Abstract
BACKGROUND & AIMS:
The fecal immunochemical test (FIT) is an alternative to colonoscopy and can increase overall screening for colorectal cancer (CRC). However, little is known about the frequency of and reasons for mishandled FIT samples.
METHODS:
We performed a prospective study, nested within a randomized controlled trial of patients, recruited from December 2015 through August 2017, who were not up to date with colorectal cancer screening (50–75 years old). The patients were randomly assigned to usual care or outreach groups that received a mailed FIT with low literacy level instructions or a reminder call, or both. We examined frequency of and reasons for mishandled FIT samples, including absence of collection date; time from collection to laboratory receipt of more than 14 days; or mishandling of stool, buffer, or cap. The outcomes were the frequency of mishandled FIT samples, effects of outreach on mishandling, and positive results from the FIT among proper and mishandled samples.
RESULTS:
FIT samples were returned from 1871 patients assigned to usual care and 3045 who received the low literacy level instructions and a reminder call. In total, 19.8% of samples were mishandled; most of these (93.7%) had not labeled the date of stool collection but were still processed. Of the received samples, 1.2% of were not processed because the time from patient collection to laboratory receipt was more than 14 days. Outreach was associated with a lower proportion of mishandled samples (16.5% vs 25.0% for usual care; P < .0001). The proportion of mishandled samples was lowest among patients who received the low literacy level instruction and a reminder call (12.8%, P < .0001). There was no significant difference in proportions of positive results between properly processed samples (7.5%) and improperly processed samples (6.2%) (P = .14).
CONCLUSION:
In a prospective study of patients who were not up to date with colorectal cancer screening, we found that almost 20% of FIT samples were mishandled, with most patients missing the stool collection date. Patient outreach was associated with a lower proportion of mishandled samples, but there was no difference in proportions of positive results between properly and improperly handled samples. Our findings indicate that routine processing of undated FIT samples is associated with similar rates of positive results. There are limited data on test characteristics for FIT samples beyond the 14 days of stool acquisition. The inclusion of low literacy level instructions with reminder calls was associated with improved patient handling of the FIT sample. ClincialTrials.gov no:
Keywords: Colon Cancer, Detection, Compliance, Home Test
Colorectal cancer (CRC) is the second leading cause of cancer deaths in the United States.1,2 Despite evidence that screening is effective in reducing CRC mortality,3 screening is underutilized in the general population.4,5 Screening options include noninvasive methods such as the fecal immunochemical test (FIT).
The safety-net health system is an integrated network comprised of publicly subsidized health services to the low-income population. Within safety-net health systems, FIT has been a preferred option for population-level screening.5 However, low-income, recent immigrants, and non–English-speaking populations who often receive care from safety-net health systems may experience difficulty comprehending and completing FIT using word-based and English instructions.6–8 Despite effectiveness of FIT for population-level screening for CRC,2,9 screening relies on patients properly completing FIT tests for laboratory processing.6,10,11 Studies have examined FIT mailing to improve screening rates,12–15 but little has been reported on the frequency of and reasons for mishandled FIT specimens and the impact of FIT completion errors on the test result. Moreover, the performance characteristics of FIT may be altered by mishandling of FIT specimens, such that positivity rates decrease with delays in processing and exposure to higher temperature.11,16–19
Our objective was to examine the frequency of and reasons for mishandled FIT samples. To examine this issue, we performed a subanalysis within a randomized trial of mailed FIT outreach in the form of low-literacy instructions (LLI) and reminder phone calls in a large integrated safety-net health system. We also examined the rate of positive FIT results in mishandled vs properly completed samples.
