Abstract
This study aimed to determine whether there is an influence of interleukin 6 (IL-6) gene promoter polymorphisms on IL-6 plasma levels and its role in the development of ischemic stroke in young Indians. One hundred young patients with ischemic stroke (age ≥ 45 years) and equal number of age- and sex-matched controls were genotyped for 174G>C, −572G>C, and −597G>A promoter polymorphisms by polymerase chain reaction–restriction fragment length polymorphism. Plasma IL-6 levels were measured by enzyme-linked immunosorbent assay. Plasma IL-6 levels were significantly higher in patients as compared to controls (patients: 28.61 ± 8.61 pg/mL, controls: 7.60 ± 4.10 pg/mL, P = .001). Both −174G>C (allelic χ2/P value: 4.79/.028, genotypic χ2/P value: 5.3/.021) and −572G>C (allelic χ2/P value: 9.63/.00113 Genotypic χ2/P value: 74/.0002) polymorphisms exhibited genotypic as well as allelic significant association with the disease phenotype. Comparison was made between patients and controls for all 3 polymorphisms using a recessive model with respect to plasma IL-6 levels; no polymorphism showed any significant correlative association with the increased IL-6 levels (P = .31, .51, .32). Interleukin 6 is an inflammatory marker that is considerably influenced by nongenetic factors and is not a good candidate gene for studying genetic components associated with ischemic stroke. It seems that the variability in IL-6 levels is an integrated effect of nongenetic influences and the inflammatory events that follow ischemic stroke instead of being its cause. It is suggested that there is no direct association between −174G>C, −572G>C, and −597G>A polymorphisms and elevated IL-6 levels in the development of ischemic stroke.
Keywords: young ischemic stroke, IL-6 promoter polymorphisms, IL-6 plasma levels
Introduction
Ischemic stroke (IS) is the most common kind of stroke and ranks among the leading causes of mortality and morbidity in India. The incidence rate of stroke in India differs as per the geographical distribution.1 Stroke in young poses a major health and socioeconomic burden on the country due to high rate of morbidity. A prospective community-based study by Prassad and Singhal, 20102 found that the proportion of the young patients with stroke was 8.8%. However, other hospital-based studies3,4 showed high prevalence of young stroke that ranges from 15% to 30%, but it may be biased due to preferential admission policy in the hospitals.
Ischemic stroke is a complex polygenic disorder. Interleukin 6 (IL-6) is pro-inflammatory cytokine that contributes to inflammation and growth of the plaque that ultimately leads to atherosclerosis.5–7 Several studies reveal its role in cardiovascular diseases,8–11 indicative of its central role in the inflammatory response to cerebral ischemia.12 Raised IL-6 levels are found in the cerebrospinal fluids and serum after IS.13–16 Elevated IL-6 levels worsen the clinical outcome and increased infarct size.17,18 The susceptibility of the patients to stroke and the subsequent prognosis are clinically determined by systemic inflammatory processes. Poor clinical outcomes are observed in patients with stroke with systemic inflammation.19,20
Hence, the single-nucleotide polymorphisms occurring in this pro-inflammatory cytokine may modify levels in plasma and biologic activity of IL-6, influencing the disease process and clinical outcome. The transcription is the regulatory point for the expression of IL-6 and the promoter of the human IL-6 gene has several polymorphisms that influence IL-6 gene transcription in certain cell types.21 The −174 G/C polymorphism is associated with numerous diseases, such as Alzheimer and lacunar infarcts.22,23 However, it is still unknown whether these polymorphisms have any effect on IL-6 induction in neural cells.23 There are several studies showing association of IL-6 polymorphisms (−174G>C, −572G>C, and −597G>A) with IS.24,25 A recent study by Kumar et al25 found the association of −174G>C, −572G>C polymorphisms with the IS in Indian population but lacked the association of these polymorphisms with IL-6 levels.
To the best of our knowledge, there are no data available on the association of IL-6 levels and its promoter polymorphisms with the development of IS in young Indians; hence, this study was undertaken to look for the association of −174G>C, −572G>C, and −597G>A polymorphisms and plasma IL-6 levels in young IS patients.
