Abstract
Heparin and its low-molecular-weight heparin derivatives are widely used clinical anticoagulants. These drugs are critical for the practice of medicine in applications, including kidney dialysis, cardiopulmonary bypass, and in the management of venous thromboembolism. Currently, these drugs are derived from livestock, primarily porcine intestine and less frequently bovine intestine and bovine lung. The worldwide dependence on the pig as a single dominant animal species has made the supply chain for this critical drug quite fragile, leading to the search for other sources of these drugs, including the expanded use of bovine tissues. A number of laboratories are now also examining the similarities between heparin and low-molecular-weight heparins prepared from porcine and ovine tissues. This study was designed to compare low-molecular-weight heparin prepared from ovine heparin through chemical β-elimination, a process currently used to prepare the low-molecular-weight heparin, enoxaparin. Using top-down, bottom-up, and compositional analyses as well as bioassays, low-molecular-weight heparin derived from ovine intestine was shown to closely resemble enoxaparin. Moreover, the compositions of daughter low-molecular-weight heparins prepared from three unfractionated ovine parent heparins were compared. Ovine enoxaparins had similar molecular weight and in vitro anticoagulant activities as Lovenox. Some disaccharide compositional, oligosaccharide composition at the reducing and nonreducing ends and intact chain compositional differences could be observed between porcine enoxaparin and ovine low-molecular-weight heparin. The similarity of these ovine and porcine heparin products suggests that their preclinical evaluation and ultimately clinical assessment is warranted.
Keywords: ovine heparin, enoxaparin, chemical β-elimination, mass spectrometry, nuclear magnetic resonance spectroscopy, bioassay
Introduction
Heparin is a critically important anticoagulant–antithrombotic drug without which the practice of modern medicine would not be possible.1 Heparin, a natural product, is extracted in ton quantities from the tissues of food animals.2 Porcine intestinal tissue is the principal source of heparin worldwide.2,3 The worldwide dependence on the pig as a single dominant animal species has made the supply chain for this critical drug quite fragile, leading to the search for other sources of these drugs, including the expanded use of bovine tissues.4 Moreover, the production of bioengineered heparins prepared through a combination of microbial fermentation and chemoenzymatic synthesis5 or using metabolic engineering6 is now under extensive study.
This reexamination of the sourcing of heparin is largely motivated by the 2007 to 2008 heparin contamination crisis in which much of the world’s supply of porcine intestinal heparin was adulterated with oversulfated chondroitin sulfate. Oversulfated chondroitin sulfate resulted in the biochemical activation of multiple pathways in the circulation leading to hypotension and death of a number of patients receiving contaminated heparin.7,8 This crisis resulted in the introduction of new analytical methodology for heparin9-17 as well as revised, more rigorous pharmacopeial monographs for this class of drugs.18
Heparin is a linear, polydisperse, microheterogenous, polysaccharide comprised repeating disaccharide units of α-l-iduronic (IdoA) or β-d-glucuronic acid (GlcA) 1,4-glycosidically linked to α-d-glucosamine residues (GlcN) selectively modified with O- and N-sulfo (S) and N-acetyl (Ac) groups (Table 1).3 This polycomponent agent has a very complex structure and exhibits multiple biological and pharmacological activities. Heparin produced from animal tissues, referred to as unfractionated heparin, is primarily used as an intravenous agent with a relatively short half-life.19 Low-molecular-weight heparins (LMWHs), derived from unfractionated heparin through its controlled chemical or enzymatic depolymerization, are subcutaneously active and display significantly longer half-lives.19 The most widely used LMWH, enoxaparin, is prepared from unfractionated heparin through a controlled chemical β-elimination reaction.20 This chemistry reduces both the average molecular weight (MW) of heparin from approximately 18 000 Da to approximately 4000 Da and introduces certain structural artifacts, an unsaturated uronic acid residue (ΔUA) and 1,6-anhydrosugar residues (1,6-AnH), into some of the polysaccharide chains of the resulting LMWH. These constitute signature structures of the enoxaparin product which are referred to in the enoxaparin monograph.21
Table 1.
Preparation of Low-Molecular-Weight Heparin Analog of Enoxaparin From Ovine Heparins.
