Abstract
Jellyfish have existed on the earth for around 600 million years and have evolved in response to environmental changes. Hydrozoan jellyfish, members of phylum Cnidaria, exist in multiple life stages, including planula larvae, vegetatively-propagating polyps, and sexually-reproducing medusae. Although free-swimming medusae display complex morphology and exhibit increase in body size and regenerative ability, their underlying cellular mechanisms are poorly understood. Here, we investigate the roles of cell proliferation in body-size growth, appendage morphogenesis, and regeneration using Cladonema pacificum as a hydrozoan jellyfish model. By examining the distribution of S phase cells and mitotic cells, we revealed spatially distinct proliferating cell populations in medusae, uniform cell proliferation in the umbrella, and clustered cell proliferation in tentacles. Blocking cell proliferation by hydroxyurea caused inhibition of body size growth and defects in tentacle branching, nematocyte differentiation, and regeneration. Local cell proliferation in tentacle bulbs is observed in medusae of two other hydrozoan species, Cytaeis uchidae and Rathkea octopunctata, indicating that it may be a conserved feature among hydrozoan jellyfish. Altogether, our results suggest that hydrozoan medusae possess actively proliferating cells and provide experimental evidence regarding the role of cell proliferation in body-size control, tentacle morphogenesis, and regeneration.
Keywords: Cell proliferation, Body size control, Hydrozoan jellyfish, Cladonema pacificum, Tentacle morphogenesis, Regeneration
Introduction
Cell proliferation lies at the core of controlling cell number in Metazoa and thus contributes to the growth and the maintenance of animal body and organs (Leevers & McNeill, 2005; Penzo-Méndez & Stanger, 2015). During development, cell proliferation plays a critical role in body-size increase by adding cells into tissue layers, and it further generates cellular resources for different cell types by multiplying progenitors (Gillies & Cabernard, 2011; Hardwick et al., 2015). Later in adults, proliferating cells are required for physiological cell turnover and for the replacement of damaged cells after tissue injury (King & Newmark, 2012; Pellettieri & Sanchez Alvarado, 2007). These roles of cell proliferation in multicellularity must be conserved throughout evolution: indeed, sponges, one of the earliest metazoan organisms, have acquired mechanisms to allow cell turnover by controlling proliferative capacities (Alexander et al., 2014; Kahn & Leys, 2016).
As the sister group of bilaterians and early-branching metazoans, cnidarians have been studied as a model to understand evolutionary development (Genikhovich & Technau, 2017). Cnidarians are diploblastic and radially symmetric animals that include diverse species such as corals, sea anemones, hydroids, and jellyfish (Technau & Steele, 2011). During the embryonic development of the sea anemone Nematostella vectensis, cell proliferation is coordinated with epithelial organization and is involved in tentacle development (Fritz et al., 2013; Ragkousi et al., 2017). Cnidarians are also known for their regenerative abilities: for instance, Hydra polyps have been used for a century to investigate mechanisms of metazoan regeneration (Fujisawa, 2003; Galliot & Schmid, 2002). The basal head regeneration of Hydra relies on cell proliferation triggered by dying cells (Chera et al., 2009b; Galliot & Chera, 2010). Hydractinia polyps regenerate through cell proliferation and the migration of stem-like cells (Bradshaw, Thompson & Frank, 2015; Gahan et al., 2016). Although much has been learned about mechanisms controlling embryogenesis and growth during regeneration, it is unclear how cnidarians integrate cell proliferation to control their body size and maintain tissue homeostasis under normal physiological conditions.
Among cnidarians, hydrozoan jellyfish have a complex life cycle including planula larvae, sessile polyps, and free-swimming medusae. While polyps undergo asexual reproduction to grow vegetatively, medusae generate gametes to perform sexual reproduction. Despite the limited life span compared to the long-lived or possibly immortal polyps, the size of medusae increases dramatically (Hansson, 1997; Miyake, Iwao & Kakinuma, 1997). Furthermore, medusae maintain their regenerative capacity for missing body parts by integrating dedifferentiation and transdifferentiation (Schmid & Alder, 1984; Schmid et al., 1988; Schmid, Wydler & Alder, 1982). Recent studies using the hydrozoan jellyfish Clytia hemisphaerica have provided mechanistic insights into embryogenesis, nematogenesis, and egg maturation (Denker et al., 2008; Momose, Derelle & Houliston, 2008; Quiroga Artigas et al., 2018). However, little is known about the mechanism that controls body size growth in medusae. It is also unclear whether cell proliferation is required for tentacle morphogenesis and regeneration of hydrozoan jellyfish.
The hydrozoan jellyfish Cladonema is an emerging model, with easy lab maintenance and a high spawning rate, that is suitable for studying diverse aspects of biology including development, regeneration, and physiology (Fujiki et al., 2019; Graziussi et al., 2012; Suga et al., 2010; Takeda et al., 2018; Weber, 1981). Cladonema is characterized by small-sized medusae with branched tentacles. Using specialized adhesive tentacles, Cladonema can adhere to different substrata, such as seaweed, in the field. The species Cladonema pacificum, originally found along coastal areas in Japan, have nine main tentacles with a stereotyped branching pattern (Figs. 1A–1C). During the Cladonema medusa’s maturation, body size increases, and each main tentacle grows and exhibits branching morphology (Fujiki et al., 2019), providing an ideal system to dissect the cellular mechanisms associated with jellyfish growth and morphogenesis.
Figure 1. Cell proliferation patterns in young Cladonema medusa.
(A) Young medusa of Cladonema pacificum. (B) Sexually-matured medusa of Cladonema pacificum. (C) The scheme of Cladonema medusa development. (D–K, N, O) Distribution of S-phase cells in the Cladonema pacificum medusa (1 day old) revealed by EdU staining (20 μM, 24 h incubation). (D, E) Distribution of S-phase cells (EdU+) in a medusa whole body. (F, G) Distribution of S-phase cells (EdU+) in a medusa manubrium. (H, I) Distribution of S-phase cells (EdU+) in a medusa exumbrella. (J, K) Distribution of S-phase cells (EdU+) in a medusa subumbrella. (L, M) Mitotic cells detected by anti-PH3 in a medusa umbrella (8 day old). (N, O) Distribution of S-phase cells (EdU+) in medusa tentacles. (P) Mitotic cells (PH3+) in medusa tentacle bulbs (1 day old). Arrows indicate EdU-positive (H–K) and PH3-positive (L, P) cells, respectively. Scale bars: (A, B) one mm, (D, E, H–K, N, O) 200 μm, (F, G) 100 μm, (L, M, P) 50 μm.
In this study, we investigate the role of cell proliferation in medusa growth and morphogenesis, using Cladonema pacificum as a model of hydrozoan jellyfish. We show that cell proliferation occurs evenly across the medusa body, including the umbrella and manubrium, with the exception of the tentacles, where cell proliferation is spatially clustered. Blocking cell-cycle progression with a pharmacological assay inhibits the increase of body size, tentacle branching, and nematocyte differentiation, which suggests that cell proliferation is necessary for growth and tentacle morphogenesis. We further show that cell proliferation is required for tentacle regeneration in Cladonema medusae. Our findings reveal cell proliferation’s critical roles in the development and maintenance of the Cladonema body and appendages and provide a basis for understanding growth-control mechanisms in hydrozoan jellyfish.
Materials and Methods
Animal cultures
We used Cladonema pacificum (strains 6W and UN2) (Figs. 1–5; Figs. S1–S3), Cytaeis uchidae (strain ♀17) (Fig. 6) and Rathkea octopunctata (strain MF-1) (Fig. 6) medusae for this research. The medusae were cultured in plastic cups (V-type container, V-7 and V-8, AS ONE, Osaka, Japan) at 20 °C (Cladonema and Cytaeis) or 4 °C (Rathkea), and their polyps were maintained in the cups (V-7) at 20 or 4 °C in darkness. Vietnamese brine shrimp (A&A Marine LLC, Elk Rapids, MI, USA) were fed to medusae and polyps every other day ad libitum, with water renewed immediately after feeding. Artificial sea water (ASW) was prepared by SEA LIFE (Marin Tech, Tokyo, Japan). Pictures of medusae were taken through a LEICA S8APO microscope with a Nikon digital camera (D5600).
Figure 5. Cell proliferation is necessary for tentacle regeneration.