Materials and Methods-
Study Participants (Inclusion and Exclusion Criteria)
The participants for the study were young adults (18-45 years). Patients (N = 123) presenting with clinically and radiologically diagnosed IS (computed tomography/magnetic resonance imaging) diagnosed were recruited for the study. Patients of North Indian origin were exclusively included in the study in order to maintain the geographical homogeneity. Patients were excluded if they presented with any history of transient ischemic attack, rheumatologic disease, fever, any autoimmune disease, any chronic or acute infection, hemorrhagic stroke proven by computed tomography or magnetic resonance imaging, and any immunosuppressive or analgesic therapies. Also, no such patients were recruited who had suffered trauma or had any surgery in the past 30 days. During sampling, 23 could not fulfill the inclusion criteria. Finally, 100 patient samples were included in the study. The control group comprised of 100 healthy participants, age- (+3 years) and sex-matched, with no relationship with patients but from the same demographic area. The control population has to fulfill a questionnaire to ascertain that they were stroke-free.26
A standardized interview was conducted to get details about the smoking habits, use of medication, and a personal history of the disease. Body mass index was determined as weight in kilograms divided by height in square meters. Persons who were cognizant of having hypertension, were taking antihypertensive medication, and/or had blood pressure values 160/90 mm Hg at baseline were categorized as being actual hypertensive. A participant was defined as a regular smoker when at least 5 cigarettes per day were currently being smoked and alcohol consumption habits were 2 to 4 days in a week. The study participants, both patients and controls, joined the study voluntarily and gave a written consent for the same. The local ethics committee of the institute approved the above study and was conducted in accordance with the Declaration of Helsinki.
Sample Collection
Venous blood samples were collected within 72 hours of the onset of symptoms. Samples were obtained from each participant after at least 12-hour fasting. Samples were collected between 7:30 am and 9:00 <SC>AM</SC> from all participants for avoiding diurnal variation. Plasma was separated as soon as possible by centrifugation for 15 minutes at 3500g and stored in 80°C for the pending analysis. Extraction of genomic DNA from peripheral blood leukocytes was done by using standard methods (phenol chloroform method).
Interleukin 6 Measurement
A commercially available kit did evaluation of IL-6 antigen levels (Sigma-Aldrich, St Louis, Missouri). Enzyme-linked immunosorbent assay was performed as per the kit manufacturer’s instructions. Plasma IL-6 levels were evaluated for patients [acute phase] as well as controls. The minimum detectable dose of IL-6 is typically less than 3 pg/mL.
Genotyping the −174G>C, −572G>C, and −597G>A Polymorphisms
All these 3 polymorphisms were genotyped by polymerase chain reaction-restriction fragment length polymorphism. The primers used for −174G>C polymorphism were: 5-TGACTTCAGCTTTACTCTTTGT-3 and 5-CTGATTGGAAACCTTATTAAG-3, and the primers for -5-72G/C and -597G>A polymorphisms were 5-GGAGACGCCTTGAAGTAACTGC-3 and 5-GAGTTTCCTCTGACTCCATCGCAG-3. The PCR product size were 198 and 163 base pair. Restriction digestion was done as described by Fernandez-Real et al27 and Brull et al28 with some minor modifications.
Statistical Analysis
Data were presented as mean (SD) and frequency (%); categorical variables were compared among groups by χ2 test or Fisher exact test. Normality of quantitative variables was assessed by Shapiro-Wilk normality test. Continuous variables following normal distribution were compared between groups by independent t-test and continuous variables not following normal distribution were compared among the groups by Kruskal-Wallis test followed by multiple comparison by Dunn test. Analysis of covariance was applied to compare IL-6 between case and control after adjusting for alcohol, hypertension, high-density lipoprotein (HDL), and low-density lipoprotein (LDL). All analyses were done by Stata (StataCorp, College Station, Texas) and P value less than .05 was considered as statistical significant. We investigated the association of the 3 polymorphisms with the levels of IL-6 and with the disease phenotype using a recessive model with multivariate analysis of variance. We adjusted for the covariates that were found individually associated with the disease cohort.
