Abstract
The objectives of this study were to examine venous thromboembolism (VTE) prophylaxis patterns and risk for VTE events during hospitalization and in the outpatient continuum of care among patients hospitalized for acute illnesses in the United States with stratification by different age groups and renal disease status. Acutely ill hospitalized patients were identified from the MarketScan databases (January 1, 2012-June 30, 2015) and grouped by age (<65, 65-74, ≥75 years old) and whether or not they had a baseline diagnosis of renal disease, separately. Of acutely ill hospitalized patients, 60.1% (n = 10 748) were <65 years old, 15.7% (n = 2803) were 65 to 74 years old, and 24.3% (n = 4344) were ≥75 years old; 32.9% (n = 5892) had baseline renal disease. Among the study cohorts, the majority of patients received no VTE prophylaxis regardless of age or baseline renal status (52.1%-63.6%). Rates of VTE during hospitalization and in the 6 months postdischarge were 4.7%, 4.6%, and 4.5% for patients <65, 65 to 74, and ≥75 years old, respectively, and 6.3% and 3.8% for patients with and without baseline renal disease. The risk for VTE was elevated for 30 to 40 days after index admission regardless of age and renal disease status.
Keywords: venous thromboembolism, acute medical illness, hospitalized patients, VTE prophylaxis, inpatient and outpatient continuum of care, elderly, renal disease
Introduction
Annually, in the United States, approximately 500 000 venous thromboembolism (VTE) events occur, and in 2011 total health-care costs of patients with VTE were estimated to range between US$13.5 and US$27.2 billion, with US$4.5 to US$14.2 billion predicted as preventable hospital-acquired costs.1,2 Patients hospitalized for acute medical illnesses have a significant risk for VTE, which can extend for several weeks to months after discharge from the hospital.1,3,4 The cost of a VTE event related to hospitalization for another acute illness within the first 3 months from the index hospitalization has been estimated to increase medical costs by approximately US$17 000 (2011 USD) per patient, compared to control cases without a VTE event.5
Studies have shown that patients aged older than 75 and those with kidney disease have an elevated risk of VTE over the general population.4,6–9 However, VTE prophylaxis among older hospitalized patients and those with impaired renal function may be compromised and ineffective for reducing VTE risk in these patient populations.6,9 Additionally, pharmacologic prophylaxis for such populations may increase the risk of adverse events, including major bleeding.10 Venous thromboembolism thromboprophylaxis in the inpatient setting has been shown in some studies to reduce the risk of VTE during hospitalization among both older patients and those with renal disease.11,12 Although other studies show conflicting data on the benefit of inpatient VTE thromboprophylaxis for such patient populations as well as other patient populations with other VTE risk levels.1,6,13–15 Also, there is scant information on the effectiveness of VTE thromboprophylaxis for reducing the risk of VTE after hospital discharge among older patients and those with renal disease.
The health-care and economic burden of VTE is substantial, and the uncertainty regarding appropriate prevention strategies, especially among patients who are older or have compromised renal function, warrant further study. Thus, we examined VTE prophylaxis treatment patterns and VTE risk during hospitalization and in the 6 months following hospital discharge as well as the frequency of VTE-related hospital readmissions among patients hospitalized for acute medical illnesses and stratified by different age groups and presence or absence of baseline renal disease.
Methods
Study Population
This was a retrospective database analysis to evaluate the patterns of pharmacologic VTE prophylaxis among patients hospitalized for acute medical illnesses in the real-world setting. Patients hospitalized for acute medical illnesses of cancer, heart failure, infectious diseases, ischemic stroke, respiratory diseases, and rheumatic diseases, as the primary hospital discharge diagnosis were identified from the Truven Health Analytics MarketScan databases between January 1, 2012, and June 30, 2015. Medical illnesses were identified by International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes. The acute medical illnesses were based on the American College of Chest Physician (ACCP) guidelines and other existing clinical trial populations.16–18 The databases consist of health-care claims data from >100 different insurance companies, Blue Cross Blue Shield plans, and third-party administrators. The claims data include inpatient and outpatient information, laboratory data, and detailed hospital drug data, reflecting real-world treatment patterns and costs. In compliance with the Health Insurance Portability and Accountability Act of 1996, the databases consist of fully deidentified data sets, with synthetic identifiers applied to patient-level and provider-level data to protect the identities of both the patients and the data contributors. The MarketScan claims databases were further linked to the MarketScan Hospital Drug Database to provide the details of health-care services, resource utilization, and costs in both inpatient and outpatient settings.
The earliest hospitalization for acute medical illnesses to occur during the index identification period was defined as the index hospitalization. Patients were required to have 6 months of continuous medical and prescription insurance coverage prior to the index hospitalization (baseline period). Patients were additionally required to have 6 months of continuous insurance coverage after the index admission date (follow-up period). Patients were excluded if they had a pregnancy diagnosis during the baseline period or at the index hospitalization, death during the index hospitalization, or hip or knee replacement surgery during the index hospitalization. The study population was separately grouped into 3 cohorts by age (<65 years, 65-74 years, and ≥75 years) and 2 cohorts based on whether or not they had a diagnosis of renal disease during the baseline period.
Demographics, Patient Clinical Characteristics, and Hospital Characteristics
Patient demographics and clinical characteristics, including age, gender, health plan type, Charlson Comorbidity Index (CCI) score, and hospital length of stay (LOS), were evaluated during the 6-month baseline period and index hospitalization for each of the study cohorts. Hospital characteristics (ie, year of hospitalization, geographic region, urban/rural status, teaching status, and bed size) were additionally evaluated.