(A–D) Tentacle regenerative processes after amputation in an adult medusa. Series of pictures show the growing tentacle over 4 days. (E, F) Mitotic cells (PH3+) in tentacle bulbs of the unremoved control and the dissected medusa. Arrowheads indicate PH3-positive cells. (G) Quantification of proliferative cells in tentacle bulbs for control and after amputation. Control: n = 26, Amputation: n = 11. Error bar: SD. Unpaired two-tailed t-test. t(35) = 6.246, ****p < 0.0001. (H) Quantification of tentacle length after amputation in control (HU−) and 10 mM HU treatment (HU+). Unpaired two-tailed t-test. Day 1 t(46) = 9.227, day 2 t(46) = 10.29, day 3 t(46) = 14.1, day 4 t(46) = 20.5, day 5 t(46) = 22.49, day 6 t(45) = 17.11, day 7 t(45) = 15.36, ****p < 0.0001. Scale bars: (A–D) one mm, (E, F) 100 μm.
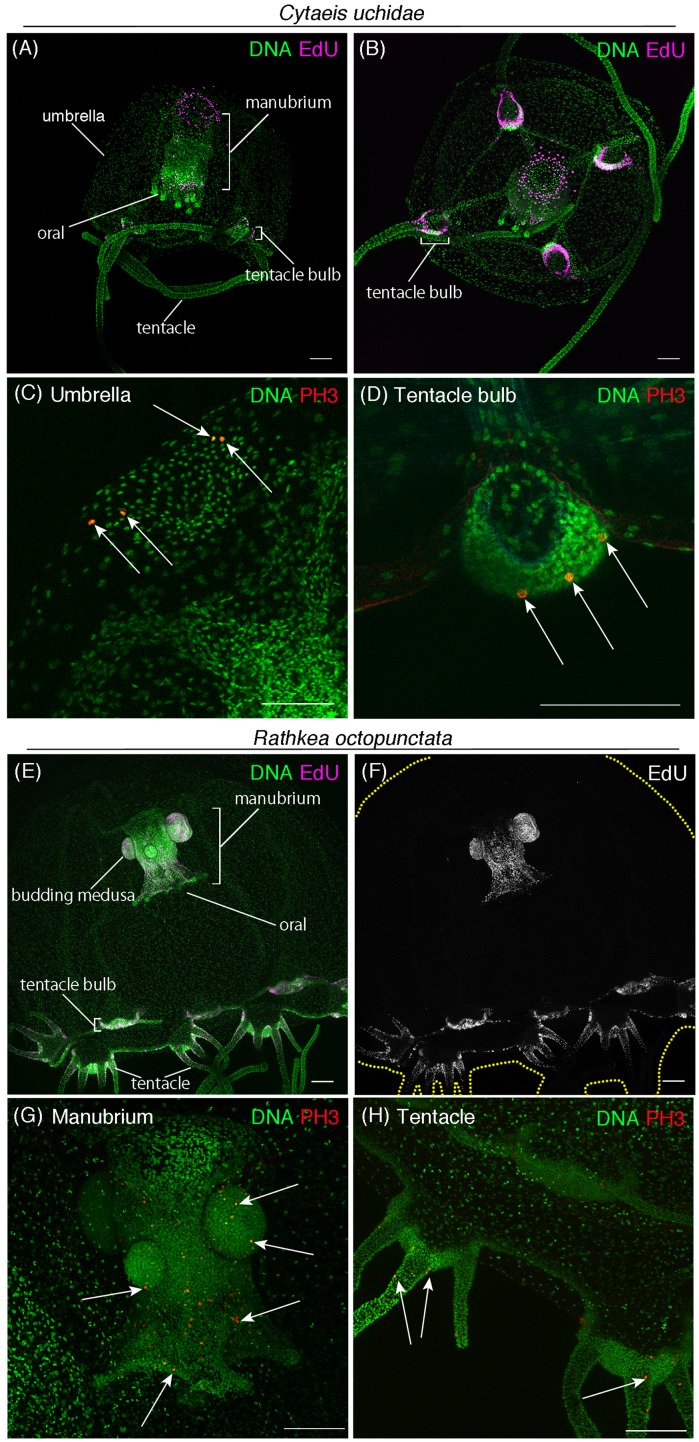
Figure 6. Cell proliferation patterns across different hydrozoan jellyfish.
(A) Distribution of S-phase cells in the Cytaeis uchidae medusa (30 day old) shown with EdU staining (EdU: 20 μM, 24 h). (B) Distribution of S-phase cells (EdU+) in Cytaeis medusa (11 day old). (C) Mitotic cells (PH3+) in the umbrella of Cytaeis medusa (30 day old). (D) Mitotic cells in Cytaeis medusa tentacle bulbs (30 day old). (E, F) Distribution of S-phase cells (EdU+) in the Rathkea octpunctata juvenile medusa (EdU: 20 μM, 24 h). (G) Mitotic cells (PH3+) in a manubrium of Rathkea juvenile medusa. (H) Mitotic cells (PH3+) in Rathkea juvenile medusa tentacles. Arrows indicate PH3-positive mitotic cells. Scale bars: 100 μm.
Immunofluorescence
The medusae were anesthetized with 7% MgCl2 in ASW for 10 min and fixed 4% paraformaldehyde (PFA) in ASW for 1 h. After fixation, the samples were rinsed in 1× PBS and washed three times (10 min each) in PBS containing 0.1% Triton X-100 (0.1% PBT). The samples were incubated in primary antibodies in 0.1% PBT overnight at 4 °C. The antibodies used were rabbit anti-Phospho-Histone H3 (Ser10) (1:500; 06–570, Upstate, Lake Placid, NY, USA) and mouse anti-α-Tubulin (1:500; T6199, Sigma-Aldrich, St Louis, MO, USA). After the primary antibody incubation, the samples were washed three times (10 min each) in 0.1% PBT and incubated in secondary antibodies (1:500; ALEXA FLUOR Goat anti-mouse IgG, ALEXA FLUOR Goat anti-rabbit IgG; Thermo Fisher Scientific, Waltham, MA, USA) and Hoechst 33342 (1:250; Thermo Fisher Scientific, Waltham, MA, USA) in 0.1% PBT for 1 h in dark. After four washes (10 min each) in 0.1% PBT, the samples were mounted on slides with 70% glycerol. Confocal images were collected through Leica SP8 or SP5 confocal microscopes. Z-stack images were performed using ImageJ/Fiji software.
EdU labeling
The medusae were incubated with 20 μM 5-ethynyl-2′-deoxyuridine (EdU; EdU kit; 1836341; Invitrogen, Carlsbad, CA, USA) in ASW for 24 h (Figs. 1–3 and 6) or 150 μM for 1 h (Fig. S1). After EdU treatment, the medusae were anesthetized with 7% MgCl2 in ASW for 10 min and fixed 4% PFA in ASW for 1 h. After fixation, the samples were rinsed in 1× PBS and washed three times (10 min each) in 0.1% PBT. The samples were incubated with a EdU reaction cocktail (1× reaction buffer, CuSO4, Alexa Fluor azide, and 1× reaction buffer additive; all included in EdU kit; 1836341; Invitrogen, Carlsbad, CA, USA) for 30 min in the dark. After the EdU reaction, the samples were washed three times (10 min each) in 0.1% PBT and Hoechst 33342 (1:250; Thermo Fisher Scientific, Waltham, MA, USA) in 0.1% PBT for 1 h in dark. The samples were washed four times (10 min each) in 0.1% PBT and were mounted on slides with 70% glycerol.
Figure 3. Cell proliferation is necessary for body-size growth.
