Abstract
Recombinant coagulation factor Xa (FXa), inactivated Zh-zo, also known as andexanet alfa (AA), is a modified version of human FXa that has been developed to neutralize FXa inhibitors. We studied the reversal effect of AA for these inhibitors in various anticoagulant and thrombin generation (TG) assays. Individual aliquots of normal human plasma containing 1 µg/mL of apixaban, betrixaban, edoxaban, and rivaroxaban, were supplemented with saline or AA at a concentration of 100 µg/mL. Clotting profiles include prothrombinase-induced clotting time, activated partial thromboplastin time, and prothrombin time. Factor Xa activity was measured using an amidolytic method. Thrombin generation was measured using a calibrated automated thrombogram. Differential neutralization of all 4 anticoagulants was noted in the activated clotting time and other clotting tests. The FXa activity reversal profile varied with an observed decrease in apixaban (22%), betrixaban (56%), edoxaban (28%), and rivaroxaban (49%). Andexanet alfa also led to an increased TG in comparison to saline. The peak thrombin was higher (40%), area under the curve (AUC) increased (15%), whereas the lag time (LT) decreased (17%). Andexanet alfa added at 100 µg/mL to various FXa supplemented systems resulted in reversal of the inhibitory effects, restoring the TG profile; AUC, LT, and peak thrombin levels were comparable to those of unsupplemented samples. Andexanet alfa is capable of reversing anti-Xa activity of different oral FXa inhibitors but overshoots thrombogenesis in both the saline and FXa inhibitor supplemented systems. The degree of neutralization of Xa inhibitor is specific to each agent.
Keywords: factor Xa inhibitors, antidote, thrombin generation, reversal
Introduction
Currently, 4 anti-Xa agents (apixaban, betrixaban, edoxaban, and rivaroxaban) are available for clinical use. Factor Xa (FXa) inhibitors are approved for stroke prevention in patients with nonvalvular atrial fibrillation and for the treatment/prevention of venous thromboembolism.1 They are also currently being developed for additional indications including acute coronary syndrome, peripheral arterial disease, and cancer-associated thrombosis (CAT). Similar to the other anticoagulants, FXa inhibitors may cause life-threatening or uncontrolled bleeding which would be difficult to treat without having an antidote.2
Andexanet alfa (AA, coagulation FXa recombinant, inactivated Zh-zo; Portola Pharmaceuticals, South San Francisco, California) is a recombinant FXa decoy protein which is designed to reverse the inhibition of FXa bleeding associated with newer oral anti-Xa agents.3,4 In this recombinant protein, the serine active site is replaced by alanine and the γ-carboxyglutamic acid domain is removed to restrict its assemblage into prothrombinase complex.5 Food and Drug Administration approved AA in May 2018 as an antidote for apixaban and rivaroxaban.6 Andexanet alfa may also neutralize the anti-Xa effects of the other oral anti-Xa agents, betrixaban and edoxaban.
Although AA is not an active procoagulant, its use has been reported to result in thromboembolic events.2,3 It was hypothesized that AA may enhance TG in human plasma which may contribute to the reported thrombotic complications. To confirm this hypothesis, the relative neutralization profiles of each of the 4 anti-Xa inhibitors by AA were studied in various laboratory assays.
Materials and Method
Whole Blood Analysis
Whole blood activated clotting time (ACT) was measured using the Hemochron whole blood coagulation system (Accriva Diagnostics, Inc, San Diego, California). Whole blood was supplemented with the factor Xa inhibitors at a final concentration of 2.5 µg/mL as control and transferred into celite ACT tube. For reversal studies, blood was supplemented with the factor Xa inhibitors at a final concentration of 2.5 µg/mL and AA at a final concentration of 100 µg/mL and transferred into celite ACT tubes. Results were recorded in seconds and compiled in terms of means (standard deviation [SD]).
Clot-Based Assays
Factor Xa inhibitors were supplemented in citrated plasma over a concentration range of 1.0 to 0.062 µg/mL. Either saline as a control or AA at a final concentration of 100 µg/mL was added to individual aliquots of plasma supplemented with each inhibitor. Samples were analyzed using the 1-stage prothrombinase-induced clotting time (PiCT), activated partial thromboplastin time (aPTT), and prothrombin time (PT) assays. The PiCT assay reagents were obtained from PentaPharm (Basel, Switzerland). The TriniCLOT aPTT reagent was obtained from Diagnostica Stago (Parsippany, New Jersey). The PT, HemosIL reagent was obtained from Instrumentation Laboratory (Bedford, Massachusetts). All reagents were reconstituted according to the manufacturer’s instructions. Activated-partial thromboplastin time and PT were measured using an ACL-Elite (Instrumentation Laboratory). Prothrombinase-induced clotting time was measured using an ST4 instrument from Diagnostica Stago (Parsippany, New Jersey). Results were compiled in terms of mean (SD).