Prophylaxis Patterns of VTE
The proportions of patients who received and did not receive inpatient and/or outpatient VTE pharmacologic prophylaxis were determined for each of the study cohorts. Venous thromboembolism prophylaxis in the inpatient setting was determined based on pharmacy records for enoxaparin, warfarin, direct-acting oral anticoagulants (DOACs: apixaban, dabigatran, rivaroxaban, and edoxaban), fondaparinux, or unfractionated heparin (UFH) during the index hospitalization. Venous thromboembolism prophylaxis in the outpatient setting was determined based on pharmacy claims for the above-listed anticoagulants within 15 days after VTE diagnosis. Among patients who received inpatient and/or outpatient prophylaxis, the proportions of patients who received enoxaparin only, warfarin only, enoxaparin and warfarin combined, a DOAC only, and “other” VTE prophylactic drug combinations or drugs (eg, other anticoagulant combinations, fondaparinux) were evaluated.
VTE Events
The proportions of patients with VTE events during the index hospitalization and within 6 months of hospital discharge were evaluated for each of the study cohorts. A VTE event during the index hospitalization was based on the presence of an ICD-9-CM code for deep vein thrombosis (DVT) and/or pulmonary embolism (PE) at either primary or secondary position of discharge diagnosis codes. A VTE event during the postdischarge follow-up period was defined by the presence of a primary or secondary ICD-9-CM code for DVT and/or PE during an emergency department or inpatient admission or on an outpatient claim with 1 or more of the following confirmatory events: a pharmacy claim for enoxaparin, fondaparinux, or UFH within 15 days after VTE diagnosis or a pharmacy claim for warfarin or DOACs (apixaban, dabigatran, rivaroxaban, edoxaban) within 15 days after VTE diagnosis, and no evidence of atrial fibrillation or atrial flutter in the 6 months preceding the outpatient diagnosis for DVT and/or PE.19
All-Cause and VTE-Related Hospital Readmissions
The proportions of patients with all-cause and VTE-related hospital readmissions in the 6-month postdischarge follow-up period were determined for each of the study cohorts.
Statistical Analyses
Descriptive statistics were utilized to evaluate differences in demographics, clinical characteristics, and hospital characteristics among the study cohorts stratified by age groups and among the study cohorts stratified by baseline renal disease status, separately. Analysis of variance and χ2 tests were used to detect statistically significant differences in continuous and categorical variables, respectively. Multivariable logistic regression analyses were conducted to evaluate the influence of age and baseline renal disease status on the likelihood of receiving any (inpatient or outpatient) VTE prophylaxis in the inpatient or outpatient setting. Covariates in the regression models included age, gender, region, index acute medical illness, preindex comorbidities (atrial fibrillation, VTE, major bleeding), and hospital characteristics, including admission source, urban/rural, teaching status, and bed size. Values of P were determined by Maximum Likelihood Estimates for the regression analyses. The VTE event rates occurring after the index hospital admission date were evaluated using Kaplan-Meier analysis for all study cohorts. A critical value of 0.05 was used to determine statistical significance. All statistical analyses were carried out using SAS version 9.4.
Results
Study Population
Patient demographics and clinical characteristics of the study cohorts are shown in Table 1. Of acutely ill hospitalized patients (n = 17 895), 60.1% (n = 10 748; mean age: 45.5 years) were <65 years of age, 15.7% (n = 2803, mean age: 69.5 years) were 65 to 74 years of age, and 24.3% (n = 4344; mean age: 83.0 years) were ≥75 years of age. Patients aged 65 years and older (66%-67%) were more likely to have comprehensive health plan coverage than patients <65 years of age (39.0%). Mean CCI score, measured at the index hospitalization, was highest for patients aged 65 to 74 years (CCI = 2.6). The mean index hospitalization LOSs were 4.6, 5.1, and 5.1 days for patients <65, 65 to 74, and ≥75 years of age, respectively.
Table 1.
Demographics and Clinical Characteristics of Study Cohorts.