(A) Cladonema pacificum newborn medusa (0 day old). (B) Cladonema pacificum juvenile medusa (8 day old). (C) Quantification of umbrella size in control and starved medusae. Control medusae were fed every other day. Error bar: SD. Unpaired two-tailed t-test. Day 1 t(36) = 4.545, day 3 t(36) = 9.888, day 6 t(36) = 12.56, ****p < 0.0001. (D–G) Distribution of S-phase cells in control medusa and starved medusa with EdU staining (20 μM, 24 h incubation). (H) Quantification of the number of S-phase cells (EdU+) in control and starved medusae. Unpaired two-tailed t-test. *p < 0.05 (p = 0.0127), t = 3.194 df = 8. (I–L) Distribution of S-phase cells in medusa of control (HU−) and hydroxyurea (HU+) treatment detected by EdU staining (20 μM, 24 h). No S-phase cells were detected in HU+ medusae. (M) Quantification of body size in control and in HU conditions. HU suppresses body-size growth. HU−: control medusae incubated in ASW, HU+: medusae incubated in HU 10 mM ASW. Both HU+ and HU− were fed every other day. Error bar: SD. Unpaired two-tailed t-test. Day 1 t(93) = 3.561, day 2 t(90) = 4.079, day 3 t(81) = 3.657, day 5 t(85) = 6.329, day 6 t(52) = 4.105, day 7 t(79) = 7.319, day 8 t(71) = 9.201, day 9 t(59) = 8.826, ***p < 0.0005, ****p < 0.0001. Scale bars: (A, B) one mm, (D–G and I–L) 100 μm.
Hydroxyurea treatment
The live medusae were incubated with 10 mM hydroxyurea (HU) (085-06653; Wako, Osaka, Japan) in ASW (ASW only for control) (Figs. 3–5; Fig. S3). HU incubation was continued for a maximum of 9 days. Medusae were fed every other day, and HU solution or ASW was renewed after feeding. The medusae treated with HU were able to ingest prey like controls, demonstrating that HU treatment had no effect on feeding behavior (Figs. S3A and S3B).
Measurement of umbrella size and tentacle length
Pictures of medusae were taken with a Nikon D5600, and umbrella size was measured using polygon selections with ImageJ software (Fig. 3C). We measured the length and width of medusae under the microscope using an ocular micrometer and multiplied the length and width to generate a value for umbrella size (Fig. 3M). Tentacle length was measured daily under the microscope with an ocular micrometer (Fig. 5H).
DAPI poly-γ-glutamate staining
This protocol was adapted from Szczepanek, Cikala & David (2002): The medusae were anesthetized with 7% MgCl2 in ASW for 10 min and fixed with 4% PFA in ASW for 1 h. After fixation, the samples were rinsed in 1× PBS and washed three times (10 min each) in 0.1% PBT. The samples were incubated in DAPI (1:500; Polysciences, Inc., Warrington, PA, USA) in PBT for 60 min. After the DAPI incubation, samples were washed four times (10 min each) in PBT and mounted on slides with 70% glycerol in DW. Samples were scanned with a combination of 488 nm excitation and 555 nm emission filter using either Leica SP8 or SP5 confocal microscopes. Using ImageJ, we performed Z-stacks and counted nematocysts. Empty nematocysts were counted manually.
Dissection of tentacles for regeneration
Tentacles’ basal sides were dissected with small scissors, leaving the tentacle bulbs intact. Amputated medusae were fed every other day.
Statistical test
An unpaired two-tailed t-test was performed on the data shown in Figs. 3–5.
Average nearest neighbor distance (spatial statistics)
We performed statistical analysis for the proliferating cells’ distribution in umbrellas and tentacles by applying the nearest neighbor distance (NND) test to EdU positive cells (Table S1). Here, we used the images of 1-day-old medusae that had been incubated with EdU 150 μM for 1 h. This analysis was applied to the umbrella area, except for tentacle bulb and manubrium, while the same analysis was applied to the entire main tentacle. The area (S), the signal number (N), and NND in analyzed areas were obtained using ImageJ/Fiji. The average of NND (W), the expectation value of W (E[W]), and the normalized average of NND (w = W/E[W]) were calculated. In this analysis, w > 1 means that EdU signals are distributed uniformly or randomly. In contrast, w < 1 means that EdU signals are distributed clustered or randomly. The spatial distribution of EdU signals were determined by Z score.
Results
Cell proliferation patterns in the medusa Cladonema pacificum
To understand the spatial pattern of cell proliferation in Cladonema medusa, we performed EdU staining, which labels S-phase or the former S-phase cells (Salic & Mitchison, 2008). Given that Cladonema medusa dramatically increases in size and exhibits tentacle branching during development (Figs. 1A–1C), distribution of proliferating cells could change throughout maturation. We thus investigated cell proliferation patterns in both young (day 1) and sexually mature (day 45) medusae.
In young medusae, EdU-positive cells were broadly detected in the whole medusa body including the umbrella, the manubrium (a supporting organ of the oral in medusae), and the tentacles regardless of the incubating time of EdU (Figs. 1D–1K and 1N–1O, EdU: 20 μM for 24 h; Fig. S1, EdU: 150 μM for 1 h). While small numbers of EdU positive cells were detected in the manubrium (Figs. 1F and 1G), EdU positive cells were uniformly distributed in the umbrella (Average NND: uniform, n = 6/7, random, n = 1/7; Table S1), especially in the exumbrella region (Figs. 1H–1K). By contrast, in the tentacles, large numbers of EdU positive cells were identified as clustered (Figs. 1N and 1O; NND: clustered, n = 7/7, Table S1). We further confirmed that these EdU-positive cells were proliferating cells using the mitotic marker, anti-Phospho-Histone 3 (PH3) antibody. PH3-positive cells were detected in both the umbrella and the tentacle bulbs (Figs. 1L and 1P). In tentacles, mitotic cells were primarily detected in the ectoderm (Fig. 1P; Fig. S2), whereas in the umbrella, proliferating cells were located in the exumbrella, which was confirmed by the presence of mitotic spindles, detected with an anti-α Tubulin antibody in PH3-positive cells (Fig. 1M).
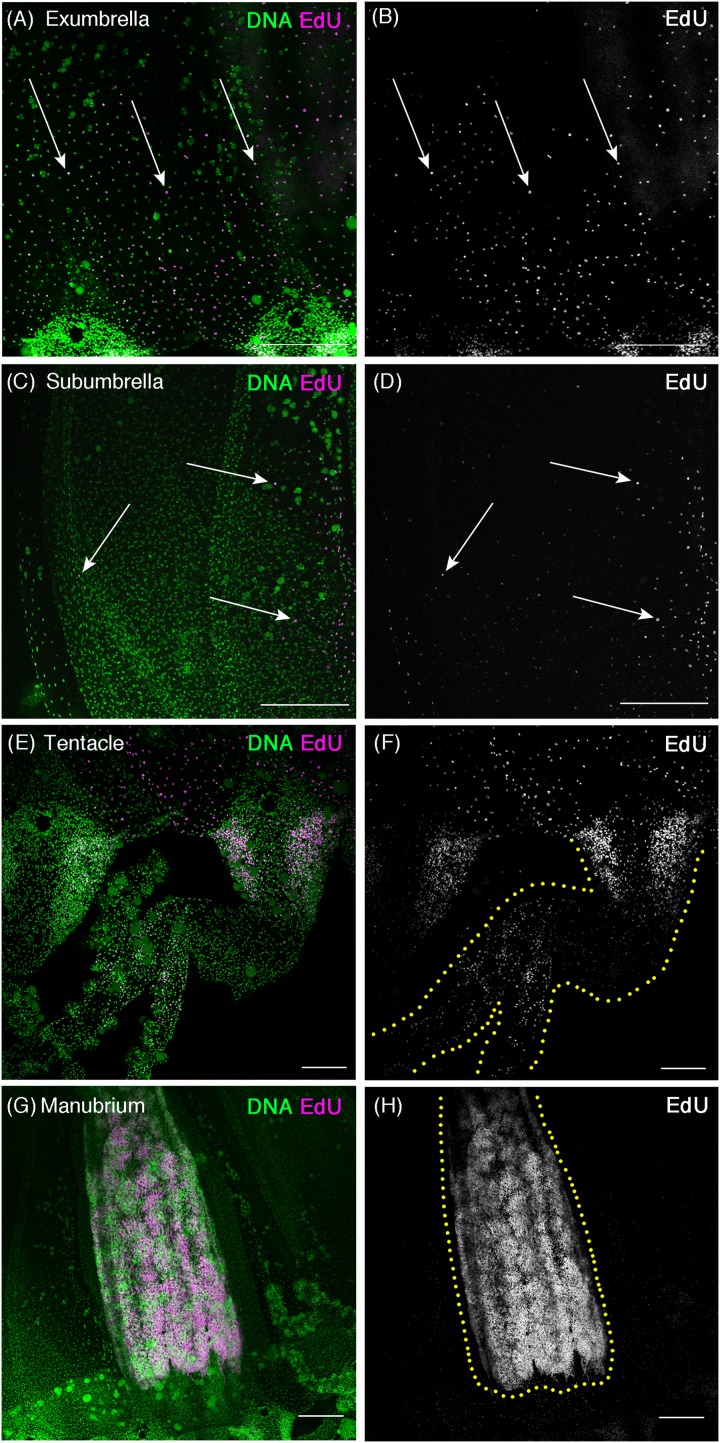
As observed in young medusae, EdU-positive cells were broadly detected in the entire body of mature medusae (Fig. 2). In the umbrella, EdU-positive cells were more often located in the exumbrella than in the subumbrella, which is similar to the case of young medusae (Figs. 2A–2D). By contrast, in tentacles, EdU-positive cells were restricted to their base, called the tentacle bulb, where two apparent “clusters” are located on both sides of the bulb (Figs. 2E and 2F). These clusters were also observed in the tentacle bulb of young medusae (Figs. 1N and 1O), suggesting that tentacle bulbs may behave as a proliferation zone throughout the medusa stage. Interestingly, in the manubrium of matured medusae, large numbers of EdU-positive cells were detected (Figs. 2G and 2H). This result likely reflects the presence of germ cells that are produced in the manubrium, a feature of the sexually matured Cladonema medusa (Takeda et al., 2018).