Results
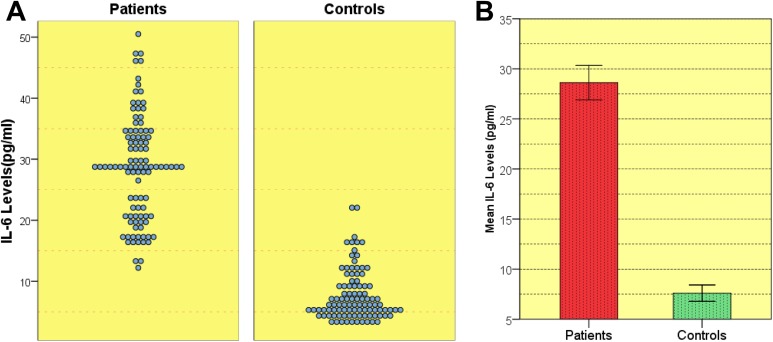
Table 1 summarizes the demographical and clinical features of the patients and controls. Significantly higher IL-6 levels were observed in patients as compared to controls (Table 2, Figure 1). Difference between IL-6 levels in stroke of arterial origin (n = 75) and venous origin (n = 25; arterial: 29.08 ± 8.62 pg/mL, venous: 27.24 ± 8.61 pg/mL, P = .17) was not significant. Also, no significant difference was observed in the IL-6 levels between recurrent (n = 22) and nonrecurrent strokes (n = 78; 31.03 ± 9.59 pg/mL, 27. 94 ± 8.25 pg/mL; P = .93). A significant positive correlation of IL-6 levels with LDL (P = .001), HDL (P = .001), very low–density lipoprotein (VLDL; P = .001) was observed. After colinearity was considered among the other variables and adjusted for alcohol and LDL, an independent association of IL-6 levels with the stroke phenotype was observed (Table 2). The intra- and interassay coefficients of variation were 3.1% and 2.3% for the IL-6 assay, respectively.
Table 1.
Clinical Baseline Characteristics and Demographic Data of the Study Patients.
| Parameters | Patients (100) | Controls (100) | P Value |
|---|---|---|---|
| Men, n (%) | 62 (62%) | 63 (63%) | .8 |
| Women, n (%) | 38 (38%) | 37 (37%) | |
| Age (mean ± SD) | 33.22 ± 5.72 | 32.55 ± 4.97 | .378 |
| Smoker, n (%) | 32 (32%) | 24 (24%) | .2 |
| Nonsmoker, n (%) | 68 (68%) | 76 (76%) | |
| Alcoholic, n (%) | 23 (23%) | 7 (7%) | .002 |
| Nonalcoholic, n (%) | 77 (77%) | 93 (93%) | |
| Hypertensive, n (%) | 11 (11%) | 4 (4%) | .06 |
| Nonhypertensive, n (%) | 89 (89%) | 96 (96%) | |
| HDL (mean ± SD) mg/dL | 40.14 ± 5.54 | 43.9 ± 8.41 | .000 |
| LDL (mean ± SD) mg/dL | 90.66 ± 15.33 | 50.03 ± 11.46 | .000 |
| VLDL (mean ± SD) mg/dL | 31.39 ± 10.49 | 20.05 ± 6.49 | .000 |
| Triglyceride (mean ± SD) mg/dL | 106.95 ± 33.22 | 107.08 ± 25.19 | .975 |
| BMI (mean ± SD) | 20.87 ± 1.199 | 20.80 ± 1.33 | .700 |
| HB (mean ± SD) | 13.09 ± 0.91 | 14.87 ± 12.56 | .162 |
| PLT (mean ± SD) | 167.99 ± 5.99 | 169.16 ± 9.50 | .299 |
| TLC (mean ± SD) | 6.19 ± 0.86 | 6.29 ± 0.86 | .415 |
Abbreviations; BMI, body mass index; HB, hemoglobin; HDL, high-density lipoprotein; LDL, Low-density lipoprotein; PLT, platelets; TLC, total leukocyte count; VLDL, very-low-density lipoprotein.
Table 2.