| Stratified by Age-Group | Stratified by Baseline Renal Disease Status | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <65 Years, n = 10 748 | 65-74 Years, n = 2803 | ≥75 years, n = 4344 | P Value | With, n = 5892 | Without, n = 12 003 | P Value | ||||||
| Age, years | ||||||||||||
| Mean (SD) | 45.5 (17.3) | 69.5 (2.9) | 83.0 (5.6) | <.001 | 64.1 (17.4) | 55.6 (22.5) | <.001 | |||||
| Median | 51 | 69 | 82 | 64 | 59 | |||||||
| n | % | n | % | n | % | n | % | n | % | |||
| Age-group, years | <.001 | <.001 | ||||||||||
| <18 | 1093 | 10.2 | 0 | 0.0 | 0 | 0.0 | 44 | 0.8 | 1049 | 8.7 | ||
| 18-29 | 796 | 7.4 | 0 | 0.0 | 0 | 0.0 | 199 | 3.4 | 597 | 5.0 | ||
| 30-39 | 1022 | 9.5 | 0 | 0.0 | 0 | 0.0 | 298 | 5.1 | 724 | 6.0 | ||
| 40-49 | 1883 | 17.5 | 0 | 0.0 | 0 | 0.0 | 531 | 9.0 | 1352 | 11.3 | ||
| 50-59 | 3635 | 33.8 | 0 | 0.0 | 0 | 0.0 | 1107 | 18.8 | 2528 | 21.1 | ||
| 60-64 | 2319 | 21.6 | 0 | 0.0 | 0 | 0.0 | 809 | 13.7 | 1510 | 12.6 | ||
| 65-69 | 0 | 0.0 | 1410 | 50.3 | 0 | 0.0 | 528 | 9.0 | 882 | 7.4 | ||
| 70-74 | 0 | 0.0 | 1393 | 49.7 | 0 | 0.0 | 560 | 9.5 | 833 | 6.9 | ||
| 75-79 | 0 | 0.0 | 0 | 0.0 | 1381 | 31.8 | 543 | 9.2 | 838 | 7.0 | ||
| ≥80 | 0 | 0.0 | 0 | 0.0 | 2963 | 68.2 | 1273 | 21.6 | 1690 | 14.1 | ||
| Gender | <.001 | <.001 | ||||||||||
| Female | 5908 | 55.0 | 1481 | 52.8 | 2525 | 58.1 | 3158 | 53.6 | 6756 | 56.3 | ||
| Male | 4840 | 45.0 | 1322 | 47.2 | 1819 | 41.9 | 2734 | 46.4 | 5247 | 43.7 | ||
| Health plan | <.001 | <.001 | ||||||||||
| Comprehensive | 4196 | 39.0 | 1879 | 67.0 | 2881 | 66.3 | 3713 | 63.0 | 5243 | 43.7 | ||
| HMO | 1684 | 15.7 | 69 | 2.5 | 31 | 0.7 | 381 | 6.5 | 1403 | 11.7 | ||
| CDHP | 968 | 9.0 | 21 | 0.8 | 20 | 0.5 | 238 | 4.0 | 771 | 6.4 | ||
| EPO | 12 | 0.1 | 0 | 0.0 | 0 | 0.0 | 1 | 0.0 | 11 | 0.1 | ||
| POS | 614 | 5.7 | 52 | 1.9 | 85 | 2.0 | 160 | 2.7 | 591 | 4.9 | ||
| POS w/ Capitation | 101 | 0.9 | 0 | 0.0 | 0 | 0.0 | 39 | 0.7 | 62 | 0.5 | ||
| PPO | 2618 | 24.4 | 741 | 26.4 | 1258 | 29.0 | 1193 | 20.3 | 3424 | 28.5 | ||
| Missing/Unknown | 555 | 5.2 | 41 | 1.5 | 69 | 1.6 | 167 | 2.8 | 498 | 4.2 | ||
| Index Hospitalization LOS, days | <.001 | |||||||||||
| Mean (SD) | 4.6 (4.7) | 5.1 (4.5) | 5.1 (3.9) | 6.0 (5.7) | 4.2 (3.7) | <.001 | ||||||
| Median | 3 | 4 | 4 | 4 | 3 | |||||||
| Charlson Comorbidity Index, Pre-index Hospitalization | <.001 | |||||||||||
| Mean (SD) | 2.0 (2.4) | 2.6 (2.4) | 2.4 (2.2) | 3.2 (2.6) | 1.7 (2.1) | <.001 | ||||||
| Median | 1 | 2 | 2 | 3 | 1 | |||||||
| n | % | n | % | n | % | n | % | n | % | |||
| CCI Score Group | <.001 | <.001 | ||||||||||
| CCI = 0 | 3642 | 33.9 | 568 | 20.3 | 915 | 21.1 | 947 | 16.1 | 4178 | 34.8 | ||
| CCI = 1-2 | 4007 | 37.3 | 1091 | 38.9 | 1770 | 40.8 | 1745 | 29.6 | 5123 | 42.7 | ||
| CCI = 3-4 | 1592 | 14.8 | 662 | 23.6 | 1011 | 23.3 | 1501 | 25.5 | 1764 | 14.7 | ||
| CCI ≥ 5 | 1507 | 14.0 | 482 | 17.2 | 648 | 14.9 | 1699 | 28.8 | 938 | 7.8 | ||
Abbreviations: CCI, Charlson Comorbidity Index; CDHP, Consumer Driven Health Plan; EPO, Exclusive Provider Organization; HMO, Health Maintenance Organization; LOS, length of stay; POS, point-of-service; PPO, Preferred Provider Organization; SD, standard deviation.
Of the overall study population, 32.9% (n = 5892) had a diagnosis of baseline renal disease and 67.1% (n = 12 003) did not. Patients with renal disease were older (64.1 vs 55.6 years, P < .001), more likely to have comprehensive health plan coverage (63.0% vs 43.7%, P < .001), and had higher CCI scores (3.2 vs 1.7, P < .001) than patients without renal disease.
Hospital characteristics of the study cohorts are shown in Table 2. For study cohorts grouped by age, most hospitals were urban (84%-89%), nonteaching (95%-96%), of large size (300 to ≥500 beds: 60%-71%), and located in the South Census region (74%-80%) reflecting the distribution of hospital records contained in the database. Similarly, patients with and without renal disease received care mostly from urban (87%-88%), nonteaching (95%-96%), large-size hospitals (67%-69%) located in the South Census region (75%-80%).
Table 2.