Figure 2. Cell proliferation patterns in sexually mature Cladonema medusa.
(A–H) Distribution of S-phase cells in the Cladonema pacificum medusa (45 day old) shown with EdU staining (20 μM, 24 h incubation). (A, B) Distribution of S-phase cells (EdU+) in a medusa exumbrella. (C, D) Distribution of S-phase cells (EdU+) in a medusa subumbrella. (E, F) Distribution of S-phase cells (EdU+) in medusa tentacles. (G, H) Distribution of S-phase cells (EdU+) in a medusa manubrium. Arrows indicate EdU-positive (A–D) cells. Scale bars: (A–D, G, H) 200 μm, (E, F) 100 μm.
Altogether, our results suggest that cell proliferation may occur uniformly in the medusa umbrella, while a subset of cell proliferation could occur locally in tentacles. Based on these observations, we hypothesized that uniform cell proliferation may control body size growth and tissue homeostasis while clustered cell proliferation in tentacles may contribute to tentacle morphogenesis.
Cell proliferation is necessary for the control of body size
Animal body size increases upon intake of nutrition because nutrition influences cell proliferation and cell growth (Bohnsack & Hirschi, 2004). We first monitored the body size of juvenile medusae by focusing on the size of their umbrella because the umbrella grows in direct proportion with whole body size. Under normal feeding conditions, the medusa umbrella size increased dramatically by 54.8%, from 0.62 ± 0.02 to 0.96 ± 0.02 mm2 during the first 24 h, with a subsequent minor increase observed over the following 5 days (0.98 ± 0.03 mm2) (Figs. 3A–3C). By contrast, under starved conditions, the size of medusa umbrella did not increase, compared to controls, and rather gradually decreased over the following 5 days (Fig. 3C). Moreover, fewer EdU positive cells were detected in the starved medusae than in fed controls (Figs. 3D–3H; Control: 1,240.6 ± 214.3, Starved: 433.6 ± 133, t(8) = 3.194, *p < 0.05 (p = 0.0127)), suggesting that, at the cellular level, nutrition affects cell proliferation in medusae. These results indicate that body-size growth in juvenile medusae depends on available nutrition.
To test the hypothesis that uniform cell proliferation in medusae contributes to body-size increase, we performed a pharmacological assay to block cell-cycle progression using HU, a cell-cycle inhibitor that causes G1 arrest (Koç et al., 2004). Under HU treatment, S phase cells detected by EdU staining disappeared from the medusa body (Figs. 3I–3L). By tracking the size of umbrella, we found that HU-treated medusae did not exhibit the size increase that was observed in controls (Fig. 3M). Together, these results suggest that cell-cycle progression affects body size in Cladonema medusae.
Cell proliferation is necessary for tentacle morphogenesis
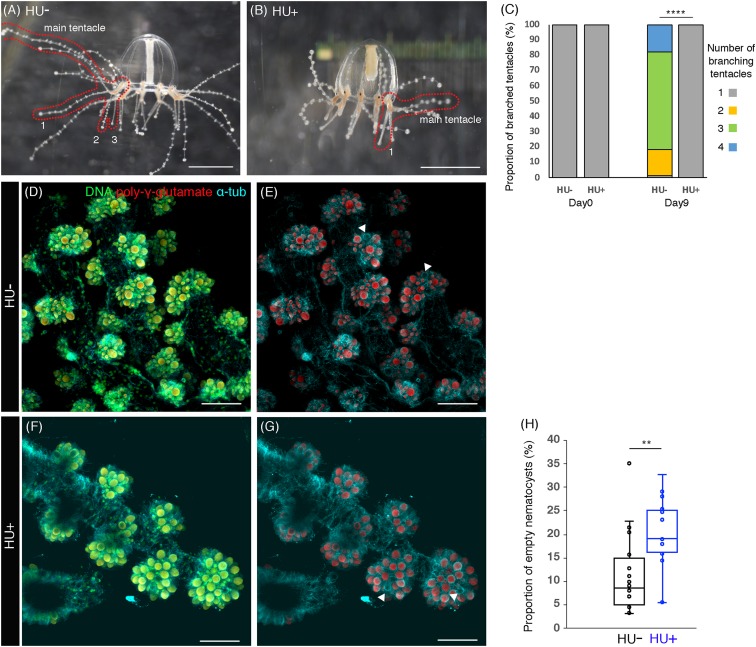
In Clytia hemisphaerica, another hydrozoan jellyfish, stem-like cells or progenitors are proposed to exist in tentacle bulbs (Denker et al., 2008). The clustered or local cell proliferation observed in tentacles, including those in the bulb, of the Cladonema medusa may reflect such stem or progenitor cell populations (Figs. 1N–1O and 2E–2F; Fig. S1D). Furthermore, many EdU positive cells were frequently detected in small branched tentacles, which were the most recently branched (Figs. 3D–3E and 3I–3J). To test the hypothesis that local cell proliferation in tentacles contributes to tentacle morphogenesis, we first focused on tentacle branching. Although the initial tentacles have one branch in juvenile medusae, the number of branches gradually increases during medusa maturation (Fujiki et al., 2019). In our normal feeding condition, the branching number reached approximately three (2.98 ± 0.05 per tentacle) by day 9 (Figs. 4A and 4C). By contrast, when cell proliferation was blocked with HU, none of the medusae exhibited the typical increase in branched tentacles; rather, all maintained only one branch (Figs. 4B and 4C). Importantly, upon removal of HU, these animals showed an increase in tentacle branching similar to controls, suggesting that the effects of the drug treatment are reversible (Fig. S3C). Combined, these results point to cell proliferation in tentacles as a necessary component for normal tentacle branching.
Figure 4. Cell proliferation is necessary for tentacle morphogenesis.
(A) Control (HU−) medusa incubated in ASW for 9 days. The picture shows the representative image of medusae with three branched tentacles. (B) The medusa incubated in 10 mM HU (HU+) ASW for 9 days. The picture shows the representative image of medusae with one branched tentacle. (C) Quantification of branching numbers per tentacle at day 0 and day 9. HU+: n = 313, HU− condition: n = 199. Error bars: SD. Unpaired two-tailed t-test. t(510) = 54.49, ****p < 0.0001. (D–G) Nematocytes in tentacles labeled by DAPI (poly-γ-glutamate) in the 8 day old medusa incubated in ASW (HU−) or 10 mM HU ASW (HU+). Arrowheads indicate empty nematocysts. (H) The proportion of empty nematocysts in HU− and HU+ medusa. HU+: n = 19, HU−: n = 18. Unpaired two-tailed t-test. t(31) = 2.869, **p < 0.01 (p = 0.0074). Scale bar: (D–G) 50 μm.
Cnidarian tentacles have nematocysts, organelles specific to the cnidarian phylum that are utilized for food capture and defense against predators (Kass-Simon & Scappaticci, 2002). In Clytia hemisphaerica, stem-like cells or progenitors in tentacle bulbs seem to supply nematocysts at the tips of tentacles via cell proliferation, migration to the tip, and differentiation (Denker et al., 2008). This evidence raises the possibility that cell proliferation also controls nematocyte development or nematogenesis in hydrozoan jellyfish. To monitor nematocytes in Cladonema tentacles, we utilized DAPI, a nuclear staining dye that can label poly-γ-glutamate synthesized in the nematocyst wall (Szczepanek, Cikala & David, 2002). Using poly-γ-glutamate staining, we discovered nematocyte size variations ranging from 2 to 110 μm2 (Figs. 4D–4G). We also found that some of the nematocysts were empty, suggesting that such nematocytes had been depleted (Figs. 4D–4G).