IL-6 Levels in Patients and Controls.
| Patients (100) | Controls (100) | P Value | Adjusted P Valuea | |
|---|---|---|---|---|
| IL-6 levels (mean ± SD) | 28.61 ± 8.61 | 7.60 ± 4.10 | >.00 | >.00 |
Abbreviation: IL-6, interleukin 6; HDL, high-density lipoprotein; LDL, low-density lipoprotein; VLDL, very-low-density lipoprotein.
a P value after adjusting for alcohol, hypertension, HDL, LDL, and VLDL. P < .05 is considered significant.
Figure 1.
Distribution of interleukin 6 (IL-6) levels in patients and controls. A, Representation of the IL-6 levels in patients versus control. B, Standard error bar representing the mean IL-6 levels and standard deviation in patients and controls.
All 3 polymorphisms evaluated in this study were in Hardy-Weinberg equilibrium. The genotyping for −174G>C, −572G>C, and −597G>A showed the prevalence: 47%, 65%, and 26% in patients and 35%, 30%, and 27% in controls. Out of the 3 polymorphisms, only 2 showed show significant differences between patients and controls, that is, −174G>C and −572G>C (Table 3.). A significant allelic and genotypic association with the disease phenotype was shown by these 2 polymorphisms (Table 3). No association of the −597G>A polymorphisms with the disease phenotype was seen at all (Table 3).
Table 3.
Allelic and Genotypic Associations.a
| Polymorphisms | Allelic Frequencies | Genotypic Frequencies | ||||
|---|---|---|---|---|---|---|
| Case | Control | Allelic χ2/P Value | Case | Control | Genotypic χ2/P Value | |
| −174G>C | G = 0.73 | G = 0.80 | 4.79/.028 | GG = 0.51 | GG = 0.65 | 5.3/.021 |
| r-s22727026 | C = 0.27 | C = 0.20 | GC = 0.42 | GC = 0.30 | ||
| CC = 0.07 | CC = 0.05 | |||||
| −572G>C | G = 0.63 | G = 0.75 | 9.63/.001 | GG = 0.37 | GG = 0.59 | 13.74/.0002 |
| rs22726646 | C = 0.37 | C = 0.25 | GC = 0.51 | GC = 0.32 | ||
| CC = 0.12 | CC = 0.09 | |||||
| −597G>A | G = 0.86 | G = 0.85 | 0.13/.71 | GG = 0.75 | GG = 0.73 | 0.16/.68 |
| rs22726602 | C = 0.14 | C = 0.15 | GA = 0.23 | GA = 0.25 | ||
| AA = 0.02 | AA = 0.02 | |||||
aCase–control χ2 and P values for genotypic and allelic associations observed for −174G>C, −572G>C, and −597G>A analyzed in this study.
Only one of the polymorphisms, that is, −174G>C showed significant association of dosage dependent increase in plasma IL-6 levels with the rare allele (C) for the entire study population (patient and controls). The −572G>C polymorphism also showed similar dose-dependent association; however, the heterozygous and the rare genotypes for this polymorphism did not differ from each other in terms of mean plasma Il-6 levels (P = .99) as much as that observed for −174G>C (P = .067; trend of association; Table 4). For the −597G>A polymorphism, a reverse trend was observed with the rarer allele/genotype showing higher plasma IL-6 levels than the common one, although the association was not significant (Table 4). However, when the 3 polymorphisms were compared between patients and controls using a recessive model with respect to plasma IL-6 levels and adjusted for associated covariates such as alcohol, smoking, hypertension, HDL, LDL, and VLDL, none of them showed any significant correlative association (Table 5).
Table 4.