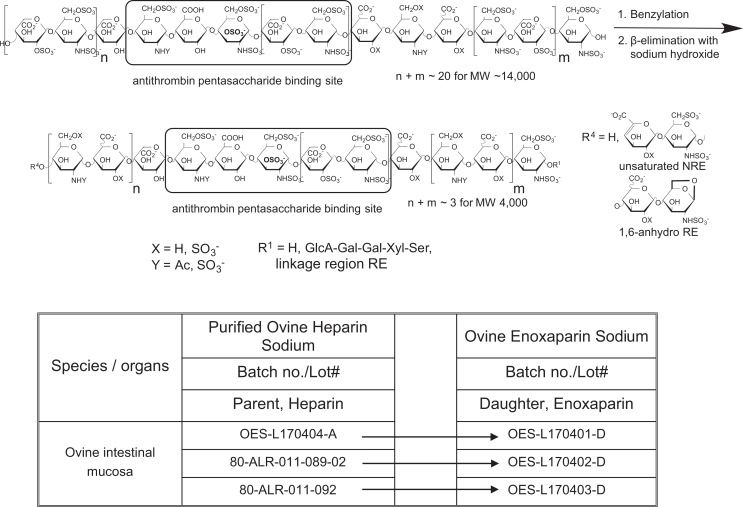
|
In a previous study, we examined the use of bovine intestinal heparin to prepare a bovine enoxaparin product using a controlled chemical β-elimination reaction.15,22 Worldwide there are 1.4 billion cattle, 1.9 billion sheep and goats, and 980 million pigs.23 Sheep might provide alternate source of heparin in the form of ovine intestinal heparin. Ovine intestinal heparin has been used in the past to produce unfractionated heparin and has recently been suggested as a source for the production of LMWHs, such as enoxaparin.10,23-25 In the current study, we have prepared 3 batches of unfractionated heparin from ovine intestine. These batches were then used to prepare 3 batches of ovine enoxaparin (Table 1). These ovine-derived heparin products were chemically analyzed using state-of-the-art methods and their biological/pharmacological activities were evaluated. The structure and activity of unfractionated ovine intestinal heparin and ovine enoxaparin were compared to the porcine-derived products.
Materials and Methods
Materials
The ovine heparins and ovine-derived LMWHs were obtained from Ronnsi (Jiangsu, China). Porcine intestinal heparin reference standard was obtained from the US Pharmacopeial Convention (USP). Lovenox from Sanofi-Aventis (Bridgewater, New Jersey) was obtained from commercial suppliers. All samples were analyzed prior to their expiration dates. Heparin disaccharide standards (0S: ΔUA [1 → 4] GlcNAc; NS: ΔUA [1 → 4] GlcNS; 6S: ΔUA [1 → 4] GlcNAc6S; 2S: ΔUA2S [1 → 4] GlcNAc; NS2S: ΔUA2S [1 → 4] GlcNS; NS6S: ΔUA [1 → 4] GlcNS6S; 2S6S: ΔUA2S [1 → 4] GlcNAc6S; and TriS: ΔUA2S [1 → 4] GlcNS6S, where ΔUA is 4-deoxy-β-l-threo-hex-4-enopyranosiduronic acid, GlcN is glucosamine, Ac is acetyl, and S is sulfo) were from Iduron (Manchester, United Kingdom). Tributylamine was from Sigma Chemical (St Louis, Missouri). Ammonium acetate, calcium chloride, acetic acid, water, and acetonitrile were of high-performance liquid chromatography grade (Fisher Scientific, Springfield, New Jersey). Microcon YM-3 centrifugal filter units were obtained from Millipore (Bedford, Massachusetts). Escherichia coli expression and purification of recombinant flavobacterial heparin lyase I, II, and III (Enzyme Commission #s 4.2.2.7, 4.2.2.X, and 4.2.2.8) were performed in our laboratory as described previously.26 The LMWHs were desalted by dialysis using 1-kDa MW cutoff dialysis tube (Spectrum Laboratories, Rancho Dominguez, California), lyophilized before NMR analysis, and redissolved in distilled water into stock solution (20 mg/mL) for liquid chromatography (LC)-mass spectrometry (MS) analysis.
Chemical β-Elimination of Ovine Heparin
Chemical β-elimination of porcine and ovine heparins to prepare LMWHs has been described in detail previously.2 Sodium heparin was converted into benzethonium salt and was recovered by precipitation. The heparin benzethonium salt was dissolved in methylene chloride and benzylated using benzyl chloride. The benzyl ester of ovine heparin was recovered and treated with aqueous sodium hydroxide resulting in its alkaline depolymerization and debenzylation. Recovery by methanol precipitation and dialysis provided the ovine enoxaparin.
Potency Evaluation
Ovine heparin and enoxaparin were supplemented in pooled normal human plasma and tested in a concentration range of 0 to 10 μg/mL to determine potency. The amidolytic anti-IIa and anti-Xa assays were performed on an ACL ELITE (Instrumentation Laboratory, Lexington, Massachusetts) using bovine Xa and human thrombin (TH) from Enzyme Research Laboratories (South Bend, Indiana). Chromogenic substrates, Spectrozyme Xa, and TH were obtained from American Diagnostica (Stamford, Connecticut) or using kits from Aniara Diagnostica (West Chester, Ohio). The potency of the ovine heparin was calculated using the USP standard for heparin (lot FOI 187). The potency of each heparin was calculated based on the calibration curve prepared with the USP heparin standard. The potency of the enoxaparin was calculated using the National Institute for Biological Standards and Control (01/608; Potters Bar, England) standard.