In order to investigate whether cell proliferation in tentacle also contributes to nematocyte maturation, we examined the emptiness of nematocytes after cell-cycle blocking with HU. We detected that the proportion of the empty nematocysts was higher in the medusae with HU treatment than in controls (HU−: 11.4% ± 2.0%; HU+: 19.7% ± 2.0%, Figs. 4D–4G and 4H). This result indicates that even after discharge, nematocytes are still actively supplied by progenitor cell proliferation and that this refill is prevented when cell proliferation is blocked. Taken together, our data suggest that cell proliferation in tentacle plays an important role in both tentacle branching and nematogenesis.
Cell proliferation is necessary for tentacle regeneration
Cnidarians are known to have a high regenerative capacity (Galliot & Schmid, 2002; Holstein, Hobmayer & Technau, 2003), and the hydrozoan jellyfish Cladonema species exemplifies this typical regenerative ability (Weber, 1981). Given the localization of proliferative cells in the tentacle bulb of matured Cladonema medusae (Figs. 2E and 2F), we decided to investigate the nature of tentacle regeneration. After dissecting tentacles at their base, we monitored the process of tentacle regeneration (Figs. 5A–5D). During the first 24 h, wound healing occurred at the dissected area (Fig. 5B). Subsequently, the tip of tentacle became elongated and started branching on day 2 (Fig. 5C). At day 4, fully branched tentacles were observed (Fig. 5D), suggesting that tentacle regeneration may follow normal tentacle morphogenesis after elongation.
To examine the initial stage of tentacle regeneration, we examined the distribution of proliferating cells using PH3 staining to visualize mitotic cells. While dividing cells were frequently observed near the amputated area, mitotic cells were dispersed in uncut control tentacle bulbs (Figs. 5E and 5F). We quantified the number of PH3-positive cells present in the tentacle bulbs and found a significant increase in PH3-positive cells in the tentacle bulbs of amputee medusae, compared to controls (Fig. 5G). These observations indicate that initial regenerative responses accompany the active increase of cell proliferation in tentacle bulbs.
In order to test the role of cell proliferation in tentacle regeneration, we blocked cell-cycle progression using HU after dissection and monitored the length of regenerating tentacles. While the tentacles continued to elongate from the bulb structure after dissection in controls, tentacles in animals treated with HU were not able to elongate despite displaying normal wound healing (Fig. 5H). These results demonstrate that cell proliferation in tentacle bulbs is required for proper tentacle regeneration.
Cell proliferation patterns across different hydrozoan jellyfish
Hydrozoan jellyfish constitute the most broadly varied class of cnidarian jellyfish with approximately 1,150 species worldwide featuring highly diverse morphological and physiological characteristics (Cartwright & Nawrocki, 2010; Schuchert, 2019). For instance, Cytaeis uchidae has four tentacles, and their polyps live exclusively on one type of shell: Niotha livescens (Takeda, Deguchi & Itabashi, 2018; Takeda et al., 2013). Another species, R. octopunctata, has eight grouped-tentacles, and their juvenile medusae asexually produce medusae that grow out of the manubrium (Berrill, 1952; Schuchert, 2007).
To gain insight into the conserved and diversified nature of cell proliferation in hydrozoan jellyfish, we investigated the spatial pattern of cell proliferation in Cytaeis and Rathkea medusae. In Cytaeis medusa, EdU-positive cells were observed in manubrium, tentacle bulbs, and at the top of the umbrella (Figs. 6A and 6B). PH3-positive cells were also detected in the same regions, suggesting that proliferating cells in Cytaeis are distributed in a pattern similar to that observed in Cladonema, although there are some discrepancies (Figs. 6C and 6D). By contrast, in R. octopunctata, EdU-positive cells and PH3-positive cells were mostly restricted to the manubrium and tentacle bulbs (Figs. 6E–6H). Of note, proliferating cells were frequently detected in the medusa buds that grew out of the manubrium (Figs. 6E–6G), which may reflect asexual reproduction in Rathkea medusae. These results suggest that cell proliferation may occur in tentacle bulbs across hydrozoan medusae commonly, while cell proliferation patterns may vary in a species-specific manner with physiology.
Discussion
In this study, we show that the body size of Cladonema medusae is influenced by cell proliferation following uptake of nutrition. Without nutrition and under the blocking of cell-cycle progression, body-size increase is inhibited (Fig. 3). Intriguingly, despite the significant differences between fed and starved animals and between HU-treated and -untreated animals, the body size of Cladonema medusae increases during the first 24 h regardless of condition (Fig. 3). A similar body size increase under starved conditions is also reported in the medusa of Cladonema californicum Hyman (Costello, 1998). These observations can be explained by cell growth via protein synthesis (Schiaffino et al., 2013) or accretionary growth, in which cells secrete extracellular matrix to increase extracellular regions, as has been suggested in the growth of cartilage and bone (Karsenty, Kronenberg & Settembre, 2009; Wang, Rigueur & Lyons, 2014). Given the large amount of collagen that jellyfish contain (Khong et al., 2016; Miura & Kimura, 1985), extracellular matrix may increase their size during the initial growth of juvenile medusae.
Another interesting feature we observed is that the body size of the starved medusae gradually decreases after 24 h (Fig. 3C). Similarly, upon starvation, Hydra polyps cease asexual budding and decrease their size (Buzgariu, Chera & Galliot, 2008; Chera et al., 2009a), suggesting that cnidarian animals are sensitive to nutrition availability and adapt to metabolic changes. At the organ and tissue level, such size reduction can occur via autophagy or cell death during starvation in diverse phyla (Jeschke et al., 2000; O’Brien et al., 2011; Thongrod et al., 2018; Tracy & Baehrecke, 2013). Cnidarians thus may utilize similar mechanisms to reduce cell size and/or cell number to adjust their body size in response to environmental changes. Molecularly, TOR and Hippo signaling are conserved machinery that control organ size, and, as such, these molecules may also play an important role in cnidarian growth control (Coste et al., 2016; Ikmi et al., 2014; Loewith & Hall, 2011; Van Dam et al., 2011).
Hydrozoan animals are known to possess interstitial stem cell populations, called i-cells. In Hydra and Hydractinia polyps, i-cells are localized to the body column, mostly in ectoderm, and have the potential to differentiate into several cell types including nematocytes, nerve cells, and gametes (Gold & Jacobs, 2013; Hemmrich et al., 2012; Hobmayer et al., 2012; Künzel et al., 2010; Müller, Teo & Frank, 2004). By contrast, the current understanding of the localization and roles of stem-like cells or i-cells in hydrozoan jellyfish are limited (Leclère et al., 2012). In Cladonema medusa, although mitotic cells are localized in the ectoderm, clusters of proliferative cells are distributed throughout the tentacle, except for the tip region (Figs. S1 and S2). This finding contrasts with the case of Clytia, where i-cells are primarily clustered in the tentacle bulb (Denker et al., 2008), implying that the i-cells or progenitors of Cladonema may be more broadly localized. Our pharmacological experiments confirmed that cell proliferation contributes to tentacle branching, nematogenesis, and tentacle regeneration in Cladonema (Figs. 4 and 5), suggesting that these proliferative cells may behave as progenitors or stem-like cells. We further found similar distribution of proliferative cells in tentacle bulbs of Cytaeis uchidae and R. octopunctata (Fig. 6). Together, these results suggest that the distribution of proliferative cells in tentacle bulbs are widely conserved in hydrozoan jellyfish, while such cells might exist in other tissue to allow body-size increase and species-specific life styles.
