Abstract
The neuropeptide oxytocin (OT) promotes social behavior and attenuates stress responsivity in mammals. Recent clinical evidence suggests OT concentrations may be dysregulated in major depression. This study extends previous research by testing whether: 1) OT concentrations vary systematically in depressive disorders with and without hypercortisolemia, 2) gender differences in OT concentrations are observed in depressed vs. healthy control participants, and 3) OT concentrations are predictive of clinical phenotypes. Plasma OT concentrations of psychotic major depressive (PMD; n = 14: 10 female, 4 male), non-psychotic major depressive (NPMD; n = 17: 12 female, 5 male), and non-depressed, healthy control (n = 19: 11 female, 8 male) participants were assayed at 2000, 2400, 0400, and 0800 h. Plasma cortisol concentrations were quantified at 2300 h, and clinical phenotypes were determined. As expected, PMD participants, compared to NPMD and healthy control participants, showed higher plasma cortisol concentrations. Although both depressed groups showed similar OT concentrations, a significant interaction effect between group and gender was observed. Specifically, depressed females exhibited lower mean OT concentrations than depressed males. Further, depressed vs. healthy control female participants exhibited lower mean OT concentrations, whereas depressed vs. healthy control male participants showed a trend in the opposite direction. OT concentrations were also predictive of desirability, drug dependence, and compulsivity scores as measured by the Million Clinical Multiaxial Inventory-III. All findings were independent of cortisol. These data suggest that OT signaling may provide a mechanism by which to better understand female-biased risk to develop depressive disorders and that plasma OT concentrations may be a useful biomarker of certain clinical phenotypes.
Keywords: Clinical phenotypes, Cortisol, Gender, Major depression, Plasma oxytocin, Psychotic major depression
1. Introduction
Individuals with depressive disorders often exhibit social difficulties, anxiety symptoms, and dysregulation of the hypothalamic—pituitary—adrenal (HPA) axis (Heuser et al., 1994). A common feature of HPA axis dysregulation is hypercortisolemia, which is present in 40—60% of depressed patients (Gold et al., 1986; Parker et al., 2003). Depressive disorders are also twice as prevalent in female compared to male patients (Nestler et al., 2002; Young, 1998).
The pathophysiology of depressive disorders and enhanced female risk to develop them are not well understood. It has been hypothesized that the neuropeptide oxytocin (OT) may play a role in these phenomena (Frasch et al., 1995). OT is a neuropeptide of hypothalamic origin with broad ranging central and peripheral nervous system effects. OT is released during positive social interactions (Knox and Uvnas-Moberg, 1998; Young et al., 2008), and exhibits anxiolytic properties in preclinical animal models (Amico et al., 2004). OT also exerts a tonic inhibitory influence over the HPA axis (Neumann et al., 2000). However, stressful social experiences, which are frequent precipitants of depressive disorders, activate the HPA axis. Often these experiences involve social isolation or loss, which may decrease OT signaling, thereby diminishing OT’s ability to inhibit HPA axis drive. Females may be particularly vulnerable under these stressful circumstances, as they respond to social rejection (Stroud et al., 2002) and marital conflict (Kiecolt-Glaser et al., 1996,1998) with greater HPA axis responses compared to men.
Preliminary research has shown that some depressed patients exhibit lower OT concentrations compared to healthy controls and that OT concentrations are lower in patients with moderate compared to mild depression (Frasch et al., 1995; Garcia et al., 2011; Ozsoy et al., 2009). Several pilot studies have concomitantly measured cortisol and OT concentrations in major depressed patients (Garcia et al., 2011; Parker et al., 2010), but no research has evaluated OT concentrations in patients with psychotic major depression (PMD), who typically exhibit greater depressive severity, and who are more likely to exhibit hypercortisolemia than patients with non-psychotic major depression (NPMD) (Belanoff et al., 2001).
In addition to the well-described roles of OT biology in human behavioral functioning, there is emerging evidence that OT concentrations are also associated with an array of clinical phenotypes and personality traits. For example, cerebrospinal fluid (CSF) OT concentrations are associated with a history of aggression (Lee et al., 2009), childhood trauma (Heim et al., 2009), and suicide intentionality (Jokinen et al., 2012). CSF OT concentrations are likewise negatively correlated with anxiety concentrations in patients with major depression (Scantamburlo et al., 2007) and plasma OT concentrations are lower in female patients with borderline personality disorder compared to healthy female controls (Bertsch et al., 2013). Mothers who used cocaine during pregnancy have lower plasma OT concentrations and greater hostility and depressed mood, as well as less social support and fewer adaptive strategies for dealing with stressful life events compared to cocaine naïve mothers (Light et al., 2004). In contrast, higher plasma OT concentrations are associated with measures of positive personality traits including increased extroversion in healthy male and female participants (Andari et al., 2012) and secure attachment and lower compulsive temperament in first-time mothers (Strathearn et al., 2009, 2012). Increased plasma OT concentrations are also associated with secure attachments and lower plasma cortisol and anxiety concentrations in healthy premenopausal women (Tops et al., 2007).
The present study extends previous research efforts in several respects. We measured plasma OT and cortisol concentrations in both male and female PMD, NPMD, and healthy control participants to test whether OT concentrations differ in participants with PMD vs. NPMD, or in depressed participants with and without hypercortisolism (regardless of depressive subtype). We also sought to examine whether female and male depressed participants differed in OT concentrations, and whether cortisol concentrations influenced these differences. Finally, we explored whether OT concentrations were associated with depression severity and anxiety symptomology (Hamilton Depression Rating Scale), as well as personality and clinical syndromes (Million Clinical Multiaxial Inventory-III) in depressed participants.