Activity and MW Assays
The activity and MW assays for heparins and LMWHs were comparable to USP methods.27
Disaccharide Analysis
Each sample (100 μg) was dissolved in 100 μL digestion buffer (50 mmol/L NH4OAc, 2 mmol/L CaCl2, pH 7.0). A mixture of heparin lyase I, II, and III (10 mU each in Tris–HCl buffer, pH 7.0) was added and the samples were digested in 37°C water bath for 12 hours. The lyase enzymes were removed using a YM-3 centrifugal filter unit (Millipore, Billerica, Massachusetts) to terminate the reaction. The filtrates were lyophilized and redissolved in distilled water at a concentration of 1 mg/mL. Reversed-phase, ion-pairing LC with online electrospray ion (ESI)-trap MS analysis relied on an Agilent 1200 LC/MSD instrument (Agilent Technologies, Wilmington, Delaware) equipped with a 6300 ion-trap and a binary pump. A solution of disaccharide standards was prepared (each disaccharide at 100 μg/μL) for relative quantification.
Bottom-Up Analysis of Heparins and LMWHs
Each sample (100 μg) was added to 100 μL digestion buffer (50 mmol/L pH 7.0). Heparin lyase II (10 mU in Tris–HCl buffer, pH 7.0) was added and the sample was digested in 37°C water bath for 12 hours to produce fragments. Enzymatic digestion was terminated by removing the enzymes using an YM-3 centrifugal filter unit. The filtrates were lyophilized and redissolved in 100 µL of distilled water at a concentration of 1 µg/mL. Online hydrophilic interaction chromatography (HILIC) Fourier transform mass spectrometry (FTMS) was used to analyze the resulting oligosaccharides.12 A Luna HILIC column (2.0 mm2 × 50 mm2, 200 Å; Phenomenex, Torrance, California) was connected online to the standard ESI source of LTQ-Orbitrap XL FTMS (Thermo Fisher Scientific, San Jose, California). Mass spectra were acquired at a resolution 60 000 with 200 to 1800 m/z range.
Top-Down Analysis of LMWHs
Online HILIC-FTMS was applied to analyze the intact chains.13 A Luna HILIC column (2.0 mm2 × 150 mm2, 200 Å; Phenomenex, Torrance, California) was used. Mass spectra were acquired at a resolution 60 000 with 200 to 2000 Da mass range.
Bioinformatics Used in Processing Bottom-Up and Top-Down Data
Charge deconvolution was autoprocessed by DeconTools software (web source from PNNL at http://omics.pnl.gov/). The LMWH structural assignment was done by automatic processing using GlycReSoft 1.0 software developed at Boston University (http://code.google.com/p/glycresoft/downloads/list).28 A theoretical database was generated by GlycReSoft 1.0 as described previously.13 All of the relative quantitative data were normalized to the total identified oligosaccharides peak area (in the format of percentage).
Nuclear Magnetic Resonance Analysis
One-dimensional (1D) 1H and 13C NMR spectra were obtained at 600 MHz on 2H2O exchanged heparin (20 mg/mL in 2H2O) using a Bruker Advance II 600 MHz spectrometer (Bruker BioSpin, Billerica, Massachusetts) with Topspin 3.2 software (Bruker BioSpin) for signal integration. Two-dimensional HSQC-NMR spectra were obtained at 800 MHz on 2H2O exchanged heparin (20 mg/mL in 2H2O) using a Bruker Advance II 800 MHz spectrometer.10
Results and Discussion
Three batches of ovine heparin were prepared from ovine intestinal mucosa (Table 1). The MWs of the ovine heparins were somewhat lower than those for USP heparin produced from porcine intestine (Table 2).
Table 2.