Chromogenic Assay
Anti-factor Xa activity was measured by a kinetic amidolytic method on the ACL-Elite instrument. The factor Xa inhibitors were supplemented in normal human plasma (NHP) over a concentration range of 1.0 to 0.062 µg/mL. Either saline or AA at a final concentration of 100 µg/mL was added to individual aliquots of plasma supplemented with each factor Xa inhibitor. Bovine factor-Xa (Enzyme Research Laboratories, South Bend, Indiana) was diluted in 50 nM Tris buffer (pH = 8.4) to a concentration of 1.25 IU/mL, and factor Xa substrate 2.5 µM (BioMedica Diagnostics, Stanford, Connecticut) was used in this assay and reconstituted in sterile water. The inhibitory potency of each of these agents was calculated as an concentration of drug where the response is reduced by half (IC50) in terms of ng/mL or µM concentrations. The extent of reversal by AA was also calculated.
Inhibition of Thrombin Generation
Inhibition of thrombin generation was measured using Fluoroskan Ascent fluorometer and calibrated automated thrombogram (CAT; Diagnostica Stago). Factor Xa inhibitors were supplemented in normal pooled plasma to obtain concentration ranges from 1.0 to 0.062 µg/mL. Saline or AA at a final concentration of 100 µg/mL was added to individual aliquots of plasma supplemented with each factor Xa inhibitor. Reagents used in this assay included the fluo-substrate, fluo-buffer, tissue factor (TF) high reagent (mixture of TF and phospholipids), and a thrombin calibrator. The thrombin generation assay was carried out in 96-well Immulon 2HB transparent round bottom plates. The thrombin generation potential was measured in terms of the peak thrombin concentration, lag time, and endogenous thrombin potential (ETP)/area under the curve (AUC). % Peak thrombin and % ETP were calculated for samples supplemented with AA. The potency of the factor Xa inhibitors was measured in terms of IC50 in ng/mL and µM. Results were compiled in terms of mean (SD).
Results
Activated Clotting Time Test (Whole Blood)
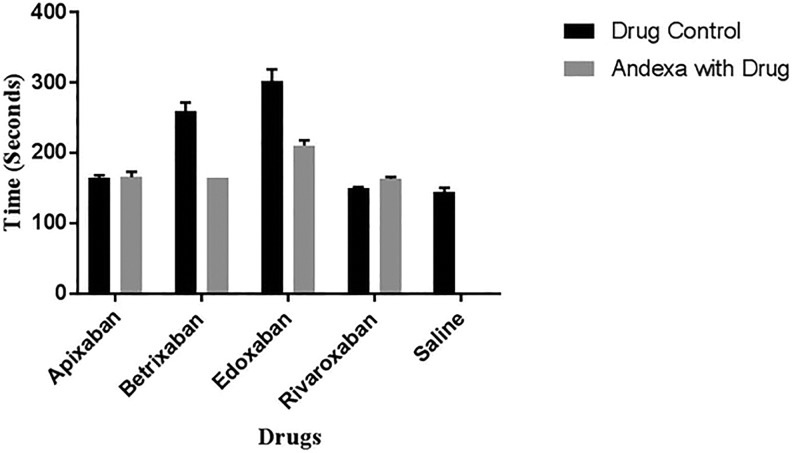
The prolongation of the ACT with each of the FXa inhibitors and its neutralization following supplementation with 100 µg/mL AA is depicted on Figure 1. In the ACT assay, at a concentration of 2.5 µg/mL, edoxaban produced a stronger anticoagulant effect followed by betrixaban, whereas apixaban and rivaroxaban produced marginal effects. The difference between apixaban and rivaroxaban was minimal. The ACT values for the anti-Xa agents ranged from 150 to 303 seconds in comparison to saline control of 144 seconds. Betrixaban was almost completely neutralized by AA and edoxaban was partially neutralized. Andexanet alfa did not produce any effect on apixaban and rivaroxaban in this assay.
Figure 1.
A comparison of factor Xa (FXa) inhibitors and their neutralization by andexanet alfa in whole blood activated clotting time (ACT).
