047, CI: 0.019-0.114; P < .0001), HAS-BLED score = 1 or 2 (OR: 0.131, CI: 0.018-0.942; P = .043), HAS-BLED score ≥3 (OR: 0.100, CI: 0.016-0.623; P = .014), hypertension (OR: 0.193, CI: 0.106-0.349; P < .0001), diabetes mellitus (OR: 0.198, CI: 0.106-0.349; P < .0001), and alcohol (OR: 0.028, CI: 0.003-0.314; P = .004) were independently associated with anticoagulant prescribing before catheter ablation (Table 5). However, we did not find significant factors associated with the postablation anticoagulation use.
Table 5.
Factors Associated With Anticoagulant Prescribing Pre- and Postablation.
| Variable | No OAC vs OAC Before Ablation | NOACs vs Warfarin After ablation | ||
|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Sex | 0.972 (0.928-1.018) | .230 | 1.183 (0.719-1.946) | .508 |
| Age<65 | Reference | Reference | ||
| Age (65-75) | 7.523 (1.76-32.163) | .006 | 1.263 (0.480-3.319) | 1.263 |
| Age (≥75) | 1.452 (0.503-4.191) | .490 | 1.147 (0.502-2.619) | .746 |
| Patterns of AF | 0.959 (0.499-1.844) | .90 | 0.999 (0.608-1.642) | .996 |
| Insurance | 0.849 (0.570-1.266) | .93 | 1.040 (0.714-1.516) | .837 |
| CHA2DS2-VASc score | ||||
| SCORE = 0 | Reference | Reference | ||
| Score = 1 | 0.001 (0.001-0.004) | <.0001 | 0.876 (0.3-22-2.385) | .795 |
| Score ≥2 | 0.047 (0.019-0.114) | <.00001 | 1.333 (0.661-2.686) | .422 |
| HAS-BLED score | ||||
| Score = 0 | Reference | Reference | ||
| Score 1 or 2 | 0.131 (0.018-0.942) | .043 | 0.707 (0.166-3.019) | .64 |
| Score ≥3 | 0.100 (0.016-0.623) | .014 | 1.005 (0.267-3.775) | .995 |
| CHF | 1.096 (0.421-2.858) | .851 | 1.871 (0.833-4.199) | .129 |
| Hypertension | 0.193 (0.106-0.349) | <.0001 | 1.153 (0.680-1.955) | .598 |
| DM | 0.198 (0.093-0.442) | <.0001 | 0.939 (0.510-1.731) | .841 |
| Previous stroke, TIA or TE | 0.623 (0.205-1.896) | .405 | 1.041 (0.472-2.294) | .92 |
| CHD | 0.989 (0.436-2.244) | .979 | 1.211 (0.628-2.336) | .568 |
| Alcohol | 0.028 (0.003-0.314) | .004 | 1.436 (0.617-3.343) | .401 |
Abbreviations: CHD, coronary heart disease; CHF, congestive heart failure; CI, confidence interval; DM, diabetes mellitus; NOACs, novel oral anticoagulants; OAC, oral anticoagulant; OR, odds ration; TE, thromboembolism; TIA, transient ischemic attacks.
Discussion
Our study found that of 475 patients, 303 were high risk for thrombosis, and they were eligible for OACs before ablation according to the guidelines and accounting for the presence of contraindications, but only 147 (48.51%) received an anticoagulant. Underutilization of anticoagulant therapy of these eligible high-risk patients was observed in our study. This finding is in line with the China15 and International16–19 studies reporting that anticoagulants are commonly underutilized in AF patients. Antiplatelet therapy cannot be recommended for stroke prevention in NVAF patients. But, in our population, there was a little preference for lone antiplatelet therapy, with 22 of our high-risk population receiving this therapy. Of the 172 patients who did not need anticoagulation, 65 (37.79%) received an anticoagulant and 19 (11.05%) received lone antiplatelet therapy. However, only 7 of them received antiplatelet therapy after ablation.