Molecular Weight and Potency of Ovine Heparin Sodium and Ovine Enoxaparin Sodium.
| Lot# | Ovine Heparin Sodium | HP std | Ovine Enoxaparin Sodium | LMWH std | ||||
|---|---|---|---|---|---|---|---|---|
| 80-ALR-089 | 80-ALR-092 | OES-404A | Medefil Hep | OES-401D | OES-402D | OES-403D | Lovenox | |
| MW (Da) | ||||||||
| MW (UV) | 13 624 | 13 558 | 14 459 | 18 853 | 3778 | 3761 | 3820 | 4026 |
| MW (RI) | 13 407 | 13 499 | 14 299 | 18 856 | 4245 | 4192 | 4274 | 4486 |
| Mn (UV) | 10 113 | 10 025 | 10 772 | 14 781 | 3260 | 3242 | 3311 | 3403 |
| Mn (RI) | 9794 | 9762 | 10 525 | 15 405 | 3725 | 3686 | 3739 | 3823 |
| USP potency | ||||||||
| Xa (U/mg) | 201 | 196 | 205 | 190 | 105 | 103 | 118 | 107 |
| IIa (U/mg) | 201 | 193 | 201 | 191 | 42 | 41 | 43 | 39 |
| Xa/IIa ratio | 1.00 | 1.02 | 1.02 | 0.99 | 2.5 | 2.5 | 2.7 | 2.7 |
Abbreviations: MW, molecular weight; RI, refractive index.
Characterization of the MW and Activity Properties
The USP porcine heparin had a weight average MW of 18 900 with a polydispersity index (PI) of 1.23, and the ovine heparins had an average MW of 13 800 and an average PI of 1.38. Each parent ovine heparin was then converted to their benzyl esters and chemically β-eliminated to afford 3 daughter ovine enoxaparins. The ovine enoxaparin had an average MW of 4200 and an average PI of 1.14 very similar to porcine enoxaparin MW of 4300 and a PI of 1.19 (Table 2 and SI 1-4). The anticoagulant activities, as determined using antifactor Xa and IIa assays, of unfractionated USP porcine heparin and ovine heparins and the porcine and ovine enoxaparins were very similar showing no major differences (Table 2 and SI 5-6).
Nuclear Magnetic Resonance Analysis
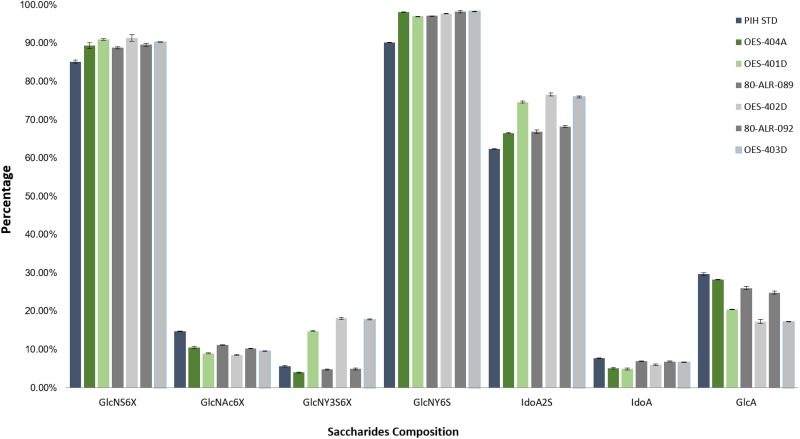
The NMR analysis of the unfractionated ovine and porcine heparins and the ovine and porcine enoxaparins was next undertaken. The 3 batches of parent ovine heparin and the 3 batches of daughter heparin were very similar to one another, indicating that the batch-to-batch variability of the ovine heparin preparation and ovine enoxaparin preparation was very low (Figure 1).
Figure 1.
Composition by nuclear magnetic resonance (NMR) integration. The error bars indicate the analytical variability in the method.
As anticipated, the parent unfractionated heparin differed from the daughter enoxaparins. The ovine parent heparins (OES-404A, 80-ALR-089, and 80-ALR-092) all showed higher GlcA content but lower GlcNY3S6X and IdoA2S content when compared to the daughter samples (OES-401D, OES-402D, and OES-403D). The ovine parent samples had a saccharide composition that was very similar to a standard USP porcine intestinal heparin.
Disaccharide Analysis
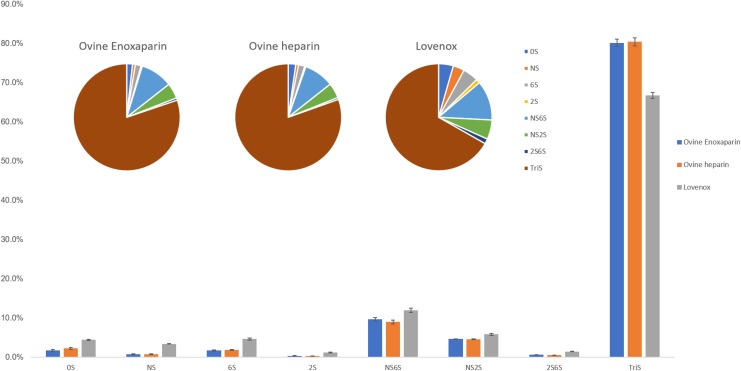
Disaccharide analysis uses a cocktail of heparin lyase enzymes to convert either unfractionated heparin or LMWH to a mixture of disaccharides that are then determined by LC-MS analysis. The disaccharide compositional analysis of the parent ovine heparins, the daughter ovine enoxaparins, and porcine enoxaparin (Lovenox) was examined (Figure 2). The disaccharide composition of 3 batches of parent heparins and their daughter LMWHs was similar and consistent with the NMR data as shown in Figure 1. Interestingly, both the ovine heparin and ovine enoxaparin had a significantly higher percentage of Tris than did porcine enoxaparin, Lovenox, suggesting that ovine enoxaparins were richer in IdoA2S(1 → 4)GlcNS6S sequences.