Materials and Methods
Study Setting
This study was based in the San Francisco Health Network (SFHN). SFHN is a publicly funded, integrated safety-net health system comprising 12 community- and hospital-based adult primary care clinics and 1 specialty referral center, Zuckerberg San Francisco General Hospital. SFHN clinics share an integrated electronic health records platform,20 a clinical laboratory, and 1 gastroenterology referral unit at Zuckerberg San Francisco General Hospital.21
Study Population
Our examination of FIT samples is nested within a randomized controlled trial of patients recruited from December 2015 to August, 2017. This study was approved by the University of California, San Francisco’s institutional review board (#14–14861; ). The parent study enrolled patients not up to date with colorectal cancer screening (Supplementary Appendix). This included men and women aged 50–75 years who had not completed a FIT within 365 days, sigmoidoscopy within 5 years, or colonoscopy within 10 years. Patients were excluded from the parent clinical trial if they were homeless, had abnormal FIT but no follow-up colonoscopy completed, had a diagnosis of CRC or colectomy, or had limited life expectancy. Eligible patients were randomized from 8 participating clinics to usual care or outreach with mailed FIT kits. All kits were returned by mail to 1 hospital-based clinical laboratory. For our subanalysis of FIT samples for this manuscript, we examined data from the 4916 FIT samples that were returned to the laboratory. A total of 1871 were returned from patients assigned to usual care, and 3045 were returned from patients assigned to the outreach intervention.
Study Design
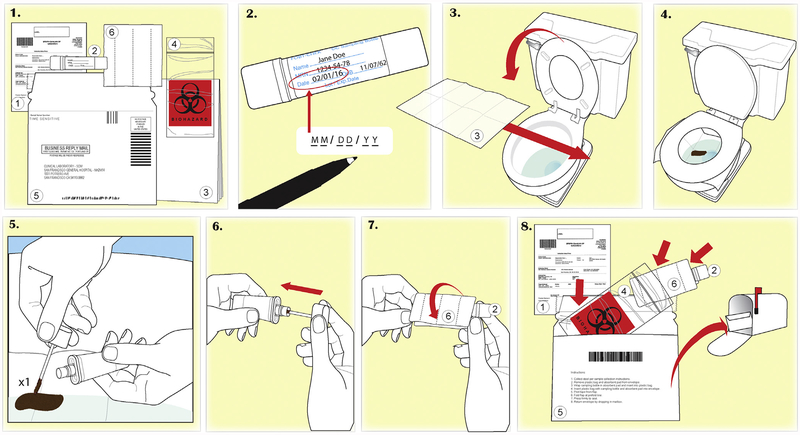
Patients were randomized to receive an outreach intervention which included a mailed FIT kit or usual care. The outreach intervention included a prompting postcard and phone call 2 weeks before the mailed FIT kit, a low-literacy wordless FIT instruction accompanying the mailed FIT kit (Figure 1) or up to 2 reminder phone calls if the FIT kit was not returned after 2 weeks, or both. Patients assigned to usual care were not mailed a FIT kit. Patients receiving usual care obtained their FIT kit with standard manufacturer instructions during a clinic visit. However, some patients (n = 503) who were assigned to the outreach arm received their FIT kits during an encounter with their provider preceding receipt of the FIT kit through the outreach intervention, despite being assigned to outreach. These patients did not receive a low-literacy wordless instruction, and may have only received a reminder phone call. All outreach intervention components were developed and tested in patient advisory boards.
Figure 1.
Low literacy wordless instruction accompanying the mailed fecal immunochemical test kit.
Study Variables
Demographic information and clinical laboratory data were extracted from the electronic medical records (eClinicalWorks) and managed using Microsoft Access (version 2016; Microsoft, Redmond, WA).
The FIT kits (Polymedco CDP, Cortlandt Manor, NY) used for usual care and outreach were the OC-Light (before April 2016) and the OC-Auto FIT (from April 2016 to August 2017). Usual care received manufacturer instructions while outreach received low-literacy wordless instructions. Mishandled samples were defined by absence of stool collection date on the FIT label, inappropriate quantity of stool, absence of buffer, improperly capped test tube, or test tube tampering. Among returned FIT samples, the clinical laboratory noted improperly handled specimen and recorded the test result if they were able to process the test. These notes were captured during data extraction and curated into the specific categories. Per manufacturer guidelines, FIT samples with stool collection dates exceeding 14 days to the time of receipt at the laboratory were not processed and were cancelled. Laboratory processing for returned FIT was based on the difference of date of clinical laboratory receipt and date of stool collection date being less than 14 days. FIT samples with unknown stool collection dates were also allowed to be processed by the clinical laboratory; however, the clinical laboratory inserted a note stating, “Collection date unknown. Samples may have exceeded 14 day stability period.” The clinical laboratory was blinded to study interventions.