Hospital Characteristics of Study Cohorts.
| Stratified by Age-Group | Stratified by Baseline Renal Disease Status | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <65 Years, n = 10 748 | 65-74 Years, n = 2803 | ≥75 years, n = 4344 | P Value | With, n = 5892 | Without, n = 12 003 | P Value | ||||||
| n | % | n | % | n | % | n | % | n | % | |||
| Census Region | <.001 | <.001 | ||||||||||
| South | 8317 | 77.4 | 2246 | 80.1 | 3198 | 73.6 | 4709 | 79.9 | 9052 | 75.4 | ||
| North Central | 1438 | 13.4 | 398 | 14.2 | 682 | 15.7 | 729 | 12.4 | 1789 | 14.9 | ||
| West | 720 | 6.7 | 140 | 5.0 | 407 | 9.4 | 364 | 6.2 | 903 | 7.5 | ||
| Northeast | 273 | 2.5 | 19 | 0.7 | 57 | 1.3 | 90 | 1.5 | 259 | 2.2 | ||
| Urban/Rural Status | <.001 | .20 | ||||||||||
| Urban | 9579 | 89.1 | 2365 | 84.4 | 3658 | 84.2 | 5164 | 87.6 | 10 438 | 87.0 | ||
| Rural | 1169 | 10.9 | 438 | 15.6 | 686 | 15.8 | 728 | 12.4 | 1565 | 13.0 | ||
| Teaching Status | <.001 | <.001 | ||||||||||
| Yes | 568 | 5.3 | 102 | 3.6 | 159 | 3.7 | 209 | 3.6 | 620 | 5.2 | ||
| No | 10 180 | 94.7 | 2701 | 96.4 | 4185 | 96.3 | 5683 | 96.5 | 11 383 | 94.8 | ||
| Number of Beds | <.001 | <.001 | ||||||||||
| <200 | 1679 | 15.6 | 534 | 19.1 | 971 | 22.4 | 1068 | 18.1 | 2116 | 17.6 | ||
| 200-299 | 1439 | 13.4 | 431 | 15.4 | 749 | 17.2 | 730 | 12.4 | 1889 | 15.7 | ||
| 300-499 | 5032 | 46.8 | 1191 | 42.5 | 1593 | 36.7 | 2804 | 47.6 | 5012 | 41.8 | ||
| ≥500 | 2598 | 24.2 | 647 | 23.1 | 1031 | 23.7 | 1290 | 21.9 | 2986 | 24.9 | ||
Venous Thromboembolism Prophylaxis
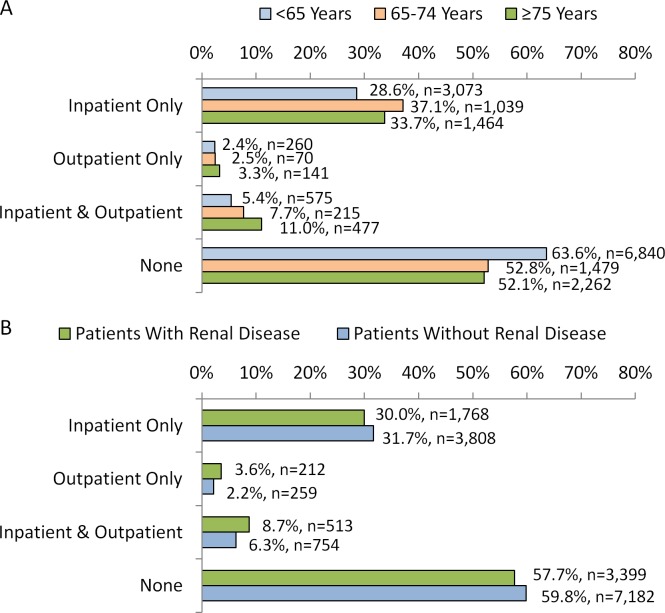
Figure 1A and B shows the proportions of acutely ill hospitalized patients who received VTE pharmacologic prophylaxis in the inpatient and outpatient settings as well as those who did not receive any. A greater proportion of patients <65 years of age (63.6%) did not receive any VTE prophylaxis in comparison to cohorts of patients 65 to 74 years of age (52.8%) and ≥75 years of age (52.1%; P < .001). More patients ≥75 years of age (11.0%) received both inpatient and outpatient VTE prophylaxis than patients <65 (5.4%) and 65 to 74 (7.7%) years of age. Of those who received inpatient prophylaxis, most patients of any age-group received enoxaparin only (<65: 84.5%; 65-74: 73.0%; ≥75: 64.6%; P < .001; Table 3). In the outpatient setting, warfarin only was the most frequent anticoagulant patients received (<65: 35.8%; 65-74: 42.5%; ≥75: 55.2%, P < .001; Table 3). After controlling for differences in patient and hospital characteristics, the findings of the regression analysis showed that patients ≥75 years of age and between 65 and 74 years of age were 1.4-fold (confidence interval [CI]: 1.3 -1.5, P < .001) and 1.2-fold (CI: 1.2 -1.3, P < .001), respectively, more likely to have received any VTE prophylaxis than patients <65 years of age.
Figure 1.
Proportions of hospitalized patients who received VTE prophylaxis in the inpatient and outpatient settings with (A) stratification by age-group and (B) stratification by baseline renal disease status. All P values <.001 for comparisons of cohorts stratified by age-group and cohorts stratified by baseline renal disease status. VTE indicates venous thromboembolism.
Table 3.