2. Materials and methods
2.1. Study participants
Adult participants were recruited through Stanford University Medical Center, as well as through online and print advertisements, as part of a larger research program, which investigates HPA axis physiology in depressive disorders. The present study was initiated after the completion of two recruitment rounds of a larger parent study, and resulted in available samples from 50 participants: 14 PMD (10 female, 4 male), 17 NPMD (12 female, 5 male), and 19 healthy controls (11 female, 8 male). Participants enrolled in the present study did not overlap with those in our previously published OT and depression study (Parker et al., 2010). However, participants’ cortisol data have been reported previously (Keller et al., 2006; Schatzberg et al., 2013). This research was conducted in accordance with the Declaration of Helsinki, and the Stanford University Institutional Review Board approved the study design. Informed written consent was obtained from all participants after the study procedures had been fully explained.
All PMD and NPMD participants met DSM-IV-TR criteria for a current major depressive episode, with or without psychotic features and provided Hamilton (1960) Depression Rating Scale (HDRS) scores ranging from severe to very severe depression. PMD participants were required to have a minimum score of 5 on the positive symptom subscale of the Brief Psychiatric Rating Scale (BPRS) (Overall and Gorham, 1962). This subscale consists of the following four items: conceptual disorganization, suspiciousness, hallucinations, and un-usual thought content. Healthy control participants were required to have a score <6 on the HDRS, have no psychotic symptoms as measured by the BPRS positive symptom subscale, and have no history of Axis I disorders as determined by the Structured Clinical Interview forDSM-IV-TR (Firstet al., 1997). Depressed participants were allowed to remain on psychiatric medications (see Table 1) provided the dose had not been adjusted in the last week. Healthy control participants were free of psychiatric medications. Additional study exclusion criteria for all participants were as follows: electroconvulsive shock therapy or substance abuse problems in the last six months, current drug use (evaluated by urinary toxicology screening), major medical illness, history of seizures, major head trauma, abnormal clinical laboratory tests, unstable or untreated hypertension, cardiovascular disease, endocrine disorders, pregnancy, or lactation.
Table 1.
Concomitant psychiatric medications for participants with non-psychotic major depression (NPMD) and psychotic major depression (PMD).
| NPMD (N = 14) |
PMD (N = 13) |
|
|---|---|---|
| No psychiatric medications | 2 | 8 |
| Antidepressant only | 3 | 4 |
| Antidepressant and antipsychotic | 1 | 0 |
| Antidepressant and anxiolytic | 1 | 1 |
| Antidepressant and mood stabilizer | 0 | 1 |
| Antidepressant, antipsychotic, and anxiolytic | 3 | 0 |
| Antidepressant, antipsychotic, and mood stabilizer | 3 | 0 |
| Antipsychotic and mood stabilizer | 1 | 0 |
| Breakdown of psychiatric medications | ||
| Any antidepressant | 10 | 6 |
| Any antipsychotic | 8 | 0 |
| Any anxiolytic | 4 | 1 |
| Any mood stabilizer | 4 | 1 |
2.2. Blood collection procedures
Participants were admitted to the Stanford University Hospital General Clinical Research Center at 1500 h. At 1600 h, an intravenous line, kept patent with saline infusion, was started in one arm and 4 mL of whole blood was drawn into heparinized vacutainer tubes once every hour between 1800 and 0900 h. Participants were required to be supine in bed 15 min prior to each blood sample collection. No food or sleep restrictions were imposed. Blood samples were subsequently centrifuged and the plasma fraction was stored at −70 °C prior to hormone quantification. Blood aliquots were available for OT analyses at four time points (2000, 2400, 0400, and 0800 h).
2.3. Plasma hormone quantification
Plasma OT concentrations were quantified using a commercially available enzyme immunoassay kit (Enzo Life Sciences, Inc., Farmingdale, NY). This kit has been validated for use in humans, and is highly specific and exclusively recognizes OT and not related peptides (Carter et al., 2007). Per Enzo Life Sciences literature, the limit of assay sensitivity where the curve is no longer linear is 10 pg/mL. Sample extraction and concentration procedures were initiated by thawing plasma samples in an ice bath before acidification with 0.1% trifluoroacetic acid (TFA). Acidified samples were then centrifuged at 17,000g for 15 min at 4 °C. Phenomenex Strata-X columns (Phenomenex Inc., Torrance, CA) were activated with 4 mL of HPLC grade methanol followed by 4 mL of molecular biology grade water. Following column activation, the sample supernatants were applied and drawn through the columns by vacuum. Peptides were then eluted by applying 4 mL of pre-elution buffer (89:10:1 water:acetonitrile:TFA) followed by 4 mL of elution buffer (80:20 acetonitrile:water). Elutes were evaporated at room temperature using compressed nitrogen, and reconstituted in assay buffer prior to quantification. Samples were assayed in duplicate with a tunable microplate reader for 96-well format according to manufacturer’s instructions. A trained technician without knowledge of experimental conditions performed all assays. Intra- and inter-assay coefficients of variation were below 10%.
Plasma cortisol concentrations were quantified for all participants using the Access Immunoassay System (Beckman Coulter, Chaska, MN) as part of the larger parent study. The sensitivity of this assay is 0.4 ug/dL (11 nmol/L). Cortisol concentrations in our study were evaluated at 2300 h, a time well-validated for determination of hypercortisolemia (Boscaro et al., 2001; Raff et al., 1998).
2.4. Phenotypic measurements
Depression severity and anxiety symptomology were determined using the HDRS total score and the HDRS anxiety subscale, respectively. The HDRS is a widely used and well-validated, investigator administered measure of symptom severity and determination of clinical subtypes for depression (Hamilton, 1960). Personality characteristics and clinical syndromes were evaluated with the Million Clinical Multiaxial Inventory (MCMI)-II or -III. These characteristics correspond to Axis II diagnoses in the DSM-IV. The MCMI was administered to depressed participants only, as it was standardized and developed for exclusive use in clinical populations (Million et al., 2009). In addition to assessing a wide range of personality disorders and clinical syndromes, the MCMI includes three indices that modify a participant’s Base Rate scores: Disclosure, Desirability, Debasement; and, two random response indicators: Validity and Inconsistency.