After ablation, 466 (98.11%) of the whole patients turned to anticoagulant therapy. This was in line with the updated consensus statement on catheter and surgical ablation of AF,20 which recommend that patients should be anticoagulated for at least 2 months postablation, regardless of their CHA2DS2-VASc score or rhythm status,20 but was odds with the consensus of 2012 year,8 which recommended that decisions regarding the use of systemic anticoagulation more than 2 months following ablation should be based on the patient’s stoke risk, and to continue anticoagulation treatment in patients with high stroke risk.8 Accordingly, those studies before 2017 were assessed by the consensus of 2012, which indicated irrational utilization of anticoagulation after ablation.21,22
Ultimately, 185 (38.95%) patients turned to NOACs therapy, of which 42 (22.7%) transitioned from warfarin to NOAC therapy. Nevertheless, only 3 (0.63%) patients changed from NOACs to warfarin therapy. Novel oral anticoagulants were increasingly used in our study, both for initial non-anticoagulation and warfarin pretreated patients. Reasons why patients switch from one OAC to another after catheter ablation are many. Patients turned to NOAC therapy may be influenced by aspects such as intended greater safety and efficacy but possibly also practicability due to no INR monitoring. Patients on dabigatran with gastrointestinal symptoms or minor bleeding may desire to switch to warfarin or a different NOAC.
Results showed that 83.47% of patients received NOAC therapy oral dabigatran, which was different from the study of Jobski et al23 and Providencia et al.24 Possible reason for underuse of rivaroxaban was that dabigatran was approved for preventing stork in NVAF patients in the year of 2013, while rivaroxaban was in the year of 2015. Furthermore, rivaroxaban is much more expensive than dabigatran. Dabigatran 110 mg twice daily was given to the patients who received dabigatran therapy, while dabigatran 150 mg twice daily was not observed in our population. We identified physician’s decision to be the most common reason for taking gastrointestinal bleeding into account. Of the 39 patients who received rivaroxaban therapy, 32 (82.51%) were given 20 mg once daily, and another 7 (17.95%) patients with no renal insufficiency were given dose below 20 mg once daily, who received insufficient anticoagulant doses.
The utilization rate of NOACs increased significantly after ablation, from 54 (11.37%) to 236 (49.68%), exceeding that of warfarin, which from 158 (33.26%) increased to 230 (48.42%). The obvious increase in NOACs use may be attributed to the ease of NOACs use, marketing efforts around the time of NOACs approved by China Food and Drug Administration, and adoption of recommendations published in the ESC guidelines on the use of NOACs in patients with NVAF. The European guidelines have indicated a preference for NOACs over warfarin in embolism prevention for AF patients, based on their clinical benefit. Meanwhile, NOACs are considered to be preferentially indicated in Asians, owing to their significantly lower relative risk than VKA for ICH and bleeding in Asians, while maintaining their efficacy profile.7,25
Factors associated with anticoagulant prescribing before and after AF ablation are different. CHA2DS2-VASc score as a significant predictor of anticoagulant prescribing before catheter ablation in our population was observed. The higher CHA2DS2-VASc score was associated with a higher anticoagulation prescription preablation, which was inconsistent with the discovery by Bista et al.17,21 Furthermore, HAS-BLED score, age, hypertension, and diabetes mellitus were also related to the use of anticoagulant before catheter ablation. Although those factors were observed as negative predictors for anticoagulant prescribing after catheter ablation, we found that the higher CHA2DS2-VASc score was associated with less frequent prescription of NOACs, which may illustrate that practitioner reluctant to use a newer therapy for higher embolism risk patients.
Our study presents some limitations that must be pointed out. First of all, the retrospective nature of the study could have resulted in an overestimation of utilization of anticoagulants. Secondly, as a single-center retrospective study, the small sample size and limited study duration might be not enough to explain absolute conclusion. Additionally, this study also potentially limited by the quality of documentation within HIS system. Interactions between NOACs and other medications were not illuminated in the study. However, to our knowledge, this study is the exceptional investigation of anticoagulation utilization in Chinese NVAF patients before and after catheter ablation.