Figure 2.
Disaccharide analysis by liquid chromatography mass spectrometry (LC-MS) presented by bar chart and pie chart.
Top-Down Analysis
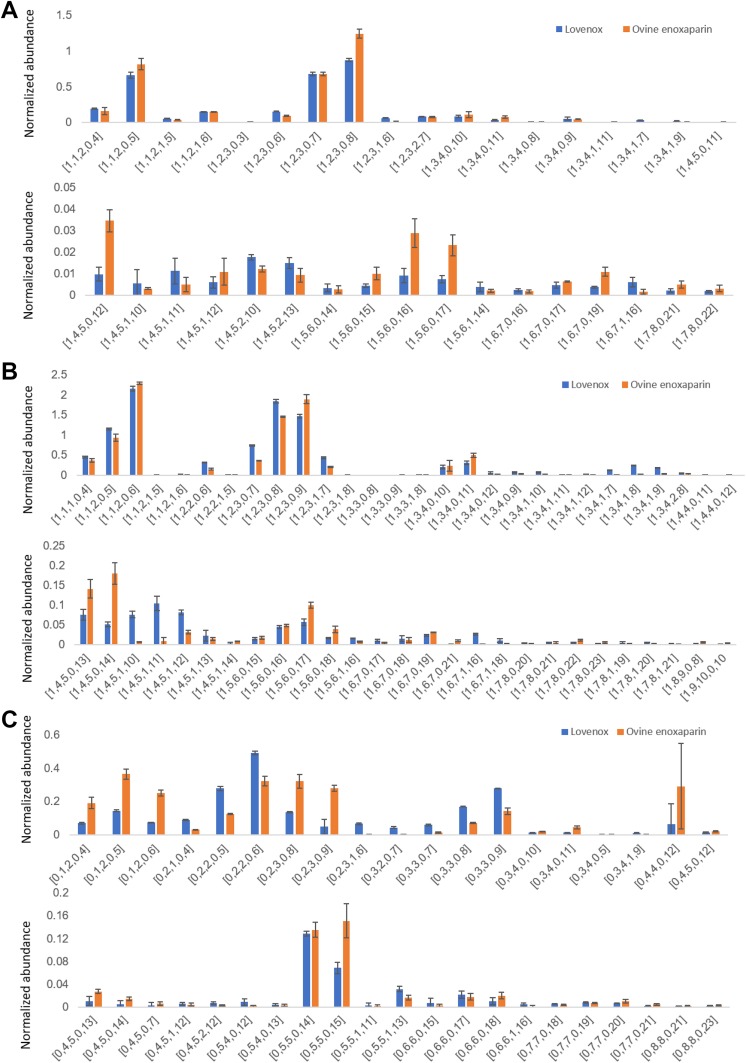
Top-down analysis uses LC-MS to examine intact oligosaccharide chains found within an LMWH. The top-down analysis of LMWHs showed that ovine enoxaparin contains 227 oligosaccharide species (Figure 3), while 225 oligosaccharide species were identified in Lovenox, and 130 of the same oligosaccharide species were present in both ovine enoxaparin and Lovenox (Figure 4). The highly sulfated oligosaccharides, [1, 2, 3, 0 8], [1, 4, 5, 0, 12], [1, 5, 6, 0, 16], [1, 5, 6, 0, 17], and [1, 6, 7, 0, 17], in ovine enoxaparin were in higher abundance than in Lovenox. The less sulfated N-acetylated oligosaccharides, [1, 4, 5, 1, 10], [1, 4, 5, 1, 11], and [1, 4, 5, 1, 12], was in higher abundance in ovine enoxaparin than in Lovenox. These analyses were in agreement with the disaccharide analysis, showing that ovine enoxaparins were richer in IdoA2S(1 → 4)GlcNS6S sequences (Figure 2).
Figure 3.
Top-down analysis of low-molecular-weight heparins (LMWHs). For the 3 ovine enoxaparin intact chains detected in top-down analysis whose (A) reducing ends are 1, 6-anhydro, (B) nonreducing ends are unsaturated, and (C) nonreducing ends are saturated. The error bars indicate sample variability.