Clotting Assay
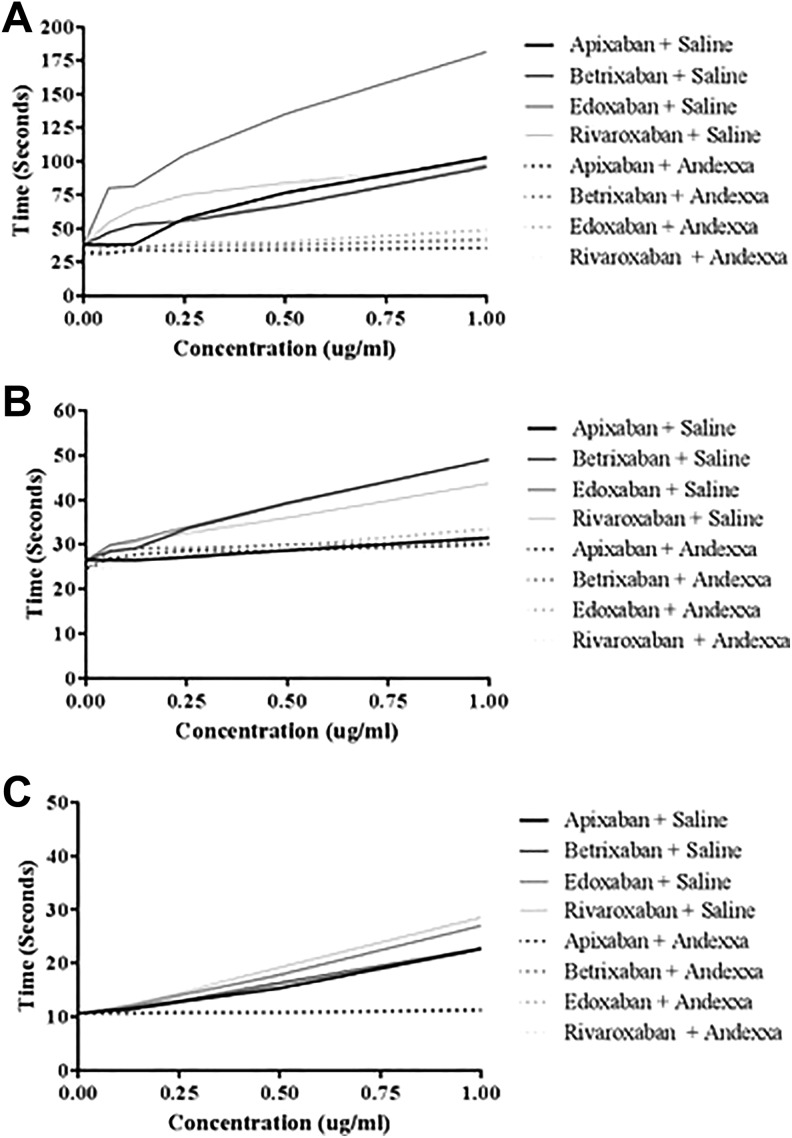
Figure 2 shows a comparison of the effects of various anti-Xa agents when supplemented in NHP as measured by PiCT, aPTT, and PT assays. All drugs produced concentration-dependent anticoagulant effects in these assays. Assay-dependent variations in the anticoagulant activities of these agents were also noted. In the PiCT test, the rank order of the anticoagulant activity in control samples was edoxaban > apixaban = rivaroxaban = betrixaban. All drugs were almost completely neutralized by AA with the ranked order edoxaban > rivaroxaban = betrixaban > apixaban. In the aPTT assay, betrixaban and edoxaban produced comparable anticoagulant effects; however, rivaroxaban showed a relatively weaker response. Apixaban produced minimal anticoagulant effect in this assay. The anticoagulant effects of each of the FXa inhibitors were completely neutralized by AA at a final concentration of 100 µg/mL, with the exception of apixaban, due to its low anticoagulant effect on this particular assay. In the PT assay, rivaroxaban and edoxaban produced a comparable and stronger response followed by apixaban and betrixaban. Prolongation of the PT by each of the FXa inhibitors was completely neutralized by AA at a concentration of 100 µg/mL.
Figure 2.
A comparison of factor Xa (FXa) inhibitors and their neutralization by andexanet alfa in clotting assays. A, Prothrombinase-induced clotting time. B, Activated partial thromboplastin time. C, Prothrombin time.
Anti-Xa Activity
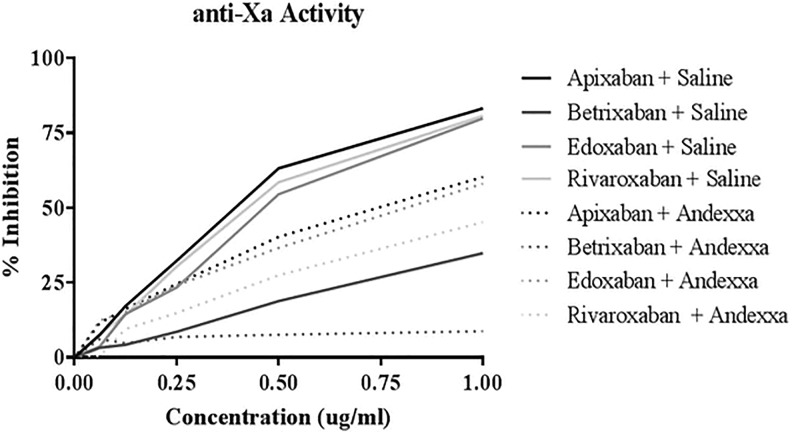
Figure 3 compares the effect of various FXa inhibitors in an amidolytic anti-Xa assay in NHP. All agents produced a concentration-dependent inhibition of FXa. The IC50 values for control samples followed the ranked order apixaban < edoxaban < rivaroxaban < betrixaban and are in the range of 0.74 > 2.88 µM. Andexanet alfa partially neutralized all agents. The rank order for the IC50 in the presence of AA was edoxaban < apixaban < rivaroxaban < betrixaban. The IC50 ranged from 1.48 to >15.5 µM. The FXa activity reversal profile with AA varied with the specific agents, apixaban (23.4%), betrixaban (55.5%), edoxaban (28.8%), and rivaroxaban (50.5%) decrease.