Analytic Plan
Patient characteristics were described as proportions and means, and compared using chi-square test and Student’s t test as appropriate. Patients who were included in our analyses had all returned the FIT kits to the laboratory. The primary outcome was the rate of mishandled FIT samples received by the clinical lab out of all FIT kits received in the usual care group compared with the outreach intervention group. To determine the effect of specific intervention components on the rate of mishandled FIT samples, we examined the subgroup of patients in the intervention group who received their FIT kit by mail and those that received their FIT kit during a clinic visit after being assigned to the outreach intervention, and compared this to usual care. Similarly, we examined the subgroup of patients who received the FIT kit by mail, which contained the LLI, but returned the FIT kit before a reminder call (RC). Last, we examined the subgroup who received the FIT kit with the LLI but also received the RC. These were all compared with usual care to examine the effect of each component of the outreach intervention. We also evaluated the rate of positive or abnormal FIT results among properly completed and mishandled samples and how it differed by outreach and usual care. Other analyses included the association of outreach with the frequency of mishandled FIT samples stratified by patient demographics (language, race) and a history of completing a prior FIT.
Statistically significant results were considered when P < .05 using a 2-tailed test. Displayed 95% confidence intervals (CIs) were calculated by the Wald method. Stata/IC (version 15.0; StataCorp, College Station, TX) statistical software was used for all analyses.
All authors had access to the study data and reviewed and approved the final manuscript.
Results
Patient Demographics
The mean age of patients completing the FIT was 59 years, and 49% were men. The cohort was racially diverse, with 19% White, 24% Asian, 19% Black, and 23% Hispanic (Table 1) participants and 50% primary English speakers, 20% Spanish speakers, and 9% who identified Chinese as their primary language. The mean age and sex distribution were similar in the usual care and outreach groups (Table 1). Patients in the outreach group were slightly more likely to be white (20.0% in outreach group, 17.4% in usual care group; P = .02). Among the patients whose language was known, the percentage who spoke English, Spanish, or Chinese was similar in the usual care and outreach groups.
Table 1.
Demographics of patients who returned FIT samples
| Usual care (n = 1871) | Outreach (n = 3045) | |
|---|---|---|
| Age, y | 59 | 59 |
| Sex | ||
| Male | 48.8 (913) | 49.8 (1517) |
| Female | 51.2 (958) | 50.2 (1528) |
| Race | ||
| White | 17.4 (325) | 20.0 (609) |
| Black | 19.1 (357) | 19.0 (579) |
| Hispanic | 24.1 (451) | 23.0 (701) |
| Asian | 24.7 (462) | 23.4 (712) |
| Other | 14.7 (275) | 14.6 (444) |
| Language | ||
| English | 39.2 (734) | 57.3 (1745) |
| Spanish | 13.5 (252) | 23.2 (707) |
| Chinese | 6.5 (122) | 9.8 (297) |
| Other/unknowna | 40.8 (763) | 9.7 (296) |
NOTE. Values are mean or % (n).
FIT, fecal immunochemical test.
Outreach provided an opportunity to confirm preferred language, which resulted in fewer patients grouped in other/unknown.