Venous Thromboembolism Prophylaxis of Study Cohorts.
| Stratified by Age-Group | Stratified by Baseline Renal Status | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <65 Years | 65-74 Years | ≥75 years | With | Without | ||||||||
| n | % | n | % | n | % | P Value | n | % | n | % | P Value | |
| Inpatient VTE prophylaxis | 3648 | 33.9 | 1254 | 44.7 | 1941 | 44.7 | <.001 | 2281 | 38.7 | 4562 | 38.0 | .36 |
| Anticoagulants | <.001 | <.001 | ||||||||||
| Enoxaparin only | 3083 | 84.5 | 915 | 73.0 | 1253 | 64.6 | 1554 | 68.1 | 3697 | 81.0 | ||
| Enoxaparin + Warfarin | 143 | 3.9 | 77 | 6.1 | 142 | 7.3 | 138 | 6.1 | 224 | 4.9 | ||
| DOAC only | 50 | 1.4 | 27 | 2.2 | 57 | 2.9 | 51 | 2.2 | 83 | 1.8 | ||
| Warfarin only | 348 | 9.5 | 224 | 17.9 | 471 | 24.3 | 511 | 22.4 | 532 | 11.7 | ||
| Othera | 24 | 0.7 | 11 | 0.9 | 18 | 0.9 | 27 | 1.2 | 26 | 0.6 | ||
| Outpatient VTE prophylaxis | 835 | 7.8 | 285 | 10.2 | 618 | 14.2 | <.001 | 725 | 12.3 | 1013 | 8.4 | <.001 |
| Anticoagulants | <.001 | <.001 | ||||||||||
| Enoxaparin only | 122 | 14.6 | 20 | 7.0 | 34 | 5.5 | 49 | 6.8 | 127 | 12.5 | ||
| Enoxaparin + Warfarin | 70 | 8.4 | 28 | 9.8 | 34 | 5.5 | 46 | 6.3 | 86 | 8.5 | ||
| DOAC only | 65 | 7.8 | 57 | 20.0 | 116 | 18.8 | 88 | 12.1 | 150 | 14.8 | ||
| Warfarin only | 299 | 35.8 | 121 | 42.5 | 341 | 55.2 | 317 | 43.7 | 444 | 43.8 | ||
| Othera | 279 | 33.4 | 59 | 20.7 | 93 | 15.1 | 225 | 31.0 | 206 | 20.3 | ||
Abbreviations: DOAC, direct oral anticoagulant; VTE, venous thromboembolism.
a Includes other anticoagulant combinations not listed above as well as the use of other anticoagulants, such as fondaparinux.
A greater proportion of patients with renal disease did not receive any VTE prophylaxis than patients without renal disease (59.8% vs 57.7%, P < .001). Only 8.7% and 6.3% of patients with and without renal disease, respectively received VTE prophylaxis in the inpatient and outpatient continuum of care. Of those who received inpatient prophylaxis, most patients with and without renal disease received enoxaparin only (with: 68.1%, without: 81.0%; P < .001;Table 3). In the outpatient setting, warfarin only was the most frequent anticoagulant received (with: 43.7%; without: 43.8%, P < .001; Table 3). After controlling for differences in patient and hospital characteristics, the findings of the regression analysis showed that patients with baseline renal disease were less likely to have received any VTE prophylaxis than patients without renal disease (odds ratio: 0.8, CI: 0.8-0.9, P < .001).
VTE Events
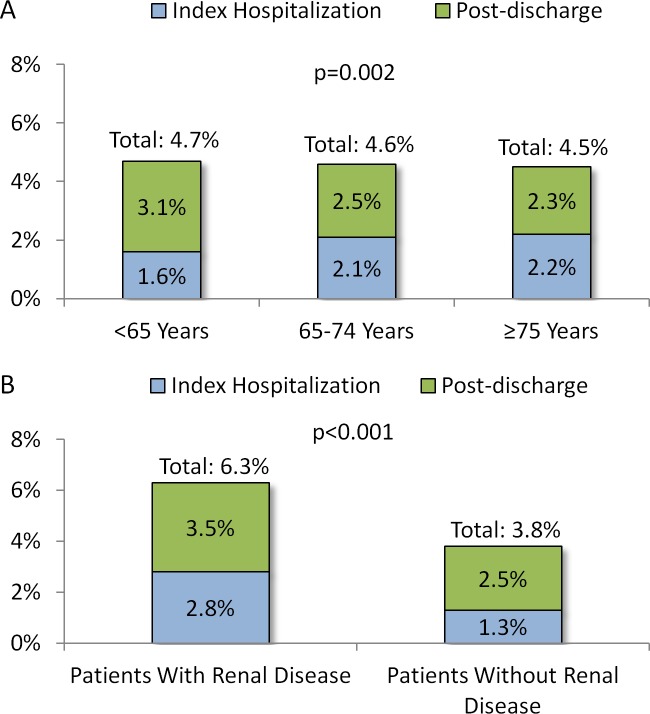
The proportions of patients with VTE events during the index hospitalization and within 6 months of hospital discharge are shown in Figure 2A and B. The VTE event rate in the inpatient setting and within 6 months of hospital discharge was 4.7% for patients <65 years of age, with 66.9% having occurred postdischarge. For patients 65 to 74 years of age, VTE event rate in the inpatient setting and within 6 months of hospital discharge was 4.6%, with 54.2% having occurred postdischarge. For patients ≥75 years of age, VTE event rate in the inpatient setting and within 6 months of hospital discharge was 4.5%, with 51.8% having occurred postdischarge. The VTE event rate was slightly higher among younger patients than older age-groups (P = .002).
Figure 2.
Proportions of patients with VTE events during the index hospitalization and within 6 months of hospital discharge with (A) stratification by age group and (B) stratification by baseline renal disease status. P values are for comparisons of cohorts stratified by age-group and cohorts stratified by baseline renal disease status, separately. VTE indicates venous thromboembolism.
The VTE event rate in the inpatient setting and within 6 months of hospital discharge was higher for patients with versus without baseline renal disease (6.3% vs 3.8%, P < .001). Among patients with and without baseline renal disease, 66.1% and 55.4% of VTE events, respectively, occurred postdischarge.