2.5. Statistical analyses
The plasma OT data were severely skewed, following an exponential distribution. For use as an independent (predictor) variable, we took the mean of the log of the 2000, 2400, 0400 and 0800 h measures (which prevents a single data point skewing the mean). For use as a dependent (predicted) variable, we calculated the arithmetic mean of the four time points, and then modeled both the mean and the skewed error distribution directly in a Generalized Linear Model (GLM; gamma error distribution, reciprocal link function) implemented using JMP v10 for Windows, and PROC GENMOD in SAS v9.3 for Windows as needed for detailed post-hoc tests. This approach is preferable to simple transformation and modeling in a least-squares framework, particularly because it is more agnostic and flexible in accommodating the error distribution of the data (Littell et al., 2002). The 2300 h plasma cortisol data showed the same skew, and thus were log transformed for use as a predictor, and treated in the same GLM framework for use as a dependent variable.
We first tested whether Group (PMD, NPMD, and healthy control) and Gender (female, male), predicted 2300 h plasma cortisol concentrations. This analysis is essentially a positive control as PMD patients are known to show elevated 2300 h cortisol concentrations over NPMD patients (Belanoff et al., 2001). Group was nested within Depressed, to explicitly test whether Depressed and Non-depressed participants differed on average; and then whether NPMD and PMD participants differed from each other; Gender and all interactions were included. Least-squares means and Bonferroni-corrected orthogonal planned contrasts were calculated for post-hoc-testing. Assumptions of the model were tested graphically. In addition, this model estimated overdispersion (no significant evidence for overdispersion was observed).
An identical analysis was used to predict mean plasma OT; and then also with 2300 h cortisol included as a covariate, yielding identical results. The simpler model is presented in the Results section. The post-hoc tests, and model-fit tests discussed above were employed.
Finally, the predictive relationships of plasma OT and cortisol concentrations to measures of 1) depressive severity (HDRS), 2) anxiety symptoms (HDRS anxiety subscale), and 3) personality dimensions (MCMI base rate scores) were each tested with the same multiple linear regression models implemented as a GLM in JMP v10 for Windows. These analyses excluded healthy controls, because they either scored low and without variation, or because no score could be obtained on the instrument. The models were blocked by Group, Gender, and their interaction, and the log of the 2300 h plasma cortisol, and its interaction with Group. We tested for an overall effect of the mean of the log of the plasma OT concentration, and its interaction with Group (i.e., whether the effect of OT differed in PMD and NPMD participants). This model therefore tested within each blocking factor for consistent effects of OT across the blocking factors (e.g., testing whether the effect of OT within each gender is consistent for both genders). The interaction explicitly tests whether the effect of OT is different in the two patient groups. Bonferroni planned contrasts were calculated as required for post-hoc tests. The assumptions of GLM were tested graphically, and no transformations were required.
3. Results
Demographic and clinical characteristics for PMD, NPMD, and healthy control groups are summarized in Table 2. Because age differed significantly by group [F2,41 = 3.889; p = 0.028], with NPMD participants being older than healthy control participants (p = 0.027; no other groups differed significantly), age was included as a statistical covariate in subsequent analyses as appropriate.
Table 2.
Participant demographics and clinical characteristics.
| HC (N = 17) |
NPMD (N = 14) |
PMD (N = 13) |
Test | |
|---|---|---|---|---|
| Age | 37.12 (12.71)ab | 47.57 (11.99)a | 35.69 (12.22)b | F41,2 = 3.889; |
| p = 0.028 | ||||
| Education | 15.62 (1.62)a | 15.29 (1.44)a | 15.15 (3.00)a | F41,2 = 0.203; |
| p = 0.817 | ||||
| Gender | ||||
| Male | 8 | 5 | 8 | LRχ2 = 0.899; |
| Female | 9 | 9 | 9 | p = 0.638 |
| HDRS | 0.71 (1.21)a | 22.14 (3.70)b | 31.31 (6.12)c | F41,2 = 237.6; |
| p < 0.001 | ||||
| HDRS Anxiety | 0.47 (1.01)a | 14.57 (3.27)b | 15.54 (3.86)c | F41,2 = 135.9; |
| p < 0.001 | ||||
| BPRS | 18.94 (1.56)a | 31.86 (4.29)b | 47.75 (6.62)c | F40,2 = 153.7; |
| p < 0.001 | ||||
| BPRS Positive | 4.18 (0.53)a | 4.36 (0.74)a | 11.83 (1.99)b | F40,2 = 179.5; |
| Symptom Scale | p < 0.001 | |||
| 11pm plasma | 2.32 (1.54)a | 2.71 (2.40)a | 6.50 (6.45)b,d | F40,2 = 4.916; |
| cortisol (μg/dL) | p = 0.012 | |||
HC, healthy control; NPMD, non-psychotic major depression; PMD, psychotic major depression; HDRS, Hamilton Depression Rating Scale; BPRS, Brief Psychiatric Rating Scale.
Means with the same superscript are not significantly different according to Tukey’s post-hoc test.
One PMD participant had a missing 11pm plasma cortisol value.
Of the 50 participants with available plasma samples, one participant was omitted for a missing plasma OT value. We also excluded all female participants prescribed estrogen due to the known modulatory effects of estrogen on OT (Ochedalski et al., 2007) and cortisol (White et al., 2006) signaling. These exclusions yielded a final sample size of 44 participants [13 PMD (9 female, 4 male), 14 NPMD (9 female, 5 male), and 17 healthy controls (9 female, 8 male)] for the analysis.