Conclusions
This study reveals the spatial patterns of cell proliferation during the growth and morphogenesis of the medusae Cladonema pacificum at different stages of maturation. Using a cell-cycle inhibitor assay, we show that uniform cell proliferation in the umbrella is responsible for overall body-size increase, while clustered cell proliferation in tentacles contributes to branching morphogenesis, nematocyte differentiation, and regeneration. We further provide evidence for conserved and species-specific cell proliferation patterns in hydrozoan jellyfish by examining two other hydrozoan species, Cytaeis uchidae and R. octopunctata. The clustered cell proliferation in the tentacle bulbs of these hydrozoan medusae evinces the possibility of a progenitor or stem-like cell population, although its existence will need to be confirmed in future studies. On the whole, our work establishes the basis for understanding the cellular mechanisms of jellyfish growth and homeostasis, which will facilitate future work to dissect the molecular mechanisms underlying these processes.
Supplemental Information
(A–D) Distribution of S-phase cells in the Cladonema pacificum medusa (1 day old) shown by EdU staining with short incubation time (150 μM, 1 h incubation). (A) Distribution of S-phase cells (EdU+) a whole medusa body. (B) Distribution of S-phase cells (EdU+) in a medusa manubrium. (C) Distribution of S-phase cells (EdU+) in a medusa umbrella. (D) Distribution of S-phase cells (EdU+) in medusa tentacles. Scale bar: (A, C) 200 μm, (B, D) 100 μm.
(A, B) Cross sections of medusa tentacle bulbs. (A) Mitotic cells (PH3+) located in ectoderm in medusa tentacle bulbs (70 days old). (B) Mitotic cells (PH3+) located in ectoderm in medusa tentacle bulbs (30 days old). Scale bars: (A–B) 50 μm.
(A) Cladonema pacificum medusa (2 days old) before feeding (left image) and Cladonema pacificum medusa (2 days old) after feeding (right image). (B) Cladonema pacificum medusa (2 days old) with 48 h HU treatment before feeding (left image) and Cladonema pacificum medusa (2 days old) with 48 h HU treatment after feeding (right image). (C) Quantification of the number of tentacle branching in control and HU-treated medusa, with HU washed off, after 48 h treatment. Error bar: SD. Scale bars: (A, B) one mm.
Statistical analysis for the proliferating cells’ distribution in umbrellas and tentacles was performed by applying the nearest neighbor distance test to EdU positive cells.
Acknowledgments
We thank R. Deguchi (Miyagi Univ. Education, Japan) for sharing jellyfish species and helpful discussion. We thank S. Tasaki for helping statistical analyses. We thank H. Takashima for technical assistance. We thank Kuranaga lab members for discussion.
Funding Statement
This work was supported by the Naito Foundation, the Takeda Science Foundation, the Kanae Foundation for the Promotion of Medical Science, the Daiichi Sankyo Foundation of Life Science, and the JSPS KAKENHI Grant Numbers JP17H05004 and 17H06332 (to Yu-ichiro Nakajima). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
Sosuke Fujita conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Erina Kuranaga contributed reagents/materials/analysis tools, approved the final draft.
Yu-ichiro Nakajima conceived and designed the experiments, analyzed the data, contributed reagents/materials/analysis tools, authored or reviewed drafts of the paper, approved the final draft.
Data Availability
The following information was supplied regarding data availability:
The raw data (pictures) are available in Figshare: Fujita, Sosuke; Nakajima, Yuichiro; Kuranaga, Erina (2019): Raw data for paper (SF-EK-YN). figshare. Dataset. https://doi.org/10.6084/m9.figshare.7935197.v4.
References
- Alexander et al. (2014).Alexander BE, Liebrand K, Osinga R, Van Der Geest HG, Admiraal W, Cleutjens JP, Schutte B, Verheyen F, Ribes M, Van Loon E, De Goeij JM. Cell turnover and detritus production in marine sponges from tropical and temperate benthic ecosystems. PLOS ONE. 2014;9(10):e109486. doi: 10.1371/journal.pone.0109486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrill (1952).Berrill NJ. Growth and form in gymnoblastic hydroids. II. Sexual and asexual reproduction in rathkea. III. Hydranth and gonophore development in pennaria and acaulis. IV. Relative growth in eudendrium. Journal of Morphology. 1952;90(1):1–32. doi: 10.1002/jmor.1050900102. [DOI] [Google Scholar]
- Bohnsack & Hirschi (2004).Bohnsack BL, Hirschi KK. Nutrient regulation of cell cycle progression. Annual Review of Nutrition. 2004;24(1):433–453. doi: 10.1146/annurev.nutr.23.011702.073203. [DOI] [PubMed] [Google Scholar]
- Bradshaw, Thompson & Frank (2015).Bradshaw B, Thompson K, Frank U. Distinct mechanisms underlie oral vs aboral regeneration in the cnidarian Hydractinia echinata. eLife. 2015;4:e05506. doi: 10.7554/eLife.05506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzgariu, Chera & Galliot (2008).Buzgariu W, Chera S, Galliot B. Methods to investigate autophagy during starvation and regeneration in hydra. Methods in Enzymology. 2008;451:409–437. doi: 10.1016/S0076-6879(08)03226-6. [DOI] [PubMed] [Google Scholar]
- Cartwright & Nawrocki (2010).Cartwright P, Nawrocki AM. Character evolution in Hydrozoa (phylum Cnidaria) Integrative and Comparative Biology. 2010;50(3):456–472. doi: 10.1093/icb/icq089. [DOI] [PubMed] [Google Scholar]
- Chera et al. (2009a).Chera S, Buzgariu W, Ghila L, Galliot B. Autophagy in Hydra: a response to starvation and stress in early animal evolution. Biochimica et Biophysica Acta. 2009a;1793(9):1432–1443. doi: 10.1016/j.bbamcr.2009.03.010. [DOI] [PubMed] [Google Scholar]
- Chera et al. (2009b).Chera S, Ghila L, Dobretz K, Wenger Y, Bauer C, Buzgariu W, Martinou J-C, Galliot B. Apoptotic cells provide an unexpected source of Wnt3 signaling to drive hydra head regeneration. Developmental Cell. 2009b;17(2):279–289. doi: 10.1016/j.devcel.2009.07.014. [DOI] [PubMed] [Google Scholar]
- Coste et al. (2016).Coste A, Jager M, Chambon J-P, Manuel M. Comparative study of Hippo pathway genes in cellular conveyor belts of a ctenophore and a cnidarian. EvoDevo. 2016;7(1):4. doi: 10.1186/s13227-016-0041-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello (1998).Costello J. Physiological response of the hydromedusa Cladonema californicum Hyman (Anthomedusa: Cladonemidae) to starvation and renewed feeding. Journal of Experimental Marine Biology and Ecology. 1998;225(1):13–28. doi: 10.1016/S0022-0981(97)00204-9. [DOI] [Google Scholar]
- Denker et al. (2008).Denker E, Manuel M, Leclere L, Le Guyader H, Rabet N. Ordered progression of nematogenesis from stem cells through differentiation stages in the tentacle bulb of Clytia hemisphaerica (Hydrozoa, Cnidaria) Developmental Biology. 2008;315(1):99–113. doi: 10.1016/j.ydbio.2007.12.023. [DOI] [PubMed] [Google Scholar]
- Fritz et al. (2013).Fritz AE, Ikmi A, Seidel C, Paulson A, Gibson MC. Mechanisms of tentacle morphogenesis in the sea anemone Nematostella vectensis. Development. 2013;140(10):2212–2223. doi: 10.1242/dev.088260. [DOI] [PubMed] [Google Scholar]
- Fujiki et al. (2019).Fujiki A, Hou S, Nakamoto A, Kumano G. Branching pattern and morphogenesis of medusa tentacles in the jellyfish Cladonema pacificum (Hydrozoa, Cnidaria) Zoological Letters. 2019;5(1):12. doi: 10.1186/s40851-019-0124-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa (2003).Fujisawa T. Hydra regeneration and epitheliopeptides. Developmental Dynamics. 2003;226(2):182–189. doi: 10.1002/dvdy.10221. [DOI] [PubMed] [Google Scholar]