Association of Plasma IL-6 Levels With IL-Genotypes.a
| Genotype | N | IL-6 Levels,b Mean ± SD | P Value | Post Hoc P Value | ||
|---|---|---|---|---|---|---|
| GG vs C | GG vs C | GC vs C | ||||
| −174G>C | ||||||
| GG | 118 | 15.17 ± 10.95 | .0001 | .002 | .008 | .067 |
| GC | 73 | 21.04 ± 12.75 | ||||
| CC | 9 | 32.76 ± 14.80 | ||||
| −572G>C | GG vs C | GG vs C | GC vs C | |||
| GG | 99 | 13.8 ± 10.91 | .0001 | .001 | .002 | .99 |
| GC | 80 | 22.42 ± 13.10 | ||||
| CC | 21 | 21.99 ± 10.54 | ||||
| −597G>A | GG vs A | GG vs A | GA vs A | |||
| GG | 147 | 18.21 ± 12.83 | .28 | .999 | .19 | .17 |
| GA | 48 | 16.80 ± 10.90 | ||||
| AA | 5 | 27.4 ± 15.47 | ||||
Abbreviation: IL-6, interleukin 6.
aThe −174G>C and −572G>C single-nucleotide polymorphisms show association with IL-6 levels.
bIL-6 levels in pg/mL.
Table 5.
Association of Plasma IL-6 Levels With IL Genotypes Across Patients and Controls Under a Recessive Model and Adjusted for Alcohol, Smoking, Hypertension, HDL, LDL, and VLDL.
| Genotype | Total No. | Patient (IL-6 Levels,a Mean ± SD) N | Control (IL-6 Levels,a Mean ± SD) N | P Value |
|---|---|---|---|---|
| −174G>C | ||||
| GG | 123 | 25.3 ± 8.1 (55) | 6.87 ± 3.0 (68) | .311 |
| GC + CC | 77 | 32.7 ± 7.5 (45) | 8.69 ± 4.6 (32) | |
| −572G>C | ||||
| GG | 104 | 27.4 ± 6.6 (40) | 6.4 ± 2.8 (64) | .510 |
| GC + CC | 96 | 29.4 ± 9.7 (60) | 12.1 ± 6.1 (36) | |
| −597G>A | ||||
| GG | 148 | 28.9 ± 8.8 (75) | 7.3 ± 3.5 (73) | .321 |
| GA + AA | 52 | 27.6 ± 8.1 (25) | 8.7 ±5.2 (27) |
Abbreviation: IL-6, interleukin 6; HDL, high-density lipoprotein; LDL, low-density lipoprotein; VLDL, very-low-density lipoprotein.
aIL-6 levels in pg/mL.
Discussion
There is an increasing corroboration that inflammatory cell infiltration is primarily deleterious in the early phase after IS.29,30 Interleukin 6 is among the chief regulators of the in vivo inflammatory reactions that are related to atherosclerotic disease and is likewise a prime modulator in the inflammatory response to cerebral ischemia.30 Also, it has been reported that the serum level of the cytokine IL-6 increases significantly in IS.31 There is scaling corroboration that confirms that IL-6 is a pivotal modulator of inflammatory mechanisms that are significant in the pathophysiology and development of IS.32 In the present study, we observed significantly raised baseline plasma levels of IL-6 of patients with IS when compared to controls (Table 2, Figure 1). After adjusting for traditional risk factor which found associated with the disease, the IL-6 levels found still significantly higher in patients (Table 2), which is in confirmation with other studies that have observed increased IL-6 plasma levels with risk of IS.33–35 A study by Bharosay et al36 from central province of India also found higher IL-6 levels in patients with acute IS as compared to controls. However, in IL-6 levels between recurrent and nonrecurrent IS, no significant difference was seen (31.03 ± 9.59 pg/mL and 27.94 ± 8.25 pg/mL; P = .93), indicating that predictability of stroke cannot be aforesaid based only on IL-6 level values. However, it has been found that higher levels of IL-6 are associated with severity of stroke, its clinical outcome, and the extent of the brain damage.16–18 It seems that IL-6 levels may be one of the strong initial markers for events in acute IS. However, studies contradictory to our findings have revealed that early IL-6 levels is neuroprotective and not an indicative of the marker of disease progression with an inverse relationship between initial IL-6 levels, size of the lesion, and final outcome of the patient. Such observations might be biased by the poor sample size of the study.37