As expected, 2300 h cortisol concentrations differed by group. Specifically the PMD participants showed significantly higher concentrations than the NPMD participants [Group-nested-within-depressed Likelihood Ratio (LR) χ2 = 7.76; p = 0.0053; see Table 2], and further post-hoc tests showed that healthy controls did not differ from NPMD, but did differ from PMD participants. No other significant main or interaction effects were observed.
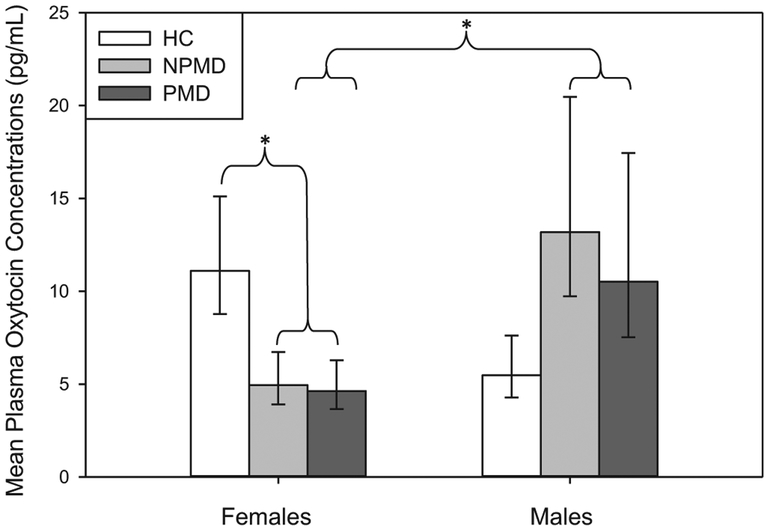
Initial descriptive statistics and subsequent follow up analyses revealed that PMD and NPMD participants did not differ in plasma OT concentrations [Group-nested-within-depressed LR χ2 = 0.14; p = 0.7125]. Instead, the only predictor of plasma OT concentrations was a highly significant interaction between depressed group and gender [Depressed*Gender LR χ2 = 9.54; p = 0.0020]. Males and females showed opposite patterns between depressed and non-depressed participants. Depressed females had significantly lower plasma OT concentrations than healthy control females [Healthy Control Females vs. Depressed Females: LR χ2 = 6.76; p = 0.0093; Bonferroni critical alpha correction set at 0.025]. Male participants generally showed greater OT variability, which rendered some post-hoc tests non-significant [Healthy Control Males vs. Depressed Males: LR χ2 = 3.52; p = 0.0605; Bonferroni critical alpha correction set at 0.025]. Male and female depressed participants significantly differed [Depressed Males vs. Depressed Females: LR χ2 = 7.33; p = 0.0068; Bonferroni critical alpha correction set at 0.025], but their control counterparts did not [Healthy Control Males vs. Healthy Control Females: LR χ2 = 3.14; p = 0.0762; Bonferroni critical alpha correction set at 0.025] (see Fig. 1).
Fig. 1.
Mean plasma oxytocin (OT) concentrations differ by group and sex. Post-hoc tests revealed that depressed females have lower OT concentrations than healthy control females, and depressed females have lower OT concentrations than depressed males. Data show fitted means and standard errors. * indicates a significant finding after correction for multiple comparisons (p < 0.025). HC, healthy control; NPMD, non-psychotic major depression; PMD, psychotic major depression.
For the phenotypic data we only report significant results for OT, or its interactions, since the presence of cortisol in the model did not alter the findings as mentioned above. The MCMI Desirability Score increased with higher mean log plasma OT [F1,12 = 5.470; p = 0.0375; Fig. 2a]. The MCMI Drug Dependence Base Rate Score decreased with higher mean log plasma OT (F1,12 = 6.428; p = 0.0262; Fig. 2b). Finally the MCMI Compulsive Base Rate Score, was predicted by the oxytocin*group interaction (F1,12 = 9.498; p = 0.0095; Fig. 2c). For this last result, post-hoc analysis showed that OT positively predicted compulsivity in the PMD group only [F1,12 = 17.19; p = 0.0014; Bonferroni critical alpha correction set at 0.025], but not the NPMD group [F1,12 = 0.14; p = 0.1361; Bonferroni critical alpha correction set at 0.025].
Fig. 2.
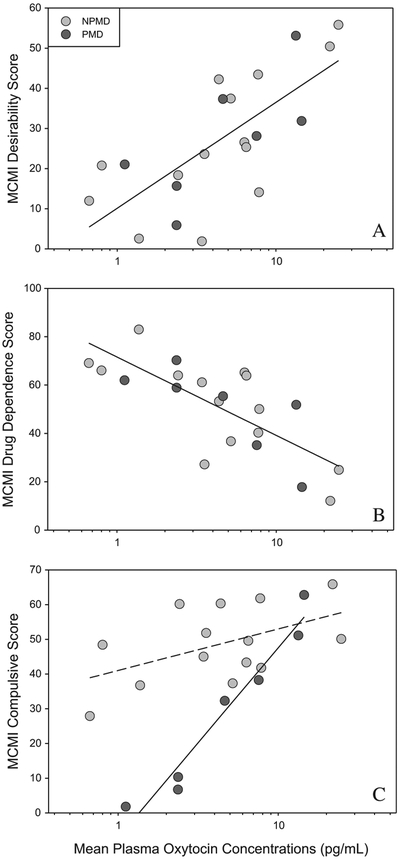
Mean plasma OT concentrations: positively predict MCMI Desirability scores in depressed participants (Panel A), negatively predict MCMI Drug Dependence Base Rate scores in depressed participants (Panel B), and positively predict MCMI Compulsivity Base Rate scores in PMD, but not NPMD, participants (Panel C). To correct for skew, mean plasma OT concentration is calculated as the mean of logged titers. To correct for the controlling variables in the analysis, the regression line is partialed (controlled) for other variables in the analysis, and calculated at the mean value of those variables. Similarly, data are shown as residuals from this mean regression line. NPMD, non-psychotic major depression; PMD, psychotic major depression.