Mishandled FIT Samples
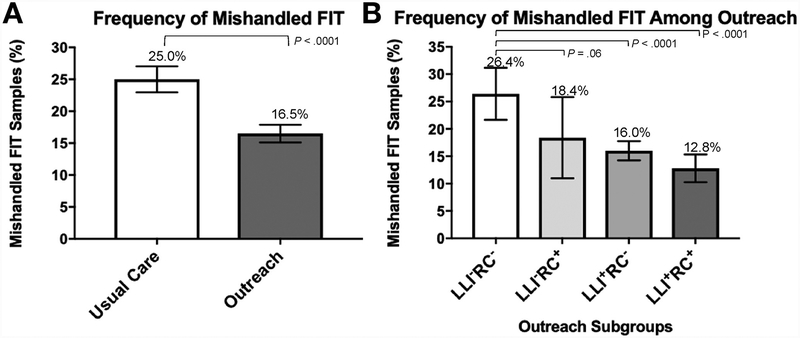
Of the 4916 FIT samples returned, there were 971(19.8%) mishandled samples according to clinical laboratory notes. Of 971 mishandled samples, 59 samples with stool collection dates were not processed by the clinical laboratory (1.2% of all returned kits) due to date of returned FIT exceeding 14 days. Overall, the vast majority of mishandled samples were due to missing date of stool collection (93.7%) (Table 2). Compared with usual care, the group receiving FIT distributed through mailed outreach had a lower frequency of mishandled samples (25.0% vs 16.5%, P < .0001) (Figure 2A).
Table 2.
Reasons for mishandled FIT samples
| Usual care (n = 468) | Outreach (n = 503) | |
|---|---|---|
| Unknown date | 94.7 (443) | 92.8 (467) |
| Test tube tampering | 0.2 (1) | 0.0 (0) |
| Other/unknown | 0.2 (1) | 0.2 (1) |
| Cancelled (exceeding 14 days) | 4.9 (23) | 7.0 (36) |
NOTE. Values are % (n). Inappropriate quantity of stool and absence of buffer were not observed in our study sample. Percentages are percent of total mishandled in usual care or outreach group.
FIT, fecal immunochemical test.
Figure 2.
Frequency of mishandled fecal immunochemical test (FIT) tests by usual care or exposure to different components of the intervention. (A) Compared with usual care, outreach was associated with decreased percentage of mishandled FIT samples returned to the clinical laboratory. (B) In the outreach arm, components of the study intervention were associated with reduced rate of mishandled FIT samples. LLI = instructions; RC = reminder call.
Association of Outreach With Mishandled FIT Samples
To determine the association of specific outreach intervention components with the rate of mishandled FIT samples, we examined the subgroups of patient who received partial vs all components of the intervention (Figure 2B).
In the intervention group of patients (n = 503) who received the FIT kit in the clinic before outreach could be initiated, the frequency of mishandled FIT samples from patients who received neither LLI nor an RC (LLI−RC−) (n = 367) was 26.4% (95% CI, 21.7%–31.2%, P = .66 vs usual care); this decreased to 18.4% among patients who received only a RC (LLI−RC+) (n = 136) (95% CI, 11.0%–25.8%, P = .061 vs LLI−RC−).
In the intervention group (n = 2542) who received the FIT kit instructions by mail, which was accompanied by LLI, patients could have returned the FIT kit before or following an RC. In the subgroup that received the LLI but returned their kit before the RC (LLI+RC−) (n = 1766), the frequency of mishandled FIT samples was 16.0% (95% CI, 14.3%–17.8%, P < .0001 vs LLI−RC−). Patients who received both LLI and the RC (LLI+RC+) (n = 776) had the lowest rate of mishandled FIT samples at 12.8% (95% CI, 10.3%–15.3%, P < .0001 vs LLI−RC−).
Mishandled FIT Samples and Positivity Rates
Among samples that were processed, FIT positivity rate was slightly higher for the samples that were mishandled than for the other FIT samples, but this difference was not statistically significant (7.5% vs 6.2%; P = .14). The rate of FIT positivity in mishandled FIT samples was similar between patients assigned to usual care and outreach (7.5% vs 7.6%; P = 0.97).
The outreach group had a higher proportion of patients who had no prior FIT testing (26.4% vs 22.6%, P = .002). Patients who had no prior FIT testing had a modestly higher rate of mishandled FIT samples compared with patients who previously completed the test (usual care 28.7% vs 23.9%, P = .055; outreach 17.2% vs 16.3%, P = .58); however, the rate of mishandled FIT samples was higher for the usual care group both among those with and without prior FIT testing. FIT positivity was higher in patients who had no prior FIT testing (9.9% vs 5.4%, P < .0001). Among mishandled FIT samples, the FIT positivity rate in those with no prior FIT tests and those with prior FIT tests was 10.9% vs 6.3% (P = .02), whereas among appropriately handled FIT samples, the FIT positivity rate was 9.6% in those with no prior FIT tests and 5.1% in those with a prior FIT test (P < .0001).