Figure 4.
Top-down analysis of low-molecular-weight heparins (LMWHs). Comparison of ovine enoxaparin and Lovenox about intact chains detected in top-down analysis whose (A) reducing ends are 1,6-anhydro, (B) nonreducing ends are unsaturated, and (C) nonreducing ends are saturated.
Bottom-Up Analysis
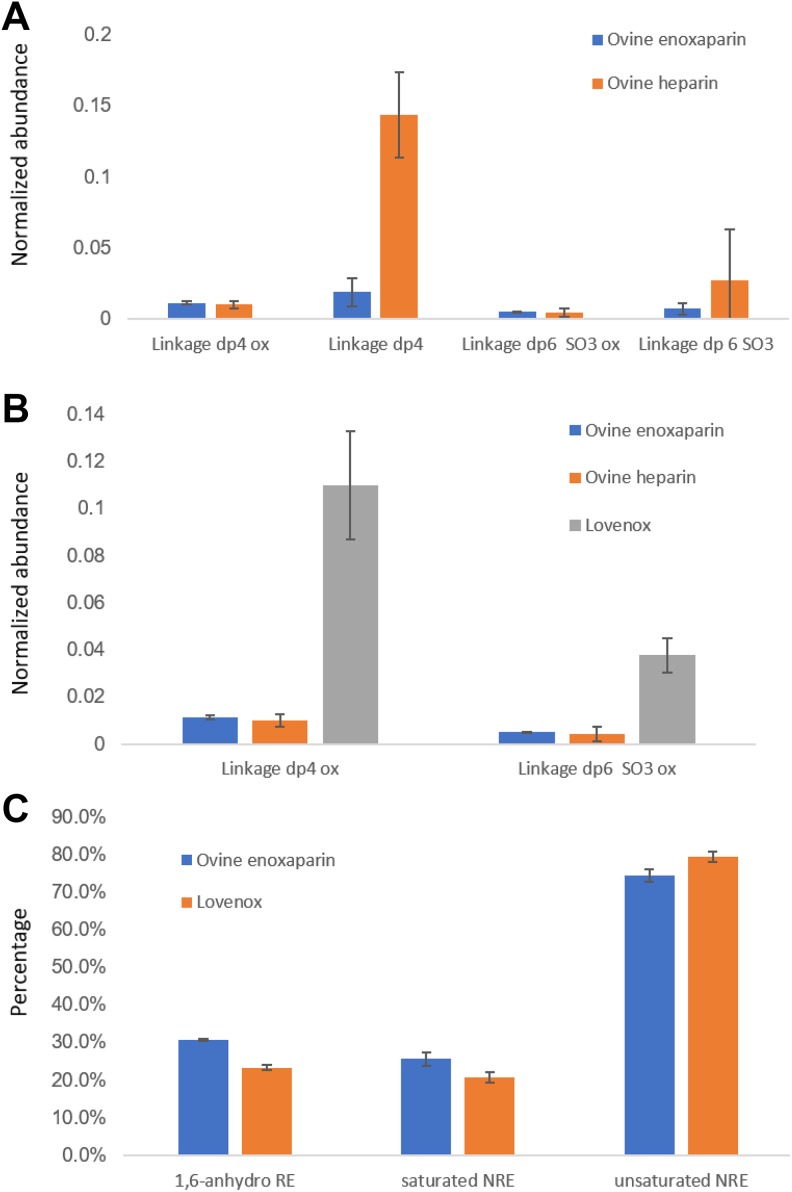
Treatment of LMWH sample with a single heparin lyase, heparin lyase II, affords resistant oligosaccharides that provide a bottom-up analysis. Ovine enoxaparins and Lovenox were compared to one another using this approach (Figure 5A and B).
Figure 5.
A and B, Comparison of the oligosaccharides generated from heparin-protein linkage region (bottom-up analysis). C, Composition of the daughters and Lovenox on terminal structure composition (top-down analysis). Error bars indicate sample variability (Linkage dp4: ΔUA-Gal-Gal-Xyl-O-Ser; Linkage dp4 ox: ΔUA-Gal-Gal-Xyl-O-SerOX; Linkage dp6 SO3: ΔUA-GlcNAc-GlcA-Gal-Gal-Xyl-O-Ser; Linkage dp6 SO3 OX: ΔUA-GlcNAc-GlcA-Gal-Gal-Xyl-O-SerOX;).