4. Discussion
This pilot study was designed to investigate whether plasma OT concentrations differed in the context of hypercortisolemia in PMD, NPMD, and healthy control participants. We secondarily tested the relationship between plasma OT concentrations and clinical and personality syndromes in depressed participants. As expected, PMD participants exhibited significantly higher cortisol concentrations compared to NPMD and healthy control participants. However, PMD and NPMD participants did not differ in plasma OT concentrations. Interestingly, subsequent analyses revealed a significant group by gender interaction effect, such that depressed females exhibited lower OT concentrations compared to depressed males, irrespective of psychotic status. Further, depressed females, irrespective of psychotic status, exhibited lower OT concentrations compared to healthy control females. Depressed males, irrespective of psychotic status, exhibited a strong trend (before controlling for multiple comparisons), toward higher plasma OT concentrations compared to healthy control males. These findings were all independent of cortisol concentrations. A positive relationship was found between OT concentrations and MCMI Desirability scores for both male and female depressed participants, irrespective of psychotic status. Further, an inverse relationship between OT concentrations and MCMI Drug Dependence Base Rate scores was found for both male and female depressed participants, irrespective of psychotic status. Finally, OT concentrations were positively correlated with MCMI Compulsivity Base Rate scores in PMD, but not NPMD, participants.
The findings that OT concentrations: 1) differed significantly between female and male depressed participants; and, 2) were decreased in depressed females but increased (non-significantly) in depressed males compared to gender-matched healthy controls were not predicted. One post-hoc explanation is that in females, OT plays its previously demonstrated role of promoting social bonding (Lim and Young, 2006; Pedersen et al., 1982; Pedersen and Prange, 1979; Witt et al., 1992), and when females experience social isolation or loss, as frequently occurs prior to onset of depression (Bruce and Hoff, 1994), they experience diminished OT release. In contrast, OT may function as a stress hormone in males, as exogenous OT suppresses cortisol release in response to psychological stress when paired with social support in males (Heinrichs et al., 2003). This suggests that males may experience elevated OT release as a mechanism to reduce stress by promoting social behavior, increasing social support and suppressing cortisol release. This interpretation may help to explain the disparity in findings from previous studies examining plasma OT in depression, which have shown both elevated (Parker et al., 2010) and diminished (Frasch et al., 1995) plasma OT concentrations in depressed participants. These previous studies consisted of small sample sizes, and were not powered to examine gender differences in OT concentrations, raising the possibility that the gender most represented in each study was perhaps responsible for driving the observed effects.
Although PMD and NPMD females presented with and without hypercortisolemia, respectively, they did not differ in OT concentrations, with both groups exhibiting equally diminished OT concentrations compared to healthy control female participants. There are two possible explanations for these findings. First, OT biology may be differentially regulated in females with PMD vs. NPMD. Specifically, the hypercortisolism that characterizes PMD may suppress OT release, whereas in NPMD, low circulating OT concentrations may be regulated via a cortisol-independent (and as yet unknown) mechanism. In contrast, it is also possible that diminished OT concentrations occur via cortisol-independent mechanisms in females for both depressive disorders. The available data unfortunately do not allow us to differentiate between these two possibilities, and indicate a clear need for follow up studies to rigorously test these competing hypotheses.
OT concentrations were significantly correlated with depressed participants’ concentrations of Desirability scores on the MCMI. This item is one of the “Modifying Indices” of the MCMI and aims to measure a participant’s response style to determine whether they have a propensity to present themselves in a positive light, essentially controlling for the subjective nature of this self-report measure (Million et al., 2009). Interestingly, it has been hypothesized that intranasal administration of OT may inflate a person’s desire to report socially relevant information in a positive manner, thus altering their responses on self-report measures and interview questionnaires (Guastella and MacLeod, 2012). Our findings of a positive correlation between endogenous OT concentrations and increased MCMI Desirability scores suggest that higher plasma OT concentrations represent a heightened drive to be viewed positively by others, at least for depressed participants. This finding also aligns with the established evidence that OT is critical in the expression of prosocial and affiliative behaviors (Young, 2009).
Our finding of an inverse relationship between OT plasma concentrations and MCMI Drug Dependence scores are supported by increasing evidence that suggests dysregulation in OT signaling may play a significant role in the development of substance abuse problems and associated psychosocial issues, including depression (see Carson et al., 2013; McGregor and Bowen, 2012; McGregor et al., 2008 for review). For example, chronic administration of cocaine to pregnant and post-partum rats results in aberrant mothering behavior and increased aggression toward a resident intruder and these behavioral changes are associated with decreased OT concentrations in the medial preoptic area, ventral tegmental area, and hippocampus, as well as reduced OT receptor binding density in the ventral medial hypothalamus and the bed nucleus of the stria terminalis (Jarrett et al., 2006; Johns et al., 1997a,b). Importantly, this research is supported by evidence in humans. As outlined in the Introduction, women who used cocaine during pregnancy show blunted plasma OT concentrations, experience increased hostility and depressed mood, and have less social support and adaptive coping strategies for stressful life events than do healthy non-cocaine using mothers (Light et al., 2004). In other relevant preclinical studies repeat cocaine self-administration results in decreased hippocampal and hypothalamic OT concentrations, chronic exposure to opiates decreases central OT synthesis (You et al., 2000), and chronic exposure to alcohol is associated with degeneration of hypothalamic magnocellular OT containing neurons in both humans and rats (Silva et al., 2002; Sivukhina et al., 2006).