Figure 3.
A comparison of factor Xa (FXa) inhibitors and their neutralization by andexanet alfa in amidolytic anti-Xa activity.
Thrombin Generation
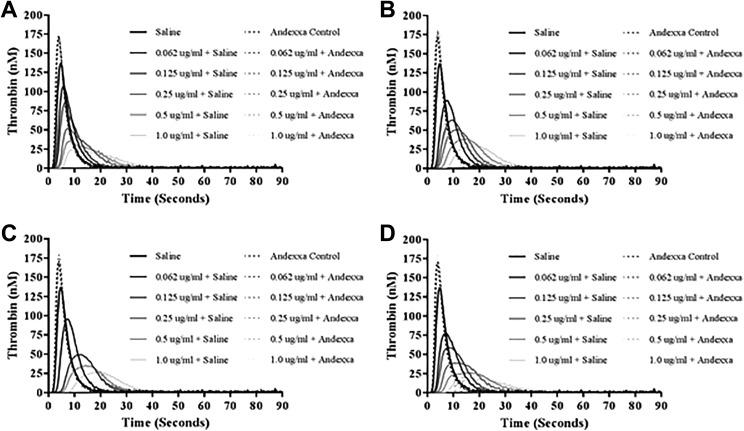
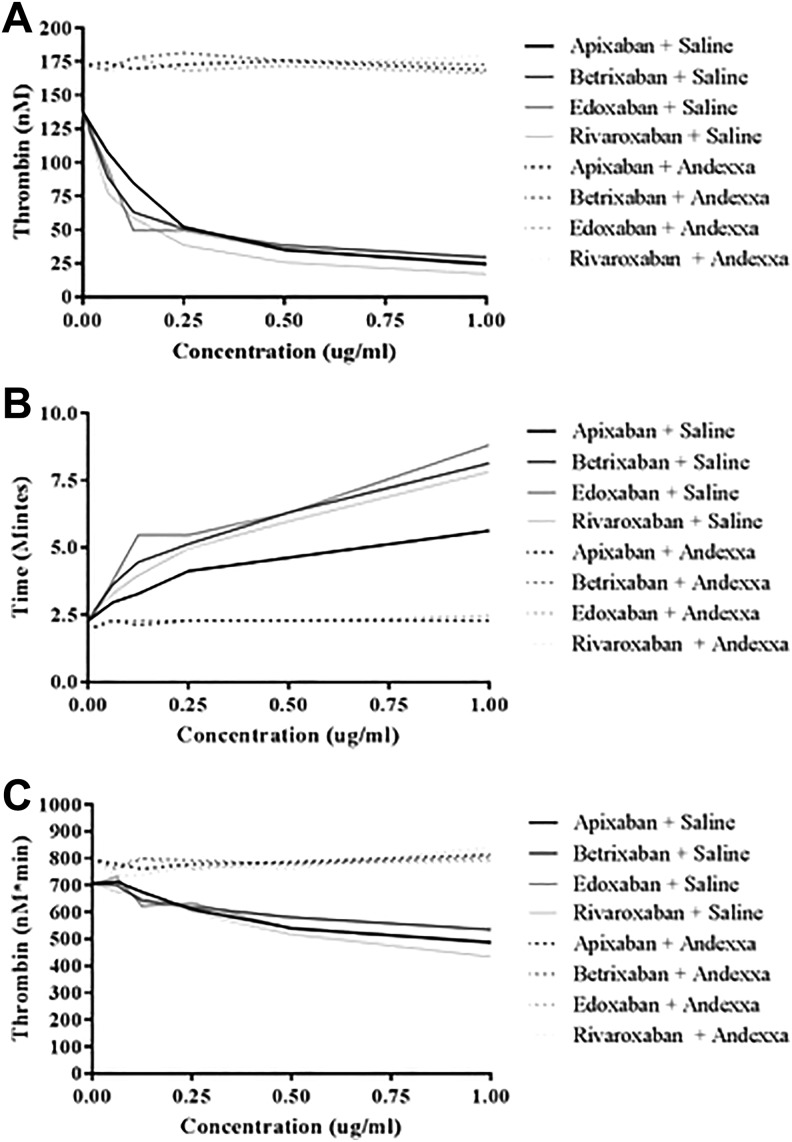
Figure 4A to D shows the thrombokinetograms obtained in the thrombin generation on the CAT assay. All of the FXa inhibitors produced a concentration-dependent inhibition of TG. Apixaban, betrixaban, and edoxaban exhibited comparable inhibitory effects, whereas rivaroxaban produced relatively stronger effects. The peak thrombin was in the range of 17.14 to 29.56 nM at inhibitor concentration of 1 µg/mL. Andexanet alfa at a final concentration of 100 µg/mL completely reversed all 4 FXa inhibitors at all concentrations and the peak thrombin was in the range of 166.17 to 179.49 nM. Andexanet alfa alone and when added to FXa inhibitor supplemented plasmas showed more thrombin generation as compared to saline control with an increase in peak thrombin and AUC.