VTE-Related Hospital Readmissions
Within 6 months of hospital discharge, 29.4%, 23.7%, and 22.3% of patients <65, 65-74, and ≥75 years of age, respectively had a hospital readmission for any cause (P < .001); of which 7.5%, 6.2%, and 5.9% were VTE-related (P = .14; Figure 3A).
Figure 3.
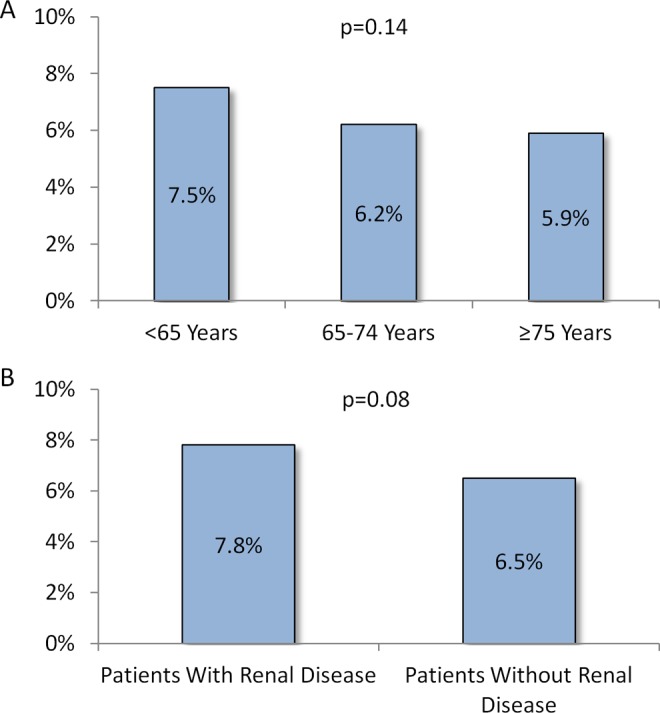
Proportions* of VTE-related hospital readmissions in the follow-up period with (A) stratification by age-group and (B) stratification by baseline renal disease status. *These are percentages of all-cause hospital readmissions that were VTE related. P values are for comparisons of cohorts stratified by age-group and cohorts stratified by baseline renal disease status, separately. VTE indicates venous thromboembolism.
Hospital readmission rate for any cause was higher among patients with baseline renal disease versus those without (23.0% vs 34.5%, P < .001). Among patients with renal disease, 7.8% of readmissions were VTE-related and among those without renal disease, 6.5% were VTE-related (P = .08; Figure 3B).
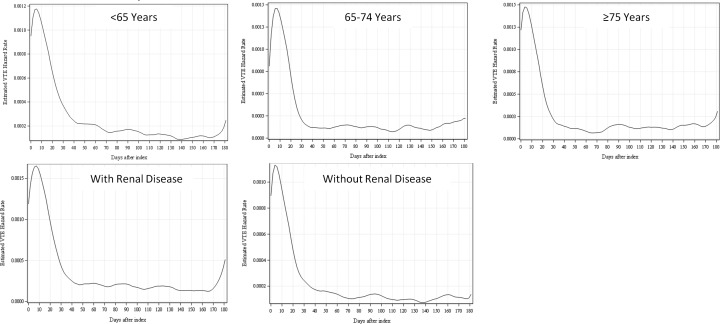
VTE Event Risk
The risk of VTE events within 6 months of hospital discharge is shown in Figure 4 for the study cohorts. For all study cohorts, VTE risk remained elevated up to 30 to 40 days after hospital admission.
Figure 4.
Risk of VTE events (hazard function) by days after the index hospital admission date of the study population with stratification by age-group and baseline renal disease status. VTE indicates venous thromboembolism.
Discussion
In this retrospective analysis of nearly 18 000 patients with acute medical illnesses who received care in US hospitals, nearly one-quarter were ≥75 years of age and one-third had renal disease. Patients between 65 and 74 years of age and ≥75 years of age were more likely to have received any VTE pharmacologic prophylaxis than younger patients; however, for all age groups more than half did not receive any VTE pharmacologic prophylaxis and few (<11%) received both inpatient and outpatient prophylaxis. When patients were stratified by baseline renal disease status, those with renal disease were found to have a lower likelihood of having received any VTE pharmacologic prophylaxis compared to patients without renal disease, although approximately 60% of either cohort did not receive any VTE pharmacologic prophylaxis and less than 10% received both inpatient and outpatient prophylaxis.