The finding that OT concentrations were positively correlated with MCMI Compulsivity scores in PMD, but not NPMD, participants are intriguing. Preclinical studies have shown that both central and peripheral injections of OT to rodents result in repetitive and compulsive grooming behavior (Sarnyai, 1998). Further, Leckman et al. (1994) provided evidence for elevated CSF OT concentrations in patients with obsessive—compulsive disorder (OCD) compared to patients with Tourette’s syndrome and healthy controls. They further showed that CSF OT concentrations were positively correlated with current severity of OCD symptoms. However, given the evidence that higher plasma OT concentrations and intranasal administration of OT are associated with reduced positive symptoms in schizophrenia it is difficult to posit why plasma OT might be related to compulsivity in PMD, but not NPMD, participants (Pedersen et al., 2011; Rubin et al., 2010).
5. Limitations and conclusions
While producing interesting results, our study has several limitations. First, we did not experimentally control menstrual cyclicity, which is problematic given the known interaction of female reproductive hormones and OT on modulating the stress response (Ochedalski et al., 2007; Taylor et al., 2006). This study was also limited by the availability of social behavioral data, which precluded detailed analysis of OT concentrations in relation to social functioning. In addition, much like other studies in this field (Frasch et al., 1995; Garcia et al., 2011; Parker et al., 2010) our sample size was relatively small, particularly for males, so may not have been statistically powered to find significant relationships between OT concentrations in both males and females. Research with more participants, especially males, is necessary to bring greater clarity to gender effects on OT biology in depression. Research with more PMD participants is also necessary in order to determine whether low OT and high cortisol concentrations are associated. Finally, central and peripheral OT concentrations can be independently regulated under certain conditions (Landgraf and Neumann, 2004; Ludwig and Leng, 2006) thus leaving the possibility that clinical symptoms in our study population reflect dysregulation in brain OT systems that are not reflected in plasma samples. Future studies would benefit from assessing the relationship between concomitantly collected blood and CSF samples to more precisely determine the role of OT biology in depressive disorders.
In conclusion, these findings further implicate a role for dysregulated OT biology in patients with major depression, and extend this knowledge for the first time to patients with psychotic major depression. Evidence from this study indicates that OT signaling may provide a mechanism by which to better understand female-biased risk to develop depressive disorders, and suggests that intranasal OT “replacement” therapy may be efficacious in female patients with known impairments in OT signaling. Finally, data from this study suggest that plasma OT concentrations may be a useful biomarker for the presence of certain clinical and personality syndromes.
Acknowledgments
This research program was supported by grants MH50604, MH66537, and RR-00070 from the National Institutes of Health, Bethesda, MD, a Stanford University Undergraduate Advising and Research Student Major Grant, and Stanford University’s Bio-X Program. We also thank Dr. Robert Sapolsky for his thoughtful comments on Kaeli Yuen’s honor’s thesis, from which this research report is derived.
Role of Funding Source
Funding for this study was provided by Stanford University and The National Institutes of Health, Bethesda, MD (grants MH50604, MH66537, and RR-00070). The funding sources had no further role in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the manuscript for publication.
Footnotes
Conflict of Interest
Ms. Yuen, Dr. Garner, Dr. Carson, Dr. Keller, Dr. Lembke, Ms. Hyde, Ms. Kenna, Dr. Tennakoon, Dr. Schatzberg, and Dr. Parker report no biomedical financial interests or potential conflicts of interest.
References
- Amico JA, Mantella RC, Vollmer RR, Li X. Anxiety and stress responses in female oxytocin deficient mice. Journal of Neuroendocrinology 2004;16:319–24. [DOI] [PubMed] [Google Scholar]
- Andari E, Schneider FC, Mottolese R, Vindras P, Sirigu A. Oxytocin’s fingerprint in personality traits and regional brain volume. Cerebral Cortex 2012. 10.1093/cercor/bhs328. [DOI] [PubMed] [Google Scholar]
- Belanoff JK, Kalehzan M, Sund B, Ficek SKF, Schatzberg AF. Cortisol activity and cognitive changes in psychotic major depression. American Journal of Psychiatry 2001;158:1612–6. [DOI] [PubMed] [Google Scholar]
- Bertsch K, Schmidinger I, Neumann ID, Herpertz SC. Reduced plasma oxytocin levels in female patients with borderline personality disorder. Hormones and Behavior 2013;63:424–9. [DOI] [PubMed] [Google Scholar]
- Boscaro M, Barzon L, Fallo F, Sonino N. Cushing’s syndrome. Lancet 2001;357:783–91. [DOI] [PubMed] [Google Scholar]
- Bruce ML, Hoff RA. Social and physical health risk-factors for 1st-onset major depressive disorder in a community sample. Social Psychiatry and Psychiatric Epidemiology 1994;29:165–71. [DOI] [PubMed] [Google Scholar]
- Carson DS, Guastella AJ, Taylor ER, McGregor IS. A brief history of oxytocin and its role in modulating psychostimulant effects. Journal of Psychopharmacology 2013;27:231–47. [DOI] [PubMed] [Google Scholar]
- Carter CS, Pournajafi-Nazarloo H, Kramer KM, Ziegler TE, White-Traut R, Bello D, et al. Oxytocin: behavioral associations and potential as a salivary biomarker. Annals of the New York Academy of Sciences 2007;1098:312–22. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV-TR Axis I disorders (Patient Edition). SCID-I/P, 2/2001 revision ed. Washington, DC: American Psychiatric Press; 1997. [Google Scholar]