Figure 4.
A comparison of factor Xa (FXa) inhibitors and their neutralization by andexanet alfa in inhibition of thrombin generation. A, Apixaban. B, Betrixaban. C, Edoxaban. D, Rivaroxaban.
Figure 5A to C and Table 1 show the composite of the individual TG parameters for the 4 anti-FXa agents as measured by peak thrombin, AUC, and lag time. As shown in Figure 5A, the peak thrombin values showed comparable inhibitory patterns with apixaban, betrixaban, and edoxaban, whereas rivaroxaban produced weaker effects. Andexanet alfa as a reversal agent completely neutralized all 4 of the FXa inhibitors, with peak thrombin values at a concentration of 1.0 µg/mL increased from 24.26 nM to 168.56 nM for apixaban, 29.56 nM to 172.83 nM for betrixaban, 25.9 nM to 166.17 nM for edoxaban, and 17.14 to 179.49 nM for rivaroxaban. Figure 5B shows the effects of these agents on lag time values. Betrixaban, edoxaban, and rivaroxaban showed delayed lag time in a comparable fashion; however, apixaban produced weaker response. Andexanet alfa at final concentration of 100 µg/mL completely neutralized all 4 anti-FXa agents in a comparable fashion. Figure 5C shows ETP in terms of AUC. Rivaroxaban produced stronger inhibition of thrombin generation, whereas edoxaban and apixaban produced moderate and comparable response, and betrixaban produced weaker response. The AUC values at a 1.0 µg/mL concentration ranged from 434.14 to 535.49 nM/min. All anti-FXa agents were completely neutralized by AA at 100 µg/mL, and their AUC values at 1.0 µg/mL ranged from 790.3 to 842.8 nM/min.
Figure 5.
A comparison of FXa inhibitors and their neutralization by andexanet alfa in inhibition of thrombin generation and their parameters. A, Peak thrombin. B, Lag time. C, Area under the curve (AUC).
Table 1.
Relative Neutralization of the Direct Anti-Xa Agents by Andexanet Alfa on Thrombin Generation.
| Peak Thrombin | ||||||||
|---|---|---|---|---|---|---|---|---|
| Drug | Apixaban | Betrixaban | Edoxaban | Rivaroxaban | ||||
| Saline | Andexxa | Saline | Andexxa | Saline | Andexxa | Saline | Andexxa | |
| 0 | 137.68 | 172.51 | 137.68 | 172.51 | 137.68 | 172.51 | 137.68 | 172.51 |
| 0.062 | 107.8 | 174.05 | 89.08 | 168.9 | 95.89 | 170.09 | 77.36 | 168.08 |
| 0.125 | 84.99 | 169.58 | 63.3 | 177.81 | 49.55 | 178.37 | 58.96 | 169.71 |
| 0.25 | 52.2 | 172.87 | 50.7 | 181.41 | 49.48 | 168.13 | 38.63 | 170.02 |
| 0.5 | 35.18 | 175.57 | 38.31 | 175.82 | 34.82 | 171.98 | 25.74 | 172.82 |
| 1.0 | 24.26 | 168.56 | 29.56 | 172.83 | 25.9 | 166.17 | 17.14 | 179.49 |
| Area Under the Curve (AUC) | ||||||||
| Drug | Apixaban | Betrixaban | Edoxaban | Rivaroxaban | ||||
| Saline | Andexxa | Saline | Andexxa | Saline | Andexxa | Saline | Andexxa | |
| 0 | 705.82 | 797.36 | 705.82 | 797.36 | 705.82 | 797.36 | 705.82 | 797.36 |
| 0.062 | 713.25 | 777.92 | 699.49 | 760.2 | 731.17 | 779.04 | 676.22 | 735.77 |
| 0.125 | 675.34 | 760.15 | 642.91 | 798.83 | 621.97 | 801.83 | 658.58 | 738.15 |
| 0.25 | 610.72 | 778.22 | 619.74 | 791.91 | 634.58 | 762.26 | 598.63 | 771.24 |
| 0.5 | 540.46 | 786.11 | 580.93 | 777.88 | 538.05 | 784.11 | 516.84 | 762.39 |
| 1.0 | 487.07 | 812.8 | 535.49 | 804.72 | 492.4 | 790.3 | 434.14 | 842.85 |
| Lag Time | ||||||||
| Drug | Apixaban | Betrixaban | Edoxaban | Rivaroxaban | ||||
| Saline | Andexxa | Saline | Andexxa | Saline | Andexxa | Saline | Andexxa | |
| 0 | 2.29 | 1.95 | 2.29 | 1.95 | 2.29 | 1.95 | 2.29 | 1.95 |
| 0.062 | 2.96 | 2.29 | 3.63 | 2.29 | 3.79 | 2.29 | 3.29 | 2.29 |
| 0.125 | 3.29 | 2.12 | 4.46 | 2.29 | 5.47 | 2.29 | 3.96 | 2.12 |
| 0.25 | 4.13 | 2.29 | 5.13 | 2.29 | 5.47 | 2.29 | 4.96 | 2.29 |
| 0.5 | 4.63 | 2.29 | 6.3 | 2.29 | 6.3 | 2.29 | 5.97 | 2.29 |
| 1.0 | 5.63 | 2.29 | 8.14 | 2.29 | 8.81 | 2.46 | 7.81 | 2.29 |
Discussion