Mishandled FIT Samples by Language and Race
Outreach interventions were associated with a lower rate of mishandled FIT samples across all language and race categories. Outreach was associated with lower frequency of mishandled samples among patients who identified English as their preferred language (27.1% vs 18.1%, P < .0001) and among non-English speakers (Spanish speaking, 20.2% vs 13.7%, P = .014; Chinese speaking, 21.3% vs 14.5%, P = .087). Within racial and ethnic groups, outreach also was associated with lower frequency of mishandled samples: Whites (30.2% vs 17.1%, P < .0001), Blacks (35.3% vs 24.4%, P < .0001), Hispanics (21.1% vs 12.6%, P < .0001), and Asians (18.8% vs 14.2%, P = .03).
OC-Auto and OC-Light FIT Tests
Compared with usual care, outreach was associated with lower rate of mishandled FIT samples for both OC-Light and OC-Auto FIT kits (33.2% vs 20.1% for OC-Light, P < .0001; 22.9% vs 15.3% for OC-Auto, P < .0001). Comparing the 2 different FIT kits, OC-Auto was less likely to be mishandled (24.7% vs 18.4%, P < .0001).
Discussion
As clinical laboratory practices vary and some labs discard mishandled FIT samples, properly completing FIT for lab processing is an important first step in stool-based screening.11,22–24 Minimizing patient errors when completing at home FIT is important to achieving maximal screening participation.7,8 To our knowledge, our study is the first to describe the various reasons for mishandled FIT within a safety-net population and explore whether LLI and phone reminders are associated with the quality of FIT handling. We show that mishandled FITs are common, with the vast majority due to missing stool collection date, which were still processed in our clinical setting. This allowed us to examine whether the absence of a stool collection date was associated with a lower FIT positivity rate. Furthermore, we show that a combination of LLI and an RC was associated with a reduction in the quality of handling of FIT samples by patients.
Previous literature has raised concerns over the rate of degradation of hemoglobin over time and by temperature leading to reduced FIT sensitivity.11,16,17,25 In our study, mishandled FIT samples, in the form of a missing stool collection date, were not associated with a higher rate of FIT positivity. Patients who did and did not properly label the stool collection date may have returned their FIT kits at similar times following sample collection.
This study underscores the value of LLI and timely use of prompts and reminders to educate and engage patients in testing. Pictorial-based instructions increase FIT test completion and return rate8,26; in the same way, we show that low-literacy wordless FIT instruction was associated with a reduction in mishandled samples. Specifically, each component of the outreach intervention appeared to be valuable. Moreover, the LLI was associated with a lower rate of mishandled samples within each major racial and ethnic group and among patients who preferred non-English language.
There are several limitations of this study. First, as this was a pragmatic study, clinics were allowed to choose their own method to educate and instruct patients. Different clinics may have different practices, and the heterogeneity between clinic practices was not captured in this study. However, we did observe a reduction in mishandling for those enrolled in the outreach intervention. Second, we only analyzed the subgroup who returned FIT kits, and the results could be confounded by other patient characteristics. Third, there remains a significant proportion of patients that still do not adhere to the wordless instructions and do not put a date on the stool collection bottle. Qualitative approaches would be needed to identify the root causes. Fourth, this study was a secondary analysis. As the intervention is multicomponent, there could be some overlapping effect that cannot be teased apart. The impact of the LLI and RC might be evaluated more directly with a randomized control trial that randomizes each intervention component. Last, ambient temperature conditions were not collected for each sample. Further studies should be performed regarding the stability of the samples over time as there is data suggesting instability in the presence of high temperature.11,16–19
In conclusion, mishandled FIT samples received by the clinical laboratory are common. The most frequent cause is the absence of a stool collection date. There is concern for hemoglobin degradation with time; however, there is little evidence that positivity rates are altered by the absence of collection date which may reassure providers and clinical laboratories that FIT results are valid even when collection date is not recorded and should be processed. However, we remain mindful that the practice of processing undated FIT specimens may not be representative of individuals with false negatives from mishandled samples greater than 14 days of age. Hence, the routine processing of undated FIT samples is not associated with lower positivity rates. Further studies should be performed regarding the stability of the samples over time as there are data suggesting instability in the presence of high temperature. Our data show that low-literacy wordless instructions and reminder phone calls may be helpful in reducing missing information in FIT samples returned by mail, even among non–English-speaking and racially and ethnically diverse populations. Standardized materials and communication with patient feedback as used in this outreach initiative underscore how health systems can enhance the patient experience.