In the current study, we did observe significant genotypic as well as allelic association of −174G>C and −572G>C with IS (Table 3). The higher prevalence of −174G>C and −572G>C genotype in our patient population supports the earlier reports in different ethnic populations.9–11,38–40 A recent study by Kumar et al25 from India also reported findings similar to ours, although their study cohort was a little bit different than ours in that we studied only patients with young stroke (18-45 years). Besides, we also attempted to correlate IL-6 plasma levels in all study patients with the promoter polymorphisms that were lacking in the earlier studies. Sharma et al40 correlated the IL-6 levels with the deep vein thrombosis patients, but their study cohort as well as inclusion criteria are different from our study. However, while we did find 2 of the polymorphisms, more particularly the −174G>C polymorphism influencing plasma IL-6 levels in a dose-dependent manner, we could not establish a direct correlation with disease association. A comparison made when adjusted for associated covariates in a recessive model of inheritance showed a nonsignificant association for all 3 polymorphisms (Table 5). Studies that have pursued to study a correlation between IL-6 promoter polymorphisms and plasma Il-6 levels with IS have often reported very contradictory findings.20 Our study therefore suggests that IL-6, being an inflammatory marker, is heavily influenced by nongenetic influences and is not a good candidate gene for studying genetic components associated with IS. It appears that the variability in IL-6 levels is more the combined effect of nongenetic influences and the inflammatory events following IS rather than being its cause. This is of course a distinct possibility with IS itself being a highly inflammatory condition. While some in vitro studies have in fact reported an additive effect of promoter polymorphisms in IL-6 gene on plasma levels,41 these studies cannot account for the nongenetic influences (like diet, smoking, alcohol intake) that can mask or overcome such contributions in vivo.
To summarize, we report that although preliminary genetic-epidemiological reports had suggested the association of IL-6 promoter polymorphisms with IS, our study which looked into this association more comprehensively at the phenotype level (ie, IL-6 plasma levels) suggests that there is no direct correlation between plasma IL-6 levels, IL-6 promoter polymorphisms, and IS. If anything, the IL-6 gene is a poor candidate gene when it comes to studying association with IS since its levels are heavily influenced by nongenetic influences.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Pandian JD, Sudhan P. Stroke epidemiology and stroke care services in India. J Stroke. 2013;15(3):128–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Prassad K, Singhal KK. Stroke in young: an Indian perspective. Neurol India. 2010;58(3):343–350. [DOI] [PubMed] [Google Scholar]
- 3. Tripathi M, Vibha D. Stroke in young in India. Stroke Res Treat. 2010;2011: 368629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang Q, Tang XN, Yenari MA. The inflammatory response in stroke. J Neuroimmunol. 2007;184(1-2):53–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Huber SA, Sakkinen P, Conze D, Hardin N, Tracy R. Interleukin-6 exacerbates early atherosclerosis in mice. Arterioscler ThrombVasc Biol. 1999;19(10):2364–2367. [DOI] [PubMed] [Google Scholar]
- 6. Schieffer B, Schieffer E, Hilfiker-Kleiner D, et al. Expression of angiotensin II and interleukin 6 in human coronary atherosclerotic plaques: potential implications for inflammation and plaque instability. Circulation. 2000;101(12):1372–1378. [DOI] [PubMed] [Google Scholar]
- 7. Biasucci LM, Liuzzo G, Fantuzzi G, et al. Increasing levels of interleukin (IL)-1Ra and IL-6 during the first 2 days of hospitalization in unstable angina are associated with increased risk of in-hospital coronary events. Circulation. 1999;99(16):2079–2084. [DOI] [PubMed] [Google Scholar]