Conclusion
The utilization of anticoagulant therapy in NVAF patients before catheter ablation in China was still inadequate. CHA2DS2-VASc score, HAS-BLED score, age, hypertension, and diabetes mellitus were significant factors influencing the anticoagulant utilization before catheter ablation. In contrast to that, nearly all the patients received anticoagulant therapy after ablation. The number of patients switching from warfarin to NOACs was much more than those switching from NOACs to warfarin, and use of NOACs increased in our study, especially of dabigatran, which implied that more physicians prefer to prescribe NOACs for NVAF patients after ablation in our country, because of the aspects of NOACs such as intended greater safety and efficacy but possibly also practicability due to no INR monitoring. Although the CHA2DS2-VASc score was not a significant factor influencing the anticoagulation postablation, the higher CHA2DS2-VASc score was observed associated with less frequent prescription of NOACs after ablation.
Acknowledgments
Cao Lei and Xu Jianan (Zhongshan Hospital of Fudan University) are acknowledged for valuable help with data collection.
Authors’ Note: The authors declare that the paper was not presented orally at a professional meeting.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Chen Tingting, BS  https://orcid.org/0000-0003-0197-1987
https://orcid.org/0000-0003-0197-1987
References
- 1. Wilke T, Groth A, Mueller S, et al. Incidence and prevalence of atrial fibrillation: an analysis based on 8.3 million patients. Europace. 2013;15(4):486–493. [DOI] [PubMed] [Google Scholar]
- 2. Patel NJ, Deshmukh A, Pant S, et al. Contemporary trends of hospitalization for atrial fibrillation in the United States, 2000 through 2010: implications for healthcare planning. Circulation. 2014;129(23):2371–2379. [DOI] [PubMed] [Google Scholar]
- 3. Chugh SS, Havmoeller R, Narayanan K, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129(8):837–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37(38):2893–2962. [DOI] [PubMed] [Google Scholar]
- 5. Banerjee A, Lane DA, Torp-Pedersen C, Lip GY. Net clinical benefit of new oral anticoagulants (dabigatran, rivaroxaban, apixaban) versus no treatment in a ‘real world’ atrial fibrillation population: a modelling analysis based on a nationwide cohort study. Thromb Haemost. 2012;107(3):584–589. [DOI] [PubMed] [Google Scholar]
- 6. Heidbuchel H, Verhamme P, Alings M, et al. Updated European Heart Rhythm Association practical guide on the use of non-vitamin-K antagonist anticoagulants in patients with non-valvular atrial fibrillation: executive summary. Eur Heart J. 2017;38(27):2137–2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chiang CE, Wang KL, Lip GY. Stroke prevention in atrial fibrillation: an Asian perspective. Thromb Haemost. 2014;111(5):789–797. [DOI] [PubMed] [Google Scholar]
- 8. Calkins H, Kuck KH, Cappato R, et al. ; Heart Rhythm Society Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design: a report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Developed in partnership with the European Heart Rhythm Association (EHRA), a registered branch of the European Society of Cardiology (ESC) and the European Cardiac Arrhythmia Society (ECAS); and in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), the Asia Pacific Heart Rhythm Society (APHRS), and the Society of Thoracic Surgeons (STS). Endorsed by the governing bodies of the American College of Cardiology Foundation, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, the Asia Pacific Heart Rhythm Society, and the Heart Rhythm Society. Heart Rhythm. 2012;9(4):632–696.e621. [DOI] [PubMed] [Google Scholar]
- 9. Arbelo E, Brugada J, Hindricks G, et al. The atrial fibrillation ablation pilot study: a European Survey on Methodology and results of catheter ablation for atrial fibrillation conducted by the European Heart Rhythm Association. Eur Heart J. 2014;35(22):1466–1478. [DOI] [PubMed] [Google Scholar]