VTE event rate in the inpatient and outpatient continuum of care (6 months post-discharge) was higher for patients <65 years of age (4.7%) than for patients 65 to 74 years of age (4.6%) and patients ≥75 years of age (4.5%). However, the differences in VTE event rates across the different age groups were relatively minor. Younger (<65) patients also more frequently had a VTE event after hospital discharge (66.9% vs 51.8%-54.2% of patients of older age groups). Patients who were diagnosed with renal disease were nearly twice as likely as those without renal disease to have a VTE event (6.3% vs 3.8%), and two-thirds of the events for patients with renal disease occurred after hospital discharge. We would like to note that not all hospitalized acutely medically ill patients in this study may have been considered at increased risk for VTE based on the ACCP criteria.16
The frequency of inpatient VTE prophylaxis observed in our study is similar to the findings of Mahan et al, who reported an inpatient VTE prophylaxis rate of 41.5% among 141 628 hospitalized medically ill patients between 2005 and 2009.15 In the study by Mahan et al, older patients and those with renal failure/insufficiency were more likely to have received VTE prophylaxis, and thus our results are consistent with regard to age, but not renal disease status.15 The lower frequency of VTE prophylaxis for patients with renal disease observed in our study may be reflective of the greater uncertainty in the benefit versus risk for this patient population and the lack of more specific treatment guidelines among patients with renal diseases. The frequency of inpatient VTE prophylaxis in the current study was also fairly similar to that of another older study of 11 139 medically ill hospitalized patients between 2005 and 2008, which was 46.7% in the inpatient setting (majority treated with enoxaparin) and 8.8% in the outpatient setting (majority treated with warfarin).19 In this latter study, VTE event rate was 3.3%, of which 56.6% occurred after hospital discharge.19 The results of our study compared to other earlier conducted studies show that VTE prophylaxis patterns have remained fairly unchanged over the last decade. Moreover, there has not been any evidence of a decline in VTE event rate among hospitalized acutely medically ill patients during hospitalization or after discharge.15,19
Few studies have examined VTE prophylaxis patterns and VTE risk specifically among older patients and those with renal disease. A recent study conducted in France of patients hospitalized for acute medical illness found that patients ≥75 years of age who received thromboprophylaxis did not have a reduced risk of VTE, although those <75 years of age did have a VTE risk reduction.6 Another study of older patients (33% received VTE prophylaxis) from the Acute Decompensated Heart Failure Registry also found that standard pharmacologic VTE prophylaxis regimens during hospitalization did not affect 30-day postdischarge outcomes, including the occurrence of thromboembolic events.14 Neither of these studies evaluated the efficacy of continuation of VTE prophylaxis in the outpatient setting.6,14 Furthermore, a US single-institution study found that among elderly hospitalized patients who experienced a VTE event within 30 days of hospital discharge versus patients who did not that there had been less adherence to the ACCP guidelines for VTE prophylaxis.11 These studies provide further evidence that current inpatient VTE prophylaxis strategies may not be optimal, particularly for older patients hospitalized for acute medical illness and that there is an unmet medical need for alternative prophylaxis regimens. The Acute Medically Ill VTE Prevention With Extended-Duration Betrixaban trial evaluated the efficacy and safety of betrixaban and included both elderly patients and patients with moderate and severe renal insufficiency.17 In this trial, extended-duration betrixaban compared to standard-duration enoxaparin was associated with reduced risk for VTE (5.3% vs 7.0%; relative risk: 0.76; CI: 0.63-0.92, P = .006) and no significant increase in the risk for major bleeding (0.7% vs 0.6%; relative risk: 1.19; 95% CI: 0.67-2.12; P = .55).17 Betrixaban may thus be an alternative option for VTE prophylaxis for acutely medically ill hospitalized patients.
In this study, we examined VTE prophylaxis patterns and VTE event rates during and after hospitalization for an acute medical illness, stratified by age-group and renal disease status. We did not evaluate the impact of VTE prophylaxis on the risk for VTE events during hospitalization or within 6 months of hospital discharge. This is an interesting topic for future studies in the real-world setting, especially in the case of certain patient groups with acute illnesses. It will be important for upcoming studies to further examine the most appropriate strategies for VTE prophylaxis in the inpatient and outpatient continuum of care. This is emphasized by the inconsistent results across studies of the impact of in particular inpatient VTE prophylaxis on VTE risk reduction, most notably among certain patient populations at high risk of VTE events.6,12–15
Potential Limitations
Older age has been shown to be a risk factor for VTE risk in some studies,4,15 but among the study population of the current study, this was not observed as such. This could be potentially because health insurance claims information may not fully capture the occurrence of VTE, especially in outpatient settings. Also, there may have been differences in the characteristics of the other study populations, such as age-group distribution, acute illness distribution, comorbidity, and VTE prophylaxis patterns. The VTE prophylaxis rates may be higher than reported in this study, since the frequency of mechanical VTE prophylaxis was not measured in this study, as the data sources do not contain reliable information on mechanical VTE prophylaxis. Additionally, claims and hospitalization records in the MarketScan databases are subject to coding errors, coding for the purpose of rule out rather than actual disease, and undercoding, either by the health-care provider or due to limitations imposed by the database. Furthermore, the MarketScan databases may not be representative of the US population as a whole; for example, this study used claims data from MarketScan commercial and Medicare supplemental databases, which may not generalize to patients insured by Medicaid. Also, the majority of claims in the MarketScan databases are from patients located in the South Census region and thus may not be generalizable to other geographic regions in the United States, particularly the Northeast of which in the databases there is low representation. Despite the potential limitations, the MarketScan databases are generally robust in data, which likely represent real-world patterns of routine clinical practice in the United States.
Conclusion
In this large-scale retrospective analysis, among patients with acute medical illnesses who were hospitalized in the United States, nearly one-quarter were 75 years of age and older and one-third had baseline renal disease. Regardless of age or baseline renal disease, the majority of patients in all study cohorts did not receive any VTE prophylaxis. Among patients who had VTE events, across all age-groups, more than half of VTE events occurred after hospital discharge. The presence of renal disease might have influenced the likelihood of a patient receiving VTE prophylaxis. The results of this real-world study provide further evidence of the need for better optimization of VTE prophylaxis across all age-groups and renal disease status.