Andexanet alfa is effective in the neutralization of the anticoagulant effects of various FXa inhibitors as measured by various laboratory assays such as the whole blood ACT, PT, aPTT, and PiCT clotting time. Oral FXa inhibitors inhibit prothrombin activation by FXa which is bound to factor Va in the prothrombinase complex. Andexanet alfa, which is an active site modified variant of FXa, is devoid of membrane-binding domain. It competes with Xa for stoichiometric binding to FXa inhibitors, rendering these agents inactive.7 Another FXa variant that retains its Gla domain and only expresses activity upon incorporation into prothrombinase complex has been shown to inhibit antithrombotic and bleeding effects of rivaroxaban in mice.8 Most likely the increased thrombin generation in the presence of AA is a result of the reactivation of FXa leading to increased TG. However, this increase is not measurable by routine clot-based assays. For this reason, TG studies were carried out to determine the effect of AA for the formation of thrombin.
The anticoagulant effects of various FXa agents at a concentration of 2.5 µg/mL in the whole blood clotting assay such as the ACT were variable. Edoxaban and betrixaban produced much stronger anticoagulant effects in comparison to the apixaban and rivaroxaban. Upon addition of AA at a concentration of 100 µg/mL, the anticoagulant effects were completely reversed for betrixaban and edoxaban. No change was observed in the case of apixaban and rivaroxaban.
In the citrated plasma systems, each of these drugs produced a concentration-dependent anticoagulant effect in the PiCT, aPTT, and PT assay with the exception of apixaban, which did not produce any measurable effects in the aPTT assay. Andexanet alfa at 100 µg/mL readily neutralized the anticoagulant effects of each of these drugs as measured by these 3 assays. Since these tests measure activation of the coagulation cascade at different points, these studies clearly show that FXa plays a role in the activation processes measured by these tests. The results of these tests are different than the one observed in the whole blood ACT. This may be due to the presence of cells in the whole blood.
Although AA is approved as an antidote on the basis of its ability to reverse the FXa inhibitory effects of various agents, in this study where AA was supplemented to plasmas containing each of these drugs, only betrixaban was completely reversed, whereas apixaban, edoxaban, and rivaroxaban produced variable anti-Xa activities which were only partially neutralized by AA. This is in contrast to the effect of AA in the clot-based assay, where a restoration to baseline of anticoagulant activity was noted. Thus, there is a discordance between the neutralization of the anticoagulant effects as measured by PiCT, aPTT, and PT test and the amidolytic anti-Xa activity of these agents. This observation is in contrast to the earlier findings where a restoration to baseline anti-Xa activity of these agents was reported.9 The composite results of the neutralization profiles of each anti-Xa agents are shown in Table 2.
Table 2.
Relative Neutralization of the Direct Anti-Xa Agents by Andexanet Alfa.
| Test | Apixaban | Betrixaban | Edoxaban | Rivaroxaban | ||||
|---|---|---|---|---|---|---|---|---|
| Saline | Andexxa | Saline | Andexxa | Saline | Andexxa | Saline | Andexxa | |
| Thrombin generation assay—IC50 | 0.28 µg/mL (0.61 µM) | Not definite | 0.26 µg/mL (0.57 µM) | Not definite | 0.12 µg/mL (0.26 µM) | Not definite | 0.18 µg/mL (0.39 µM) | Not definite |
| ACT (2.5 µg/mL) | 164.5 | 165.5 | 259 | 164 | 302.5 | 210.5 | 149.5 | 163 |
| PICT test (1 µg/mL) | 198.8 | 67.5 | 180.1 | 69.0 | 300 | 94.7 | 221.8 | 82.9 |
| aPTT (1 µg/mL) | 40.7 | 38.2 | 62.5 | 38.7 | 63.7 | 41 | 59.2 | 40 |
| PT test (1 µg/mL) | 26.2 | 12.5 | 27.1 | 12.4 | 29 | 12.5 | 33.5 | 12.8 |
| Anti-Xa activity—IC50 ng/mL (µM) | 0.34 µg/mL (0.74 µM) | 0.52 µg/mL (1.13 µM) | 1.3 µg/mLa (>2.88 µM) | 5.5 µg/mLa (>12.17 µM) | 0.37 µg/mL (0.68 µM) | 0.75 µg/mL (1.37 µM) | 0.635 µg/mL (1.46 µM) | 1.42 µg/mLa (>3.26 µM) |
Abbreviations: ACT, activated clotting time; aPTT, activated partial thromboplastin time; PiCT, prothrombinase-induced clotting time; PT, prothrombin time.
aThe values represent the apparent IC50 based on the extrapolated values.