- Gahan et al. (2016).Gahan JM, Bradshaw B, Flici H, Frank U. The interstitial stem cells in Hydractinia and their role in regeneration. Current Opinion in Genetics & Development. 2016;40:65–73. doi: 10.1016/j.gde.2016.06.006. [DOI] [PubMed] [Google Scholar]
- Galliot & Chera (2010).Galliot B, Chera S. The Hydra model: disclosing an apoptosis-driven generator of Wnt-based regeneration. Trends in Cell Biology. 2010;20(9):514–523. doi: 10.1016/j.tcb.2010.05.006. [DOI] [PubMed] [Google Scholar]
- Galliot & Schmid (2002).Galliot B, Schmid V. Cnidarians as a model system for understanding evolution and regeneration. International Journal of Developmental Biology. 2002;46(1):39–48. [PubMed] [Google Scholar]
- Genikhovich & Technau (2017).Genikhovich G, Technau U. On the evolution of bilaterality. Development. 2017;144(19):3392–3404. doi: 10.1242/dev.141507. [DOI] [PubMed] [Google Scholar]
- Gillies & Cabernard (2011).Gillies TE, Cabernard C. Cell division orientation in animals. Current Biology. 2011;21(15):R599–R609. doi: 10.1016/j.cub.2011.06.055. [DOI] [PubMed] [Google Scholar]
- Gold & Jacobs (2013).Gold DA, Jacobs DK. Stem cell dynamics in Cnidaria: are there unifying principles? Development Genes and Evolution. 2013;223(1–2):53–66. doi: 10.1007/s00427-012-0429-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziussi et al. (2012).Graziussi DF, Suga H, Schmid V, Gehring WJ. The “eyes absent” (eya) gene in the eye-bearing hydrozoan jellyfish Cladonema radiatum: conservation of the retinal determination network. Journal of Experimental Zoology Part B: Molecular and Developmental Evolution. 2012;318(4):257–267. doi: 10.1002/jez.b.22442. [DOI] [PubMed] [Google Scholar]
- Hansson (1997).Hansson LJ. Effect of temperature on growth rate of Aurelia aurita (Cnidaria, Scyphozoa) from Gullmarsfjorden, Sweden. Marine Ecology Progress Series. 1997;161:145–153. doi: 10.3354/meps161145. [DOI] [Google Scholar]
- Hardwick et al. (2015).Hardwick LJA, Ali FR, Azzarelli R, Philpott A. Cell cycle regulation of proliferation versus differentiation in the central nervous system. Cell and Tissue Research. 2015;359(1):187–200. doi: 10.1007/s00441-014-1895-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmrich et al. (2012).Hemmrich G, Khalturin K, Boehm AM, Puchert M, Anton-Erxleben F, Wittlieb J, Klostermeier UC, Rosenstiel P, Oberg HH, Domazet-Loso T, Sugimoto T, Niwa H, Bosch TC. Molecular signatures of the three stem cell lineages in hydra and the emergence of stem cell function at the base of multicellularity. Molecular Biology and Evolution. 2012;29(11):3267–3280. doi: 10.1093/molbev/mss134. [DOI] [PubMed] [Google Scholar]
- Hobmayer et al. (2012).Hobmayer B, Jenewein M, Eder D, Eder MK, Glasauer S, Gufler S, Hartl M, Salvenmoser W. Stemness in Hydra - a current perspective. International Journal of Developmental Biology. 2012;56(6–7–8):509–517. doi: 10.1387/ijdb.113426bh. [DOI] [PubMed] [Google Scholar]
- Holstein, Hobmayer & Technau (2003).Holstein TW, Hobmayer E, Technau U. Cnidarians: an evolutionarily conserved model system for regeneration? Developmental Dynamics. 2003;226(2):257–267. doi: 10.1002/dvdy.10227. [DOI] [PubMed] [Google Scholar]
- Ikmi et al. (2014).Ikmi A, Gaertner B, Seidel C, Srivastava M, Zeitlinger J, Gibson MC. Molecular evolution of the Yap/Yorkie proto-oncogene and elucidation of its core transcriptional program. Molecular Biology and Evolution. 2014;31(6):1375–1390. doi: 10.1093/molbev/msu071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeschke et al. (2000).Jeschke MG, Debroy MA, Wolf SE, Rajaraman S, Thompson JC. Burn and starvation increase programmed cell death in small bowel epithelial cells. Digestive Diseases and Sciences. 2000;45(2):415–420. doi: 10.1023/A:1005445501016. [DOI] [PubMed] [Google Scholar]
- Kahn & Leys (2016).Kahn AS, Leys SP. The role of cell replacement in benthic–pelagic coupling by suspension feeders. Royal Society Open Science. 2016;3(11):160484. doi: 10.1098/rsos.160484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsenty, Kronenberg & Settembre (2009).Karsenty G, Kronenberg HM, Settembre C. Genetic control of bone formation. Annual Review of Cell and Developmental Biology. 2009;25(1):629–648. doi: 10.1146/annurev.cellbio.042308.113308. [DOI] [PubMed] [Google Scholar]
- Kass-Simon & Scappaticci (2002).Kass-Simon G, Scappaticci AA., Jr The behavioral and developmental physiology of nematocysts. Canadian Journal of Zoology. 2002;80(10):1772–1794. doi: 10.1139/z02-135. [DOI] [Google Scholar]
- Khong et al. (2016).Khong NMH, Yusoff FM, Jamilah B, Basri M, Maznah I, Chan KW, Nishikawa J. Nutritional composition and total collagen content of three commercially important edible jellyfish. Food Chemistry. 2016;196:953–960. doi: 10.1016/j.foodchem.2015.09.094. [DOI] [PubMed] [Google Scholar]
- King & Newmark (2012).King RS, Newmark PA. The cell biology of regeneration. Journal of Cell Biology. 2012;196(5):553–562. doi: 10.1083/jcb.201105099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koç et al. (2004).Koç A, Wheeler LJ, Mathews CK, Merrill GF. Hydroxyurea arrests DNA replication by a mechanism that preserves basal dNTP pools. Journal of Biological Chemistry. 2004;279(1):223–230. doi: 10.1074/jbc.M303952200. [DOI] [PubMed] [Google Scholar]
- Künzel et al. (2010).Künzel T, Heiermann R, Frank U, Müller W, Tilmann W, Bause M, Nonn A, Helling M, Schwarz RS, Plickert G. Migration and differentiation potential of stem cells in the cnidarian Hydractinia analysed in eGFP-transgenic animals and chimeras. Developmental Biology. 2010;348(1):120–129. doi: 10.1016/j.ydbio.2010.08.017. [DOI] [PubMed] [Google Scholar]
- Leclère et al. (2012).Leclère L, Jager M, Barreau C, Chang P, Le Guyader H, Manuel M, Houliston E. Maternally localized germ plasm mRNAs and germ cell/stem cell formation in the cnidarian Clytia. Developmental Biology. 2012;364(2):236–248. doi: 10.1016/j.ydbio.2012.01.018. [DOI] [PubMed] [Google Scholar]
- Leevers & McNeill (2005).Leevers SJ, McNeill H. Controlling the size of organs and organisms. Current Opinion in Cell Biology. 2005;17(6):604–609. doi: 10.1016/j.ceb.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Loewith & Hall (2011).Loewith R, Hall MN. Target of rapamycin (TOR) in nutrient signaling and growth control. Genetics. 2011;189(4):1177–1201. doi: 10.1534/genetics.111.133363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura & Kimura (1985).Miura S, Kimura S. Jellyfish mesogloea collagen. Characterization of molecules as alpha 1 alpha 2 alpha 3 heterotrimers. Journal of Biological Chemistry. 1985;260:15352–15356. [PubMed] [Google Scholar]
- Miyake, Iwao & Kakinuma (1997).Miyake H, Iwao K, Kakinuma Y. Life history and environment of Aurelia aurita. South Pacific Study. 1997;17:273–285. [Google Scholar]
- Momose, Derelle & Houliston (2008).Momose T, Derelle R, Houliston E. A maternally localised Wnt ligand required for axial patterning in the cnidarian Clytia hemisphaerica. Development. 2008;135(12):2105–2113. doi: 10.1242/dev.021543. [DOI] [PubMed] [Google Scholar]
- Müller, Teo & Frank (2004).Müller WA, Teo R, Frank U. Totipotent migratory stem cells in a hydroid. Developmental Biology. 2004;275(1):215–224. doi: 10.1016/j.ydbio.2004.08.006. [DOI] [PubMed] [Google Scholar]
- O’Brien et al. (2011).O’Brien LE, Soliman SS, Li X, Bilder D. Altered modes of stem cell division drive adaptive intestinal growth. Cell. 2011;147(3):603–614. doi: 10.1016/j.cell.2011.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellettieri & Sanchez Alvarado (2007).Pellettieri J, Sanchez Alvarado A. Cell turnover and adult tissue homeostasis: from humans to planarians. Annual Review of Genetics. 2007;41(1):83–105. doi: 10.1146/annurev.genet.41.110306.130244. [DOI] [PubMed] [Google Scholar]