Supplementary Material
What You Need to Know.
Background
Home fecal immunochemical test (FIT) can increase colorectal cancer screening, but it is not clear that patients mishandle their samples. We collected data from patients randomly assigned to groups that received a mailed FIT with low literacy level instructions and a reminder call (outreach) or usual care.
Findings
Almost 20% samples were mishandled; most of these (93.7%) had not labeled the date of stool collection, which was still processed by the clinical laboratory. Outreach was associated with a lower proportion of mishandled samples. There was no significant difference in the rate of abnormal results between properly handled and mishandled samples.
Implications for patient care
We recommend routine processing of undated FIT samples and inclusion of low literacy level instructions with reminder calls to improve sample handling by patients.
Acknowledgments
The authors appreciate the support of the University of California, San Francisco, Academic Research System services, specifically Marshal Jackson, who supported the data extraction. They are grateful to Gloria D. Coronado, PhD (Center for Health Research, Kaiser Permanente Northwest, Portland, OR), for allowing them to adapt their wordless instruction for the study, and Helen Landicho (Regulatory Affairs, Polymedco, Cortlandt Manor, NY, USA) for her insight and data on OC-Light and OC-Auto products. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Funding
This study was supported in part by grants from the Centers for Disease Control and Prevention (U48DP004998, SIP 14–012 [to Ma Somsouk]) and the SF Cancer Initiative. This study was supported by University of California, San Francisco, Academic Research Systems, and by the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSFCTSI Grant Number UL1 TR991872.
Abbreviations used in this paper:
- CI
confidence interval
- CRC
colorectal cancer
- FIT
fecal immunochemical test
- LLI
low-literacy instructions
- RC
reminder call
- SFHN
San Francisco Health Network
Footnotes
Supplementary Material
Note: To access the supplementary material accompanying this article, visit the online version of Clinical Gastroenterology and Hepatology at www.cghjournal.org, and at https://doi.org/10.1016/j.cgh.2018.11.050.
Conflicts of interest
The authors disclose no conflicts.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Mandel JS, Bond JH, Church TR, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. N Engl J Med 1993; 328:1365–1371. [DOI] [PubMed] [Google Scholar]
- 3.Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for colorectal cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2016;315:2564–2575. [DOI] [PubMed] [Google Scholar]
- 4.Inadomi JM, Vijan S, Janz NK, et al. Adherence to colorectal cancer screening: a randomized clinical trial of competing strategies. Arch Intern Med 2012;172:575–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta S, Sussman DA, Doubeni CA, et al. Challenges and possible solutions to colorectal cancer screening for the underserved. J Natl Cancer Instit 2014;106:dju032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pham R, Cross S, Fernandez B, et al. “Finding the right FIT”: rural patient preferences for fecal immunochemical test (FIT) characteristics. J Am Board Fam Med 2017;30:632–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bapuji SB, Lobchuk MM, McClement SE, et al. Fecal occult blood testing instructions and impact on patient adherence. Cancer Epidemiol 2012;36:e258–e264. [DOI] [PubMed] [Google Scholar]
- 8.Coronado GD, Sanchez J, Petrik A, et al. Advantages of wordless instructions on how to complete a fecal immunochemical test: lessons from patient advisory council members of a federally qualified health center. J Cancer Educ 2014; 29:86–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Selby JV, Friedman GD, Quesenberry CP Jr, et al. Effect of fecal occult blood testing on mortality from colorectal cancer. A case-control study. Ann Intern Med 1993;118:1–6. [DOI] [PubMed] [Google Scholar]