- 10. Hunter RJ, McCready J, Diab I, et al. Maintenance of sinus rhythm with an ablation strategy in patients with atrial fibrillation is associated with a lower risk of stroke and death. Heart. 2012;98(1):48–53. [DOI] [PubMed] [Google Scholar]
- 11. Karasoy D, Gislason GH, Hansen J, et al. Oral anticoagulation therapy after radiofrequency ablation of atrial fibrillation and the risk of thromboembolism and serious bleeding: long-term follow-up in nationwide cohort of Denmark. Eur Heart J. 2015;36(5):307–314a. [DOI] [PubMed] [Google Scholar]
- 12. Themistoclakis S, Corrado A, Marchlinski FE, et al. The risk of thromboembolism and need for oral anticoagulation after successful atrial fibrillation ablation. J Am Coll Cardiol. 2010;55(8):735–743. [DOI] [PubMed] [Google Scholar]
- 13. Bunch TJ, May HT, Bair TL, et al. Atrial fibrillation ablation patients have long-term stroke rates similar to patients without atrial fibrillation regardless of CHADS2 score. Heart Rhythm. 2013;10(9):1272–1277. [DOI] [PubMed] [Google Scholar]
- 14. Reynolds MR, Gunnarsson CL, Hunter TD, et al. Health outcomes with catheter ablation or antiarrhythmic drug therapy in atrial fibrillation: results of a propensity-matched analysis. Circ Cardiovasc Qual Outcomes. 2012;5(2):171–181. [DOI] [PubMed] [Google Scholar]
- 15. Zhao S, Zhao H, Wang X, et al. A prospective study investigating the causes of warfarin under-utilization in Chinese patients. Int J Clin Pharm. 2016;38(5):1286–1293. [DOI] [PubMed] [Google Scholar]
- 16. Frain B, Castelino R, Bereznicki LR. The utilization of antithrombotic therapy in older patients in aged care facilities with atrial fibrillation. Clin Appl Thromb Hemost. 2018;24(3):519–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bista D, Chalmers L, Peterson GM, Bereznicki LRE. Anticoagulant use in patients with nonvalvular atrial fibrillation: has prescribing improved? Clin Appl Thromb Hemost. 2016;23(6):573–578. [DOI] [PubMed] [Google Scholar]
- 18. Bahri O, Roca F, Lechani T, et al. Underuse of oral anticoagulation for individuals with atrial fibrillation in a nursing home setting in France: comparisons of resident characteristics and physician attitude. J Am Geriatr Soc. 2015;63(1):71–76. [DOI] [PubMed] [Google Scholar]
- 19. Spivey CA, Qiao Y, Liu X, et al. Discontinuation/interruption of warfarin therapy in patients with nonvalvular atrial fibrillation. J Manag Care Spec Pharm. 2015;21(7):596–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Europace. 2018;20(1):e1–e160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. van Breugel HN, Gelsomino S, Lozekoot PW, et al. Guideline adherence in antithrombotic treatment after concomitant ablation surgery in atrial fibrillation patients. Interact Cardiovasc Thorac Surg. 2014;18(3):313–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dagres N, Hindricks G, Kottkamp H, et al. Real-life anticoagulation treatment of atrial fibrillation after catheter ablation: possible overtreatment of low-risk patients. Thromb Haemost. 2009;102(4):754–758. [DOI] [PubMed] [Google Scholar]
- 23. Jobski K, Hoffmann F, Herget-Rosenthal S, Dorks M. Use of oral anticoagulants in German nursing home residents: drug use patterns and predictors for treatment choice. Br J Clin Pharmacol. 2018;84(3):590–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Providencia R, Marijon E, Albenque JP, et al. Rivaroxaban and dabigatran in patients undergoing catheter ablation of atrial fibrillation. Europace. 2014;16(8):1137–1144. [DOI] [PubMed] [Google Scholar]
- 25. Heidbuchel H, Verhamme P, Alings M, et al. Updated European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist anticoagulants in patients with non-valvular atrial fibrillation. Europace. 2015;17(10):1467–1507. [DOI] [PubMed] [Google Scholar]