- Penzo-Méndez & Stanger (2015).Penzo-Méndez AI, Stanger BZ. Organ-size regulation in mammals. Cold Spring Harbor Perspectives in Biology. 2015;7(9):a019240. doi: 10.1101/cshperspect.a019240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiroga Artigas et al. (2018).Quiroga Artigas G, Lapébie P, Leclère L, Takeda N, Deguchi R, Jékely G, Momose T, Houliston E. A gonad-expressed opsin mediates light-induced spawning in the jellyfish Clytia. eLife. 2018;7:e29555. doi: 10.7554/eLife.29555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragkousi et al. (2017).Ragkousi K, Marr K, McKinney S, Ellington L, Gibson MC. Cell-cycle-coupled oscillations in apical polarity and intercellular contact maintain order in embryonic Epithelia. Current Biology. 2017;27(9):1381–1386. doi: 10.1016/j.cub.2017.03.064. [DOI] [PubMed] [Google Scholar]
- Salic & Mitchison (2008).Salic A, Mitchison TJ. A chemical method for fast and sensitive detection of DNA synthesis in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(7):2415–2420. doi: 10.1073/pnas.0712168105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiaffino et al. (2013).Schiaffino S, Dyar KA, Ciciliot S, Blaauw B, Sandri M. Mechanisms regulating skeletal muscle growth and atrophy. FEBS Journal. 2013;280(17):4294–4314. doi: 10.1111/febs.12253. [DOI] [PubMed] [Google Scholar]
- Schmid & Alder (1984).Schmid V, Alder H. Isolated, mononucleated, striated muscle can undergo pluripotent transdifferentiation and form a complex regenerate. Cell. 1984;38(3):801–809. doi: 10.1016/0092-8674(84)90275-7. [DOI] [PubMed] [Google Scholar]
- Schmid et al. (1988).Schmid V, Alder H, Plickert G, Weber C. Transdifferentiation from striated muscle of medusae in vitro. Cell Differentiation and Development. 1988;25(Suppl):137–146. doi: 10.1016/0922-3371(88)90110-4. [DOI] [PubMed] [Google Scholar]
- Schmid, Wydler & Alder (1982).Schmid V, Wydler M, Alder H. Transdifferentiation and regeneration in vitro. Developmental Biology. 1982;92(2):476–488. doi: 10.1016/0012-1606(82)90193-2. [DOI] [PubMed] [Google Scholar]
- Schuchert (2007).Schuchert P. The European Athecate hydroids and their medusae (Hydrozoa, Cnidaria): filifera part 2. Revue Suisse De Zoologie. 2007;114:195–396. doi: 10.5962/bhl.part.80395. [DOI] [Google Scholar]
- Schuchert (2019).Schuchert P. World hydrozoa database. 2019. http://www.marinespecies.org/hydrozoa http://www.marinespecies.org/hydrozoa
- Suga et al. (2010).Suga H, Tschopp P, Graziussi DF, Stierwald M, Schmid V, Gehring WJ. Flexibly deployed Pax genes in eye development at the early evolution of animals demonstrated by studies on a hydrozoan jellyfish. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(32):14263–14268. doi: 10.1073/pnas.1008389107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczepanek, Cikala & David (2002).Szczepanek S, Cikala M, David CN. Poly-gamma-glutamate synthesis during formation of nematocyst capsules in Hydra. Journal of Cell Science. 2002;115(Pt 4):745–751. doi: 10.1242/jcs.115.4.745. [DOI] [PubMed] [Google Scholar]
- Takeda, Deguchi & Itabashi (2018).Takeda N, Deguchi R, Itabashi T. Reproductive strategies in marine hydrozoan jellyfish: sexual medusae and asexual polyps. In: Kobayashi K, Kitano T, Iwao Y, Kondo M, editors. Reproductive and Developmental Strategies: The Continuity of Life. Tokyo: Springer Japan; 2018. pp. 157–174. [Google Scholar]
- Takeda et al. (2018).Takeda N, Kon Y, Quiroga Artigas G, Lapebie P, Barreau C, Koizumi O, Kishimoto T, Tachibana K, Houliston E, Deguchi R. Identification of jellyfish neuropeptides that act directly as oocyte maturation-inducing hormones. Development. 2018;145(2):dev156786. doi: 10.1242/dev.156786. [DOI] [PubMed] [Google Scholar]
- Takeda et al. (2013).Takeda N, Nakajima Y, Koizumi O, Fujisawa T, Takahashi T, Matsumoto M, Deguchi R. Neuropeptides trigger oocyte maturation and subsequent spawning in the hydrozoan jellyfish Cytaeis uchidae. Molecular Reproduction and Development. 2013;80(3):223–232. doi: 10.1002/mrd.22154. [DOI] [PubMed] [Google Scholar]
- Technau & Steele (2011).Technau U, Steele RE. Evolutionary crossroads in developmental biology: cnidaria. Development. 2011;138(8):1447–1458. doi: 10.1242/dev.048959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thongrod et al. (2018).Thongrod S, Wanichanon C, Kankuan W, Siangcham T, Phadngam S, Morani F, Isidoro C, Sobhon P. Autophagy-associated shrinkage of the hepatopancreas in fasting male Macrobrachium rosenbergii is rescued by Neuropeptide F. Frontiers in Physiology. 2018;9:613. doi: 10.3389/fphys.2018.00613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracy & Baehrecke (2013).Tracy K, Baehrecke EH. The role of autophagy in Drosophila metamorphosis. Current Topics in Developmental Biology. 2013;103:101–125. doi: 10.1016/B978-0-12-385979-2.00004-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dam et al. (2011).Van Dam TJ, Zwartkruis FJ, Bos JL, Snel B. Evolution of the TOR pathway. Journal of Molecular Evolution. 2011;73(3–4):209–220. doi: 10.1007/s00239-011-9469-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Rigueur & Lyons (2014).Wang W, Rigueur D, Lyons KM. TGFβ signaling in cartilage development and maintenance. Birth Defects Research Part C: Embryo Today: Reviews. 2014;102(1):37–51. doi: 10.1002/bdrc.21058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber (1981).Weber C. Structure, histochemistry, ontogenetic development, and regeneration of the ocellus of Cladonema radiatum dujardin (cnidaria, hydrozoa, anthomedusae) Journal of Morphology. 1981;167(3):313–331. doi: 10.1002/jmor.1051670306. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A–D) Distribution of S-phase cells in the Cladonema pacificum medusa (1 day old) shown by EdU staining with short incubation time (150 μM, 1 h incubation). (A) Distribution of S-phase cells (EdU+) a whole medusa body. (B) Distribution of S-phase cells (EdU+) in a medusa manubrium. (C) Distribution of S-phase cells (EdU+) in a medusa umbrella. (D) Distribution of S-phase cells (EdU+) in medusa tentacles. Scale bar: (A, C) 200 μm, (B, D) 100 μm.
(A, B) Cross sections of medusa tentacle bulbs. (A) Mitotic cells (PH3+) located in ectoderm in medusa tentacle bulbs (70 days old). (B) Mitotic cells (PH3+) located in ectoderm in medusa tentacle bulbs (30 days old). Scale bars: (A–B) 50 μm.
(A) Cladonema pacificum medusa (2 days old) before feeding (left image) and Cladonema pacificum medusa (2 days old) after feeding (right image). (B) Cladonema pacificum medusa (2 days old) with 48 h HU treatment before feeding (left image) and Cladonema pacificum medusa (2 days old) with 48 h HU treatment after feeding (right image). (C) Quantification of the number of tentacle branching in control and HU-treated medusa, with HU washed off, after 48 h treatment. Error bar: SD. Scale bars: (A, B) one mm.
Statistical analysis for the proliferating cells’ distribution in umbrellas and tentacles was performed by applying the nearest neighbor distance test to EdU positive cells.
Data Availability Statement
The following information was supplied regarding data availability:
The raw data (pictures) are available in Figshare: Fujita, Sosuke; Nakajima, Yuichiro; Kuranaga, Erina (2019): Raw data for paper (SF-EK-YN). figshare. Dataset. https://doi.org/10.6084/m9.figshare.7935197.v4.