- 10.Chambers JA, Callander AS, Grangeret R, et al. Attitudes towards the Faecal Occult Blood Test (FOBT) versus the Faecal Immunochemical Test (FIT) for colorectal cancer screening: perceived ease of completion and disgust. BMC Cancer 2016; 16:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doubeni CA, Jensen CD, Fedewa SA, et al. Fecal Immunochemical Test (FIT) for colon cancer screening: variable performance with ambient temperature. J Am Board Fam Med 2016; 29:672–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rachocki C, Laleau V, Garcia D, et al. Mailed FIT to improve colorectal cancer screening in an integrated safety-net system. Gastroenterology 2017;152:S75. [Google Scholar]
- 13.Coronado GD, Schneider JL, Sanchez JJ, et al. Reasons for non-response to a direct-mailed FIT kit program: lessons learned from a pragmatic colorectal-cancer screening study in a federally sponsored health center. Transl Behav Med 2015; 5:60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levy BT, Daly JM, Xu Y, et al. Mailed fecal immunochemical tests plus educational materials to improve colon cancer screening rates in Iowa Research Network (IRENE) practices. J Am Board Fam Med 2012;25:73–82. [DOI] [PubMed] [Google Scholar]
- 15.Daly JM, Levy BT, Merchant ML, et al. Mailed fecalimmunochemical test for colon cancer screening. J Community Health 2010;35:235–239. [DOI] [PubMed] [Google Scholar]
- 16.van Roon AHC, Hol L, van Vuuren AJ, et al. Are fecal immuno-chemical test characteristics influenced by sample return time? A population-based colorectal cancer screening trial. Am J Gastroenterol 2011;107:99–107. [DOI] [PubMed] [Google Scholar]
- 17.Dancourt V, Hamza S, Manfredi S, et al. Influence of sample return time and ambient temperature on the performance of an immunochemical faecal occult blood test with a new buffer for colorectal cancer screening. Eur J Cancer Prev 2016; 25:109–114. [DOI] [PubMed] [Google Scholar]
- 18.Polymedco Cancer Diagnostic Products. Instructions for Use: OC-Light S FIT 2015. Cortlandt Manor, NY: Polymedco, 2015. [Google Scholar]
- 19.Polymedco Cancer Diagnostic Products L. Instructions for Use: OC-Auto FIT 2016. Cortlandt Manor, NY: Polymedco, 2015. [Google Scholar]
- 20.Chen AH, Murphy EJ, Yee HF Jr. eReferral–a new model for integrated care. N Engl J Med 2013;368:2450–2453. [DOI] [PubMed] [Google Scholar]
- 21.Katz MH, Brigham TM. Transforming a traditional safety net into a coordinated care system: lessons from healthy San Francisco. Health Aff (Millwood) 2011;30:237–245. [DOI] [PubMed] [Google Scholar]
- 22.Allison JE, Fraser CG, Halloran SP, et al. Population screening for colorectal cancer means getting FIT: the past, present, and future of colorectal cancer screening using the fecal immuno-chemical test for hemoglobin (FIT). Gut and Liver 2014; 8:117–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robertson DJ, Lee JK, Boland CR, et al. Recommendations on Fecal Immunochemical Testing to Screen for Colorectal Neoplasia: a consensus statement by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2017;152:1217–1237.e3. [DOI] [PubMed] [Google Scholar]
- 24.Imperiale TF, Ransohoff DF, Itzkowitz SH, et al. Multitarget stool DNA testing for colorectal-cancer screening. N Engl J Med 2014;370:1287–1297. [DOI] [PubMed] [Google Scholar]
- 25.Shapiro JA, Bobo JK, Church TR, et al. A comparison of fecal immunochemical and high-sensitivity guaiac tests for colorectal cancer screening. Am J Gastroenterol 2017;112:1728–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bui D, Nakamura C, Bray BE, et al. Automated illustration of patients instructions. AMIA Annu Symp Proc 2012; 2012:1158–1167. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.