Lovenox had a higher normalized abundance of linkage region oligosaccharide than did the ovine enoxaparins. The 1,6-anhydro at reducing end in ovine enoxaparin (30.7 ± 0.3%) was higher than Lovenox, 23.2 ± 0.7%, as determined by mass spectrometry (Figures 3 –5). The nonreducing end saturated oligosaccharides in ovine enoxaparins (25.6% ± 1.7%) was slightly higher in ovine enoxaparins than in Lovenox (20.6 ± 1.4%) and the unsaturated oligosaccharides of ovine enoxaparin (74.4 ± 1.7%) was lower than Lovenox (79.4 ± 1.4%; Figure 5C). These differences were expected based on the lower MW of the ovine intestinal heparins.
Conclusion
There is a clear need to develop new sources for heparin and LMWH to diversify the supply of these critical life-saving drugs. The current study examines ovine intestinal heparin and ovine enoxaparin as potential replacements for porcine intestinal heparin and porcine enoxaparin or Lovenox. Unfractionated ovine intestinal heparin, while having some compositional differences and MW differences from unfractionated porcine heparin, has a similar profile of in vitro anticoagulant activities. Ovine enoxaparins had similar MW and in vitro anticoagulant activities as Lovenox. Some disaccharide compositional, oligosaccharide composition at the reducing and nonreducing ends and intact chain compositional differences could be observed between porcine enoxaparin and ovine LMWH. The similarity of these ovine and porcine heparin products suggests that their preclinical evaluation and ultimately clinical assessment is warranted.
Supplemental Material
Supplemental Material, SI_figures for Comparison of Low-Molecular-Weight Heparins Prepared From Ovine Heparins With Enoxaparin by Jianle Chen, Yanlei Yu, Jawed Fareed, Debra Hoppensteadt, Walter Jeske, Ahmed Kouta, Caijuan Jin, Yongsheng Jin, Yiming Yao, Ke Xia, Fuming Zhang, Shiguo Chen, Xingqian Ye and Robert J. Linhardt in Clinical and Applied Thrombosis/Hemostasis
Footnotes
Authors’ Note: Prepared for Clinical and Applied Thrombosis/Hemostasis, August 2018, revised February 2019. Our institution does not require ethical approval for in vitro assays. Informed consent for patient information to be published in this article was not obtained because no patient samples were used in this study. Pooled plasma used in the assays was purchased from a commercial supplier.
Author Contribution: Jianle Chen and Yanlei Yu contributed equally to this work.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Caijuan Jin, Yongsheng Jin, and Yiming Yao are employees of Ronnsi Pharma Co, Ltd.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by Grants from the National Institutes of Health in the form of Grants DK111958, HL125371, CA207717, NS088496, and HL62244.
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Bigelow WG. Mysterious Heparin: The Key to Open Heart Surgery. Toronto, Canada, Canada: McGraw-Hill Ryerson; 1990. [Google Scholar]
- 2. Linhardt RJ, Gunay NS. Production and chemical processing of low molecular weight heparins. Semin Thromb Hemost. 1999;25(suppl 3):5–16. [PubMed] [Google Scholar]
- 3. Linhardt RJ. 2003 Claude S. Hudson Award address in carbohydrate chemistry. Heparin: structure and activity. J Med Chem. 2003;46(13):2551–2564. [DOI] [PubMed] [Google Scholar]
- 4. Chase C, Elaine G, Paulo M, et al. Diversifying the global heparin supply chain: reintroduction of bovine heparin in the United States. Pharm Technol. 2015;39(11):28–35. [Google Scholar]
- 5. Fu L, Suflita M, Linhardt RJ. Bioengineered heparins and heparan sulfates. Adv Drug Delivery Rev. 2016;97:237–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Badri A, Williams A, Linhardt RJ, Koffas M. The road to animal-free glycosaminoglycan production: current efforts and bottlenecks. Curr Opin Biotechn. 2018;53:85–92. [DOI] [PubMed] [Google Scholar]
- 7. Guerrini M, Shriver Z, Naggi A, et al. Oversulfated chondroitin sulfate is not the sole contaminant in heparin. Nat Biotechnol. 2010;28(3):207–211; author reply 207–211 [DOI] [PubMed] [Google Scholar]
- 8. Li B, Suwan J, Martin JG, et al. Oversulfated chondroitin sulfate interaction with heparin-binding proteins: new insights into adverse reactions from contaminated heparins. Biochem Pharmacol. 2009;78(3):292–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang Z, Li B, Suwan J, et al. Analysis of pharmaceutical heparins and potential contaminants using 1H-NMR and PAGE. J Pharm Sci. 2009;98(11):4017–4026. [DOI] [PubMed] [Google Scholar]