- Frasch A, Zetzsche T, Steiger A, Jirikowski GF. Reduction of plasma oxytocin levels in patients suffering from major depression. Advances in Experimental Medicine and Biology 1995;395:257–8. [PubMed] [Google Scholar]
- Garcia FD, Coquerel Q, Kiive E, Dechelotte P, Harro J, Fetissov SO. Autoantibodies reacting with vasopressin and oxytocin in relation to cortisol secretion in mild and moderate depression. Progress in Neuro-Psychopharmacology & Biological Psychiatry 2011;35:118–25. [DOI] [PubMed] [Google Scholar]
- Gold PW, Loriaux DL, Roy A, Kling MA, Calabrese JR, Kellner CH, et al. Responses to corticotropin-releasing hormone in the hypercortisolism of depression and Cushing’s disease. Pathophysiologic and diagnostic implications. The New England Journal of Medicine 1986;314:1329–35. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, MacLeod C. A critical review of the influence of oxytocin nasal spray on social cognition in humans: evidence and future directions. Hormones and Behavior 2012;61:410–8. [DOI] [PubMed] [Google Scholar]
- Hamilton M A rating scale for depression. Journal of Neurololgy, Neurosurgery and Psychiatry 1960;23:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Young LJ, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. Lower CSF oxytocin concentrations in women with a history of childhood abuse. Molecular Psychiatry 2009;14:954–8. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biological Psychiatry 2003;54:1389–98. [DOI] [PubMed] [Google Scholar]
- Heuser I, Yassouridis A, Holsboer F. The combined dexamethasone/CRH test: a refined laboratory test for psychiatric disorders. Journal of Psychiatric Research 1994;28:341–56. [DOI] [PubMed] [Google Scholar]
- Jarrett TM, McMurray MS, Walker CH, Johns JM. Cocaine treatment alters oxytocin receptor binding but not mRNA production in postpartum rat dams. Neuropeptides 2006;40:161–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns JM, Lubin DA, Walker CH, Meter KE, Mason GA. Chronic gestational cocaine treatment decreases oxytocin levels in the medial preoptic area, ventral tegmental area and hippocampus in Sprague-Dawley rats. Neuropeptides 1997a;31:439–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns JM, Noonan LR, Zimmerman LI, Li L, Pedersen CA. Effects of short- and long-term withdrawal from gestational cocaine treatment on maternal behavior and aggression in Sprague-Dawley rats. Developmental Neuroscience 1997b;19: 368–74. [DOI] [PubMed] [Google Scholar]
- Jokinen J, Chatzittofis A, Hellstrom C, Nordstrom P, Uvnas-Moberg K, Asberg M. Low CSF oxytocin reflects high intent in suicide attempters. Psychoneuroendocrinology 2012;37:482–90. [DOI] [PubMed] [Google Scholar]
- Keller J, Flores B, Gomez RG, Solvason HB, Kenna H, Williams GH, et al. Cortisol circadian rhythm alterations in psychotic major depression. Biological Psychiatry 2006;60:275–81. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Glaser R, Cacioppo JT, Malarkey WB. Marital stress: immunologic, neuroendocrine, and autonomic correlates. Annals of the New York Academy of Sciences 1998;840:656–63. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Newton T, Cacioppo JT, MacCallum RC, Glaser R, Malarkey WB. Marital conflict and endocrine function: are men really more physiologically affected than women? Journal of Consulting and Clinical Psychology 1996;64: 324–32. [DOI] [PubMed] [Google Scholar]
- Knox SS, Uvnas-Moberg K. Social isolation and cardiovascular disease: an atherosclerotic pathway? Psychoneuroendocrinology 1998;23:877–90. [DOI] [PubMed] [Google Scholar]
- Landgraf R, Neumann ID. Vasopressin and oxytocin release within the brain: a dynamic concept of multiple and variable modes of neuropeptide communication. Frontiers in Neuroendocrinology 2004;25:150–76. [DOI] [PubMed] [Google Scholar]
- Leckman JF, Goodman WK, North WG, Chappell PB, Price LH, Pauls DL, et al. Elevated cerebrospinal-fluid levels of oxytocin in obsessive-compulsive disorder – comparison with Tourettes-syndrome and healthy controls. Archives of General Psychiatry 1994;51:782–92. [DOI] [PubMed] [Google Scholar]
- Lee R, Ferris C, Van de Kar LD, Coccaro EF. Cerebrospinal fluid oxytocin, life history of aggression, and personality disorder. Psychoneuroendocrinology 2009;34: 1567–73. [DOI] [PubMed] [Google Scholar]
- Light KC, Grewen KM, Amico JA, Boccia M, Brownley KA, Johns JM. Deficits in plasma oxytocin responses and increased negative affect, stress, and blood pressure in mothers with cocaine exposure during pregnancy. Addictive Behaviors 2004;29:1541–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim MM, Young LJ. Neuropeptidergic regulation of affiliative behavior and social bonding in animals. Hormones and Behavior 2006;50:506–17. [DOI] [PubMed] [Google Scholar]
- Littell RC, Stroup WW, Freund RJ. SAS for linear models. 4th ed. Cary, NC: SAS Institute; 2002. [Google Scholar]
- Ludwig M, Leng G. Dendritic peptide release and peptide-dependent behaviours. Nature Reviews Neuroscience 2006;7:126–36. [DOI] [PubMed] [Google Scholar]
- McGregor IS, Bowen MT. Breaking the loop: oxytocin as a potential treatment for drug addiction. Hormones and Behavior 2012;61:331–9. [DOI] [PubMed] [Google Scholar]
- McGregor IS, Callaghan PD, Hunt GE. From ultrasocial to antisocial: a role for oxytocin in the acute reinforcing effects and long-term adverse consequences of drug use? British Journal of Pharmacology 2008;154:358–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Million T, Million C, Davis R, Grossman S. MCMI-III manual. Minneapolis, MN: Pearson Education, Inc.; 2009. [Google Scholar]
- Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron 2002;34:13–25. [DOI] [PubMed] [Google Scholar]
- Neumann ID, Kromer SA, Toschi N, Ebner K. Brain oxytocin inhibits the (re)activity of the hypothalamo-pituitary-adrenal axis in male rats: involvement of hypothalamic and limbic brain regions. Regulatory Peptides 2000;96:31–8. [DOI] [PubMed] [Google Scholar]
- Ochedalski T, Subburaju S, Wynn PC, Aguilera G. Interaction between oestrogen and oxytocin on hypothalamic-pituitary-adrenal axis activity. Journal of Neuroendocrinology 2007;19:189–97. [DOI] [PubMed] [Google Scholar]
- Overall JE, Gorham DR. The brief psychiatric rating scale. Psychological Reports 1962;10:799–812. [Google Scholar]
- Ozsoy S, Esel E, Kula M. Serum oxytocin levels in patients with depression and the effects of gender and antidepressant treatment. Psychiatry Research 2009;169: 249–52. [DOI] [PubMed] [Google Scholar]
- Parker KJ, Kenna HA, Zeitzer JM, Keller J, Blasey CM, Amico JA, et al. Preliminary evidence that plasma oxytocin levels are elevated in major depression. Psychiatry Research 2010;178:359–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KJ, Schatzberg AF, Lyons DM. Neuroendocrine aspects of hypercortisolism in major depression. Hormones and Behavior 2003;43:60–6. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Prange AJ. Induction of maternal behavior in virgin rats after intra-cerebroventricular administration of oxytocin. Proceedings of the National Academy of Sciences of the United States of America 1979;76:6661–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen CA, Ascher JA, Monroe YL, Prange AJ. Oxytocin induces maternal behavior in virgin female rats. Science 1982;216:648–50. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Gibson CM, Rau SW, Salimi K, Smedley KL, Casey RL, et al. Intranasal oxytocin reduces psychotic symptoms and improves Theory of Mind and social perception in schizophrenia. Schizophrenia Research 2011;132:50–3. [DOI] [PubMed] [Google Scholar]
- Raff H, Raff JL, Findling JW. Late-night salivary cortisol as a screening test for Cushing’s syndrome. The Journal of Clinical Endocrinology and Metabolism 1998;83:2681–6. [DOI] [PubMed] [Google Scholar]
- Rubin LH, Carter CS, Drogos L, Pournajafi-Nazarloo H, Sweeney JA, Maki PM. Peripheral oxytocin is associated with reduced symptom severity in schizophrenia. Schizophrenia Research 2010;124:13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnyai Z Oxytocin and neuroadaptation to cocaine. Progress in Brain Research 1998;119:449–66. [DOI] [PubMed] [Google Scholar]
- Scantamburlo G, Hansenne M, Fuchs S, Pitchot W, Marechal P, Pequeux C, et al. Plasma oxytocin levels and anxiety in patients with major depression. Psychoneuroendocrinology 2007;32:407–10. [DOI] [PubMed] [Google Scholar]
- Schatzberg AF, Keller J, Tennakoon L, Lembke A, Williams G, Kraemer FB, et al. HPA axis genetic variation, cortisol, and psychosis in major depression. Molecular Psychiatry 2013. October 29 10.1038/mp.2013.129. [Epub ahead of print]. PMID: 24166410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva SM, Madeira MD, Ruela C, Paula-Barbosa MM. Prolonged alcohol intake leads to irreversible loss of vasopressin and oxytocin neurons in the paraventricular nucleus of the hypothalamus. Brain Research 2002;925:76–88. [DOI] [PubMed] [Google Scholar]
- Sivukhina EV, Dolzhikov AA, Morozov IE, Jirikowski GF, Grinevich V. Effects of chronic alcoholic disease on magnocellular and parvocellular hypothalamic neurons in men. Hormone and Metabolic Research 2006;38:382–90. [DOI] [PubMed] [Google Scholar]
- Strathearn L, Fonagy P, Amico J, Montague PR. Adult attachment predicts maternal brain and oxytocin response to infant cues. Neuropsychopharmacology 2009;34:2655–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathearn L, Iyengar U, Fonagy P, Kim S. Maternal oxytocin response during mother–infant interaction: associations with adult temperament. Hormones and Behavior 2012;61:429–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroud LR, Salovey P, Epel ES. Sex differences in stress responses: social rejection versus achievement stress. Biological Psychiatry 2002;52:318–27. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Gonzaga GC, Klein LC, Hu PF, Greendale GA, Seeman TE. Relation of oxytocin to psychological stress responses and hypothalamic-pituitary-adrenocortical axis activity in older women. Psychosomatic Medicine 2006;68:238–45. [DOI] [PubMed] [Google Scholar]
- Tops M, Van Peer JM, Korf J, Wijers AA, Tucker DM. Anxiety, cortisol, and attachment predict plasma oxytocin. Psychophysiology 2007;44:444–9. [DOI] [PubMed] [Google Scholar]
- White T, Ozel B, Jain JK, Stanczyk FZ. Effects of transdermal and oral contraceptives on estrogen-sensitive hepatic proteins. Contraception 2006;74:293–6. [DOI] [PubMed] [Google Scholar]
- Witt DM, Winslow JT, Insel TR. Enhanced social interactions in rats following chronic, centrally infused oxytocin. Pharmacology Biochemistry and Behavior 1992;43:855–61. [DOI] [PubMed] [Google Scholar]
- You ZD, Li JH, Song CY, Wang CH, Lu CL. Chronic morphine treatment inhibits oxytocin synthesis in rats. Neuroreport 2000;11:3113–6. [DOI] [PubMed] [Google Scholar]
- Young EA. Sex differences and the HPA axis: implications for psychiatric disease. The Journal of Gender Specific Medicine 1998;1:21–7. [PubMed] [Google Scholar]
- Young LJ. Being human: love: neuroscience reveals all. Nature 2009;457:148. [DOI] [PubMed] [Google Scholar]
- Young KA, Liu Y, Wang Z. The neurobiology of social attachment: a comparative approach to behavioral, neuroanatomical, and neurochemical studies. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology 2008;148:401–10. [DOI] [PMC free article] [PubMed] [Google Scholar]