Thrombin generation test is now commonly used to profile anticoagulant drugs.10 In the current studies, the CAT method was used to study these agents. All of these agents produced a profound concentration-dependent inhibition of TG. Additional parameters such as the lag time or delayed time (initiation of thrombin formation) and AUC (area under thrombogram or total thrombin) are also altered by these agents. Andexanet alfa at 100 µg/mL completely neutralized the TG inhibitory effects of these drugs. The increase in lag time was also reduced to near normal. These data clearly suggest that TG inhibitory effects of various anti FXa agents are neutralized by AA. While product-specific profiles were noted for the anti-Xa inhibitory activity, the neutralization in the peak thrombin and AUC parameters was complete and comparable for all of the agents.
In addition to the complete reversal of TG inhibitory effects of anti-Xa agents, supplementation of AA also resulted in increased TG in both the saline and anti-Xa supplemented plasma systems. In the presence of AA alone in the plasma, TG was markedly increased in this assay. Thus, AA somehow augmented the increased generation of thrombin. In the CAT assay, TF is used to trigger the activation of coagulation process. The TF-Xa complex initiates the generation of FXa. Anti Xa agents are able to block the generated FXa. Andexanet alfa mimics FXa, and while acting as a decoy, neutralizing the anti-Xa effects, it may substantially augment the procoagulant effects of native FXa leading to increased TG. Andexanet alfa produced varying degree of the augmentation of the TG in plasma samples supplemented with different anti-Xa agents. These differences may be due to the varying affinities of these agents to both the native FXa and AA.
The studies reported earlier on the variations in the anti-Xa potency have clearly demonstrated the individual biochemical profile of various anti-Xa agents.11 The current study show that various anti-Xa agents differ in their anti-Xa potency. Betrixaban exhibits relatively lower anti-Xa activity and is totally neutralized by AA; on the other hand, apixaban, edoxaban, and rivaroxaban are only partially neutralized by AA. The clinical implication of this observation is unknown. This will require parallel clinical trials to determine whether different amounts of AA will be needed to neutralize the bleeding effects of individual anti-Xa agents.
Although the anti-Xa agents are considered to be single-target agents, our studies indicate that these drugs may also target other sites in the coagulation cascade as evident by their effects in the whole blood and global anticoagulant assays. Andexanet alfa is capable of neutralizing the anticoagulant effects of the anti-Xa agents. Interestingly, the anticoagulant effects of these agents are not proportional to the anti-Xa effects. Andexanet alfa neutralized the anti-Xa effects and anticoagulant effect of these anti-Xa drugs differently. This may be due to the structural and biochemical differences in the anti-Xa agents. Of interest was the observation that AA augmented TG. The exact mechanism of this augmentation remains to be explored. This could be due to AA binding to antithrombin, leaving more native FXa active. There are references that demonstrate increased TG in antithrombin-deficient patients.12 The role of increased TG in promoting thrombotic complications in AA administered to patients needs to be further investigated. These studies suggest that besides being an antidote for the anti-Xa agents, AA may also have additional effects which can promote procoagulant process. Concerns regarding AA potential prothrombotic effects were already stated in previous studies, including an increase in D-dimers induced by AA.3
A full study report of the use of AA to treat bleeding associated with FXa inhibitors reported that within 30 days, 49 (14%) patients died in a cohort of 352 patients. Thirty-four of these patients had thrombotic events. Of these, 11 patients encountered thrombotic event within 5 days after AA therapy, 11 had an event with in 6 to 14 days, and 12 had an event between 13 and 30 days. Myocardial infarction, ischemic stroke, and deep venous thrombosis (DVT) were recorded as major thrombotic complications.2 In other studies, a transient reduction in tissue factor pathway inhibitor (TFPI) activity was reported after AA therapy.8 Since TFPI is a major inhibitor of TF VIIa complex, a decrease in its activity may lead to increased thrombogenesis. Andexanet alfa also reverses the anticoagulant effects of low-molecular-weight heparin and fondaparinux by competing with FXa for binding with antithrombin.13,14 It is plausible that AA treatment may also inhibit the endogenous heparan sulfate glycosaminoglycan (GAG) complexes with endothelium-bound antithrombin.15 Taken together, AA produces additional effects on endogenous modulators which regulate hemostasis, including TFPI, antithrombin, and GAG, and may also influence other heparin-like mediators. Thus, besides neutralizing the anti-Xa agents, AA may promote a procoagulant environment by disrupting hemostasis at multiple sites.16
Conclusion
The current data support the findings of clinical trials with AA that showed increase in severe thrombotic events associated with AA therapy.1–3 These data also suggest that the AA doses required to neutralize FXa inhibitors are drug-specific. Furthermore pharmacological reversal with AA may be associated with increased thrombogenesis compared to pretreatment values. Additional studies are warranted to provide more information on AA-mediated prothrombotic effects as well as its specific doses for specific anti-FXa agents.