- 8. Hongmei Y, Yongping J, Jiyuan L. Interleukin-6 polymorphisms and risk of coronary artery diseases in a Chinese population: a case-control study. Pak J Med Sci. 2016;32(4):880–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ridker PM, Hennekens CH, Buring JE, et al. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342(12):836–843. [DOI] [PubMed] [Google Scholar]
- 10. Ridker PM, Rifai N, Stampfer MJ, et al. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000;101(15):1767–1772. [DOI] [PubMed] [Google Scholar]
- 11. Castillo J, Rodriguez I. () Biochemical changes and inflammatory response as markers for brain ischaemia: molecular markers of diagnostic utility and prognosis in human clinical practice. Cerebrovasc Dis. 2004;17(suppl 1):7–18. [DOI] [PubMed] [Google Scholar]
- 12. Beamer NB, Coull BM, Clark WM, Hazel JS, Silberger JR. Interleukin-6 and interleukin-1 receptor antagonist in acute stroke. Ann Neurol. 1995;37(6):800–805. [DOI] [PubMed] [Google Scholar]
- 13. Fassbender K, Rossol S, Kammer T, et al. Proinflammatory cytokines in serum of patients with acute cerebral ischemia: kinetics of secretion and relation to the extent of brain damage and outcome of disease. J Neurol Sci. 1994;122(2):135–139. [DOI] [PubMed] [Google Scholar]
- 14. Kim JS, Yoon SS, Kim YH, et al. Serial measurement of interleukin-6, transforming growth factor-beta, and S-100 protein in patients with acute stroke. Stroke. 1996;27(9):1553–1557. [DOI] [PubMed] [Google Scholar]
- 15. Tarkowski E, Rosengren L, Blomstrand C, et al. Intrathecal release of pro- and anti-inflammatory cytokines during stroke. Clin Exp Immunol 1997;110(3):492–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Castellanos M, Castillo J, Garcia MM, et al. Inflammation-mediated damage in progressing lacunar infarctions: a potential therapeutic target. Stroke. 2002;33(4):982–987. [DOI] [PubMed] [Google Scholar]
- 17. Smith CJ, Emsley HC, Gavin CM, et al. Peak plasma interleukin-6 and other peripheral markers of inflammation in the first week of ischaemic stroke correlate with brain infarct volume, stroke severity and long-term outcome. BMC Neurol. 2004; 4:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vila N, Castillo J, Davalos A, et al. Proinflammatory cytokines and early neurological worsening in ischemic stroke. Stroke. 2000;31(10):2325–2329. [DOI] [PubMed] [Google Scholar]
- 19. Waje-Andreassen U, Krakenes J, Ulvestad E, et al. IL-6: an early marker for outcome in acute ischemic stroke. Acta Neurol Scand. 2005;111(6):360–365. [DOI] [PubMed] [Google Scholar]
- 20. Terry F, Loukaci V, Green R. Cooperative influence of genetic polymorphisms on interleukin 6 transcriptional regulation. J Biol Chem. 2000;275(24):18138–18144. [DOI] [PubMed] [Google Scholar]
- 21. Pola R, Gaetani E, Flex A, et al. -174 G/C interleukin-6 gene polymorphism and increased risk of multi-infarct dementia: a case-control study. Exp Gerontol. 2002;37(7):949–955. [DOI] [PubMed] [Google Scholar]
- 22. Revilla M, Obach V, Cervera A, et al. A -174G/C polymorphism of the interleukin-6 gene in patients with lacunar infarction. Neurosci Lett. 2002;324(1):29–32. [DOI] [PubMed] [Google Scholar]
- 23. Yan J, J MG P AM. Interleukin 6 promoter 174 G/C polymorphisms in acute ischemic stroke: G allele is protective but not associated with IL-6 levels or stroke outcome. J Neuroimmunol. 2016;293:22–27. [DOI] [PubMed] [Google Scholar]
- 24. Yang X, Feng L, Li C, et al. Association of IL-6-174G > C and -572C > G polymorphisms with risk of young ischemic stroke patients. Gene. 2014;539(2):258–262. [DOI] [PubMed] [Google Scholar]