Footnotes
Authors’ Note: Ethics: In compliance with the Health Insurance Portability and Accountability Act of 1996 (HIPAA), the databases utilized for this study consist of fully deidentified data sets, with synthetic identifiers applied to patient-level and provider-level data to protect the identities of both the patients and the data contributors.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Alpesh Amin is a research consultant and/or speaker for Novosys Health, Portola, BI, BMS, and Pfizer. Alpesh Amin did not receive funding for manuscript development. W Richey Neuman was an employee of Portola Pharmaceuticals at the time of this study. Melissa Lingohr-Smith, Brandy Menges, and Jay Lin are employees of Novosys Health, who have received research funds from Portola Pharmaceuticals in connection with conducting this study and development of this manuscript.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Sponsorship for this study and development of this article were funded by Portola Pharmaceuticals.
References
- 1. Heit JA, Crusan DJ, Ashrani AA, Petterson TM, Bailery KR. Effect of a near-universal hospitalization-based prophylaxis regimen on annual number of venous thromboembolism events in the US. Blood. 2017;130(2):109–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mahan CE, Borrego ME, Woersching AL, et al. Venous thromboembolism: Annualised United States models for total, hospital-acquired and preventable costs utilising long-term attack rates. Thromb Haemost. 2012;108(2):291–302. [DOI] [PubMed] [Google Scholar]
- 3. Cardoso LF, Krokoscz DV, de Paiva EF, et al. Results of a venous thromboembolism prophylaxis program for hospitalized patients. Vasc Health Risk Manag. 2016;12:491–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Alikhan R, Cohen AT, Combe S, et al. Risk factors for venous thromboembolism in hospitalized patients with acute medical illness: analysis of the MEDENOX Study. Arch Intern Med. 2004;164(9):963–968. [DOI] [PubMed] [Google Scholar]
- 5. Cohoon KP, Leibson CL, Ransom JE, et al. Costs of venous thromboembolism associated with hospitalization for medical illness. Am J Manag Care. 2015;21(4):e255–e263. [PMC free article] [PubMed] [Google Scholar]
- 6. Hemon F, Fouchard R, Tromeur C, et al. Association between hospitalization for acute medical illness and VTE risk: A lower efficacy of thromboprophylaxis in elderly patients? Results from the EDITH case-control study. Eur J Intern Med. 2017;44:39–43. [DOI] [PubMed] [Google Scholar]
- 7. Massicotte-Azarniouch D, Bader Eddeen A, LazoLanger A, et al. Risk of venous thromboembolism in patients by albuminuria and estimated GFR. Am J Kidney Dis. 2017;70(6):826–833. [DOI] [PubMed] [Google Scholar]
- 8. Cheung KL, Zakai NA, Folsom AR, et al. Measures of kidney disease and the risk of venous thromboembolism in the REGARDS (Reasons for geographic and racial differences in stroke) study. Am J Kidney Dis. 2017;70(2):182–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Christiansen CF, Schmidt M, Lamberg AL, et al. Kidney disease and risk of venous thromboembolism: a nationwide population-based case-control study. J Thromb Haemost. 2014;12(9):1449–1454. [DOI] [PubMed] [Google Scholar]
- 10. Kooiman J, den Exter PL, Cannegieter SC, et al. Impact of chronic kidney disease on the risk of clinical outcomes in patients with cancer-associated venous thromboembolism during anticoagulant treatment. J Thromb Haemost. 2013;11(11):1968–1976. [DOI] [PubMed] [Google Scholar]
- 11. Suh J, Desai A, Desai A, et al. Adherence to thromboprophylaxis guidelines in elderly patients with hospital acquired venous thromboembolism: a case control study. J Thromb Thrombolysis. 2017;43(2):172–178. [DOI] [PubMed] [Google Scholar]
- 12. Ageno W, Riva N, Noris P, et al. Safety and efficacy of low-dose fondaparinux (1.5 mg) for the prevention of venous thromboembolism in acutely ill medical patients with renal impairment: the FONDAIR study. J Thromb Haemost. 2012;10(11):2291–2297. [DOI] [PubMed] [Google Scholar]
- 13. Nieto JA, Camara T, Camacho I; MEDITROM Investigators. Venous thromboembolism prophylaxis in acutely ill hospitalized medical patients. A retrospective multicenter study. Eur J Intern Med. 2014;25(8):717–723. [DOI] [PubMed] [Google Scholar]
- 14. Kocio RD, Hammil BG, Hernandez AF, et al. Pharmacologic prophylaxis for venous thromboembolism and 30-day outcomes among older patients hospitalized with heart failure: an analysis from the ADHERE National registry linked to Medicare claims. Clin Cardiol. 2011;34(11):682–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mahan CE, Fisher MD, Mills RM, et al. Thromboprophylaxis patterns, risk factors, and outcomes of care in the medically ill patient population. Thromb Res. 2013;132(5):520-526. [DOI] [PubMed] [Google Scholar]
- 16. Kahn SR, Lim W, Dunn AS, et al. Prevention of VTE in nonsurgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl 2): e195S–e226S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cohen AT, Harrington RA, Goldhaber SZ, et al. ; APEX Investigators. Extended thromboprophylaxis with betrixaban in acutely ill medical patients. N Engl J Med. 2016;375(6):534–544. [DOI] [PubMed] [Google Scholar]
- 18. Cohen AT, Spiro TE, Buller HR, et al. Rivaroxaban for thromboprophylaxis in acutely ill medical patients. N Engl J Med. 2013;368(6):513–523. [DOI] [PubMed] [Google Scholar]
- 19. Amin AN, Varker H, Princic N, et al. Duration of venous thromboembolism risk across a continuum in medically ill hospitalized patients. J Hosp Med. 2012;7(3):231–238. [DOI] [PubMed] [Google Scholar]