Acknowledgments
The authors are thankful to the staff of the Hemostasis Research Laboratory for their skillful assistance in completing this study. Special thanks to Dr Paul Riley of Diagnostica Stago (Parsippany, New Jersey) for facilitating the thrombin generation inhibition studies by providing the instrument and reagents.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was supported by internal research funds and the Hemostasis Research Laboratory and Cardiovascular Institute, Health Science Division, Loyola University Chicago.
ORCID iD: Fakiha Siddiqui  https://orcid.org/0000-0002-2219-7049
https://orcid.org/0000-0002-2219-7049
Eduardo Ramacciotti  https://orcid.org/0000-0002-5735-1333
https://orcid.org/0000-0002-5735-1333
Jawed Fareed  https://orcid.org/0000-0003-3465-2499
https://orcid.org/0000-0003-3465-2499
References
- 1. Comerota AJ, Ramacciotti E. A comprehensive overview of direct oral anticoagulants for the management of venous thromboembolism. Am J Med Sci. 2016;352(1):92–106. [DOI] [PubMed] [Google Scholar]
- 2. Connolly SJ, Crowther M, Eikelboom JW, et al. Full study report of andexanet alfa for bleeding associated with factor Xa inhibitors. N Engl J Med. 2019;380(14):1326–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Connolly SJ, Milling TJ, Jr, Eikelboom JW, et al. Andexanet alfa for acute major bleeding associated with factor Xa inhibitors. N Engl J Med. 2016;375(12):1131–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Siegal DM, Curnutte JT, Connolly SJ, et al. Andexanet alfa for the reversal of factor Xa inhibitor activity. N Engl J Med. 2015;373(25):2413–2424. [DOI] [PubMed] [Google Scholar]
- 5. Heo YA. Andexanet alfa in the treatment of acute major bleeding related to apixaban and rivaroxaban: a profile of its use in the USA. Drugs Ther Perspect. 2018;34(11):507–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Heo YA. Andexanet alfa: first global approval. Drugs. 2018;78(10):1049–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ghadimi K, Dombrowski KE, Levy JH, Welsby IJ. Andexanet alfa for the reversal of factor Xa inhibitor related anticoagulation. Expert Rev Hematol. 2016;9(2):115–122. [DOI] [PubMed] [Google Scholar]
- 8. Lu G, Hollenbach SJ, Baker DC, et al. Preclinical safety and efficacy of andexanet alfa in animal models. J Thromb Haemost. 2017;15(9):1747–1756. [DOI] [PubMed] [Google Scholar]
- 9. Lu G, DeGuzman FR, Hollenbach SJ, et al. A specific antidote for reversal of anticoagulation by direct and indirect inhibitors of coagulation factor Xa. Nat Med. 2013;19(4):446–451. [DOI] [PubMed] [Google Scholar]
- 10. Salvagno GL, Berntorp E. Thrombin generation assays (TGAs). Methods Mol Biol. 2017;1646:515–522. [DOI] [PubMed] [Google Scholar]
- 11. Siddiqui F, Hoppensteadt D, Jeske W, Iqbal O, Tafur A, Fareed J. Factor Xa inhibitory profile of apixaban, betrixaban, edoxaban, and rivaroxaban does not fully reflect their biologic spectrum. Clin Appl Thromb Hemost. 2019;25(1):107602961984752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Niederwanger C, Hell T, Hofer S, et al. Antithrombin deficiency is associated with mortality and impaired organ function in septic pediatric patients: a retrospective study. PeerJ. 2018;6:e5538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Levy JH, Ageno W, Chan NC, et al. When and how to use antidotes for the reversal of direct oral anticoagulants: guidance from the SSC of the ISTH. J Thromb Haemost. 2016;14(3):623–627. [DOI] [PubMed] [Google Scholar]
- 14. Greinacher A, Thiele T, Selleng K. Reversal of anticoagulants: an overview of current developments. Thromb Haemost. 2015;113(5):931–942. [DOI] [PubMed] [Google Scholar]
- 15. Li L, Bonventre JV. Endothelial glycocalyx: not just a sugar coat. Am J Respir Crit Care Med 2016;194(4):390–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sartori M, Cosmi B. Andexanet alfa to reverse the anticoagulant activity of factor Xa inhibitors: a review of design, development and potential place in therapy. J Thromb Thrombolysis. 2018;45(3):345–352. [DOI] [PubMed] [Google Scholar]