- 25. Kumar P, Kumar A, Sagar R, et al. Association between interleukin-6 (G174C and C572G) promoter gene polymorphisms and risk of ischemic stroke in North Indian population: a case control study. Neurol Res. 2016;38(1):69–74. [DOI] [PubMed] [Google Scholar]
- 26. Jones WJ, Williams LS, Meschia JF. Validating the Questionnaire for Verifying Stroke-Free Status (QVSFS) by neurological history and examination. Stroke. 2001;32(10):2232–2236. [DOI] [PubMed] [Google Scholar]
- 27. Fernandez-Real JM, Broch M, Vendrell J, Richart C, Ricart W. Interleukin-6 gene polymorphism and lipid abnormalities in healthy subjects. J Clin Endocrinol Metab. 2000;85(3):1334–1339. [DOI] [PubMed] [Google Scholar]
- 28. Brull DJ, Montgomery HE, Sanders J, et al. Interleukin-6 gene -174g>c and -572g>c promoter polymorphisms are strong predictors of plasma interleukin-6 levels after coronary artery bypass surgery. Arterioscler Thromb Vasc Biol. 2001;21(9):1458–1463. [DOI] [PubMed] [Google Scholar]
- 29. Pola R, Flex A, Gaetani E, et al. Synergistic effect of -174 G/C polymorphism of the interleukin-6 gene promoter and 469 E/K polymorphism of the intercellular adhesion molecule-1 gene in Italian patients with history of ischemic stroke. Stroke. 2003;34(4):881–885. [DOI] [PubMed] [Google Scholar]
- 30. Acalovschi D, Wiest T, Hartmann M, et al. Multiple levels of regulation of the interleukin-6 system in stroke. Stroke. 2003;34(8):1864–1869. [DOI] [PubMed] [Google Scholar]
- 31. Licata G, Tuttolomondo A, Di Raimondo D, et al. Immuno-inflammatory activation in acute cardio-embolic strokes in comparison with other subtypes of ischaemic stroke. Thromb Haemost. 2009;101(5):929–937. [PubMed] [Google Scholar]
- 32. Orion D, Schwammenthal Y, Reshef T, et al. Interleukin-6 and soluble intercellular adhesion molecule-1 in acute brain ischaemia. Eur J Neurol. 2008;15(4):323–328. [DOI] [PubMed] [Google Scholar]
- 33. Shenhar-Tsarfaty S, Assayag EB, Bova I, et al. Early signaling of inflammation in acute ischemic stroke: clinical and rheological implications. Thromb Res. 2008;122(2):167–173. [DOI] [PubMed] [Google Scholar]
- 34. Boehme AK, McClure LA, Zhang Y, et al. Inflammatory markers and outcomes after lacunar stroke: levels of inflammatory markers in treatment of stroke study. Stroke. 2016;47 (3):659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bharosay A, Saxena K, Varma M, et al. Correlation between proinflammatory serum markers: high sensitivity C-reactive protein, interleukin-6 with disability score in acute ischemic stroke. Indian J Clin Biochem. 2011;26(3):279–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sotgiu S, Zanda B, Marchetti B, et al. Inflammatory biomarkers in blood of patients with acute brain ischemia. Eur J Neurol. 2006;13(5):505–513. [DOI] [PubMed] [Google Scholar]
- 37. Chakraborty B, Chowdhury D, Vishnoi G, et al. Interleukin-6 gene -174 G/C promoter polymorphism predicts severity and outcome in acute ischemic stroke patients from north India. J Stroke Cerebrovasc Dis. 2012;22(5):683–689. [DOI] [PubMed] [Google Scholar]
- 38. Yang X, Feng L, Li C, Li Y. Association of IL-6 174>C and 572C>G polymorphisms with risk of young ischemic stroke. Gene. 2014;539(2):258–262. [DOI] [PubMed] [Google Scholar]
- 39. Balding J, Livingstone J, Pittock J, et al. The IL-6 G-174C polymorphism may be associated with ischaemic stroke in patients without a history of hypertension. Ir J Med Sci. 2004;173(4):200–203. [DOI] [PubMed] [Google Scholar]
- 40. Sharma A, Singh K, Biswas A, et al. Impact of interleukin 6 promoter polymorphisms (-174 G > C, -572 G > C and -597 G > A) on plasma IL-6 levels and their influence on the development of DVT: a study from India. Hematology. 2018;11:1–6. [DOI] [PubMed] [Google Scholar]
- 41. Tso R, Merino G, Warach S. Interleukin-6 174G/C polymorphism and ischemic stroke: a systematic review. Stroke. 2007;38(11):3070–3075. [DOI] [PubMed] [Google Scholar]