- 10. Fu L, Li G, Yang B, et al. Structural characterization of pharmaceutical heparins prepared from different animal tissues. J Pharm Sci. 2013;102(5):1447–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang F, Yang B, Ly M, et al. Structural characterization of heparins from different commercial sources. Anal Bioanal Chem. 2011;401(9):2793–2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li G, Steppich J, Wang Z, et al. Bottom-up low molecular weight heparin analysis using liquid chromatography-Fourier transform mass spectrometry for extensive characterization. Anal Chem. 2014;86(13):6626–6632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li L, Zhang F, Zaia J, Linhardt RJ. Top-down approach for the direct characterization of low molecular weight heparins using LC-FT-MS. Anal Chem. 2012;84(20):8822–8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ouyang Y, Zeng Y, Rong Y, et al. Profiling analysis of low molecular weight heparins by multiple heart-cutting two dimensional chromatography with quadruple time-of-flight mass spectrometry. Anal Chem. 2015;87(17):8957–8963. [DOI] [PubMed] [Google Scholar]
- 15. Guan Y, Xu X, Liu X, et al. Comparison of low-molecular-weight heparins prepared from bovine lung heparin and porcine intestine heparin. J Pharm Sci. 2016;105(6):1843–1850. [DOI] [PubMed] [Google Scholar]
- 16. St Ange K, Onishi A, Fu L, et al. Analysis of heparins derived from bovine tissues and comparison to porcine intestinal heparins. Clin Appl Thromb Hemostasis. 2016;22(6):520–527. [DOI] [PubMed] [Google Scholar]
- 17. Naggi A, Gardini C, Pedrinola G, et al. Structural peculiarity and antithrombin binding region profile of mucosal bovine and porcine heparins. J Pharm Biomed Anal. 2016;118:52–63. [DOI] [PubMed] [Google Scholar]
- 18. Szajek AY, Chess E, Johansen K, et al. The US regulatory and pharmacopeia response to the global heparin contamination crisis. Nat Biotechnol. 2016;34(6):625–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Baglin T, Barrowcliffe TW, Cohen A, Greaves M; British Committee for Standards in Haematology. Guidelines on the use and monitoring of heparin. Br Soc Haematol. 2006;133(1):19–34. [DOI] [PubMed] [Google Scholar]
- 20. Schroeder M, Hogwood J, Gray E, et al. Protamine neutralization of low molecular weight heparins and their oligosaccharide components. Anal Bioanal Chem. 2011;399(2):763–771. [DOI] [PubMed] [Google Scholar]
- 21. U.S. Pharmacopeia–National Formulary (USP–NF). Test for 1,6-Anhydroderivative for Enoxaparin Sodium. Rockville, MD: U.S. Pharmacopeial Convention; 2009. [Google Scholar]
- 22. Liu X, St Ange K, Fareed J, et al. Comparison of low molecular weight heparins prepared from bovine heparins with enoxaparin. Clin Appl Thromb Hemost. 2017;23(6):542–553. [DOI] [PubMed] [Google Scholar]
- 23. Robinson TP, Wint GR, Conchedda G, et al. Mapping the global distribution of livestock. Plos One. 2014;9(5):e96084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hoppensteadt D, Maia P, Silva A, et al. Resourcing of heparin and low molecular weight heparins from bovine, ovine, and porcine origin. studies to demonstrate the biosimilarities. Blood. 2015;126:4733. [Google Scholar]
- 25. Bouchard O, Abro S, Kahn D. A comparison of ovine and porcine heparins and enoxaparins: a case for an alternative source of heparin products. FASEB J. 2016;30(suppl 1):No. 1_supplement 1177.5. [Google Scholar]
- 26. Su H, Blain F, Musil RA, Zimmermann JJ, Gu K, Bennett DC. Isolation and expression in Escherichia coli of hepB and hepC, genes coding for the glycosaminoglycan-degrading enzymes heparinase II and heparinase III, respectively, from Flavobacterium heparinum. Appl Environ Microbiol. 1996;62(8):2723–2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. United States Pharmacopeial Convention, USP37. Official Monograph, Heparin Sodium, D. Molecular Weight Determinations. Rockville, MD: United States Pharmacopeial Convention; 2014:3224. [Google Scholar]
- 28. Maxwell E, Tan Y, Tan Y, et al. GlycReSoft: a software package for automated recognition of glycans from LC/MS data. Plos One. 2012;7(9):e45474. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, SI_figures for Comparison of Low-Molecular-Weight Heparins Prepared From Ovine Heparins With Enoxaparin by Jianle Chen, Yanlei Yu, Jawed Fareed, Debra Hoppensteadt, Walter Jeske, Ahmed Kouta, Caijuan Jin, Yongsheng Jin, Yiming Yao, Ke Xia, Fuming Zhang, Shiguo Chen, Xingqian Ye and Robert J. Linhardt in Clinical and Applied Thrombosis/Hemostasis